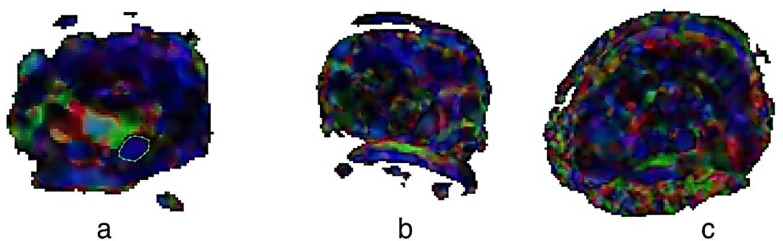Abstract
Background: Carpal tunnel syndrome (CTS) is a common peripheral nerve entrapment disorder that is diagnosed using clinical signs and symptoms and confirmed via nerve conduction studies (NCSs). While NCS is a semi-invasive procedure, magnetic resonance imaging (MRI) is a non-invasive diagnostic tool that detects macroscopic nerve abnormalities and evaluates a patient's surgical or medication treatment options. This study assessed magnetic resonance neurography (MRN)’s diagnostic and grading value by comparing it to electrodiagnostic studies in patients with CTS and healthy individuals.
Methods: This was a cross-sectional study on 27 wrists with CTS and 27 healthy wrists. After history taking and physical examination, we employed an NCS to confirm and determine the severity of CTS, then MRN and diffusion tensor imaging (DTI) were used to calculate apparent diffusion coefficient (ADC), fractional anisotropy (FA), and cross-sectional area (CSA).
Results: 18 patients with CTS (27 median nerves) and 15 healthy controls (27 median nerves) were evaluated. The mean FA in the CTS group was significantly lower (0.38 ± 0.05 vs. 0.45 ± 0.06, P < 0.001).
The mean CSA and ADC were higher in patients with CTS but not statistically significant. FA’s diagnostic cut-off was 0.42, with a sensitivity of 70.4% and a specificity of 63%.
Conclusion: MRN with DTI can be an effective and non-invasive diagnostic technique for the detection of CTS. The FA measure demonstrated adequate sensitivity and specificity for differentiating patients with CTS from healthy individuals.
Key Words: Carpal Tunnel Syndrome, Magnetic Resonance Imaging, Diffusion Tensor Imaging
Introduction
Carpal tunnel syndrome (CTS) is the most prevalent peripheral nerve entrapment disorder globally. CTS is caused by compression of the median nerve at the wrist. Paraesthesia and dysesthesias are the early symptoms of the disease, followed by weakness and thenar muscle atrophy as the disease progresses.1,2
CTS is diagnosed using clinical data and nerve conduction studies (NCSs). However, according to research, NCS sensitivity ranges from 49% to 86%, its false-negative rate is between 16% and 34%, and it is considered to be a somewhat invasive method. 3 The search for a less invasive diagnostic method with greater sensitivity and specificity appears to be more important than ever. Magnetic resonance imaging (MRI) is considered a potential non-invasive diagnostic method. Although MRI is not a typical method for diagnosing CTS, studies showed that MRI had good diagnostic value in evaluating CTS and detecting macroscopic changes in nerves and soft tissues. Additionally, MRI may determine if a patient is a candidate for surgery or medical therapy.4,5
Little research has been conducted in our country and geographical region to replace NCS with MRI as a diagnostic technique for CTS. In addition, very few regional studies have investigated the role of MRI in determining the severity of CTS. Considering the role of ethnicity in many of neurological disorders6-8 and implementing 3-Tesla MRI which most of regional studies did not use,9-12 this study assessed the diagnostic and grading significance of magnetic resonance neurography (MRN) in patients with CTS by comparing the findings of MRN in patients with CTS and healthy individuals.
Materials and Methods
Patients: We conducted a cross-sectional study using the convenience sampling method on 27 wrists with CTS diagnosis (9 mild CTS, 9 moderate CTS, 9 severe CTS according to NCS) and 27 healthy wrists of patients referred to the neurology clinic at the Imam Hospital Complex, Tehran, Iran, from January 2018 to December 2019. The study protocol was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.IKHC.REC.1400.405).
The inclusion criteria for the patient group were clinical diagnosis of primary CTS by an experienced neurologist, confirmed by NCSs. We excluded patients with CTS with secondary causes, such as pregnancy, hypothyroidism, fracture, radiculopathy, surgery, and acute trauma, as well as those with systemic neurological disease. Age and sex-matched healthy controls were recruited from the patients referred to the neurology clinic for reasons other than CTS.
Electromyography (EMG) and NCS: All participants underwent NCS testing. Motor and sensory NCSs were conducted on the ulnar and median nerves at the wrist and elbow levels. CTS severity was classified as mild [prolonged distal sensory latency (DSL) with/without a decrease in amplitude], moderate [prolonged DSL and distal motor latency (DML)], and severe [prolonged DSL and DML with a reduction/loss in sensory nerve action potential (SNAP) or compound muscle action potential (CMAP) amplitude].
MRN: MRI and diffusion tensor imaging (DTI) were performed using a 3-T scanner. An experienced radiologist, who was blinded to the clinical tests and NCSs, calculated the cross-sectional area (CSA) of the median nerve using T2-weighted images and fractional anisotropy (FA), and apparent diffusion coefficient (ADC) using DTI images (Figure 1).
Figure 1.
Representative fractional anisotropy (FA) pictures
a) FA in a patient with healthy wrist; b) FA in a patient with moderate carpal tunnel syndrome (CTS); c) FA in a patient with severe CTS
Study size: Using the Bao et al. 13 study, the significance level of α = 0.01, power of 1 – β = 0.95, and the minimum detectable difference between two groups (d = 4.6), the required sample size was calculated as 27 patients in each group.
All statistical analyses were conducted using SPSS software (version 25, IBM Corporation, Armonk, NY, USA). We used mean ± standard deviation (SD) to report quantitative variables and frequency to report qualitative variables. We had no missing data for all variables. The chi-square test (non-parametric test) was used to compare nominal variables between study groups, while the t-test and analysis of variance (ANOVA) (parametric tests) were used to evaluate quantitative variables. The receiver operating characteristic (ROC) and the measurement of the area under the curve (AUC) were used to assess the diagnostic value and define the cut-off for MRN parameters. P-value < 0.05 were considered statistically significant.
Results
Characteristics: 18 patients with CTS (27 median nerves) and 15 healthy controls (27 median nerves) were evaluated. 13 (72.2%) patients in the CTS group and 13 (86.7%) in the control group were women (P = 0.413). The mean age was 47.7 ± 5.5 for the CTS group and 48.9 ± 5.5 years for the control group (P = 0.418). 13 median nerves (48.1%) of the right hand were investigated in the CTS group, compared to 15 right median nerves (55.6%) in the control group (P = 0.586). There was no significant difference between the two groups regarding age and gender (Table 1).
Table 1 .
Participants' characteristics
| Variable | CTS | Control | P |
|---|---|---|---|
| Gender [n (%)] | 0.413 | ||
| Men | 5 (27.8) | 2 (13.3) | |
| Women | 13 (72.2) | 13 (86.7) | |
| Age (year) (mean ± SD) |
47.7 ± 5.5 | 48.9 ± 5.5 | 0.418 |
| Hand [n (%)] | 0.586 | ||
| Right | 15 (55.6) | 13 (48.1) | |
| Left | 12 (44.4) | 14 (51.9) |
CTS: Carpal tunnel syndrome; SD: Standard deviation
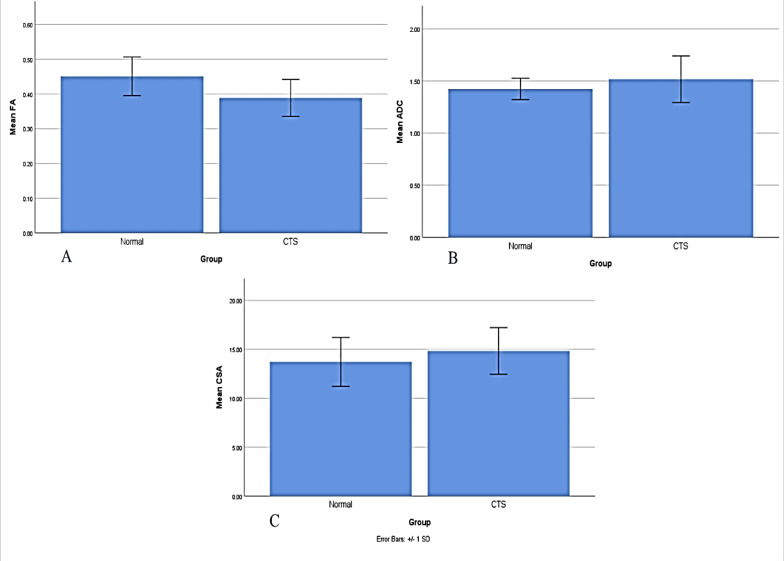
MRN parameters: The mean CSA was 14.8 ± 2.4 mm2 in the CTS group and 13.8 ± 2.5 mm2 in the control group, but this difference was not statistically significant (P = 0.137). The mean FA was significantly lower in the CTS group (0.38 ± 0.05 vs. 0.45 ± 0.06, P < 0.001). The mean ADC was 1.52 ± 0.22 × 10-3 mm2/s in the CTS group and 1.42 ± 0.10 × 10-3 mm2/s in the control group, which was also statistically not significant but by a narrow margin (P = 0.054) (Figures 1 and 2).
Figure 2.
Magnetic resonance neurography (MRN) parameters of patients with carpal tunnel syndrome (CTS) and controls
A) Comparing the mean fractional anisotropy (FA) between two groups with CTS and healthy controls; B) Comparing the mean apparent diffusion coefficient (ADC) between two groups with CTS and healthy controls; C) Comparing the mean cross-sectional area (CSA) between two groups with CTS and healthy controls
Even though all MRN parameters revealed less severe measures (lower CSA and ADC and higher FA) in individuals with mild CTS compared to those with severe CTS, ANOVA and Bonferroni tests revealed no significant association between MRN parameters and CTS severity (Table 2).
Table 2.
Relationship between magnetic resonance neurography (MRN) parameters and carpal tunnel syndrome (CTS) severity
| MRN parameters |
Mild CTS
(n = 9) (mean ± SD) |
Moderate CTS
(n = 9) (mean ± SD) |
Severe CTS
(n = 9) (mean ± SD) |
P * |
P
**
|
||
|---|---|---|---|---|---|---|---|
|
Mild vs.
moderate |
Mild vs.
severe |
Moderate
vs. severe |
|||||
| CSA | 14.30 ± 2.62 | 14.51 ± 2.53 | 15.67 ± 2.00 | 0.438 | > 0.999 | 0.710 | 0.944 |
| FA | 0.40 ± 0.04 | 0.39 ± 0.07 | 0.37 ± 0.05 | 0.341 | > 0.999 | 0.465 | 0.979 |
| ADC | 1.41 ± 0.17 | 1.52 ± 0.27 | 1.62 ± 0.18 | 0.134 | 0.796 | 0.142 | > 0.999 |
MRN: Magnetic resonance neurography; CTS: Carpal tunnel syndrome; CSA: Cross-sectional area; FA: Fractional anisotropy; ADC: Apparent diffusion coefficient; SD: Standard deviation
Analysis of variance (ANOVA);
Bonferroni post hoc
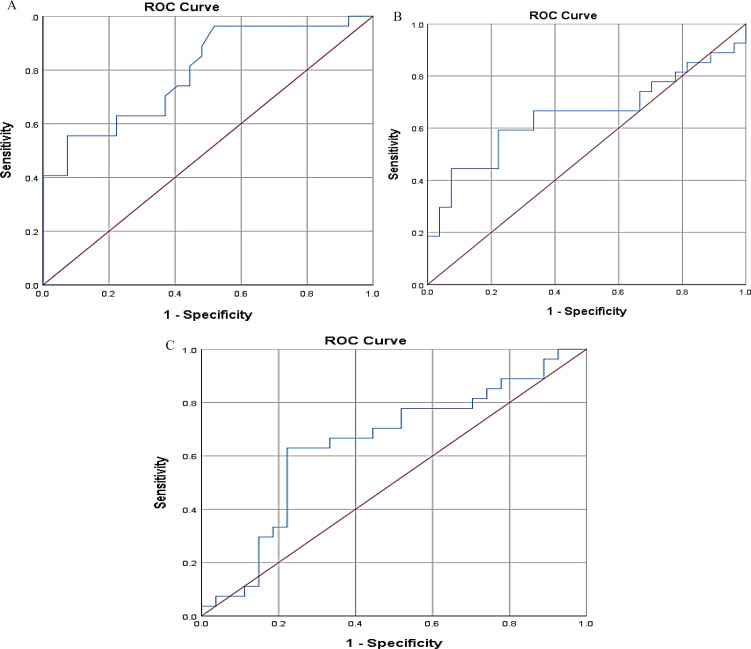
Diagnostic values of MRN parameters: Using the ROC test, AUC for the CSA and ADC was not in the significant range, indicating that the CSA and ADC had a low diagnostic value for identifying individuals with CTS [CSA: AUC = 0.645 (95% Confidence interval (CI): 0.493-0.797), P = 0.068; ADC: AUC = 0.650 (95% CI: 0.497-0.803), P = 0.058]. However, the AUC for the FA was within the significant range, indicating that it had a high diagnostic value [AUC = 0.791 (95% CI: 0.672-0.911), P = 0.001]. A diagnostic cut-off of 0.42 showed a sensitivity of 70.4% and a specificity of 63% for identifying patients with CTS (Figure 3).
Figure 3.
Receiver operating characteristic (ROC) analysis for diagnosing carpal tunnel syndrome (CTS)
A) ROC analysis for the diagnostic value of fractional anisotropy (FA); B) ROC analysis for the diagnostic value of apparent diffusion coefficient (ADC); C) ROC analysis for the diagnostic value of cross-sectional area (CSA)
Discussion
DTI has recently been proposed as a promising diagnostic tool for peripheral neuropathies. 14 We examined MRN parameters using DTI in patients with CTS. We found that CSA and ADC values were not statistically different between the CTS group and healthy controls; however, the FA was significantly lower in patients with CTS than in healthy individuals.
Similar to our work, several prior studies have demonstrated the usefulness of DTI in identifying patients with CTS from healthy individuals. However, the reported mean values for MRN parameters were slightly different. In earlier investigations, patients with CTS had a CSA value of 9.0 to 17.6 mm2, an FA value of 0.359-0.640, and an ADC value of 0.99-1.03 × 10-3 mm2/s. 9,10,15,16
In our study, participants with CTS had mean CSA, FA, and ADC values of 14.8 ± 2.4 mm2, 0.38 ± 0.05, and 1.52 ± 0.22 × 10-3 mm2/s, respectively.
In accordance with our findings, most prior investigations have demonstrated the value of FA in the diagnosis of patients with CTS. 9,16 Wafaie et al. showed that the mean FA of the median nerve in patients with CTS was significantly lower than in the healthy control group; however, in their study, the ADC values in the CTS group were also significantly higher than the control group,9 whereas in our study, even though the ADC values in the CTS group were greater, the difference was not statistically significant. Like Wafaie et al., several studies discovered significant differences in FA and ADC parameters between patients with CTS and healthy individuals. 16-19 Nonetheless, some studies support our findings that only FA was significantly different between patients and controls and that the average ADC values were comparable between patients with CTS and controls. 11,20-22 There are also discrepancies in published studies about CSA. Although a number of studies have found a significant increase in CSA in patients with CTS compared to the control group, in compliance to our findings, most studies stated that CSA had little diagnostic value for differentiating patients with CTS from healthy individuals.13,16
CTS is defined as chronic median nerve compression. Edema, fibrosis, and abnormalities in vascular permeability, especially in the first few months of the disease, are among the reasons for the observed changes in MRN and DTI parameters in patients with CTS. 13 Initial changes are caused by the blood barrier breakdown, followed by subperineural and endoneurial edema, perineural and epineural fibrosis, demyelination, and Wallerian degeneration. All these changes can affect the water diffusion process in nerve fibers and, subsequently, the DTI parameters, ADC in particular. 16 Several recent studies have shown that Wallerian degeneration can significantly reduce anisotropy while minimally increasing ADC. 23 Therefore, a more significant decrease in FA compared to a smaller increase in ADC can be anticipated in patients with CTS. As a result, FA could serve as a more sensitive parameter for diagnosing CTS than ADC.
While evaluating the association between CSA, FA, and ADC parameters with CTS severity, although the incremental pattern of CSA and ADC and the decrementing pattern of FA were observed with increasing severity of CTS, the difference was not statistically significant. In support of our findings, multiple prior studies have found a non-significant increase in ADC and decrease in FA while comparing individuals with severe CTS to those with mild and moderate CTS. 9
Few studies have investigated the association between MRN values and NCS parameters;17,19 however, selection bias in our study design prevented us from evaluating this association. In Kwon et al.’s study, the highest correlation coefficient was between FA and sensory and motor amplitude, and only FA had a significant correlation with NCS parameters and electrophysiological grading. 19 These findings suggest that advances in DTI techniques will provide further evidence on whether and to what extent DTI can detect electrophysiological changes in peripheral nerves.
In addition, we investigated the diagnostic value of MRN and DTI parameters and defined the diagnostic cut-off using ROC analysis. The diagnostic cut-off for FA was 0.42, with a sensitivity of 70.4% and a specificity of 63%. However, CSA and ADC parameters did not have a good diagnostic value for identifying patients with CTS. Consistent with our findings, earlier studies have demonstrated that FA has a higher diagnostic value than ADC and CSA in patients with CTS. 9,11,13,16,19,20
In the study by Wafaie et al., 9 the diagnostic cut-off for FA was found to be 0.54, with a sensitivity of 89.4% and a specificity of 95.7%. In the study by Guggenberger et al., 16 the diagnostic cut-off for FA was 0.47, with a sensitivity of 70% and a specificity of 71%, which is comparable to our findings. Variations in imaging protocols, the anatomical position of the studied median nerve, and software for interpreting DTI data could all affect the reported FA values of patients, leading to disparities in diagnostic cut-offs and specificity values.
The relatively small sample size was the most significant limitation of this study, as it diminished the statistical power of the analyses, particularly the intragroup comparisons of patients with CTS (comparison of CSA, FA, and ADC parameters in severity grading groups). Only one experienced radiologist assessed MRN and DTI images qualitatively and quantitatively. DTI is a technically challenging procedure, and the image quality depends on the field’s homogeneity, the coil, and the gradient systems employed. On the other hand, accurate calculation of FA and ADC values relies on the operator's precise placement of the region of interest (ROI) of the median nerve. These factors influence the reported values and contribute to intra- and inter-observer variability. In the future, multi-center studies with larger samples and more robust methodologies will be required.
Conclusion
This study indicated that DTI and 3-T MRN of the median nerve could be effective and non-invasive diagnostic tools for individuals with CTS. The FA parameter was shown to be the best parameter for diagnosing CTS. A cut-off value of 0.42 was determined to identify patients with CTS from healthy individuals with a sensitivity of 70.4% and a specificity of 63%. Although the values of CSA and ADC for patients with CTS were higher than for healthy subjects, the difference was not statistically significant, suggesting that these two variables are not very useful for identifying patients with CTS from healthy individuals.
Acknowledgments
We thank all participants for their cooperation. The study protocol was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.IKHC.REC.1400.405).
Notes:
How to cite this article: Farahmand G, Behkar A, Hashemi H, Ghajarzadeh M, Raminfard S, Shahbazi M, et al. Assessment of median nerve with magnetic resonance neurography in cases with carpal tunnel syndrome and controls. Curr J Neurol 2024; 23(2): 89-95.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Chammas M, Boretto J, Burmann LM, Ramos RM, Dos Santos Neto FC, Silva JB. Carpal tunnel syndrome - Part I (anatomy, physiology, etiology and diagnosis) Rev Bras Ortop. 2014;49(5):429–36. doi: 10.1016/j.rboe.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016;15(12):1273–84. doi: 10.1016/S1474-4422(16)30231-9. [DOI] [PubMed] [Google Scholar]
- 3.Jablecki CK, Andary MT, So YT, Wilkins DE, Williams FH. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. AAEM Quality Assurance Committee. Muscle Nerve. 1993;16(12):1392–414. doi: 10.1002/mus.880161220. [DOI] [PubMed] [Google Scholar]
- 4.Pasternack II, Malmivaara A, Tervahartiala P, Forsberg H, Vehmas T. Magnetic resonance imaging findings in respect to carpal tunnel syndrome. Scand J Work Environ Health. 2003;29(3):189–96. doi: 10.5271/sjweh.721. [DOI] [PubMed] [Google Scholar]
- 5.Vo NQ, Nguyen DD, Hoang NT, Ngo DHA, Nguyen THD, Trong BL, et al. Magnetic resonance imaging as a first-choice imaging modality in carpal tunnel syndrome: New evidence. Acta Radiol. 2023;64(2):675–83. doi: 10.1177/02841851221094227. [DOI] [PubMed] [Google Scholar]
- 6.Wolma J, Nederkoorn PJ, Goossens A, Vergouwen MD, van Schaik IN, Vermeulen M. Ethnicity a risk factor? The relation between ethnicity and large- and small-vessel disease in White people, Black people, and Asians within a hospital-based population. Eur J Neurol. 2009;16(4):522–7. doi: 10.1111/j.1468-1331.2009.02530.x. [DOI] [PubMed] [Google Scholar]
- 7.Lander RD, Jones CMC, Hammert WC. Identification of clinical and demographic predictors for treatment modality in patients with carpal tunnel syndrome. Hand (N Y) 2023;18(5):758–64. doi: 10.1177/15589447211060448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lalumandier JA, McPhee SD, Riddle S, Shulman JD, Daigle WW. Carpal tunnel syndrome: Effect on Army dental personnel. Mil Med. 2000;165(5):372–8. [PubMed] [Google Scholar]
- 9.Wafaie AM, Afifi LM, Moussa KM, Mansour AM, Abbas HM. Role of diffusion tensor imaging in carpal tunnel syndrome: A case control comparative study to electrophysiological tests and clinical assessment. The Egyptian Journal of Radiology and Nuclear Medicine. 2018;49(4):1068–75. [Google Scholar]
- 10.Naraghi A, da Gama LL, Menezes R, Khanna M, Sussman M, Anastakis D, et al. Diffusion tensor imaging of the median nerve before and after carpal tunnel release in patients with carpal tunnel syndrome: Feasibility study. Skeletal Radiol. 2013;42(10):1403–12. doi: 10.1007/s00256-013-1670-z. [DOI] [PubMed] [Google Scholar]
- 11.Khalil C, Hancart C, Le T V, Chantelot C, Chechin D, Cotten A. Diffusion tensor imaging and tractography of the median nerve in carpal tunnel syndrome: preliminary results. Eur Radiol. 2008;18(10):2283–91. doi: 10.1007/s00330-008-0971-4. [DOI] [PubMed] [Google Scholar]
- 12.Razek AAKA, Shabana AAE, El Saied TO, Alrefey N. Diffusion tensor imaging of mild-moderate carpal tunnel syndrome: correlation with nerve conduction study and clinical tests. Clin Rheumatol. 2017;36(10):2319–24. doi: 10.1007/s10067-016-3463-y. [DOI] [PubMed] [Google Scholar]
- 13.Bao H, Wu C, Wang S, Wang G, Zhang X, Zhang X, et al. Diffusion-weighted magnetic resonance neurography for the diagnosis of carpal tunnel syndrome: A pilot study. Clin Radiol. 2017;72(2):165–9. doi: 10.1016/j.crad.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Kollmer J, Bendszus M. Magnetic resonance neurography: Improved diagnosis of peripheral neuropathies. Neurotherapeutics. 2021;18(4):2368–83. doi: 10.1007/s13311-021-01166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brienza M, Pujia F, Colaiacomo MC, Anastasio MG, Pierelli F, Di Biasi C, et al. 3T diffusion tensor imaging and electroneurography of peripheral nerve: A morphofunctional analysis in carpal tunnel syndrome. J Neuroradiol. 2014;41(2):124–30. doi: 10.1016/j.neurad.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Guggenberger R, Markovic D, Eppenberger P, Chhabra A, Schiller A, Nanz D, et al. Assessment of median nerve with MR neurography by using diffusion-tensor imaging: Normative and pathologic diffusion values. Radiology. 2012;265(1):194–203. doi: 10.1148/radiol.12111403. [DOI] [PubMed] [Google Scholar]
- 17.Wang CK, Jou IM, Huang HW, Chen PY, Tsai HM, Liu YS, et al. Carpal tunnel syndrome assessed with diffusion tensor imaging: Comparison with electrophysiological studies of patients and healthy volunteers. Eur J Radiol. 2012;81(11):3378–83. doi: 10.1016/j.ejrad.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Stein D, Neufeld A, Pasternak O, Graif M, Patish H, Schwimmer E, et al. Diffusion tensor imaging of the median nerve in healthy and carpal tunnel syndrome subjects. J Magn Reson Imaging. 2009;29(3):657–62. doi: 10.1002/jmri.21553. [DOI] [PubMed] [Google Scholar]
- 19.Kwon BC, Koh SH, Hwang SY. Optimal parameters and location for diffusion-tensor imaging in the diagnosis of carpal tunnel syndrome: A prospective matched case-control study. AJR Am J Roentgenol. 2015;204(6):1248–54. doi: 10.2214/AJR.14.13371. [DOI] [PubMed] [Google Scholar]
- 20.Barcelo C, Faruch M, Lapegue F, Bayol MA, Sans N. 3-T MRI with diffusion tensor imaging and tractography of the median nerve. Eur Radiol. 2013;23(11):3124–30. doi: 10.1007/s00330-013-2955-2. [DOI] [PubMed] [Google Scholar]
- 21.Klauser AS, Abd EM, Kremser C, Taljanovic M, Schmidle G, Gabl M, et al. Carpal tunnel syndrome assessment with diffusion tensor imaging: Value of fractional anisotropy and apparent diffusion coefficient. Eur Radiol. 2018;28(3):1111–7. doi: 10.1007/s00330-017-5046-y. [DOI] [PubMed] [Google Scholar]
- 22.Tasdelen N, Gurses B, Kilickesmez O, Firat Z, Karlikaya G, Tercan M, et al. Diffusion tensor imaging in carpal tunnel syndrome. Diagn Interv Radiol. 2012;18(1):60–6. doi: 10.4261/1305-3825.DIR.3994-10.1. [DOI] [PubMed] [Google Scholar]
- 23.Pierpaoli C, Righini A, Linfante I, Tao-Cheng JH, Alger JR, Di CG. Histopathologic correlates of abnormal water diffusion in cerebral ischemia: Diffusion-weighted MR imaging and light and electron microscopic study. Radiology. 1993;189(2):439–48. doi: 10.1148/radiology.189.2.8210373. [DOI] [PubMed] [Google Scholar]