Abstract
The CAMP reaction was first described by Christie et al. (R. Christie, N. E. Atkins, and E. Munch-Petersen, Aust. J. Exp. Biol. 22:197-200, 1944) as the synergistic lysis of sheep red blood cells by Staphylococcus aureus sphingomyelinase and CAMP factor (cohemolysin), a secreted protein from group B streptococci. We observed a CAMP-like reaction when Bartonella henselae was grown in close proximity to S. aureus on 5% sheep blood agar. This study describes the cloning, sequencing, and characterization of a CAMP-like factor autotransporter gene (cfa) from B. henselae. A cosmid library of B. henselae ATCC 49793 was constructed using SuperCos1 in Escherichia coli XL1-Blue MR. Cosmids were screened for the CAMP reaction, and a quantitative cohemolysis microtiter assay was developed using purified sphingomyelinase. Cosmid clones with the strongest cohemolytic reaction had similar restriction enzyme patterns. A DNA fragment that expressed the cohemolysin determinant was subcloned in a 7,200-bp StuI-BamHI fragment which contained a 6,024-bp open reading frame. The deduced amino acid sequence showed homology to the family of autotransporters. The autotransporters are a group of proteins that mediate their own export through the outer membrane. They contain an N-terminal passenger region, the α-domain, and a C-terminal transporter region, the β-domain. The α-domain contained four, nearly identical 42-amino-acid repeats and showed homology to the family of RTX (repeat in toxin) hemolysins. The concentrated supernatant of the recombinant strain expressed a protein with a molecular mass of 180 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis consistent with the calculated molecular weight of the secreted α-domain. In conclusion, we have characterized a novel secreted cohemolysin autotransporter protein of B. henselae.
Bartonella henselae is an extremely fastidious, pleomorphic, gram-negative rod that has been found to be associated with cat scratch disease, bacillary angiomatosis, and bacillary peliosis (9, 12, 28, 48). Of these syndromes, cat scratch disease is the most common and affects an estimated 25,000 people in the United States annually, the majority of which are children (25).
Bartonella spp. are the only bacterial pathogens for humans that engage in erythrocyte parasitism (30, 38) and require erythrocytes or hemin supplements in order to grow in vitro (42). Most B. henselae isolates require more than 7 days of incubation before growth can be detected. Typically they are plated on freshly prepared enriched (chocolate- or blood-containing) medium incubated at 35 to 37°C with 5 to 10% CO2 and >40% humidity. There are two types of colony morphology for B. henselae. They can appear as dry, irregular whitish raised colonies or moist circular tan colonies, often in the same culture. B. henselae colonies are not hemolytic on blood agar (47). We, however, observed a synergistic lysis of the red cells when B. henselae was grown in close proximity to S. aureus on 5% sheep blood agar. Cohemolysis (CAMP-like reaction) has not been previously described for B. henselae. The CAMP reaction, as originally described in 1944 by Christie, Atkins, and Munch-Peterson (whose initials give rise to the eponym CAMP) (8), is the synergistic lysis of sheep erythrocytes by Staphylococcus aureus sphingomyelinase C (beta-toxin) and CAMP factor (cohemolysin), a secreted 25.3-kDa protein from group B streptococci (Streptococcus agalactiae) (36). The CAMPreaction is performed with erythrocytes whose cell membranes contain at least 45 mol% sphingomyelin. Human erythrocytes have a sphingomyelin:cholesterol ratio of 1:5 and are known not to undergo CAMP hemolysis (8).
The CAMP reaction occurs in a sequential two-step process. The first reaction is the hydrolysis of membrane sphingomyelin and phospholipids by the action of a sphingomyelinase or phospholipase (11). In the second reaction, the CAMP factor interacts with the metastable red cell membrane, leading to cell lysis (3). A number of other gram-positive and gram-negative bacteria are known to react positively in the CAMP test, including Listeria monocytogenes (33), Listeria seeligeri (37), Rhodococcus equi (34), Pasteurella spp. (15), Aeromonas spp. (14), certain Vibrio spp. (29), group G streptococci (44), and Actinobacillus pleuropneumoniae (17).
Since the cohemolysin of B. henselae causes lysis of red blood cells, it is considered a potential virulence factor. To facilitate studying the role of the CAMP reaction in the pathogenesis of B. henselae infection, we cloned and characterized the CAMP-like factor of B. henselae. The deduced amino acid sequence of this gene is homologous to the family of autotransporters. The autotransporters are a family of diverse proteins secreted by gram-negative bacteria that mediate their own export through the outer membrane. They contain an N-terminal passenger region, the α-domain, and a C-terminal transporter region, the β-domain. This study describes the cloning, sequencing, and characterization of a gene encoding a novel CAMP-like factor (Cfa) of B. henselae that possesses the characteristics of an autotransporter virulence protein.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Characteristics of the bacterial strains and plasmids used in this study are described in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Bacterial strains | ||
| B. henselae | Isolated from blood of a 31-year-old male with AIDS, Oklahoma City, OK, ATCC 49793 | ATCC |
| Staphylococcus aureus | Sphingomyelinase-producing strain used for CAMP test for Listeria monocytogenes, ATCC 25923 | ATCC |
| Group B streptococcus | Control strain used for CAMP test | Lab stock |
| Listeria monocytogenes | Control strain used for CAMP test | Lab stock |
| E. coli XL1-Blue MR | ΔmcrA183 ΔmcrCB-hsdSMR-mrr endA1 supE44 thi-1 recA1 gyrA96 relA1 lac | 5 |
| Plasmids | ||
| SuperCos1 | Cosmid cloning vector; Kmr, Apr | Stratagene |
| pCML75 | B. henselae cfa cosmid clone from B. henselae ATCC 49793 genomic library; 29.7-kb chromosomal fragment in SuperCos1; Kmr, Apr | This study |
| pCML76 | B. henselae cfa subclone of pCML75 containing 7.2-kb StuI-BamHI cfa clone in a plasmid derivative of SuperCos1 with 10× higher copy number than original cosmid; SuperCos1 derivative has a 3.4-kb fragment deleted from StuI site within SuperCos1 to the StuI site in cfa clone; the simian virus 40 promoter sequence and cos sequences were deleted; Kmr, Apr | This study |
| pCML77 | pCML76 with an internal 5.6-kb EcoRV-EcoRV deletion of the coding sequence of cfa; Kmr, Apr |
Kmr, kanamycin resistance; Apr, ampicillin resistance.
Media.
Escherichia coli strains were routinely grown in Luria-Bertani broth (LB). Kanamycin (50 μg/ml) was added as appropriate. S. aureus and L. monocytogenes were routinely grown on Columbia blood agar base (Difco, Detroit, Mich.) containing 5% defibrinated sheep blood (Difco) at 37°C. B. henselae was grown on freshly prepared Columbia 5% sheep blood agar plates for 7 days at 35°C under 5% carbon dioxide. All strains were maintained at −70°C in LB medium containing 15% glycerol.
Construction of B. henselae cosmid library.
A cosmid library of B. henselae ATCC 49793 was constructed using a SuperCos1 cosmid vector kit (Stratagene, La Jolla, Calif.).
B. henselae genomic DNA was partially digested with BamHI.
The digested DNA was sized to yield 30- to 40-kb fragments, dephosphorylated, and then ligated into the BamHI site of SuperCos1, previously linearized with XbaI. Packaging of the cosmids into phage and their subsequent infection in the E. coli strain XL1-Blue MR were performed as described by the manufacturer.
DNA manipulations and cloning.
Standard methods were followed for molecular biological techniques (41). Oligonucleotides were synthesized at the Huntsman Cancer Center Peptide and DNA facility.
CAMP test.
The CAMP test for cohemolytic activity was performed on fresh Columbia agar containing 5% sheep blood (8). The B. henselae ATCC 49793 strain was streaked on the surface of the blood agar and then incubated for 7 days at 35°C under 5% carbon dioxide. After 7 days of growth, the sphingomyelinase-producing S. aureus ATCC 25923 indicator strain was streaked on the surface of the blood agar perpendicular and juxtaposed to the B. henselae streak and incubated an additional 18 to 24 h. To test cohemolytic activity in the other test strains, the bacterial strains were streaked on Columbia 5% sheep blood agar, perpendicular and juxtaposed to the streaked S. aureus strain on the same day. The plate was incubated at 37°C for 18 to 24 h and then inspected for CAMP hemolysis.
Hemolysis and cohemolysis assay.
Assays to measure hemolysin activity were performed by a microtiter format. The hemolysis and cohemolysis assay was performed on sheep erythrocytes (Difco), human donor erythrocytes, and feline erythrocytes (gift of L. McGill, University of Utah, Animal Reference Pathology, ARUP Laboratories, Salt Lake City, Utah). Strains were grown in LB broth with the appropriate antibiotics to stationary phase with aeration at 37°C. The liquid cultures were centrifuged 5 min at 10,000 × g in a microcentrifuge. The supernatants were serially diluted twofold in modified Krebs-Ringer solution (KRT) in U-bottomed wells of 96-well microtiter plates, each well containing 100 μl of 1% (vol/vol) washed erythrocytes in KRT (19). KRT consisted of 7.5 g of NaCl, 0.383 g of KCl, 0.318 g of MgSO4 · 7H2O, and 0.305 g of CaCl2 in 1 liter of 10 mM Tris hydrochloride (pH 7.4). Erythrocytes in KRT were also pretreated with 0.025 U/ml of S. aureus sphingomyelinase (Sigma Chemical Co., St. Louis, Mo.) for 30 min at 37°C before addition of the culture supernatants (35). The microtiter plates were initially incubated at room temperature for 30 min and observed for hemagglutination. Then the plates were further incubated at 37°C for 8 h and scored for the endpoint of lysis of erythrocytes by observation of the reduction of the red cell pellet. The titer was recorded as the reciprocal of the dilution factor of the sample showing hemoglobin release.
Preparation and analysis of outer membrane, cytoplasmic, and secreted proteins.
Enriched outer membrane proteins and cytoplasmic proteins were prepared by modification of the procedure by Hantke (21). Exponentially grown cells were pelleted for 10 min at 4°C at 10,000 rpm (8,000 × g) in a Beckman JA-20 in a 50-ml tube. The pellet was resuspended in 1 ml 0.2 M Tris, pH 8.0, and transferred to a microcentrifuge tube. The bacteria were pelleted at 16,000 × g for 2 min at 4°C. After decanting of the supernatant, the pellets were resuspended in 50 μl of 0.2 M Tris (pH 8.0) on ice. In sequence on ice, 100 μl of 0.2 M Tris (pH 8.0), 1 M sucrose, 10 μl of 10 mM EDTA (pH 8.0), 10 μl of lysozyme (2 mg/ml), and 320 μl of distilled water were added with gentle mixing after each addition. The mixture was incubated for 10 min at room temperature. Then 10 μl of DNase (bovine pancreatic DNase I; 1 mg/ml in 0.15 M NaCl, 50% glycerol; Sigma) and 500 μl of 2% Triton X-100, 10 mM MgCl2, 50 mM Tris (pH 8.0) were added. The solution was centrifuged at 16,000 × g for 30 min at 4°C. The supernatants (cytoplasmic proteins) were suspended in 2× Laemmli's sample buffer. After removal of the supernatants, the pellets were washed four times with ice-cold distilled water and then resuspended in 100 μl of 2× electrophoresis sample buffer.
For purification of supernatant (secreted proteins), 100-ml cultures were incubated for 16 to 18 h at 37°C on a rotary shaker at 225 rpm. Culture supernatants were recovered by centrifugation and filtered. Ammonium sulfate was dissolved to a final concentration of 60% saturation. The precipitate was centrifuged and dissolved in 3 ml of 50 mM Tris-HCl, pH 7.5, and this preparation was dialyzed against 50 mM Tris-HCl (pH 7.5). This resulted in an approximately 10-fold concentration of the original supernatant. The protein preps were analyzed by separation on a sodium dodecyl sulfate (SDS)-4 to 15% polyacrylamide gel (Ready Gel; Bio-Rad Laboratories, Inc. Hercules, Calif.) and stained with Coomassie blue.
Western blot.
Ten microliters of proteins (approximately 6.5 mg/ml) were loaded to each well of a 4 to 15% SDS-polyacrylamide gel electrophoresis (PAGE). Electrophoresis was carried out at 200 V for 30 to 60 min. The separated proteins were then transferred to a nitrocellulose membrane at 100 V for 1 h using the Mini-Trans-Blot electrophoretic transfer cell (Bio-Rad Laboratories). The membranes were then blocked overnight at 4°C with 3% dry milk in Tris-buffered saline (TBS) solution. Serum samples were diluted 1:200 in a diluent wash (3% dry milk in TBS).
The serum positive for antibodies against B. henselae used for Western blotting was pooled from 68 cases of suspected cat scratch disease cases that were submitted to ARUP laboratories (Salt Lake City, Utah) for confirmative diagnosis. These sera had immunoglobulin G (IgG) titers ranging from 1:512 to 1:4,096 for B. henselae, as determined by indirect immunofluorescent assay (IFA) (Focus Technologies, Cypress, Calif.). The IFA titer of the pooled specimen was 1:1,024. The B. henselae antibody-negative sera used as a control for Western blotting had titers of <1:64 by IFA. The procedures followed were in accord with the ethical standards established by the University of Utah and are in accord with the Helsinki Declaration of 1975. Specimens were collected under approval by the University of Utah Institutional Review Board (IRB no. 11343). Specimens were stored at −20°C until testing commenced and were then stored at 2 to 8°C while the evaluations were performed.
The serum was preadsorbed with E. coli XL1-Blue (containing SuperCos1 vector) whole-cell lysate. The diluted primary antibody solution was incubated with a 1:10 (vol/vol) dilution of the lysate for 30 min with shaking at 37°C to reduce background antibodies reacting against E. coli proteins.
After preadsorption, the sera were reacted with the membrane containing the protein samples for 3 h on a rocking platform. The membrane was then washed three times, changing the diluent wash every 5 min. A 1:10,000 dilution of alkaline phosphatase goat anti-human IgG (γ-chain-specific) conjugate (Sigma) was then added to the membranes. The membrane was then incubated for 1 h and washed as before, before the addition of 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) substrate (Sigma). This reaction was carried out for 5 to 10 min and then stopped by the addition of distilled water.
DNA sequencing.
The DNA sequence was determined by the AB 3700 96 capillary DNA Analyzer from Applied Biosystems. Synthetic oligonucleotides used as primers for DNA sequencing were synthesized by the Huntsman Cancer Center DNA peptide facility, University of Utah.
DNA and protein database searches.
The National Center for Biotechnology Information Services were used to consult the SwissPROT, GenBank, and EMBL databases with the BLAST algorithm (1, 20). Prediction of the signal peptide cleavage site was performed using the SignalP 3.0 Server, http://www.cbs.dtu.dk/services/SignalP/ (2).
Nucleotide sequence accession number.
The GenBank accession number for the sequence presented in this article is AY695890.
RESULTS
Demonstration of the CAMP effect (cohemolysis) in a B. henselae ATCC 49793 strain.
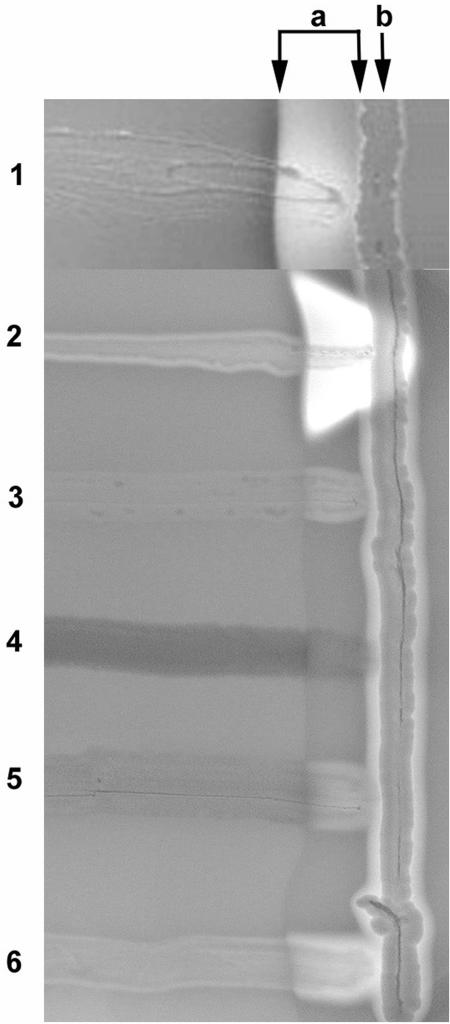
We analyzed B. henselae for cohemolytic activity by growing the organism on fresh Columbia 5% sheep blood agar plates for 7 days at 35°C under 5% carbon dioxide. After 7 days of growth, a sphingomyelinase-producing S. aureus indicator strain was streaked vertically on the surface of the blood agar perpendicular to the B. henselae streak and incubated an additional 18 h at 37°C. The B. henselae strain showed distinct cohemolytic zone of increased hemolysis within the diffusion zone (a) of the S. aureus strain after 18 h of growth of the S. aureus strain (Fig. 1, lane 1).
FIG. 1.
CAMP test with B. henselae and E. coli XL1-Blue MR recombinant strains. Strains were grown perpendicular to the S. aureus streak (b). The diffusion zone of the sphingomyelinase produced by S. aureus is indicated with two arrows (a). The B. henselae strain shows the CAMP effect with increased hemolysis next to the S. aureus streak (lane 1). The group B streptococcus control strain shows a large distinct zone with complete hemolysis next to the indicator strain (lane 2). The Listeria monocytogenes control strain shows a mild CAMP reaction (lane 3). The E. coli XL1-Blue MR strain containing the SuperCos1 cosmid vector shows no CAMP reaction (lane 4). The E. coli XL1-Blue strain containing the SuperCos1 cosmid pCML75 with the 29.7-kb insert of B. henselae chromosomal DNA shows a mild CAMP effect (lane 5). The E. coli XL1-Blue strain containing plasmid pCML76 with the 7.2-kb subclone with the entire cfa gene and promoter shows a distinct zone of complete hemolysis next to the S. aureus streak (lane 6).
Cloning and sequencing a cohemolysin gene from B. henselae ATCC 49793.
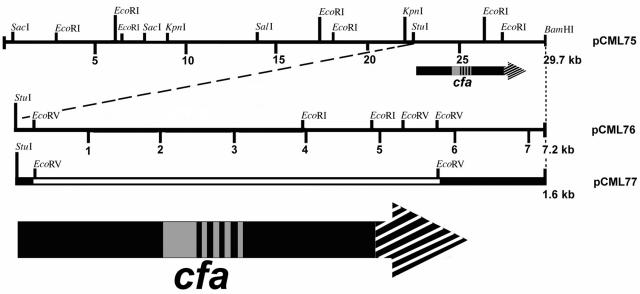
To clone the cohemolysin determinant in E. coli we constructed a cosmid library of B. henselae ATCC 49793 in SuperCos1 cosmid. Three-hundred cosmid clones were screened by the CAMP test. Eighteen cosmid clones exhibited at least a slight CAMP effect. Four cosmids showed the strongest CAMP activity similar to Listeria monocytogenes CAMP reaction and had similar restriction patterns when digested with HindIII, EcoRI, or BglII. One of these cosmids, pCML75, containing a 29.7-kb B. henselae chromosomal insert, was restriction mapped and sequenced (Fig. 2). DNA fragments of cosmid pCML75 were subcloned and tested for cohemolytic activity. Cohemolytic activity was isolated to a 7.2-kb fragment on one end of the DNA insert, from a StuI restriction site to the BamHI site within the multiple cloning site of a plasmid derivative of SuperCos1 (Table 1), pCML76 (Fig. 2).
FIG. 2.
Restriction map of the region near the cfa gene and positions of clones. The arrows represent the cfa gene with the arrowhead showing the direction of transcription. The diagonal hatch marks at the arrowhead represent the area of homology of the translated protein with the β-barrel region of proteins in the family of autotransporters. The grey area and vertical bars in the stem of the arrow represent homology of the translated protein with RTX (repeat in toxin) hemolysins. The vertical bars represent the four tandem nearly identical repeats in the amino acid sequence. Plasmid pCML77 contains the StuI-BamHI insert from pCML76 (solid dark line) with an internal 5.6-kb EcoRV-EcoRV deletion (open line) of the coding sequence of cfa.
Figure 1 illustrates examples of the CAMP effect from various bacterial species and the cosmid clones and plasmid subclones of B. henselae DNA in E. coli. The B. henselae strain exhibited CAMP activity, with an area of hemolysis within the sphingomyelinase diffusion zone. The control strain of group B streptococcus showed a large distinct zone of complete hemolysis, and the L. monocytogenes control strain showed a mild CAMP reaction. The E. coli XL1-Blue strain containing pCML75 with the 29.7-kb insert of B. henselae chromosomal DNA in SuperCos1 showed mild cohemolysis. The E. coli XL1-Blue strain containing plasmid pCML76 with the 7.2-kb subclone demonstrated moderately strong CAMP activity.
The B. henselae cohemolysin is homologous to two protein families, RTX hemolysins and autotransporters.
Sequence analysis of the 7.2-kb subclone demonstrated a 6,024-bp open reading frame coding for 2,008 amino acids. The nucleotide sequence is identical to nucleotide sequence at locus tag BH13030 of the recently sequenced B. henselae Houston strain-1 genome (GenBank accession no. YP034038). The gene was named cfa (for CAMP-like factor autotransporter). The open reading frame begins 160 bp downstream of a StuI restriction site. A putative Shine-Dalgarno sequence is located just 3 bp upstream from the initiating methionine. A signal sequence of 33 amino acids is predicted using the Signal P program.
When the deduced amino acid sequence was subjected to a BLAST similarity search the midportion of the protein and carboxy-terminal end of the protein shows separate homologies with calcium dependent RTX (repeat in toxin) hemolysins and the family of autotransporters, respectively. This homology suggests that like other autotransporters, the protein has three distinct domains: a signal peptide, a secreted α-domain, and a transporting β-domain. The precursor form of Cfa, including the leader sequence, presumed secreted α-domain and the transporting β-domain has a predicted molecular mass of 214,260 and calculated isoelectric point of 4.96. The presumed secreted α-domain has a calculated molecular mass of 179,493 and a calculated isoelectric point of 4.75. The predicted outer membrane transporting β-domain has a calculated molecular mass of 31,179 and isoelectric point of 8.98. In Fig. 2, the grey area and vertical bars in the arrow representing Cfa shows the region of homology of the translated protein with RTX family of (repeat in toxin) hemolysins and toxins. A range of 21 to 28% identity and 36 to 48% similarity is seen with a number of RTX toxins and hemolysins of Chromobacterium violaceum (GenBank accession no. AAQ57990), Novosphingobium aromaticivorans (ZP00303863), Magnetospirillum magnetotacticum (ZP00055704), Nitrosomonas europaea (CAD84072), and E. coli (AE016756), from amino acids 748 to 970. Figure 3A shows a comparison of part of this region of Cfa with the RTX hemolysins of Chromobacterium violaceum and Nitrosomonas europaea.
FIG. 3.
(A) Homology between the amino-terminal region of Cfa and RTX hemolysins of Nitrosomonas europea and Chromobacterium violaceum. (B) Homology between the carboxy-terminal region of Cfa and the autotransporters YchA of E. coli and Vag8 of B. pertussis. The numbers in parentheses indicate the position in the unprocessed protein of the first amino acid listed. Conserved amino acids between two proteins are indicated by colons, and substitutions of functionally similar amino acids are marked by periods.
From amino acid 824 to 991, there are four nearly identical tandem glycine- and serine-rich amino acid repeats comprised of the following 42 amino acids in single letter code: P-S-S-V-E-T-S-I/T-P-P-T/A-V-S-G/D-N-S-A-G-P-V-G/R-G-E/G-Q-S-A-S/P-V-A-R-S-E-S (D-G-G-V-T-V-V-L-S). Slashes and boldface lettering indicate the variant amino acids. The last 9 amino acids of the 42-amino-acid sequence (in parentheses) are only repeated three times instead of four. Although Cfa shows some similarity to RTX toxins and contains an area of glycine-rich repeats, the repeats are not similar to the glycine-rich repeat consensus sequence described for RTX family of toxins L/I/F-X-G-G-X-G-N/D-D-X (46).
A BLAST search for “short nearly exact matches” against amino acids 3 to 33 of the 42 listed above reveals 52% identity and 58% similarity to a repeating amino acid sequence found in Streptococcus gordonii platelet binding protein (serine-rich repeat protein) GspB (AAL13053).
The last 286 amino acids of the deduced amino acid sequence of cfa (from 1723 to 2008) shows homology to the family of autotransporters. A range of 23 to 28% identity and 38 to 46% similarity is seen with a number of autotransporter proteins including E. coli YchA (BAA97898), Bordetella pertussis Vag8 (AAC31247), Salmonella enterica serovar Typhimurium ShdA (AAD25110), S. enterica serovar Typhimurium MisL (AAD16954), and Yersinia pestis YapH (CAC14227). Figure 3B shows a comparison of part of this region of Cfa with the autotransporters, E. coli YchA and B. pertussis Vag8.
Expression of the cohemolysin protein in E. coli.
We developed a cohemolysin microtiter assay to quantitatively measure the amount of red blood cell hemolysis by unconcentrated bacterial supernatants in the presence of sphingomyelinase. We derived our protocol based on previously published hemolysis assays (19) and protocols for the pretreatment of tissue culture cells with sphingomyelinase to sensitize cells to the effects of toxins (35). Red blood cells untreated and treated with sphingomyelinase were tested in tandem to compare the hemolytic versus the cohemolytic activity of the bacterial supernatants (Table 2).
TABLE 2.
Hemolytic and cohemolytic activity of L. monocytogenes and E. coli containing various plasmid clones on erythrocytes of different species
| Blood source | Reactiona of erythrocytes with various bacterial species (untreated/treated with sphingomyelinase)
|
||||
|---|---|---|---|---|---|
| L. mono- cytogenes | E. coli (SuperCos1) | E. coli (pCML77) | E. coli (pCML75) | E. coli (pCML76) | |
| Sheep | 4/64 | −/− | −/− | −/32 | 8/128 |
| Human | 2/8 | −/− | −/− | −/− | −/− |
| Feline | 4/32 | −/− | −/− | −/16 | 4/32 |
Reciprocal of endpoint titer or no hemolysis (−).
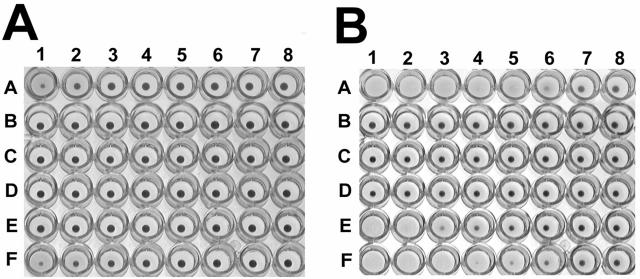
Panel A of Fig. 4 shows the results of a microtiter hemolysis assay of the bacterial supernatants without pretreatment of sheep red blood cells with sphingomyelinase. No agglutination of the sheep red cells was observed for any of the bacterial culture supernatants. Hemolytic activity of the culture supernatant from E. coli containing the original cosmid clone, pCML75 (Fig. 4A, row E), was minimal to none at a 1:2 dilution. The bacterial supernatant from L. monocytogenes showed slight hemolysis at an endpoint titer of 1:4 (Fig. 4A, row A). The bacterial supernatant from E. coli containing plasmid subclone pCML76 (cfa gene and its promoter region) showed lysis of the sheep red blood cells up to an endpoint titer of 1:8 (Fig. 4A, row F), as observed by a reduction in the size of the red blood cell pellet. No detectable hemolysis was observed with the bacterial supernatant of E. coli containing the subclone pCML77 (pCML76 with an internal 5.6-kb EcoRV-EcoRV deletion of cfa) or with the bacterial supernatant of E. coli containing the cosmid cloning vector SuperCos1.
FIG. 4.
(A) Microtiter assay of the hemolysin activity of various clones in E. coli XL1-Blue MR. Twofold serial dilutions of supernatants (except for row B) were added sequentially to columns 1 to 8 (1:2 to 1:256) in microtiter plates to a final volume of 100 μl with 1% (vol/vol) washed sheep erythrocytes in KRT. Row A contains supernatant of L. monocytogenes. Row B contains sheep erythrocytes with no added bacterial supernatant; row C contains supernatant of E. coli containing SuperCos1; row D contains supernatant of pCML77; row E contains supernatant of pCML75; row F contains supernatant of pCML76. (B) Microtiter assay of the cohemolysin activity of various clones in E. coli XL1-Blue MR. Same experiment as that for panel A, except that sheep erythrocytes were pretreated with 0.025 U/ml of sphingomyelinase for 30 min at 37°C prior to the addition of the bacterial supernatants. A and B are representative of at least three independent experiments.
Panel B of Fig. 4 shows the results of a microtiter cohemolysis assay of the bacterial supernatants with pretreatment of the sheep red blood cells with sphingomyelinase (0.025 U/ml) for 30 min. A trace amount of hemolysis was observed directly around the red blood pellet of all the treated cells, including the red cell controls without bacterial supernatant (Fig. 4B, row B), indicating a slight hemolytic effect with sphingomyelinase treatment alone. Hemolysis was observed to an endpoint titer of 1:64 for the L. monocytogenes positive control (Fig. 4B, row A). Hemolytic activity of the culture supernatant from E. coli containing the original cosmid clone, pCML75 (Fig. 4B, row E), was enhanced with sphingomyelinase treatment to an endpoint titer of 1:32. The bacterial supernatant from E. coli containing plasmid subclone pCML76 (cfa gene and its promoter region) also showed enhanced lysis with sphingomyelinase pretreatment with lysis of the sheep red blood cells up to an endpoint titer of 1:128 (Fig. 4B, row F), as observed by a reduction in the size of the red blood cell pellet. No hemolysis compared to negative control was observed with the bacterial supernatant of E. coli containing the subclone pCML77 (pCML76 with an internal deletion of cfa) or with the bacterial supernatant of E. coli containing the cosmid cloning vector SuperCos1.
We also performed the microtiter assay on human and feline erythrocytes (Table 2). Human erythrocytes have been reported not to undergo CAMP hemolysis (8). We observed only slight hemolysis of the human erythrocytes without pretreatment with sphingomyelinase at a 1:2 dilution of the L. monocytogenes supernatant. Hemolysis was enhanced in human erythrocytes treated with sphingomyelinase to an endpoint titer of 1:8 for L. monocytogenes. No detectable hemolysis of the untreated or treated human erythrocytes was observed with any of the E. coli strains containing plasmid clones. The hemolysis and cohemolysis microtiter assay using feline erythrocytes showed titers that were two- or fourfold lower than what was observed with sheep erythrocytes.
Protein and Western blot analysis of the cohemolysin protein in E. coli.
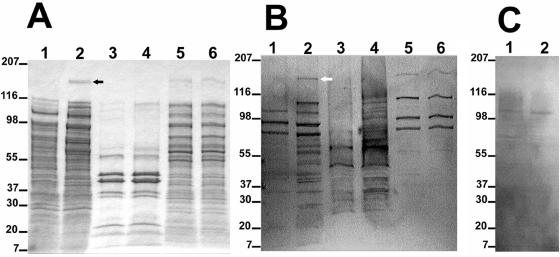
We chose to examine the expression of Cfa in E. coli by SDS-PAGE and Western blot due to the slow growth of B. henselae in liquid and on solid media. Concentrated culture supernatants, outer membrane proteins, and cytoplasmic proteins from E. coli containing the SuperCos1 cloning vector and the plasmid pCML76 containing the cfa clone was subjected to SDS-PAGE and Western blot analysis (Fig. 5). A protein band of an apparent molecular mass of 180 kDa was observed in the concentrated supernatant of the E. coli containing the cfa clone (pCML76; Fig. 5A, lane 2) not seen in the concentrated supernatant of the E. coli strain containing SuperCos1 (Fig. 5A, lane 1). A similar size band was observed in the original cosmid clone pCML75 (data not shown). The observed 180-kDa band is consistent with the molecular weight of the predicted mature secreted protein (α-domain) of Cfa. Slightly higher molecular weight bands are observed in the cytoplasmic proteins of both the E. coli containing SuperCos1 and pCML76 (Fig. 5A, lanes 5 and 6). The β-domain of Cfa with the predicted molecular mass of 31.2 kDa would be expected to be observed in the outer membrane of the bacterial cell. The outer membrane protein preparations of the E. coli containing SuperCos1 and E. coli containing pCML76, however, appeared identical with no additional proteins bands observed in this region.
FIG. 5.
(A) SDS-PAGE of secreted, outer membrane, and cytoplasmic proteins stained with Coomassie blue. Lane 1, 10× concentrated supernatant of E. coli containing SuperCos1; lane 2, 10× concentrated supernatant of E. coli containing pCML76 (cfa clone); lane 3, outer membrane protein, E. coli containing SuperCos1; lane 4, outer membrane protein, E. coli containing pCML76; lane 5, cytoplasmic protein, E. coli containing SuperCos1; lane 6, cytoplasmic protein, E. coli containing pCML76. The numbers on the left indicate the positions of protein standards (in kilodaltons). The black arrow indicates the position of the 180-kDa protein. (B) Western blot transfer of panel A probed with a sera pool from 68 patients with cat scratch disease (IgG IFA titer ranging from 1:512 to 1:4,096 for B. henselae). The white arrow indicates the position of the 180-kDa immunogenic protein. (C) Western blot transfer of lanes 1 and 2 of panel A probed with patient serum negative for B. henselae antibodies (IgG IFA titer, <1:64).
A Western blot of the same proteins, probed with sera pool from 68 patients with cat scratch disease (IgG IFA titer ranging from 1:512 to 1:4,096 for B. henselae) showed reactivity against the E. coli proteins in addition to the 180-kDa protein band unique to the concentrated bacterial supernatant of E. coli containing the cfa clone (Fig. 5B, lane 2). The same Western blot probed with sera negative for B. henselae antibodies by IFA (titer < 1:64) did not react with the 180-kDa band (Fig. 5C, lane 2).
DISCUSSION
A diverse group of toxins, proteases, and phospholipases have been identified as CAMP factors, including phospholipase C of L. monocytogenes (33), Apx toxins of A. pleuropneumoniae (17), RTX toxins of Pasteurella aerogenes (31), cholesterol oxidase of Rhodococcus equi (34), and O-sialoglycoprotein endopeptidase ofRiemerella anatipestifer (10).
The cohemolysin Cfa of B. henselae is homologous to the family of autotransporters. Autotransporters (or proteins with type V secretion pathways) have three common characteristics, an N-terminal signal sequence for translocation across the inner membrane, a passenger domain (α-domain) that is transported to the cell surface, and a conserved C-terminal transporter (β-domain) that facilitates secretion across the outer membrane through a beta-barrel porin structure (24).
Although the β-domains of autotransporters are highly conserved, the passenger α-domains are widely divergent, reflecting their many different functions (22). Virulence determinants are often secreted to the bacterial cell surface or released into the outside environment. Numerous diverse virulence factors have been classified as autotransporter proteins including toxins proteases, lipases, hemagglutinins, and adhesions (22). A cohemolysin or hemolysin has yet to be characterized as an autotransporter protein.
The α-domain of Cfa shows some homology to the RTX (repeats in structural toxin) toxins, a class of pore-forming toxins found among various species of Pasteurella (31, 32) and Actinobacillus (6, 7, 18) that play an important role in pathogenesis. Pax of P. aerogenes (31) and the ApxI, ApxII, and ApxIII RTX toxins of A. pleuropneumoniae (17) have a cohemolytic phenotype. The RTX toxins, however, have a type I secretion system in which secretion requires three accessory proteins which comprise a channel spanning both the inner and outer membranes (13). Although Cfa has a series of glycine-rich amino acid repeats in the α-domain, these repeats do not fit the repeat consensus sequence described for the RTX family of toxins (46). Therefore, without the consensus repeats and the operon consisting of the genes involved in transport and posttranslational modification, Cfa cannot be considered an RTX protein.
On Western blot, the secreted α-domain of Cfa was recognized by a sera pool of 68 patients with suspected cat scratch disease, positive for B. henselae antibodies by IFA. Cfa could be a potential candidate for immunodiagnostic test for the identification of individuals with B. henselae antibodies. Further studies need to be performed to establish the sensitivity and specificity of this antigen in diagnostic tests for B. henselae antibodies.
The carboxy-terminal domain of Cfa is highly homologous to the β-domains of autotransporters, which form porin structures in the outer membrane to facilitate the secretion of the passenger domain. Several B. henselae hypothetical proteins (BH06590, BH13160, BH13180) also show a high degree of homology with the C-terminal end of Cfa. Based on the homology of Cfa with these β-domains, an outer membrane protein of approximately 31 kDa should be visualized on examination of the outer membrane protein of the cfa clone pCML76 (Fig. 5A, lane 4). No new protein, however, was identified on SDS-PAGE of the outer membrane different than the control strain containing the cloning vector SuperCos1 (Fig. 5A, lane 3). The protein may be either rapidly degraded or poorly expressed and thus not detected with Coomassie blue stain. Conversely, since the cfa gene was expressed in E. coli and not B. henselae, the protein may not be processed properly in this heterologous host. Because of the difficulty with absorbing out anti-E. coli antibodies, we also cannot rule out that there is not a reaction of the antisera with a protein corresponding to a 31-kDa band corresponding to the C-terminal protein. The lack of detection of the β-domain outer membrane in SDS-PAGE has been previously noted in other autotransporters, including the serine protease A of Neisseria meningitidis (45). Although the B. henselae CAMP factor shows homology to regions from several proteins designated as autotransporters, because we were unable to show the C-terminal protein in outer membrane preparations, we do not yet have proof that the 180-kDa protein is secreted in this manner.
Another argument against the Cfa being an autotransporter is that the passenger domain contains nine cysteines. Most passenger domains of autotransporter proteins contain very few cysteine residues that contribute to disulfide bond formation and protein folding (26). Some controversy exists whether folded proteins can be secreted through the C-terminal transporter protein (4, 23, 26, 27). There are examples where folded proteins can be secreted in this manner. The IcsA protein of Shigella forms disulfide bonds in the periplasm and is efficiently secreted by its native translocating unit (4). The secretion of proteins with secondary structure by the autotransporter mechanism, however, probably rarely occurs (23, 26).
The precise role of the B. henselae CAMP-like factor remains to be established. Cfa could potentially be a cytotoxin with activity against various types of cells, such as endothelial cells, epithelial cells, and leukocytes, in addition to erythrocytes. For example, ApxIII of A. pleuropneumoniae is strongly cytotoxic for alveolar macrophages and neutrophils (16). Additionally, bacterial sphingomyelinase has been found to sensitize human endothelial cells to Shiga toxin of enterohemorrhagic E. coli 0157:H7 (35). It is postulated that sphingomyelinase is released in the mammalian host from activated platelets and endothelial cells (39, 40, 43). Therefore, future studies of the cytotoxic effects of Cfa on other cell types should also include sphingomyelinase pretreatment. In summary, the CAMP phenomenon in association with the sphingomyelinase of S. aureus has served as a useful screen to identify a potential virulence factor of B. henselae, a novel cohemolytic autotransporter protein.
Acknowledgments
This work was supported by Public Health Service grant AI40067 from the National Institute of Allergy and Infectious Diseases to C.M.L. and the ARUP Institute for Clinical and Experimental Pathology.
Editor: A. D. O'Brien
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. L. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., H. Nielsen, G. Von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Bernheimer, A. W., R. Linder, and L. S. Avigad. 1979. Nature and mechanism of action of the CAMP protein of group B streptococci. Infect. Immun. 23:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandon, L. D., and M. B. Goldberg. 2001. Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA. J. Bacteriol. 183:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullock, W. O., J. M. Fernández, and J. M. Short. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 4:376-378. [Google Scholar]
- 6.Chang, Y. F., J. Shi, D. P. Ma, S. J. Shin, and D. H. Lein. 1993. Molecular analysis of the Actinobacillus pleuropneumoniae RTX toxin-III gene cluster. DNA Cell. Biol. 12:351-362. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y. F., R. Young, and D. K. Struck. 1989. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA 8:635-647. [DOI] [PubMed] [Google Scholar]
- 8.Christie, R., N. E. Atkins, and E. Munch-Petersen. 1944. A note on a lytic phenomenon shown by group B streptococci. Aust. J. Exp. Biol. 22:197-200. [DOI] [PubMed] [Google Scholar]
- 9.Cockerell, C. J., and A. E. Friedman-Kien. 1988. Epithelioid angiomatosis and cat scratch disease bacillus. Lancet i:1334-1335. [DOI] [PubMed] [Google Scholar]
- 10.Crasta, K. C., K. L. Chua, S. Subramaniam, J. Frey, H. Loh, and H. M. Tan. 2002. Identification and characterization of CAMP cohemolysin as a potential virulence factor of Riemerella anatipestifer. J. Bacteriol. 184:1932-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darling, C. L. 1975. Standardization and evaluation of the CAMP reaction for the prompt, presumptive identification of Streptococcus agalactiae (Lancefield group B) in clinical material. J. Clin. Microbiol. 1:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolan, M. J., M. T. Wong, R. L. Regnery, J. H. Jorgensen, M. Garcia, J. Peters, and D. Drehner. 1993. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann. Intern. Med. 118:331-336. [DOI] [PubMed] [Google Scholar]
- 13.Fath, M. J., and R. Kolter. 1993. ABC transporters: bacterial exporters. Microbiol. Rev. 57:995-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figura, N., and P. Guglielmetti. 1987. Differentiation of motile and mesophilic Aeromonas strains into species by testing for a CAMP-like factor. J. Clin. Microbiol. 25:1341-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser, G. 1962. The hemolysis of animal erythrocytes by Pasteurella haemolytica produced in conjunction with certain staphylococcal toxins. Res. Vet. Sci. 3:104-110. [Google Scholar]
- 16.Frey, J. 1995. Eotoxins of Actinobacillus peluropneumoniae, p. 101-113. In W. Donachie, F. A. Lainson, and J. C. Hodgson (ed.), Haemophilus, Actinobacillus, and Pasteurella. Plenum Press, New York, N.Y.
- 17.Frey, J., R. Kuhn, and J. Nicolet. 1994. Association of the CAMP phenomenon in Actinobacillus pleuropneumoniae with the RTX toxins ApxI, ApxII and ApxIII. FEMS Microbiol. Lett. 124:245-251. [DOI] [PubMed] [Google Scholar]
- 18.Frey, J., R. Meier, D. Gygi, and J. Nicolet. 1991. Nucleotide sequence of the hemolysin I gene from Actinobacillus pleuropneumoniae. Infect. Immun. 59:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gish, W., and D. J. States. 1993. Identification of protein coding regions by database similarity search. Nat. Genet. 3:266-272. [DOI] [PubMed] [Google Scholar]
- 21.Hantke, K. 1981. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 182:288-292. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, L. A. 1993. Cat-scratch disease in the United States. Am. J. Public Health 83:1707-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jose, J., F. Jahnig, and T. F. Meyer. 1995. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol. Microbiol. 18:378-380. [DOI] [PubMed] [Google Scholar]
- 27.Jose, J., J. Kramer, T. Klauser, J. Pohlner, and T. F. Meyer. 1996. Absence of periplasmic DsbA oxidoreductase facilitates export of cysteine-containing passenger proteins to the Escherichia coli cell surface via the Iga beta autotransporter pathway. Gene 178:107-110. [DOI] [PubMed] [Google Scholar]
- 28.Koehler, J. E., C. A. Glaser, and J. W. Tappero. 1994. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA 271:531-535. [DOI] [PubMed] [Google Scholar]
- 29.Kohler, W. 1988. CAMP-like phenomena of vibrios. Zentralbl. Bakteriol. Mikrobiol. Hyg. Ser. 270:35-40. [DOI] [PubMed] [Google Scholar]
- 30.Kordick, D. L., and E. B. Breitschwerdt. 1995. Intraerythrocytic presence of Bartonella henselae. J. Clin. Microbiol. 33:1655-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhnert, P., B. Heyberger-Meyer, J. Nicolet, and J. Frey. 2000. Characterization of PaxA and its operon: a cohemolytic RTX toxin determinant from pathogenic Pasteurella aerogenes. Infect. Immun. 68:6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo, R. Y., C. A. Strathdee, and P. E. Shewen. 1987. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect. Immun. 55:1987-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKellar, R. C. 1994. Use of the CAMP test for identification of Listeria monocytogenes. Appl. Environ. Microbiol. 60:4219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navas, J., B. Gonzalez-Zorn, N. Ladron, P. Garrido, and J. A. Vazquez-Boland. 2001. Identification and mutagenesis by allelic exchange of choE, encoding a cholesterol oxidase from the intracellular pathogen Rhodococcus equi. J. Bacteriol. 183:4796-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obrig, T. G., R. M. Seaner, M. Bentz, C. A. Lingwood, B. Boyd, A. Smith, and W. Narrow. 2003. Induction by sphingomyelinase of Shiga toxin receptor and Shiga toxin 2 sensitivity in human microvascular endothelial cells. Infect. Immun. 71:845-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podbielski, A., O. Blankenstein, and R. Lutticken. 1994. Molecular characterization of the cfb gene encoding group B streptococcal CAMP-factor. Med. Microbiol. Immunol. (Berlin) 183:239-256. [DOI] [PubMed] [Google Scholar]
- 37.Rocourt, J., and P. A. D. Grimont. 1983. Listeria welshimeri sp. nov. and Listeria seeligeri sp. nov. Int. J. Syst. Bacteriol. 33:866-869. [Google Scholar]
- 38.Rolain, J. M., S. Novelli, P. Ventosilla, C. Maguina, H. Guerra, and D. Raoult. 2003. Immunofluorescence detection of Bartonella bacilliformis flagella in vitro and in vivo in human red blood cells as viewed by laser confocal microscopy. Ann. N. Y. Acad. Sci. 990:581-584. [DOI] [PubMed] [Google Scholar]
- 39.Romiti, E., E. Meacci, M. Tani, F. Nuti, M. Farnararo, M. Ito, and P. Bruni. 2000. Neutral/alkaline and acid ceramidase activities are actively released by murine endothelial cells. Biochem. Biophys. Res. Commun. 275:746-751. [DOI] [PubMed] [Google Scholar]
- 40.Romiti, E., E. Meacci, G. Tanzi, L. Becciolini, S. Mitsutake, M. Farnararo, M. Ito, and P. Bruni. 2001. Localization of neutral ceramidase in caveolin-enriched light membranes of murine endothelial cells. FEBS Lett. 506:163-168. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Sander, A., S. Kretzer, W. Bredt, K. Oberle, and S. Bereswill. 2000. Hemin-dependent growth and hemin binding of Bartonella henselae. FEMS Microbiol. Lett. 189:55-59. [DOI] [PubMed] [Google Scholar]
- 43.Simon, C. G., Jr., S. Chatterjee, and A. R. Gear. 1998. Sphingomyelinase activity in human platelets. Thromb. Res. 90:155-161. [DOI] [PubMed] [Google Scholar]
- 44.Soedermanto, I., and C. Lämmler. 1996. Comparative studies on streptococci of serological group G isolated from various origins. Zentbl. Vetmed. Reihe B. 43:513-523. [DOI] [PubMed] [Google Scholar]
- 45.Turner, D. P., K. G. Wooldridge, and D. A. Ala'Aldeen. 2002. Autotransported serine protease A of Neisseria meningitidis: an immunogenic, surface-exposed outer membrane, and secreted protein. Infect. Immun. 70:4447-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch, R. A., M. E. Bauer, A. D. Kent, J. A. Leeds, M. Moayeri, L. B. Regassa, and D. L. Swenson. 1995. Battling against host phagocytes: the wherefore of the RTX family of toxins? Infect. Agents Dis. 4:254-272. [PubMed] [Google Scholar]
- 47.Welch, R. A., and L. N. Slater. 1999. Bartonella and Afipia, p. 638-646. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 48.Zangwill, K. M., D. H. Hamilton, B. A. Perkins, R. L. Regnery, B. D. Plikaytis, J. L. Hadler, M. L. Cartter, and J. D. Wenger. 1993. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N. Engl. J. Med. 329:8-13. [DOI] [PubMed] [Google Scholar]