Abstract
In this study, we tested the contribution of flagellar motility, flagellin structure, and its glycosylation in Pseudomonas aeruginosa using genetically defined flagellar mutants. All mutants and their parent strains were tested in a burned-mouse model of infection. Motility and glycosylation of the flagellum appear to be important determinants of flagellar-mediated virulence in this model. This is the first report where genetically defined flagellar variants of P. aeruginosa were tested in the burned-mouse model of infection.
The flagella of bacteria are now recognized to mediate a number of functions besides motility and chemotaxis. The complexity and biologic sophistication involved in the biogenesis of this organelle and the dedication of such significant cellular resources towards its synthesis would predict that it may have also evolved to carry out a number of specialized functions or acquired these functions given its structure, being homologous to type III secretion systems (32). As more information has been gathered, it is now known that this organelle serves as a secretory system (13, 19, 34), an attachment organelle (2), and lately as an extremely potent stimulus of the innate immune response (12) and thus plays a role either in stimulating host defense or in disease causation. Besides these functions, new properties of the flagellum have come to light whose function(s) is unknown. For example, many prokaryotic flagella, especially among polar flagellated bacteria, are now known to be glycosylated (3, 6, 21, 25). However, very little is known about the role of glycosylation of flagella in virulence, if any, and indeed about the role of other properties of the flagellum besides the requirement for an intact flagellum in many models of disease (18), i.e., is it simply the presence of flagellin or motility itself that is required for virulence? In studies of Pseudomonas aeruginosa, a chemically mutagenized nonflagellated strain was shown to be less virulent than its motile counterpart in the burned-mouse model of infection (22). In addition, a genetically characterized nonflagellated mutant of P. aeruginosa was also shown to be less virulent than its isogenic parent in a mouse pneumonia model (10). Similarly, an intact flagellum was required for colonization by Campylobacter jejuni (1) and Helicobacter pylori (9). In Salmonella enteriditis serovar Typhimurium the data are more conflicting. A characterized nonmotile transposon insertion mutant of this strain was not altered in virulence in the mouse models of gut or intraperitoneal infection (20), nor was a mutant in the master regulator of its flagellar system any less virulent in the mouse model of infection (27). However, flagella and chemotaxis were shown to be required for colonization and colitis of the streptomycin-treated mouse gut (28). Thus, the requirement for motility itself versus the presence of a flagellum has not been elucidated. In regard to the role of glycosylation in virulence, even less is known. A general glycosylation system that includes flagellar glycosylation has been reported to play a role in colonization of the gut by Campylobacter jejuni 81-176 (30). In Pseudomonas syringae pv. glycinea, flagellin glycosylation is responsible for determining recognition of virulence by host plants and is involved in some way for mediating the hypersensitivity reaction to flagellin (31). Given the renewed interest in flagellar functions of P. aeruginosa, we examined the role of motility, flagellum, and glycosylation in the burned-mouse model of infection.
Characterization of flagellar and glycosylation mutants.
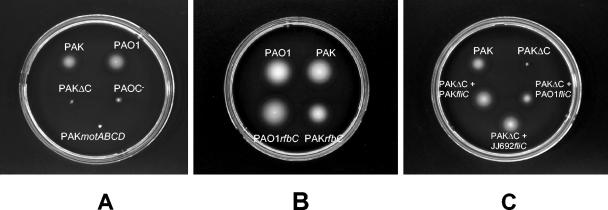
The bacterial strains and mutants used in this study are listed in Table 1. The fliC mutant strains, PAKΔC (fliC deletion) and PAOC− (gentamicin insertion), were described previously (8, 11). P. aeruginosa strain PAK possesses two sets of mot genes, now called motAB and motCD. For this study, deletion strain motABCD lacking the entire coding sequences of the individual mot paralogues was constructed by the PCR-based method of Horton et al. (17). The DNA fragments flanking individual mot genes were amplified, spliced, and cloned into pEX18Ap (15). The deleted alleles were introduced into P. aeruginosa PAK by conjugation, and the chromosomal deletions were identified following counterselection on sucrose-containing media. The PAKrfbC mutant is described by Arora et al. (3). A mutation in this gene abolishes flagellin glycosylation (3, 4). The rfbC mutant of strain PAO1 was constructed by insertional inactivation of the PAO1 rfbC gene with a gentamicin resistance cassette. The different mutant strains tested in this study were analyzed for their growth characteristics, motility, and lipopolysaccharide (LPS) phenotypes. None of the mutants had any growth defects based on their growth curves (data not shown). The motility of different P. aeruginosa strains was assessed qualitatively by examining the circular swarm from the growing motile bacterial cells on 0.325% agar plates at 37°C. The motility studies of the strains used in this study are shown in Fig. 1. The motABCD mutant of strain PAK was nonmotile, as were the fliC mutants of both PAK and PAO1 strains (Fig. 1A). Electron micrographs showed that the mot mutant possessed a flagellum (data not shown). The motility zones of the rfbC mutants were no different from those of the wild-type strains (Fig. 1B). As previously reported, the PAKrfbC mutant strain had no LPS defects (3), and similarly the PAO1rfbC mutant strain showed no apparent changes in the LPS bands (data not shown).
TABLE 1.
Bacterial strains used in this study
| Strain | Description or origin | Source or reference |
|---|---|---|
| Escherichia coli DH5α | F− φ80lacZΔM15 Δ(lacZYA-argF) U169 endA1 recA1 hsdR17 (rk−, mk+) supE44 thi-1 gyrA96 relA1 phoA | Invitrogen |
| P. aeruginosa | ||
| PAK | Wild-type laboratory strain | D. Bradley |
| PAO1 | Wild-type laboratory strain | M. Vasil |
| JJ692 | UTI isolate | S. Lory |
| PAKΔC | fliC deletion strain of P. aeruginosa PAK | 8 |
| PAOC− | Gentamicin insertion mutant in the fliC gene of P. aeruginosa PAO1 | 11 |
| PAKmotABCD | Both sets of mot genes, motAB and motCD deleted from P. aeruginosa PAK | This study |
| PAKrfbC | Gentamicin insertion mutant in the rfbC gene of P. aeruginosa PAK | 3 |
| PAOrfbC | Gentamicin insertion mutant in the rfbC gene of P. aeruginosa PAO1 | This study |
| PAKΔC + PAKfliC | PAKΔC complemented on the chromosome with the PAK fliC gene at the attB site | This study |
| PAKΔC + PAO1fliC | PAKΔC complemented on the chromosome with the PAO1 fliC gene at the attB site | This study |
| PAKΔC + JJ692fliC | PAKΔC complemented on the chromosome with the fliC gene from the P. aeruginosa clinical strain JJ692 at the attB site | This study |
| PAOC− + PAO1fliC | PAOC− complemented on the chromosome with the fliC gene from the P. aeruginosa PAO1 fliC gene at the attB site | This study |
FIG. 1.
Motility phenotype of different P. aeruginosa strains used in this study. Panel A shows the motile wild-type P. aeruginosa strains PAK and PAO1 and mutant strains that were defective in motility because of mutations in the fliC gene (PAKΔC and PAOC−) or motABCD genes (PAKmotAB). Panel B shows the glycosylation mutant strains PAKrfbC and PAOrfbC, which were both motile. Panel C shows the complemented strains in which fliC mutant strain PAKΔC was chromosomally complemented with different flagellins with their promoters.
Role of the flagellum and motility.
In order to resolve the issue of whether the flagellum itself or the swimming motility is required for P. aeruginosa virulence in this model, we performed 50% lethal dose (LD50) determinations using the fliC (flagellin) mutants of two different strains of P. aeruginosa and a motABCD mutant of strain PAK in the burned-mouse model. The burn model is a well-established murine model for studying the progression of infection from a contaminated burn wound to a systemic sepsis (24). All bacterial strains were grown in brain heart infusion medium (Becton Dickinson and Co., Sparks, MD) overnight at 37°C and were diluted to the desired challenge concentration with saline. Bacteria (0.1 ml) were injected subcutaneously under the eschar (burn) immediately after the burn was administered. Mortality was recorded daily, and on day 3 postburn, the LD50 values and 95% confidence intervals were calculated using the SYSTAT statistical program. Where LD50 values were reported as more than or less than, the 95% confidence intervals and P values were unobtainable. Consistent with the previous reports, PAO1 was extremely virulent in this model, with an LD50 of less than 100 CFU (14). As shown in Table 2, the nonmotile fliC mutants of both strains were significantly attenuated in virulence. The LD50 of PAKΔC was >4 × 103-fold that of the wild-type strain PAK, and that of PAOC− was >3 × 106-fold higher compared to the corresponding wild-type parent strain PAO1. Similarly, the LD50 of the PAKmotABCD strain having paralyzed flagella was >300-fold that of the wild-type strain PAK. These data clearly show the importance of motility in this model of P. aeruginosa virulence, since the mere presence of the flagellum as in the PAKmotABCD mutant is not sufficient to impart full virulence to this organism in this model of P. aeruginosa infection.
TABLE 2.
LD50 values of wild-type P. aeruginosa strains PAK and PAO1 compared to the flagellin mutants PAKΔC and PAOC− and paralyzed mutant strain PAKmotABCD
| P. aeruginosa strain | LD50 (CFU/mouse) (confidence interval) | Change in LD50 (fold) | P value |
|---|---|---|---|
| PAK | 5.75 × 104 (2.1 × 103-2.3 × 105) | None | None |
| PAKΔC | >2.1 × 108 | >4 × 103 | NCa |
| PAO1 | <2.3 × 101 | None | None |
| PAOC− | 6.6 × 107 (8.4 × 106-7.1 × 108) | >3 × 106 | NC |
| PAKmotABCD | >1.7 × 107 | >3 × 102 | NC |
NC, not calculable.
The burned-mouse model of infection was used earlier to explore the role of P. aeruginosa flagellum (22), but the flagellar mutants that were used in previous studies were genetically uncharacterized mutants. This model has been shown to be quite reproducible and versatile (24) and mimics aspects of the clinical development of sepsis from a small local inoculum (7, 23, 29). However, one cannot generalize the findings to other animal models of P. aeruginosa infection, since the LD50 is generally larger, especially in the case of strain PAK, and this model does not involve infection on a mucosal surface where different virulence factors may be important. However, these data definitively show the importance of swimming motility for P. aeruginosa virulence in this model but do not exclude a role for flagellin.
Role of glycosylation.
The LD50s of PAK and PAO1 glycosylation mutants, PAKrfbC and PAO1rfbC, were also determined by procedures described above. As shown in Table 3, both mutants were significantly attenuated in virulence, suggesting a role for flagellin glycosylation in P. aeruginosa virulence, unless there are other unknown glycosylation phenotypes conferred by this gene. However, flagellin modifications do seem to play a significant role in virulence since mutants whose flagellins were mainly nonglycosylated were reduced in virulence to the extent that the LD50 values increased between 35- and >10,000-fold. We have been unable to discover another phenotype accompanying the loss of glycosylation, including an examination of the LPS banding patterns of these strains to explain such marked changes in the LD50 doses. This suggests that the glycan moieties attached to the surface of the flagellin (26) might be involved in some way either to facilitate flagellin binding to the host cell receptors that trigger inflammation or do so directly, since the isolated, mainly nonglycosylated PAK flagellum shows a reduction of release of interleukin-8 (IL-8) from A549 cells compared to the wild-type PAK flagellum (33). At this time it is not possible to distinguish between whether the stimulation of inflammation is due to the sugars themselves interacting with another toll-like receptor or some facilitation of the fit of flagellin with toll-like receptor 5, since the identity of the complete glycan chains from the strains examined has not been elucidated (26).
TABLE 3.
LD50 values of wild-type P. aeruginosa strains PAK and PAO1 compared to the flagellar glycosylation mutants PAKrfbC and PAOrfbC
| P. aerugi- ginosa strain | LD50 (CFU/mouse) (confidence interval) | Change in LD50 (fold) | P value |
|---|---|---|---|
| PAK | 5.75 × 104 (2.1 × 103-2.3 × 105) | None | None |
| PAKrfbC | 2.0 × 106 (2.5 × 105-2.3 × 107) | 35 | <0.05 |
| PAO1 | <2.3 × 101 | None | None |
| PAO1rfbC | 2.2 × 106 (2.4 × 105-2.0 × 107) | >104 | NCa |
NC, not calculable when compared to the wild-type parent strain.
Importance of flagellin structure.
In P. aeruginosa, three main types of flagellins have been described (5), types A1, A2, and b. In order to examine whether these structural differences were important in virulence, the A1-, A2-, and b-type flagellin genes with their promoters were inserted into the P. aeruginosa fliC deletion mutant PAKΔC at the att site by double reciprocal recombination using the sacB system (16). The resulting strains were called PAKΔC + PAKfliC, PAKΔC + PAO1fliC, and PAKΔC + JJ692fliC, respectively, and are listed in Table 1. The LD50s of these strains was again measured. For reasons unknown, the complementation with the wild-type PAK flagellin gene into the neutral attB site did not result in an LD50 that was identical to the wild-type strain in this model despite having similar-sized motility zones (Fig. 1C). However, in spite of the structural differences in these flagellins, the LD50 values for the three complemented strains were not statistically different (Table 4), suggesting that the structural differences between A1-, A2-, and b-type flagellins did not affect the virulence of P. aeruginosa. Similarly, when we attempted to complement the PAO1 flagellin mutant PAOC− with the b-type flagellin gene in the neutral attB site, the complementation was not complete in regards to the LD50 (Table 4).
TABLE 4.
Effect of structural differences in the flagellins of P. aeruginosa on LD50 values
| P. aeruginosa strain | LD50 (CFU/mouse) (confidence interval) | Change in LD50 (fold) | P value |
|---|---|---|---|
| PAK | 5.75 × 104 (2.1 × 103-2.3 × 105) | None | None |
| PAKΔC | >2.1 × 108 | >4 × 103 | NCa |
| PAKΔC + PAKfliC | 2.6 × 106 (3.8 × 105-3.2 × 107) | 45 | <0.05 |
| PAKΔC + PAO1fliC | 2.9 × 106 (2.5 × 105-4.2 × 107) | 50 | <0.05 |
| PAKΔC + JJ692fliC | 2.1 × 106 (1.9 × 105-2.3 × 107) | 36 | <0.05 |
| PAO1 | <2.3 × 101 | None | None |
| PAOC− | 6.6 × 107 (8.4 × 106-7.1 × 108) | >3 × 106 | NC |
| PAOC− + PAO1fliC | 1.4 × 104 (2.1 × 103-1.5 × 105) | >60 | NC |
NC, not calculable.
This report demonstrates the importance of the P. aeruginosa flagellum, motility, and flagellin glycosylation in the burn wound model of P. aeruginosa infections. We propose a model for burn wound infections caused by P. aeruginosa wherein motility is essential for dissemination from the site of infection and flagellin structure and its posttranslational modifications are important for death due to sepsis. In such a model, mot mutants, flagellin mutants, and glycosylation mutants would all be less virulent.
Acknowledgments
This work was supported by NIH grant AI 47852 to R.R. and by the Shriners of North America.
Editor: V. J. DiRita
REFERENCES
- 1.Aguero-Rosenfeld, M. E., X. H. Yang, and I. Nachamkin. 1990. Infection of adult Syrian hamsters with flagellar variants of Campylobacter jejuni. Infect. Immun. 58:2214-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora, S. K., B. W. Ritchings, E. C. Almira, S. Lory, and R. Ramphal. 1998. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect. Immun. 66:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora, S., M. Bangera, S. Lory, and R. Ramphal. 2001. A genomic island in Pseudomonas aeruginosa carries the determinants of flagellin glycosylation. Proc. Natl. Acad. Sci. USA 98:9342-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora, S. K., S. M. Logan, A. Verma, M. Schirm, P. Thibault, E. Soo, and R. Ramphal. 2003. Posttranslational modification of b-type flagellin of Pseudomonas aeruginosa: structural and genetic basis. Pseudomonas International Meeting, Quebec City, Canada.
- 5.Arora, S. K., M. C. Wolfgang, S. Lory, and R. Ramphal. 2004. Sequence polymorphism in the glycosylation island and flagellins of Pseudomonas aeruginosa. J. Bacteriol. 186:2115-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brimer, C. D., and T. C. Montie. 1998. Cloning and comparison of fliC genes and identification of glycosylation in the flagellin of Pseudomonas aeruginosa a-type strains. J. Bacteriol. 180:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1984. Experimental Klebsiella pneumoniae burn wound sepsis: role of capsular polysaccharide. Infect. Immun. 43:440-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809-824. [DOI] [PubMed] [Google Scholar]
- 9.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 64:2445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig, S. M., S. K. Arora, R. Van, and R. Ramphal. 2001. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun. 69:4931-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gewirtz, A. T., T. A., Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 13.Ghelardi, E., F. Celandroni, S. Salvetti, D. J. Beecher, M. Gominet, D. Lereclus, A. C. Wong, and S. Senesi. 2002. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated proteins in Bacillus thuringiensis. J. Bacteriol. 184:6424-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg, J. B., M. J. Coyne, Jr., A. N. Neely, and I. A. Holder. 1995. Avirulence of a Pseudomonas aeruginosa algC mutant in a burned-mouse model of infection. Infect. Immun. 63:4166-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 16.Hoang, T. T., A. J. Kutchma, A. Beecher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 17.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 18.Josenhans, C., and S. Suerbaum. 2002. Role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 19.Konkel, M. E., J. D. Klena, V. Rivera-Amill, M. R. Monteville, D. Biswas, B. Raphael, and J. Michelson. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186:3296-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockman, H. A., and R. Curtiss III. 1990. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect. Immun. 58:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan, S. M., J. F. Kelly, P. Thibault, C. P. Ewing, and P. Guerry. 2002. Heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Mol. Microbiol. 46:587-597. [DOI] [PubMed] [Google Scholar]
- 22.Montie, T. C., D. Doyle-Huntzinger, R. C. Craven, and I. A. Holder. 1982. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect. Immun. 38:1296-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neely, A. N., and I. A. Holder. 1996. A murine model with aspects of clinical relevance for the study of antibiotic-induced endotoxin release in septic hosts. J. Endotoxin Res. 3:229-235. [Google Scholar]
- 24.Neely, A. N., I. A. Holder, and G. D. Warden. 1999. Then and now: studies using a burned mouse model reflect trends in burn research over the past 25 years. Burns 25:603-609. [DOI] [PubMed] [Google Scholar]
- 25.Schirm, M., E. C. Soo, A. J. Aubry, J. Austin, P. Thibault, and S. M. Logan. 2003. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 48:1579-1592. [DOI] [PubMed] [Google Scholar]
- 26.Schirm, M., S. K. Arora, A. Verma, E. Vinogradov, P. Thibault, R. Ramphal, and S. M. Logan. 2004. Structural and genetic characterization of glycosylation of type a flagellin in Pseudomonas aeruginosa. J. Bacteriol. 186:2523-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stecher, B., S. Hapfelmeier, C. Muller, M. Kremer, T. Stallmach, and W. D. Hardt. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:4138-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stieritz, D. D., and I. A. Holder. 1975. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J. Infect. Dis. 131:688-691. [DOI] [PubMed] [Google Scholar]
- 30.Szymanski, C. M., D. H. Burr, and P. Guerry. 2002. Campylobacter protein glycosylation affects host cell interactions. Infect. Immun. 70:2242-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi, K., F. Taguchi, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Flagellin glycosylation island in Pseudomonas syringae pv. glycinea and its role in host specificity. J. Bacteriol. 185:6658-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gijsegem, F., C. Gough, C. Zischek, E. Niqueux, M. Arlat, S. Genin, P. Barberis, S. German, P. Castello, and C. Boucher. 1995. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol. Microbiol. 15:1095-1114. [DOI] [PubMed] [Google Scholar]
- 33.Verma, A., S. K. Arora, and R. Ramphal. 2004. Identification of specific sites on Pseudomonas aeruginosa flagellin interacting with TLR5, Abstr. 104th Gen. Meet. Am. Soc. Microbiol 2004, abstr. B-170, p. 30. ASM Press, Washington, D.C.
- 34.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]