Abstract
During the systemic phase of murine infection with Salmonella enterica serovar Typhimurium, bacterial virulence is correlated with the ability to grow and survive within host macrophages. Salmonella pathogenicity island 2 (SPI-2), encoding a type three secretion system, has emerged as an important contributor to Salmonella intracellular growth. SPI-2 mutants have been proposed to be more accessible than wild-type Salmonella to oxyradicals generated by the NADPH phagocyte oxidase. We performed mixed infections of mice to investigate the relationship between SPI-2 and SlyA, a transcriptional regulator that confers resistance to oxyradicals. In mixed-infection experiments, the SPI-2 null mutant was severely attenuated in virulence, whereas slyA mutants were only mildly attenuated. Surprisingly, further experiments indicated that the function of SPI-2 was partially dependent on slyA. The intracellular behavior of a slyA mutant in infected cells was consistent with inefficient SPI-2 expression, as formation of Salmonella-induced filaments and the intracellular F-actin meshwork, features that depend on SPI-2, were present at abnormally low frequencies. Furthermore, the translocated levels of the SPI-2 effector SseJ were severely reduced in a strain carrying a mutation in slyA. We used flow cytometry to investigate the role of SlyA in expression of green fluorescent protein (GFP) from transcriptional fusions with promoters of either of two other SPI-2 effector genes, sifB and sifA. The slyA mutant exhibited reduced GFP expression from both promoters. Combining mutations in slyA and other regulators of SPI-2 indicated that SlyA acts through the SsrAB two-component regulatory system. SlyA exhibits partial functional redundancy with OmpR-EnvZ and contributes to the transcriptional response to low osmolarity and the absence of calcium, two environmental stimuli that promote SPI-2 gene expression.
Salmonella enterica serovar Typhimurium is the causative agent of a systemic disease in mice that resembles human typhoid fever. S. enterica serovar Typhimurium replicates within a membrane-bound compartment, the Salmonella-containing vacuole, in macrophages of the murine liver and spleen (24, 25). A pathogenicity island, Salmonella pathogenicity island 2 (SPI-2), contains genes encoding the components of a virulence-associated type three secretion system (TTSS) which translocates effector proteins into and across the Salmonella-containing vacuole membrane (23, 27, 36). Mutant strains with a defective SPI-2 TTSS do not modify their intracellular niche in the same way that wild-type Salmonella does (reviewed in reference 19), replicate poorly in macrophages, and are attenuated for virulence (6, 14, 23, 25).
Genes encoding structural components of the TTSS are located in SPI-2 (23, 27), but the genes encoding secreted effector proteins are located both in SPI-2 and elsewhere in the Salmonella chromosome (reviewed in reference 36). Expression of both sets of genes is controlled by a two-component regulatory system, SsrAB (3, 4, 6, 10, 16, 21, 37). Expression of SsrAB is in turn regulated by the OmpR-EnvZ two-component system, by direct binding of OmpR to the SsrAB promoter (17). SPI-2 gene expression is induced inside host cells (6), indicating that the regulatory systems are sensitive to the intravacuolar environment. In Salmonella cultured in vitro, SPI-2 gene expression is induced by low osmolarity (12, 17) and a low calcium concentration (9, 12) in the growth medium, conditions that may mimic the lumen of the Salmonella-containing vacuole. The induction of SPI-2 genes in response to these stimuli is completely dependent on SsrAB but only partially dependent on OmpR-EnvZ (12).
SlyA is a virulence-associated transcriptional regulator and was originally identified by its ability to induce expression of a cryptic hemolysin when it is expressed in Escherichia coli (18). A consensus DNA-binding sequence for the Salmonella SlyA protein has been defined as TTAG-N4-CTAA (31). A Salmonella strain carrying an insertional mutation in slyA was profoundly attenuated in murine infection after either oral or parenteral inoculation (18). Independent insertion mutants of slyA were impaired for the ability to lyse M cells following oral inoculation and for survival in Peyer's patches (7). In vitro, slyA mutants are compromised for intramacrophage survival (18), adherence to or extracellular survival on macrophages (31), resistance to H2O2 (5), and resistance to the antimicrobial peptides magainin 2 and polymyxin B (29). Hypersensitivity of slyA mutants to polymyxin B is due to a lack of expression of ugtL (29), whose gene product is required for modification of lipid A (28). Other proteins whose expression is positively regulated by SlyA include FliC, IroN, PagC, OmpD, and GroEL (30, 31). It is not known if these proteins and UgtL account for the attenuation of the slyA mutant.
In this paper, we report on the relationship between SlyA and SPI-2. We postulated that these two virulence systems may act redundantly to overcome oxidative stress encountered in host phagocytes. SlyA is required for resistance of Salmonella to oxidants (5), and SPI-2 mutants have been reported to be unable to exclude the NADPH phagocyte oxidase from the Salmonella-containing vacuole membrane, making them more accessible to oxidants than wild-type Salmonella is (34). However, we obtained no evidence of redundancy between SlyA and SPI-2, but we made the unexpected discovery that part of the contribution of SlyA to virulence is dependent on SPI-2 and that slyA mutants exhibit a phenotype intermediate between the wild-type phenotype and the SPI-2 mutant phenotype in infected cells. We show here that SlyA regulates SPI-2-associated genes in vitro, and we also report on the relationships between SlyA and the SsrAB and OmpR-EnvZ two-component regulatory systems.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Double- and triple-mutant strains were constructed by P22 phage-mediated transduction. Bacteria were grown in Luria-Bertani (LB) medium or a medium based on magnesium minimal salts medium (MgM). MgM contains 170 mM 2-(N-morpholino)ethanosulfonic acid (MES), pH 7.5, 5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 8 μM MgCl2, 38 mM glycerol, and 0.1% Casamino Acids (15). An MgM variant with high osmolarity also contained 20% wt/vol sucrose, and a high-calcium variant was supplemented with CaCl2 to a concentration of 2 mM. Antibiotics were added at the following concentrations, as appropriate (for strains resistant to the antibiotics [Ampr, Kmr, Cmr, and Tetr]): carbenicillin, 50 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 34 μg/ml; and tetracycline, 25 μg/ml. Bacteria were grown at 37°C with shaking (150 rpm).
TABLE 1.
Strains and plasmids used in this study
| Name | Description | Source or reference |
|---|---|---|
| S. enterica Typhimurium strains serovar | ||
| 12023s | Wild type | NTCCa |
| HH119 | ssaV::aphT (Kmr) | 9 |
| SAL1 | ΔslyA::Cmr | This study |
| SAL2 | ssaV::aphT, ΔslyA::Cmr | This study |
| P3F4 | ssrA::mTn5 (Kmr) | 13 |
| HH182 | ompR1009::Tn10 (Tetr) | 3 |
| HJG1 | ssrA::mTn5, ompR1009::Tn10 | 12 |
| SAL3 | ΔslyA::Cmr, ssrA::mTn5 | This study |
| SAL4 | ΔslyA::Cmr, ompR1009::Tn10 | This study |
| Plasmids | ||
| pKD3 | repR6K γ Apr FRT Cmr FRT | 8 |
| pKD46 | reppSC101ts Apr paraBAD γ β exo | 8 |
| pFPV25.1 | Plasmid containing gfpmut3A under control of constitutive promoter (Ampr) | 33 |
| pFPV25 | Derivative of pFPV25 with promoterless gfpmut3a (Ampr) | 32 |
| pID836 | Derivative of pFPV25, containing gfpmut3a under control of sifB promoter (Ampr) | 12 |
| pID835 | Derivative of pFPV25, containing gfpmut3a under control of sifA promoter (Ampr) | 12 |
| psseJ2HA | Derivative of pWSK29, containing sseJ under control of its own promoter (Ampr) | 35 |
NTCC, National Type Culture Collection.
Plasmid psseJ2HA is a derivative of pWSK29 (35) carrying the sseJ2HA gene under the control of the sseJ promoter. A DNA fragment including the promoter and encoding double hemagglutinin (HA)-tagged SseJ was amplified by PCR from S. enterica serovar Typhimurium HH217 (38) genomic DNA using primers sseJpt1 (5′-ATCGAATTCTCGGATGATAAGTTTGTC) and 2HAr (5′-ATGAGCTCTTACTAGAGGCTAGCATAATCAG). The PCR product, containing terminal EcoRI and SacI sites, was digested and ligated into the same sites of pWSK29, generating psseJ2HA.
Construction of the slyA mutant strain.
Deletion of the slyA gene was performed by the method of Datsenko and Wanner, using primers MUTslyA1H1P1(5′-CTAATTATAAGGAGATGAAATTGGAATCGCCACTAGGTTCGTGTAGGCTGGAGCTGCTTC-3′) and MUTslyA1H2P2 (5′-CACCTCAATCGTGAGAGTGCAATTCCATAATATTGTGTTCCATATGAATATCCTCCTTAG-3′) to amplify the Cmr cassette from plasmid pKD3 and integrate the resulting PCR product into the chromosome of S. enterica serovar Typhimurium expressing the λ red recombinase from pKD46 (8). Deletion of the slyA gene was confirmed by PCR using primers SlyA1F (5′-AACCAGGCTTTACGTGTGG-3′) and SlyA1B (5′-AGCTACAGGTGCCAAGTG-3′), which flank the deleted region.
Mouse mixed infections.
Female BALB/c mice (B and K Universal Ltd., United Kingdom) that were 7 to 12 weeks old were used in accordance with United Kingdom Home Office regulations. To prepare the inocula, bacteria were first grown overnight in LB broth and then subcultured at a dilution of 1:100 for a further 2 h. Cultures were diluted to a concentration of 5 × 105 CFU/ml in physiological saline and mixed for intraperitoneal inoculation. Viable bacteria in inocula were quantified by dilution and plating onto LB agar plates with appropriate antibiotics to distinguish between strains. Mice were killed at 48 h postinoculation by cervical dislocation. The spleens were removed aseptically and homogenized in distilled water by mechanical disruption in a Colworth stomacher. Serial dilutions were plated on LB agar for CFU enumeration. Strains were distinguished by differential counting or replica plating on antibiotic-supplemented plates.
For each mouse, the competitive index (CI) or cancelled-out index (COI) was calculated by dividing the output ratio (i.e., a− versus wild type or a−b− versus b−) divided by the input ratio. The logarithms of CI values were used to calculate means and in statistical analyses. Student's t test was used to analyze the log10 COI of the double-mutant versus single-mutant strains (i.e., a−b− versus b−) with the null hypothesis that the mean log10 COI was equal to the log10 CI of the single mutant versus the wild type (a− versus wild type). Confidence intervals of the means were also calculated to test the null hypothesis that the mean log10 CI was equal to 0. P values of <0.05 were considered significant.
Cell culture.
HeLa (clone HtTA1) cells were kindly provided by H. Bujard (Heidelberg, Germany), and the Swiss 3T3 murine fibroblast cell line was provided by G. Tran van Nhieu (Institut Pasteur, Paris, France). Cells were grown in Dulbecco's modified Eagle's medium with Glutamax supplemented with 10% fetal calf serum at 37°C in 5% CO2.
Immunofluorescence microscopy and phenotypic analysis of infected host cells.
HeLa and 3T3 cells were seeded onto glass coverslips (diameter, 12 mm) in 24-well plates at a density of 5 × 104 cells per well 24 h before infection. Bacteria were incubated for 16 h at 37°C with aeration, diluted 1:33 in fresh LB broth, and incubated in the same conditions for 3.5 h. Cultures were diluted in Earle's buffered salt solution (pH 7.4) and added to the cells at a multiplicity of infection of 100:1. The infection was allowed to proceed for 15 min at 37°C in 5% CO2. The monolayers were washed twice with Dulbecco's modified Eagle's medium containing fetal calf serum and 100 μg gentamicin ml−1 and incubated in this medium for 1 h, after which the gentamicin concentration was decreased to 16 μg ml−1 for the following 8 to 10 h; then the monolayers were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS). For F-actin and Salmonella-induced filament (Sif) detection, Salmonella strains were visualized by introduction of plasmid pFPV25.1 carrying gfpmut3a under the control of a constitutive promoter (32). For detection of translocated effectors in HeLa cells, coverslips were washed twice in PBS containing 0.1% saponin and incubated for 1 h with the HA.11 antibody (1:200 dilution; Covance, Babco) to detect the HA tag and with anti-Salmonella goat polyclonal antibody CSA-1 (3) in 10% horse serum and 0.1% saponin in PBS. Cells were washed twice with 0.1% saponin in PBS and incubated for 30 min with the secondary antibodies (donkey anti-mouse rhodamine red-X conjugated or donkey anti-goat cy2-conjugated; both at a dilution of 1:200; Jackson ImmunoResearch). Coverslips were washed twice in 0.1% saponin in PBS, once in PBS, and once in H2O and were mounted on Mowiol. Samples were analyzed using a Zeiss LSM 510 confocal laser scanning microscope. To detect Sifs, HeLa cells were labeled for LAMP-1 with mouse monoclonal antibody H4A3 and subsequently with rhodamine red-conjugated donkey anti-mouse polyclonal antibody (3). 3T3 cells were labeled for F-actin with Texas Red-conjugated phalloidin as described previously (20). Infected HeLa cells were scored for the presence of Sifs, and Salmonella microcolonies in infected 3T3 cells were scored for association with an F-actin meshwork using an Olympus epifluorescence microscope. The percentages of each strain that were positive were recorded for several independent experiments; data are reported below as means ± standard errors of the means. The percentages of slyA mutants that were positive for Sifs and F-actin were compared with percentages of other strains that were positive. A two-sided z test was used to test the null hypothesis that means were equal. P values of <0.05 were considered significant.
Flow cytometric analysis.
S. enterica serovar Typhimurium strains carrying pFPV25, pID835, or pID836 were centrifuged at 4,000 × g after overnight growth in MgM or its variants, and the pellets were resuspended in PBS. A total of 105 bacterial cells from each sample were analyzed for green fluorescent protein (GFP) fluorescence as previously described (12) using a FACS Calibur cytometer (Becton Dickinson) with CellQuest software. Geometric mean GFP fluorescence values were converted to log10 values for further analysis to facilitate comparison of differences between groups.
In experiments to investigate the relationship between different gene regulators of SPI-2, log10 differences in GFP expression between strains were calculated in each experiment. These differences between strains were averaged over several experiments, and the mean difference and associated standard error of the mean were determined from the collated data. Log10 differences between strains were expressed as means ± standard errors of the means.
When the contributions of different gene regulators were compared for strains grown in MgM and in a variant of MgM, differences between strains were quantified for each growth condition as described above. The mean difference between growth conditions was determined by subtracting one mean from the other, and the associated standard error of the difference was determined by the method of combining errors. Confidence intervals based on the t distribution were constructed from the standard error. In addition, log10 fluorescence values of strains grown in an MgM variant were subtracted from values of strains grown in MgM for each experiment in order to generate groups appropriate for statistical comparison.
In both types of experiment, groups were compared using Student's t test to test the null hypothesis that means were equal. Confidence intervals of the means were constructed to test the null hypothesis that the mean was equal to 0. P values of <0.05 were considered significant.
RESULTS
Relationship between SlyA and SPI-2 function.
We constructed a stable deletion-insertion mutant of slyA (ΔslyA) and performed CI experiments to assess its virulence in a systemic murine infection. The CI was defined as the ratio for the mutant and wild-type strains harvested from infected tissue divided by the ratio for the same strains in the inoculum and indicated how competitive a mutant was compared with the wild type. The slyA mutant was mildly attenuated for virulence by this assay; the log10 CI was −0.75 ± 0.04 (n = 6) for Salmonella recovered from spleens of BALB/c mice at 48 h postinoculation.
We sought to determine whether SlyA and SPI-2 act redundantly in Salmonella virulence or whether there is some other functional relationship between these systems. When two virulence genes are functionally dependent on each other, mutation of the second gene is unlikely to affect the virulence of a strain in which the first gene is also mutated (3). Yet when two genes contribute independently to virulence, the effects of mutation of individual genes are likely to be additive and a double mutant is likely to be more attenuated than either single mutant (26). If the effect of combining mutations in individual genes is greater than additive, this indicates that there is functional redundancy (1). Because of this, independent, dependent, and redundant virulence genes can be distinguished in vivo. To investigate whether SlyA and SPI-2 interact in vivo, we used a mixed-infection method with mice (3) called the COI, which is based on the CI (for a review see reference 2). Comparison of the CI of a strain bearing a single mutation (i.e., a− versus wild type) with the COI of a double mutant versus the single mutant (i.e., a−b− versus b−) indicates the extent and nature of any relationship between one virulence gene and another (2, 3). In these experiments we used the SPI-2 ssaV null mutant, which lacks an essential gene product of the TTSS (9), the slyA mutant, and an ssaV slyA double mutant generated by phage transduction of the ssaV mutation to the slyA mutant.
The CI of ΔslyA versus the wild type was lower than the COI of the ΔslyA ssaV double mutant versus the ssaV single mutant (P < 0.005) (Fig. 1), indicating that the function or action of slyA in virulence depends to some degree on SPI-2. However, the slyA ssaV double mutant was more attenuated than the ssaV mutant (P < 0.005) (Fig. 1), indicating that slyA also contributes to virulence independent of SPI-2. Conversely, the CI of the ssaV mutant versus the wild type was lower than the COI of the ΔslyA ssaV double mutant versus the ΔslyA mutant (P < 0.05) (Fig. 1), again indicating the possibility of a functional relationship between slyA and SPI-2.
FIG. 1.
Relationship between SlyA and SPI-2 in murine virulence examined by mixed infection. BALB/c mice were inoculated intraperitoneally with approximately 2 ×105 CFU, and CI and COI were determined at 48 h postinoculation. Mean log10 CI and COI are indicated on the y axis for the strain combinations indicated on the x axis. The error bars indicate the standard errors of the means (n = 6). Student's t test was used to test the null hypothesis that the means were equal. Confidence intervals were constructed to test the null hypothesis that the mean was 0. Three asterisks indicate that the P value is <0.001; two asterisks indicate that the P value is <0.005; and one asterisk indicates that the P value is <0.05.
The mild virulence attenuation of our ΔslyA mutant as assessed by CI appears to contrast with a previous report that a slyA insertion mutant was strongly attenuated after single-strain intraperitoneal inoculation of BALB/c mice (18). Attempts to complement a slyA mutation with a plasmid-borne copy of the wild-type allele have met with only partial success (18), possibly because overproduction of the protein leads to attenuation (7). Therefore, to confirm the CI and COI results, we generated an independent ΔslyA mutant and used this mutant to construct a double ssaV ΔslyA mutant by transduction. Using these strains, we again found that the ΔslyA mutant was mildly attenuated and that the CI of the ΔslyA mutant versus the wild type was lower than the COI of the ΔslyA ssaV double mutant versus the ssaV single mutant (P < 0.05; n = 4; log10 CI = −0.60 ± 0.02; log10 COI = −0.47 ± 0.03).
slyA mutants exhibit an intermediate phenotype in SPI 2-dependent intracellular processes.
The partial interdependence of the SlyA and SPI-2 functions in murine virulence might be explained by SlyA contributing to the expression of SPI-2 genes. To address this possibility, we examined the ΔslyA mutant for the ability to induce two phenomena in cultured mammalian cells that are known to depend on the Salmonella SPI-2 TTSS. These phenomena are the formation of Sifs, which are tubular structures containing lysosomal membrane glycoproteins (11), and the formation of an actin meshwork around bacterial microcolonies (20). For comparison, we also examined wild-type and ssaV mutant strains and a strain that has a mutation in ompR, which encodes a regulator of SPI-2, and has been shown to be defective in Sif formation (3).
At 9 h postinvasion, an average of 78% ± 2% of wild-type Salmonella-infected HeLa cells were positive for Sifs when they were labeled with an anti-LAMP-1 monoclonal antibody, but only 1% ± 1% of cells infected with a strain carrying a mutation in ssaV were scored as positive (Fig. 2A and C). ompR and ΔslyA mutants exhibited intermediate phenotypes; Sifs were detected in 14% ± 5% and 29% ± 4% of the infected cells, respectively. The percentage of ΔslyA mutant-infected cells exhibiting Sifs was significantly different from the percentage of cells infected with the wild type exhibiting Sifs (P < 0.001) and the percentage of cells infected with the ssaV mutant exhibiting Sifs (P < 0.005). An independent ΔslyA mutant also generated Sifs in infected cells with a frequency intermediate between those of the wild type and the ssaV mutant (data not shown). A wild-type strain prepared at the same time as the ΔslyA mutant but mock transformed with water instead of PCR product behaved similar to the wild type with respect to Sif formation in HeLa cells (data not shown). Bacterial microcolonies were scored for F-actin meshwork formation in Swiss 3T3 cells infected for 9 h (Fig. 2B and D); 92% ± 3% of wild-type microcolonies were surrounded by a nest of polymerized actin, but only 9% ± 3% of ssaV mutants were positive. Again, both ompR and ΔslyA mutants exhibited intermediate phenotypes; 40% ± 1% and 49% ± 6% of the microcolonies were positive, respectively. The proportion of ΔslyA mutant Salmonella associated with F-actin was significantly different (P < 0.001) from the proportions for both the wild type and the ssaV mutant. We confirmed that an independent ΔslyA mutant also had an intermediate phenotype with respect to actin polymerization and that the wild-type strain subjected to the mock mutation procedure behaved similar to the original wild-type strain (data not shown).
FIG. 2.
Sif formation and F-actin reorganization induced by Salmonella are partially dependent on slyA. The images in panels A and B were collected using a confocal laser scanning microscope (LSM 510; Zeiss). (A) HeLa cells were infected for 9 h with GFP-expressing Salmonella (green), and Sifs were visualized by labeling with anti-LAMP-1 monoclonal antibody (red). Cells were infected with the wild type (WT) and ΔslyA mutants. Sifs are indicated by arrows in the wild-type-infected cell and a ΔslyA mutant-infected cell. The other ΔslyA mutant-infected cell was negative for Sif formation. Scale bar = 5 μm. (B) Swiss 3T3 cells were infected for 9 h with GFP-expressing Salmonella (green). F-actin was visualized by labeling with phalloidin (grey in upper panel; red in lower panel). Cells were infected with the wild type and ΔslyA mutants. The wild-type-infected cell and the ΔslyA mutant-infected cell in the middle panels exhibit F-actin meshworkformation. The ΔslyA mutant-infected cell in the right panel is negative for F-actin meshwork formation. (C) Percentage of infected HeLa cells exhibiting Sifs. The results are means ± standard errors of the means from at least three independent experiments. The percentages of ΔslyA mutant-infected cells that were scored positive were compared with the percentages for other strains using a two-sided z test with Bonferronni's correction to test the null hypothesis that the means were equal. Three asterisks indicate that the P value is <0.001; two asterisks indicate that the P value is <0.005; and NS indicates that the P value is >0.05. (D) Percentage of infected cells in which microcolonies containing between 4 and 24 bacteria were surrounded with F-actin. The results are means ± standard errors of the means of at least four independent experiments. The percentages of ΔslyA mutant microcolonies that were scored positive were compared with the percentages for other strains using a two-sided z test with Bonferronni's correction, as described above for panel C.
The inability of the ΔslyA mutant to induce Sifs or SPI-2-dependent F-actin meshwork formation at wild-type levels is consistent with a requirement for SlyA for efficient expression of the SPI-2 TTSS in vitro. Formation of Sifs and the F-actin meshwork around a proportion of ΔslyA mutants indicated that even in the absence of slyA there was some expression of SPI-2 genes.
Effects of a slyA mutation on translocation of SPI-2 effectors.
Figure 2 shows that the SPI-2 function was impaired in the ΔslyA mutant. This was investigated further by examining the translocation of a plasmid-encoded, double-HA-tagged version of the SPI-2 effector SseJ (SseJ-2HA) by wild-type and ΔslyA mutant bacteria. At 10 h postinvasion, the translocated effector was detected in over 80% of the HeLa cells infected with wild-type bacteria carrying the plasmid, but it was detected in less than 10% of the cells infected with the ΔslyA mutant carrying the same plasmid (Fig. 3A and B). Furthermore, even when translocated protein was detected in cells infected with the ΔslyA mutant, the overall levels were considerably lower than those in wild-type infected cells. Similar results were obtained with strains carrying a plasmid expressing the epitope-tagged effector SseF-2HA (results not shown).
FIG. 3.
Translocation of an SPI-2 effector is affected by a slyA mutation: confocal immunofluorescence analysis of HeLa cells infected for 10 h with wild-type (wt) and ΔslyA mutant strains expressing a plasmid-encoded double-HA-tagged SseJ. (A) Percentage of infected cells with detectable SseJ-2HA following permeabilization with 0.1% saponin. The results are the means ± standard errors of three independent experiments, in which over 50 infected cells were examined per experiment. (B) Representative images of translocated SseJ-2HA (red in merged images) and bacteria (green in merged images). Bacteria were labeled with a goat anti-Salmonella antibody, and a mouse anti-HA monoclonal antibody was used to detect translocated SseJ-2HA. The upper panel shows relatively high levels of translocated effector (observed in approximately 50% of cells infected with wild-type bacteria but never in cells infected with the ΔslyA mutant). The lower two panels show relatively low levels of translocated effector (observed in cells infected with wild-type and ΔslyA mutant bacteria).
SlyA acts in partial redundancy with OmpR to drive SsrA-dependent gene expression.
As SlyA is a transcriptional regulator, we next investigated whether it affects transcription from the promoter of the gene encoding another SPI-2 effector, SifB. Although sifB is located outside SPI-2 (21), like SPI-2 genes, its expression is controlled by the two-component regulatory system SsrAB (12, 21). The sifB promoter is responsive to a range of stimuli that affect SPI-2 gene expression (12). We analyzed PsifB::gfp expression by flow cytometry of wild-type and mutant Salmonella strains carrying pID836, a plasmid containing a transcriptional fusion of the sifB promoter and the gfp gene (12). Strains were grown in MgM, which results in high expression of SPI-2 genes (9) and strong PsifB::gfp expression in wild-type Salmonella (12). To identify any relationships between slyA and the regulator genes ssrA and ompR, we compared GFP expression in the relevant single and double mutants.
In six experiments, the geometric mean PsifB::gfp fluorescence was lower in the ΔslyA mutant than in wild-type Salmonella (P < 0.001) (Fig. 4). The PsifB::gfp expression levels of two other independent ΔslyA mutants were found to be indistinguishable from the expression level of the original ΔslyA mutant (data not shown). A control strain prepared by subjecting the wild type to a mock mutagenesis procedure behaved similar to the wild type (data not shown).
FIG. 4.
GFP expression from a PsifB::gfp transcriptional fusion, analyzed by flow cytometry of Salmonella strains grown overnight at 37°C in MgM. (A) Histogram plot of fluorescence intensity due to GFP (relative units) versus number of particles for 105 bacterium-sized particles of each strain analyzed. The peaks (from right to left) are plots of the following strains carrying PsifB::gfp: wild type (black); ΔslyA mutant (black, dashed); ompR mutant (red); ompR ΔslyA double mutant (red, dashed); ssrA mutant (blue); ssrA ΔslyA double mutant (blue, dashed). The leftmost peak (grey) is the nonfluorescent negative control (wild type carrying pFPV25, a promoterless gfp construct). The results are the results of a single representative experiment. (B) Geometric mean GFP fluorescence of the strains shown in panel A, using the same color coding. The error bars indicate the standard errors of the means (n = 6). WT, wild type.
SifA, like SifB, is an effector of SPI-2 TTSS whose expression is controlled by SsrAB (12, 21). To confirm that the reduced SPI-2-associated gene expression of the ΔslyA mutant is not restricted to the sifB promoter, PsifA::gfp expression was examined in strains carrying pID835, a plasmid containing a transcriptional fusion of the sifA promoter and gfp (data not shown). Significantly lower PsifA::gfp fluorescence was measured in the ΔslyA mutant than in the wild-type strain (P < 0.001; data not shown). Independent ΔslyA mutants generated PsifA::gfp fluorescence levels similar to those of the original, and the wild-type strain subjected to the mock mutagenesis procedure behaved like the wild-type strain with respect to PsifA::gfp expression (data not shown).
To investigate the relationship between SlyA and SsrA, we compared PsifB::gfp expression in ssrA mutant and ΔslyA ssrA double-mutant strains. The geometric mean PsifB::gfp fluorescence values of these strains were indistinguishable, indicating that SlyA-dependent gene regulation is mediated by SsrA (Fig. 4). The ompR mutant also exhibited reduced PsifB::gfp expression compared to the wild type (P < 0.001) (Fig. 4), but no reduction of expression was seen in an ompR ssrA double mutant compared to the ssrA single mutant (data not shown), which confirmed previous results (12).
To determine whether SlyA and OmpR have a functional relationship, we compared the log10 difference between PsifB::gfp expression in the wild-type versus the ΔslyA mutant with the log10 difference between PsifB::gfp expression in the ompR single mutant versus the ompR ΔslyA double mutant. The difference in PsifB::gfp expression between the wild type and the single mutant (0.30 ± 0.06) was substantially lower than the difference between the single and double mutants (0.67 ± 0.02; P < 0.001), indicating that deletion of slyA has a greater effect on PsifB::gfp expression if ompR is absent than if ompR is present (P < 0.001). The converse comparison was also made between the log10 difference in PsifB::gfp expression in the wild type versus the ompR single mutant and the log10 difference in PsifB::gfp expression in the ΔslyA single mutant versus the ompR ΔslyA double mutant. Again, the difference in PsifB::gfp expression between the wild type and the single mutant (0.27 ± 0.04) was less than the difference between the single and double mutants (0.64 ± 0.03; P < 0.001), indicating that deletion of ompR has a greater effect on PsifB::gfp expression if slyA is absent than if slyA is present. Together, these results indicate that SlyA and OmpR exhibit functional redundancy and that each partially compensates in the absence of the other.
SlyA and OmpR both contribute to induction of SPI-2 gene expression in response to environmental stimuli.
Two potent environmental stimuli that induce SPI-2 gene expression are a low calcium concentration and low osmolarity (9, 12, 17). The relative contributions of SsrAB and OmpR-EnvZ regulators to the transcriptional response to these stimuli have been determined by flow cytometric analysis of the wild type and single and double Salmonella mutants carrying PsifB::gfp (12). For each strain, the relative GFP expression levels were compared after culture in MgM and after culture in MgM supplemented with 2 mM CaCl2. Fluorescence measurements for strains grown in MgM were also compared with measurements after culture in a high-osmolarity medium, MgM supplemented with 20% sucrose. SsrAB was found to be essential for the induction of SPI-2 gene expression in response to a low calcium concentration and low osmolarity, whereas OmpR-EnvZ played a minor role (12). We used the same experimental approach to investigate the role of SlyA in the SsrA-dependent transcriptional response to a low calcium concentration and low osmolarity.
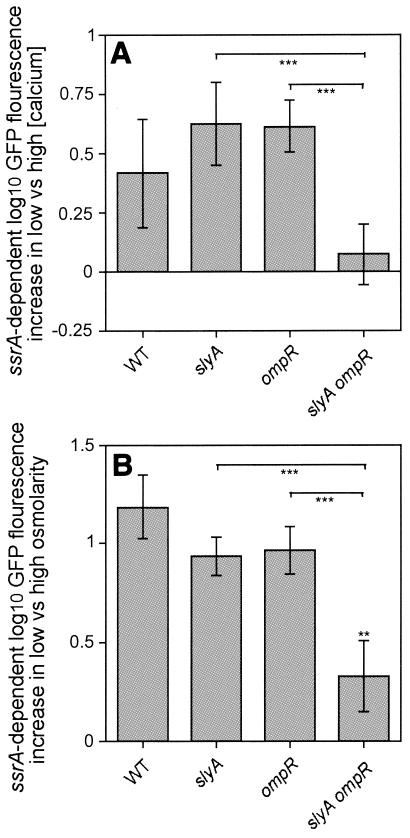
We found ssrA-dependent induction of PsifB::gfp expression in response to a low calcium concentration that was comparable in the wild-type and ΔslyA and ompR mutant strains (Fig. 5). In the ΔslyA ompR double mutant, no upregulation of PsifB::gfp expression was detectable in response to the low calcium concentration. The transcriptional response of the double mutant was significantly different from that of either single mutant (ΔslyA, P < 0.001; ompR mutant, P < 0.001), indicating that both regulators make a contribution. Only when both regulators were absent was the up-regulation of gene expression severely impaired. There was some evidence that the ΔslyA mutant was less able to respond to low osmolarity compared to the wild-type strain (P = 0.05); any reduction in the responsiveness of the ompR mutant compared to the wild type was marginal (P = 0.08). The ΔslyA ompR double mutant was weakly responsive to low osmolarity, inducing a significant (P < 0.01), albeit limited, increase in PsifB::gfp fluorescence. This increase was less than that produced by either single mutant (ΔslyA, P < 0.001; ompR mutant, P < 0.001). The loss of PsifB::gfp fluorescence between the ompR single mutant and the ΔslyA ompR double mutant (log10 difference, 0.64 ± 0.05) was greater than the loss between the wild type and the ΔslyA single mutant (log10 difference, 0.19 ± 0.08) (P < 0.005). Conversely, the ΔslyA ompR double mutant suffered a greater loss of PsifB::gfp fluorescence compared to the ΔslyA single mutant (log10 difference, 0.61 ± 0.05) than did the ompR single mutant compared to the wild type (log10 difference, 0.16 ± 0.09) (P < 0.005). Together, these data indicate that OmpR and SlyA exhibit partial redundancy in inducing transcription from the sifB promoter in response to low osmolarity.
FIG. 5.
ssrA-dependent increases in PsifB::gfp expression in MgM compared to the expression in high-calcium and high-osmolarity MgM variants for each of the following strains grown overnight at 37°C: wild type (WT), ΔslyA mutant, ompR mutant, and ΔslyA ompR double mutant. For each strain and each growth condition, the geometric mean GFP fluorescence was determined by flow cytometry. The log10 fluorescence of the ssrA mutant strain was subtracted from the log10 fluorescence values of each of the four strains to obtain a measure of the ssrA-dependent fluorescence of each strain. Differences between growth conditions were calculated as described in Materials and Methods, and the data indicate the means ± 95% confidence intervals (n = 5). Student's t test was used to test the null hypothesis that means were equal. Confidence intervals were constructed to test the null hypothesis that the mean was 0. Three asterisks indicate that the P value is <0.001, and two asterisks indicate that the P value is <0.01. (A) ssrA-dependent increases in PsifB::gfp expression in MgM compared to MgM supplemented with 2 mM Ca2+. (B) ssrA-dependent increases in PsifB::gfp expression in MgM compared to MgM supplemented with 20% sucrose.
DISCUSSION
Although SlyA has been shown to have a role in systemic virulence in mice (18) and to regulate a number of virulence determinants in vitro (29, 30, 31), it is not clear if the attenuation of the slyA mutant is caused by loss of these functions alone. In this paper we describe further phenotypic characterization of a ΔslyA mutant and show that one aspect of the SlyA function is to regulate expression of genes associated with the SPI-2 TTSS. Since slyA is not in an operon and the adjacent gene downstream of slyA is in the opposite orientation, it seems highly unlikely that deletion of the slyA gene and replacement with the Cmr cassette resulted in an effect on a downstream gene(s). The possibility that phenotypes associated with the ΔslyA mutant reported here are due to secondary unrelated mutations elsewhere in the chromosome is also highly unlikely since two independently constructed slyA mutants were found to be phenotypically indistinguishable.
The ΔslyA mutants were found to be mildly attenuated for virulence in competitive infections with the wild-type strain coinoculated via the peritoneal route. This contrasts with the high level of attenuation measured by the 50% lethal dose when a slyA mutant was administered as a single strain by the same route (18). One possible explanation for this difference is that the wild-type strain is able to complement the ΔslyA mutant in the mixed infection. If so, this would be unlikely to be mediated via SPI-2, as SPI-2 mutants are highly attenuated in mixed infections with the wild-type strain (3). Alternatively, the relatively short time in which the mixed infections are analyzed (48 h) might be insufficient for the virulence defect of the ΔslyA mutant to become fully apparent. A further possibility is that our parent wild-type strain used to construct the ΔslyA mutants carries a compensatory mutation elsewhere in the genome. In COI experiments, we found that a component of the attenuation of the ΔslyA mutant is dependent on SPI-2. Hypersensitivity to H2O2 (5) and antimicrobial peptides (29) could account for much or all of the SPI-2-independent attenuation of the ΔslyA mutant in systemic infections.
In experiments in which we quantified SPI-2-dependent effects on redistribution of host structures in infected cells, the behavior of the ΔslyA mutant was similar to that of a strain carrying a mutation in ompR, a gene encoding a known transcriptional regulator of SPI-2. Similar results emerged from experiments in which GFP expression from a transcriptional fusion with the sifB promoter was quantified by flow cytometry following growth of strains in vitro in defined SPI-2-inducing minimal medium. ΔslyA and ompR mutants exhibited reduced PsifB::gfp fluorescence compared to the wild-type strain, but they were still relatively competent in PsifB::gfp expression compared to a strain carrying a mutation in ssrA, the gene encoding the master regulator of SPI-2. The stimulatory effect of slyA on PsifB::gfp expression was completely dependent on ssrA. Likewise, ssrA is required for the actions of ompR (12; this study). ΔslyA mutants also exhibited reduced PsifA::gfp expression compared to wild-type Salmonella, as has been described previously for the ompR mutant (12).
The similarities in the phenotypes of ΔslyA and ompR mutants are linked by their convergence on SPI-2. Comparison of the abilities of wild-type and mutant Salmonella strains to induce PsifB::gfp expression indicated that slyA and ompR can partially compensate for each other. SlyA also has functional relationships with the virulence gene regulator, PhoP. SlyA and PhoP both footprint the ugtL promoter, and both are required for transcription of ugtL (29). PhoP also positively regulates expression of slyA (22) and binds to the slyA promoter (29), which itself is negatively regulated by binding of the SlyA protein (31). Biochemical analyses are required to determine how the functional redundancy between SlyA and OmpR is effected at the molecular level and whether SlyA binds to regulatory DNA sequences of ssrAB and/or the SPI-2 genes. Interestingly, Feng et al. reported recently (10) that an ssrA-lacZ fusion in E. coli can be stimulated in the presence of SlyA. Inspection of the S. enterica serovar Typhimurium genome sequence revealed the presence of a sequence, TTTAG-N8-CTAA, starting 38 bp downstream of the ssrA start codon, which is very similar to the SlyA-binding site in the ugtL promoter, TTTAG-N7-CTAA. Although it is unusual for a transcriptional activator to bind downstream of the start codon, SlyA does bind downstream of the transcription start site of ugtL (29).
The combined effects of slyA and ompR accounted for much of the induction of PsifB::gfp expression in response to two environmental stimuli, the absence of calcium and low osmolarity. However, there was significant, albeit limited, ssrA-dependent transcriptional responsiveness of the ΔslyA ompR double mutant to low osmolarity, indicating that yet another regulator must contribute to the stimulation of SPI-2 gene expression, in response to this stimulus at least. Further work is required to identify this regulator.
In this work, we showed that SlyA contributes to the regulation of SPI-2 function and that SlyA regulates expression of SPI-2-associated genes, partially overlapping in function with OmpR in dependence on SsrA. A search for evidence that SlyA might act redundantly with SPI-2 in overcoming oxidative stress encountered during murine infection led us instead to identify a partial functional dependence between SlyA and SPI-2. Further studies examining the role of SPI-2 in relation to oxidative stress avoidance will be described elsewhere.
Acknowledgments
This work was supported by grants from the Wellcome Trust and the Medical Research Council.
We thank Christoph Tang for critical reading of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Baumler, A. J., R. M. Tsolis, P. J. Valentine, T. A. Ficht, and F. Heffron. 1997. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect. Immun. 65:2254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 3:1345-1352. [DOI] [PubMed] [Google Scholar]
- 3.Beuzon, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumell, J. H., S. Kujat-Choy, N. F. Brown, B. A. Vallance, L. A. Knodler, and B. B. Finlay. 2003. SopD2 is a novel type III secreted effector of Salmonella typhimurium that targets late endocytic compartments upon delivery into host cells. Traffic 4:36-48. [DOI] [PubMed] [Google Scholar]
- 5.Buchmeier, N., S. Bossie, C. Y. Chen, F. C. Fang, D. G. Guiney, and S. J. Libby. 1997. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 65:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, J. J., I. B. Autenrieth, A. Ludwig, and W. Goebel. 1996. The gene slyA of Salmonella typhimurium is required for destruction of M cells and intracellular survival but not for invasion or colonization of the murine small intestine. Infect. Immun. 64:5075-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deiwick, J., and M. Hensel. 1999. Regulation of virulence genes by environmental signals in Salmonella typhimurium. Electrophoresis 20:813-817. [DOI] [PubMed] [Google Scholar]
- 10.Feng, X., D. Walthers, R. Oropeza, and L. J. Kenney. 2004. The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol. Microbiol. 54:823-835. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-del Portillo, F., M. B. Zwick, K. Y. Leung, and B. B. Finlay. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA 90:10544-10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garmendia, J., C. R. Beuzon, J. Ruiz-Albert, and D. W. Holden. 2003. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology 149:2385-2396. [DOI] [PubMed] [Google Scholar]
- 13.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 14.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 15.Hmiel, S. P., M. D. Snavely, C. G. Miller, and M. E. Maguire. 1986. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 168:1444-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knodler, L. A., J. Celli, W. D. Hardt, B. A. Vallance, C. Yip, and B. B. Finlay. 2002. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol. Microbiol. 43:1089-1103. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A. K., C. S. Detweiler, and S. Falkow. 2000. OmpR regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J. Bacteriol. 182:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. C. Fang, D. G. Guiney, J. G. Songer, and F. Heffron. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. USA 91:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linehan, S. A., and D. W. Holden. 2003. The interplay between Salmonella typhimurium and its macrophage host—what can it teach us about innate immunity? Immunol. Lett. 85:183-192. [DOI] [PubMed] [Google Scholar]
- 20.Meresse, S., K. E. Unsworth, A. Habermann, G. Griffiths, F. Fang, M. J. Martinez-Lorenzo, S. R. Waterman, J. P. Gorvel, and D. W. Holden. 2001. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell. Microbiol. 3:567-577. [DOI] [PubMed] [Google Scholar]
- 21.Miao, E. A., and S. I. Miller. 2000. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 97:7539-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norte, V. A., M. R. Stapleton, and J. Green. 2003. PhoP-responsive expression of the Salmonella enterica serovar Typhimurium slyA gene. J. Bacteriol. 185:3508-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salcedo, S. P., M. Noursadeghi, J. Cohen, and D. W. Holden. 2001. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell. Microbiol. 3:587-597. [DOI] [PubMed] [Google Scholar]
- 26.Shea, J. E., C. R. Beuzon, C. Gleeson, R. Mundy, and D. W. Holden. 1999. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect. Immun. 67:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi, Y., M. J. Cromie, F. F. Hsu, J. Turk, and E. A. Groisman. 2004. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53:229-241. [DOI] [PubMed] [Google Scholar]
- 29.Shi, Y., T. Latifi, M. J. Cromie, and E. A. Groisman. 2004. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J. Biol. Chem. 279:38618-38625. [DOI] [PubMed] [Google Scholar]
- 30.Spory, A., A. Bosserhoff, C. von Rhein, W. Goebel, and A. Ludwig. 2002. Differential regulation of multiple proteins of Escherichia coli and Salmonella enterica serovar Typhimurium by the transcriptional regulator SlyA. J. Bacteriol. 184:3549-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapleton, M. R., V. A. Norte, R. C. Read, and J. Green. 2002. Interaction of the Salmonella typhimurium transcription and virulence factor SlyA with target DNA and identification of members of the SlyA regulon. J. Biol. Chem. 277:17630-17637. [DOI] [PubMed] [Google Scholar]
- 32.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 33.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 35.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 36.Waterman, S. R., and D. W. Holden. 2003. Functions and effectors of the Salmonella pathogenicity island type III secretion system. Cell. Microbiol. 5:501-511. [DOI] [PubMed] [Google Scholar]
- 37.Worley, M. J., K. H. Ching, and F. Heffron. 2000. Salmonella SsrB activates a global regulon of horizontally acquired genes. Mol. Microbiol. 36:749-761. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X.-J., M. Liu, and D. W. Holden. 2004. SsaM and SpiC interact and regulate secretion of Salmonella pathogenicity island 2 type III secretion system effectors and translocators. Mol. Microbiol. 54:604-619. [DOI] [PubMed] [Google Scholar]