Abstract
Regulation/activation of the Porphyromonas gingivalis gingipains is poorly understood. A 1.2-kb open reading frame, a putative glycosyltransferase, downstream of vimE, was cloned, insertionally inactivated using the ermF-ermAM antibiotic resistance cassette, and used to create a defective mutant by allelic exchange. In contrast to the wild-type W83 strain, this mutant, designated P. gingivalis FLL95, was nonpigmented and nonhemolytic when plated on Brucella blood agar. Arginine- and lysine-specific gingipain activities were reduced by approximately 97% and 96%, respectively, relative to that of the parent strain. These activities were unaffected by the growth phase, in contrast to the vimA-defective mutant P. gingivalis FLL92. Expression of the rgpA, rgpB, and kgp gingipain genes was unaffected in P. gingivalis FLL95 in comparison to the wild-type strain. In nonactive gingipain extracellular protein fractions, multiple high-molecular-weight proteins immunoreacted with gingipain-specific antibodies. The specific gingipain-associated sugar moiety recognized by monoclonal antibody 1B5 was absent in FLL95. Taken together, these results suggest that the vimE downstream gene, designated vimF (virulence modulating gene F), which is a putative glycosyltransferase group 1, is involved in the regulation of the major virulence factors of P. gingivalis.
As a common theme, the expression of extracellular proteolytic activities is highly regulated in both prokaryotic and eukaryotic systems (reviewed in references 11 and 53). This regulation can occur at multiple levels including expression of the protease genes, secretion, processing of an inactive secreted precursor to its active form, and/or the posttranslational glycosylation of the proteins (16, 49). The multiple layers of regulation are vital to ensure that expression is tightly controlled in the appropriate temporal and spatial patterns.
Porphyromonas gingivalis, a black-pigmented, gram-negative anaerobe has been implicated as an important etiological agent in adult periodontitis (reviewed in references 26, 32, and 48). While several virulence factors have been implicated in the pathogenicity of P. gingivalis, the high proteolytic abilities of this organism are the focus of much attention as they are considered to play the most significant role in virulence (34). The major proteases, called gingipains, are both extracellular and cell associated. They consist of arginine-specific proteases (Arg-gingipain [Rgp]) and lysine-specific protease (Lys-gingipain [Kgp]) (34). Although glycosylation appears to be important for their activation (reviewed in references 16 and 41), there remains a gap in our knowledge about the regulation/activation of the P. gingivalis gingipains.
Previously, we have reported that the recA locus can affect the phenotypic expression and distribution of the gingipains in P. gingivalis (1, 2, 37). Using the cloned vimA gene, which is downstream of the recA gene and part of the bcp-recA-vimA transcriptional unit, a defective mutant was constructed by allelic exchange (1). The mutant strain, designated FLL92, was non-black-pigmented and showed increased autoaggregration in addition to a significant reduction in proteolytic, hemolytic, and hemagglutinating activities (1). Expression of the rgpA, rgpB, and kgp gingipain genes in P. gingivalis FLL92 was similar to expression in the wild-type strain (1). Furthermore, the partially processed RgpB proenzyme was secreted in P. gingivalis FLL92 (37). The appearance of the gingipain proenzyme forms and the growth phase-dependent activation of proteolytic (37) activity have raised the possibility of multiple mechanisms for gingipain activation. In other studies, we have also demonstrated that another gene, designated vimE, which is downstream of the vimA gene, is important in gingipain activation in P. gingivalis. Inactivation of this gene resulted in a similar phenotype as the vimA-defective mutant; however, gingipain activities were unaffected by the growth phase. Furthermore, in contrast to the vimA-defective mutant P. gingivalis FLL92, the gingipains, although inactive, were membrane associated in strain FLL93, the vimE-defective mutant (51). It has been suggested that vimE is needed for proper carbohydrate biogenesis (51). Collectively, these observations suggest that multiple bacteria-specific factors in P. gingivalis are involved in gingipain biogenesis.
Glycosylation, which is one of the important means in which protein maturation and other cellular processes are regulated, may be important in gingipain biogenesis in P. gingivalis. In this process, different glycosyltransferases catalyze the transfer of different carbohydrate moieties from active donors to specific acceptors (including lipids, proteins, and nucleic acids) (9). Glycosyltransferases have been classified on the basis of the reaction catalyzed, substrate specificity, and homology to other glycosyltransferases (9). In eukaryotes, it has been established that glycosylation occurs in the endoplasmic reticulum or Golgi apparatus (16, 20). However, the localization or the mechanism of glycosylation in prokaryotes has yet to be established. It has been shown in eukaryotes that the glycosylation of proteins plays a role in protein folding, protein stabilization, and protein conformation by sugar-sugar interaction or sugar-protein interactions (8, 16, 20). In addition, it plays a regulatory role in the activation of protein precursors or zymogens, resulting in active proteins. Only recently, the roles of glycosylation and glycosyltransferases in prokaryotes have been shown to be involved in the attachment of membrane proteins to bacterial membrane, for fimbriae maturation, host-cell adhesion, and also maturation of bacterial proteins (5, 43, 49).
In this report we have investigated a 1.2-kb gene downstream of the vimE gene to determine its role in protease activation in P. gingivalis. This gene, which shares homology with glycosyltransferase 1 genes from several bacteria, was inactivated in P. gingivalis by allelic exchange mutagenesis. The P. gingivalis isogenic mutant designated FLL95 exhibited reduced Arg-X- and Lys-X-specific proteolytic activities that were not affected by the phase of growth. The glycosylation of the gingipains was altered in this mutant. These results suggest an important role for this putative glycosyltransferase gene, now designated vimF, in protease maturation/activation in P. gingivalis and further confirm the requirement of multiple specific host factors in this process.
MATERIALS AND METHODS
Bacteria and growth conditions.
Strains and plasmids used in this study are listed in Table 1. P. gingivalis strains were grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI) supplemented with hemin (5 μg/ml), vitamin K (0.5 μg/ml), and cysteine (0.1%). Escherichia coli strains were grown in Luria-Bertani broth. Unless otherwise stated, all cultures were incubated at 37°C. P. gingivalis strains were maintained in an anaerobic chamber (Coy Manufacturing, Ann Arbor, MI) in 10% H2, 10% CO2, 80% N2. Growth rates for P. gingivalis and E. coli strains were determined spectrophotometrically (optical density at 600 nm [OD600]). Antibiotics were used at the following concentrations: clindamycin, 0.5 μg/ml; erythromycin, 300 μg/ml; and carbenicillin, 50 to 100 μg/ml.
TABLE 1.
Plasmids and bacterial strains used in this study
| Plasmid or strain | Phenotype and description | Source |
|---|---|---|
| Plasmids | ||
| pCR-2.1 TOPO | Apr, Kmr | Invitrogen |
| pFLL80 | pCR 2.1-TOPO PG0791:PG0792 | 50 |
| pFLL86 | pUC 19: PG0791:PG0792 | This study |
| pFLL87 | pUC19: PG0791:PG0792:: ermF-ermAM | This study |
| pVA2198 | Spr, ermF-ermAM | 15 |
| Bacterial strains | ||
| P. gingivalis | ||
| W83 | Wild type | 1 |
| FLL93 | vimE-defective | 50 |
| FLL95 | vimF-defective | This study |
| E. coli | ||
| DH5α | F−φ80dlacZΔ M15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rk−, mk+) phoA supE44 λ−thi-1 gyrA96 relA1 | Invitrogen |
| Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔ M15 ΔlacX74 recA1 ara139 Δ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
Bioinformatic analysis of vimF.
The protein sequence of vimF, obtained from the P. gingivalis genome (www.oralgen.lanl.gov), was analyzed for any homology to other proteins or any predicted class or functions using the analysis tool functions at the website. Clusters of orthologous groups (COG) searches, Pfam protein family database searches (with a cutoff strategy using an E value of 1.0), and BLAST searches were performed at the website.
DNA isolation and analysis.
P. gingivalis chromosomal DNA was prepared by the method of Marmur (31). For plasmid DNA analysis, DNA extraction was performed by the alkaline lysis procedure of Birnboim and Doly (6). For large-scale preparation, plasmids were purified using the QIAGEN (Santa Clarita, CA) plasmid maxi kit.
Generation of vimF mutant P. gingivalis strain.
A 2.5-kb fragment carrying the intact vimE and vimF downstream genes was amplified by PCR using the P1 and P6 oligonucleotide primers (Table 2). This fragment was cloned into the pCR 2.1-Topo plasmid vector (Invitrogen, Carlsbad, CA) and designated pFLL80 (51). The 2.5-kb fragment was then isolated from the EcoRI-digested pFLL80 and ligated to pUC19 linearized with EcoR1. The new recombinant plasmid was designated pFLL86. Orientation was confirmed by restriction analysis. The ermF-ermAM cassette which confers erythromycin/clindamycin resistance in E. coli and P. gingivalis (15) was PCR amplified from pVA2198 using Pfu Turbo (Stratagene, La Jolla, CA) and inserted into the EcoRV restriction site of the vimF gene. The resultant recombinant plasmid, pFLL87, was used as a donor in electroporation of P. gingivalis W83 as previously reported (15).
TABLE 2.
Primers used in this study
| Primer | Description | Sequence |
|---|---|---|
| P1 | vimE forward | 5′ ATGAATACTGAAGTAATCCAC 3′ |
| P2 | vimE reverse | 5′ TTGGTCGATGGCCGTTTCGTA 3′ |
| P3 | vimE reverse (intragenic) | 5′ TCAGCACTTCCTTGCACAAC 3′ |
| P4 | vimF (forward) | 5′ ATTCTACAAAGAAGCGGGGA 3′ |
| P5 | vimF reverse (intragenic) | 5′GCAGGATGGAGCGGGAGTATTC 3′ |
| P6 | vimF reverse | 5′ TTCACGCGAAAAAGTGGAGAT 3′ |
| P7 | vimA forward | 5′ ATGCCCATCCCTCTATACCTG 3′ |
| P8 | vimA reverse | 5′ TACCTGTTTTTGCTGACCGG 3′ |
| P10 | pro-kgp forward | 5′ ATGAGGAAATTATTATTGCTGATCG 3′ |
| P11 | pro-kgp reverse | 5′ TTGAAGAGCTGTTTATAAGCTGTTT 3′ |
| P12 | pro-rgpA forward | 5′ ATGAAAAACTTGAACAAGTTTGTTTC 3′ |
| P13 | pro-rgpA reverse | 5′ CCGGTGTGTAACGCCCTG 3′ |
| P14 | pro-rgpB forward | 5′ CGACCGATGAAGACTTCG 3′ |
| P15 | pro-rgpB reverse | 5′ TGAAGACGCTCTTATAGATATCTT 3′ |
| P16 | erythromycin forward | 5′ TATTAGGCCTATAGCTTCCGCTATT 3′ |
| P17 | erythromycin reverse | 5′ AATAGGCCTTAGTAACGTGTAACTTT 3′ |
Hemagglutination studies.
Hemagglutinin activity was determined as previously reported (17). Twenty-four-hour cultures of P. gingivalis W83, FLL93, and FLL95 cells were harvested by centrifugation (10,000 × g for 15 min). Cells were washed twice in 1× phosphate buffered saline (PBS; 0.147 m NaCl, 0.01 M sodium phosphate) and resuspended to a final OD600 of 1.5. Sheep erythrocytes were washed twice with PBS and resuspended in 1% in PBS. An aliquot (100-μl volume) of the bacterial suspension was serially diluted twofold with PBS in round-bottom 96-well microtiter plates. An equal volume (100 μl) of 1% sheep erythrocytes was mixed with each dilution and incubated at 4°C for 3 h. Hemagglutination was visually assessed, and the hemagglutination titer was determined as the last dilution that showed complete hemagglutination.
Preparation of P. gingivalis extracellular fractions and protease assays.
One-liter cultures of P. gingivalis strains FLL93, FLL95, and W83 were grown for 24 h in BHI supplemented with hemin, cysteine, and yeast extract. Cells were harvested by centrifugation at 10,000 × g for 30 min. The cell-free culture fluid was precipitated with cold (−20°C) 37.5% acetone, and the protein pellet was resuspended in 7 ml of 100 mM Tris-HCl buffer (pH 7.4), dialyzed for 24 h against the same buffer, and then stored on ice or at 0°C. The presence of Arg-X- and Lys-X-specific cysteine protease activities was determined with a microplate reader (Bio-Rad Laboratories, Hercules, CA) as previously reported (44).
Preparation of membrane fraction.
One-liter cultures of P. gingivalis strains FLL93, FLL95, and W83 were grown to OD600 of 1.3 to 1.4. Cells were harvested by centrifugation at 10,000 × g for 30 min. Membrane fractions were prepared by lysing the cells using a French pressure cell press (American Instrument Company, Silver Spring, MD) for three cycles at 109 MPa. The lysed cells were then centrifuged at 27,000 × g for 1 h. The supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h. The pellet, designated as the membrane fraction, was resuspended in 100 mM Tris HCl, pH 7.4, containing 1 mM Nα-p-tosyl-l-lysine chloromethyl ketone. The remaining supernatant was considered the cytosolic fraction.
Protein concentration determination.
Protein concentration was calculated spectrophotometrically using the Warburg formula within the protein function of the Eppendorf Biophotometer (Brinkman, Westbury, NY).
SDS-PAGE and immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 4 to 12% bis-Tris separating gel in MOPS (morpholinepropanesulfonic acid)-SDS running buffer (NuPAGE Novex gels; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Samples were prepared (65% sample, 25% 4× NuPAGE LDS sample buffer, and 10% NuPAGE reducing agent), heated at 72°C for 10 min, and then electrophoresed at 200 V for 65 min with the XCell SureLock Mini-Cell (Invitrogen, Carlsbad, CA). The separated proteins were then transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH) and processed at 15 V for 25 min with a Semi-Dry Trans-blot apparatus (Bio-Rad). The blots were probed with antibodies against specific protease domains (40) or HagA-specific antibodies (generously donated by the Progulske-Fox group). Immunoreactive proteins were detected by the procedure described in the Western Lightning Chemiluminescence Reagent Plus kit (Perkin-Elmer Life Sciences, Boston, MA). The secondary antibody was immunoglobulin G (heavy plus light chains)-horseradish peroxidase conjugate (Zymed Laboratories, Inc., South San Francisco, CA).
Fibronectin cleavage using P. gingivalis.
P. gingivalis W83 and isogenic mutants FLL92, FLL93, and FLL95 cells grown to early stationary phase (OD600 of 1.1) were harvested, washed, and resuspended in PBS to a final OD600 of 1.5. Fibronectin (75 μg) resuspended in PBS was incubated for 30 min at 37°C with 50 μl of P. gingivalis strains. The reaction mixture volume was 500 μl. After a 30-min incubation, reaction samples were centrifuged at 10,000 × g for 5 min, and cell-free supernatant from samples was analyzed by SDS-PAGE and stained with Simply Blue Safe Stain (Invitrogen, Carlsbad, CA).
Analysis of P. gingivalis vimF mutant genes by RT-PCR.
Total RNA was extracted from P. gingivalis strains grown to early stationary phase (OD600 of 1.2) using a RiboPure kit (Ambion, Austin TX). The primers used for reverse transcription-PCR (RT-PCR) analysis were specific for the rgpA, rgpB, kgp, vimE, vimA, and vimF genes (Table 2). The RT-PCR mixture (50 μl) contained 1 μg of template RNA in the Superscript One-Step RT-PCR mix (Invitrogen, Carlsbad CA). Negative controls were RT-PCR in the absence of reverse transcriptase.
LPS and polysaccharide isolation and silver staining.
Overnight cultures of P. gingivalis W83 and FLL95 were grown to stationary phase. Isolation of lipopolysaccharides (LPS) was done according to the manufacturer's protocol (Intron Biotechnology, Republic of Korea). Polysaccharides (PS) were separated from lipid A by treating the LPS preparations with 2% acetic acid for 2 h at 100°C. The soluble fraction containing the PS was purified using a Superdex 75 column (Amersham Biosciences, Piscataway, NJ). LPS were subjected to SDS-PAGE on 4 to 12% bis-Tris gel using the MOPS running buffer. PS were subjected to SDS-PAGE on 4 to 12% bis-Tris gel using the morpholineethanesulfonic acid (MES) running buffer. LPS and PS were visualized using a SilverQuest Silver Staining Kit according to manufacturer's instructions (Invitrogen, Carlsbad, CA).
RESULTS
Bioinformatic analysis of VimF.
It has been demonstrated that the vimA and vimE genes play important roles in gingipain maturation/activation (50, 51). It has also been reported that vimE can be cotranscribed with vimF and vimA (50). In addition, it has been shown that the vimA and vimE mutants display carbohydrate biogenesis defects, which may be correlated with the altered gingipain maturation/activation (51). Thus, it is our hypothesis that inactivation of a glycosyltransferase needed for modification of the gingipains would result in a phenotype similar to what is seen in the vimA- and/or vimE-defective mutants. The vimE downstream gene, vimF, may play a role in gingipain maturation/activation. A COG search on the gene sequence of vimF (Fig. 1) from www.ncbi.nih.gov revealed a COG of glycosyltransferase type 1 (COG0438) of class M, which is involved in cell envelope and outer membrane biogenesis. In addition, PSI (position-specific iterating) or local BLAST searches from tool analysis function on the www.oralgen.lanl.gov website showed that vimF has homology to other glycosyltransferases including those from the organisms Methanococcus sp. (26% identity; 42% positives), Geobacter metallireducence (26% identity; 42% positives), Haloarcula marismortui (24% identity; 44% positives), Bacillus cereus (24% identity; 42% positives), and Bacillus anthracis (24% identity; 41% positives) (PSI-BLAST). A Pfam search of vimF showed a model and description of glycosyltransferase group 1 having a significant E value of less than 0.5.
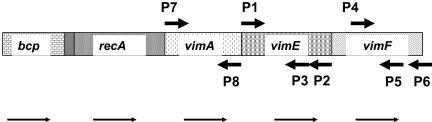
FIG. 1.
Diagram of the recA locus and the two downstream genes. Diagram shows open reading frames (taken from www.oralgen.lanl.gov.) of the recA locus and the two downstream genes, vimE and vimF. Open reading frames are shown with given primer locations. Arrows indicate gene orientation.
Inactivation of the vimF gene in P. gingivalis W83 by allelic exchange mutagenesis.
Isogenic mutants of P. gingivalis W83 defective in the PG0792 gene (designated vimF) were constructed by allelic exchange mutagenesis. The circular recombinant plasmid pFLL87, which carries the ermF-ermAM cassette in the unique EcoRV restriction site (bp 909 of the open reading frame) of the vimF gene, was used as a donor in electroporation of P. gingivalis W83 (Fig. 2A). Following electroporation and plating on selective medium (BHI containing 10 μg/ml erythromycin), we detected approximately 89 erythromycin-resistant colonies after a 5-day incubation period. To compare their phenotypic properties with those of wild-type strain W83, 20 mutants were plated on Brucella blood agar plates. In contrast to the wild-type strain, all selected mutants displayed a non-black-pigmented, non-beta-hemolytic phenotype.
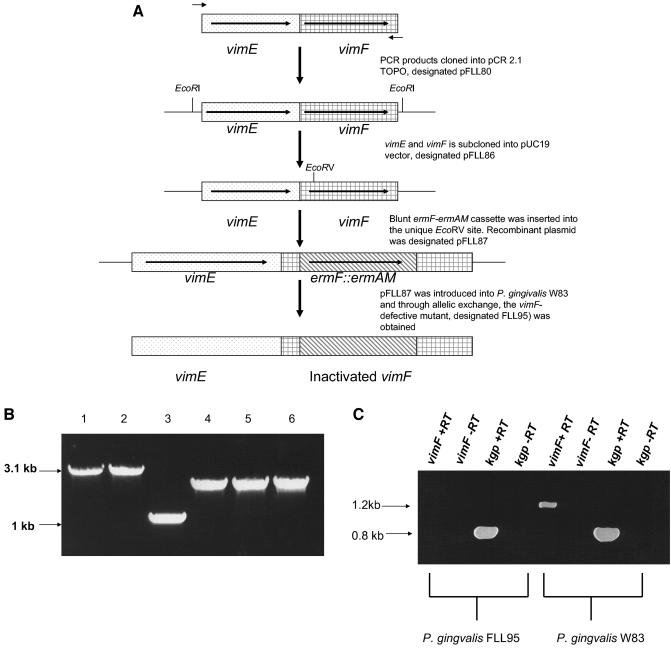
FIG. 2.
Construction and confirmation of the vimF-defective mutant. (A) Construction of mutant. Plasmid pFLL87 contains the vimF gene interrupted by an ermF-ermAM cassette (the vimF gene with flanking sequences was amplified by PCR; the ermF-ermAM cassette was purified from pVA2198). The circular recombinant plasmid pFLL87 was integrally introduced into P. gingivalis W83 by electroporation. A reciprocal recombination event between areas of homology on the chromosome and regions flanking the ermAM-ermF cassette on pFLL87 replaced the intact vimF with an inactivated copy. (B) PCR analysis of allelic exchange mutants of P. gingivalis carrying the vimF gene inactivated with the ermF-ermAM cassette. Oligonucleotide primers specific for the vimF gene or erythromycin cassette were used to amplify that gene or erythromycin from total chromosomal DNA from P. gingivalis. Lane 1, P. gingivalis FLL95.1 (vimF::ermF-ermAM; vimF primers); lane 2, P. gingivalis FLL95.2 (vimF::ermF-ermAM; vimF primers); lane 3, P. gingivalis W83 (wild type); lane 4, P. gingivalis FLL95.1 (vimF::ermF-ermAM; erythromycin primers); lane 5, P. gingivalis FLL95.2 (vimF::ermF-ermAM; erythromycin primers); lane 6, pVA2198 plasmid (plasmid carrying the erythromycin cassette; erythromycin primers) as control. (C) Transcriptional profile of vimF-defective mutant. Total RNA isolated from P. gingivalis W83 and the vimF-defective mutant isolated from stationary phase (OD600 of 1.4 to 1.5) was subjected to RT-PCR using primers for vimF and pro-kgp. No transcription or vimF was detected in P. gingivalis FLL95.
Confirmation of inactivation of vimF by PCR analysis.
Chromosomal DNA from two randomly chosen clindamycin/erythromycin-resistant colonies and the wild-type W83 strain were analyzed by PCR to confirm the inactivation in the vimF gene. If the vimF gene was interrupted by the ermF-ermAM cassette, a 3.1-kb fragment was expected to be amplified using vimF primers P4 and P5 (Table 2). In addition, a 2.1-kb fragment should be amplified from the mutant using the ermF-ermAM primers (Table 2). The expected 3.1-kb (using vimF primers) and 2.1-kb (using erythromycin primers) fragments were observed only in the erythromycin-resistant strains (Fig. 2B) in contrast to the wild-type (data not shown). The orientation of the ermF-ermAM cassette was also confirmed by restriction digest (data not shown). To further confirm the absence of the vimF transcript in the erythromycin-resistant mutant, DNase treated RNA for P. gingivalis FLL95 was subjected to RT-PCR. As shown in Fig. 2C, no vimF transcript was detected from the mutant in comparison to the wild-type W83. Using kgp-specific primers (Table 2) as a control, the expected 0.8-kb fragment was amplified from all the P. gingivalis strains. There was no amplified fragment observed from reactions in the absence of reverse transcriptase. Taken together, these results indicated that the insertional inactivation of the chromosomal vimF gene with the 2.1-kb ermF-ermAM antibiotic cassette was successful. One mutant designated P. gingivalis FLL95 was randomly chosen for further study. In both the wild-type and the vimF-defective mutant, a generation time of 3 h was determined.
The vimE and vimA genes are transcribed in P. gingivalis FLL95.
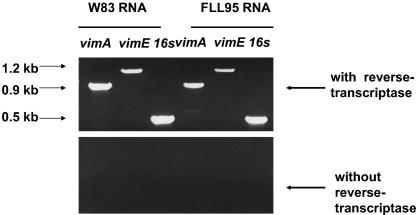
It has been shown that vimE and its downstream gene, vimF, can be cotranscribed (50). To ensure that the inactivation of the vimF gene did not prevent the transcription of vimE or vimA, DNase-treated RNA from the P. gingivalis isogenic mutant and wild type was subjected to RT-PCR. As shown in Fig. 3, the vimA and vimE fragments were amplified from the vimF-defective mutant in comparison to the wild-type W83. No amplified fragments were observed for either the wild-type or the mutant when reverse transcriptase was absent from the reaction mixture. Using 16S RNA-specific primers as a positive control, fragments of expected size were amplified from both the wild-type and the isogenic mutant.
FIG. 3.
Expression of vimE and vimA in vimF-defective mutant. Total RNA isolated from P. gingivalis W83 and the vimF-defective mutant was subjected to RT-PCR using primers for vimE, vimA, and 16S RNA as a control.
Hemagglutinin activity in P. gingivalis FLL95.
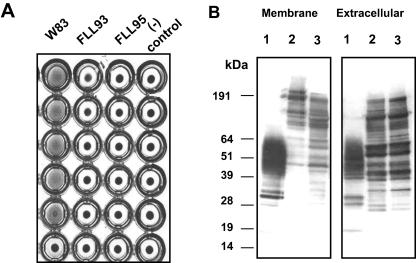
We assessed the hemagglutination potential of P. gingivalis FLL95 in comparison to P. gingivalis W83 and P. gingivalis FLL93, the nonproteolytic vimE-defective mutant. In contrast to the wild-type strain, there was a reduction in hemagglutinin activity in P. gingivalis FLL95 (Fig. 4A). This level of activity was similar to P. gingivalis FLL93 and the negative control as previously reported (51). Immunoblot analysis of membrane and extracellular fractions using monoclonal antibodies raised against HagA showed the presence of multiple high-molecular-weight immunoreactive bands ranging between 45 kDa to >191 kDa in the vimE- and vimF-defective mutant membrane fractions (Fig. 4B) and immunoreactive bands ranging between 25 to 185 kDa in the extracellular fraction (Fig. 4B). However, lower immunoreactive bands ranging between 19 to 64 kDa were observed in the wild-type membrane and extracellular fractions (Fig. 4B).
FIG. 4.
Hemagglutinin analysis of the vimF-defective mutant. (A) Hemagglutinin activities of P. gingivalis were assessed in P. gingivalis W83, FLL93, and FLL95 cells serially diluted and incubated with sheep erythrocytes at 4°C for 3 h in a round-bottom microtiter plate. The titer was defined as the last dilution that showed full agglutination. (B) Membrane and extracellular fraction preparations were assessed as described for P. gingivalis W83, FLL93, and FLL95. Proteins were analyzed by immunoblot analysis using monoclonal antibodies raised against hemagglutinin protein HagA. Lanes 1, P. gingivalis W83; lanes 2, P. gingivalis FLL93; lanes 3, P. gingivalis FLL95. Molecular mass markers are at left.
Inactivation of the vimE gene affects proteolytic activity in P. gingivalis.
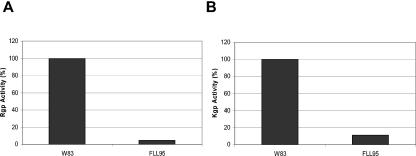
We previously reported that the proteolytic activity of the non-black-pigmented recA-defective mutant (P. gingivalis FLL32), the vimA-defective mutant (P. gingivalis FLL92), or the vimE-defective mutant (FLL93) was reduced by more that 90% in comparison to the wild-type strain (1, 2, 50). In addition, a late onset of proteolytic activity was also reported in the vimA mutant relative to the wild-type strain (37). Because hemagglutinin activities can be associated with gingipain activity in this organism (28, 45), P. gingivalis FLL95 was assayed for proteolytic activity using N-α-benzoyl-dl-arginine-p-nitroanilide for Rgp activity and acetyl-lysine-p-nitroanilide for Kgp activity. In late-exponential growth-phase cultures, both Arg-X and Lys-X protease activity levels in P. gingivalis FLL95 were very similar to activity in P. gingivalis FLL93 (51), which was reduced by approximately 94 to 96% compared to the wild-type strain (data not shown). In stationary phase cultures of P. gingivalis FLL95, there was a slight increase in Lys-X activity relative to Rgp activity (Fig. 5) and similar to that of FLL93 (51). This is in contrast to the expected late onset of proteolytic activity that was observed in P. gingivalis FLL92 (37). Taken together, these data suggest that under the same physiological conditions, the proteolytic profile for P. gingivalis FLL95 is dramatically altered by inactivation of the vimF gene in P. gingivalis.
FIG. 5.
Proteolytic activity of P. gingivalis vimF-defective mutant. P. gingivalis was grown to stationary phase in BHI broth supplemented with hemin and vitamin K. Activities against Rgp (A) and Kgp (B) were tested in whole-cell culture.
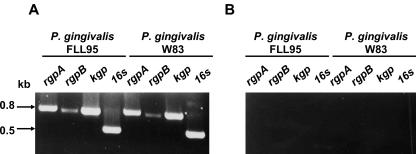
Expression of the gingipain genes in the vimF-defective mutant.
The reduced proteolytic activity in the P. gingivalis FLL95 strain could have been the result of an alteration in the transcription of the gingipain genes. There is also the possibility that the vimF gene may be involved in the posttranslational regulation of gingipain expression. In previous reports of mutations in two genes (vimA and vimE) upstream of the vimF gene, a reduction in gingipain activity was observed although the gingipains were normally expressed (1, 2, 50). Furthermore, the partially processed RgpB gingipain proenzyme was identified in the vimA-defective mutant, suggesting a defect in gingipain biogenesis at the posttranslational level (37). To determine the presence of mRNA transcripts for the gingipain genes (rgpB, rgpA, and kgp), total RNA was isolated from the wild-type W83 strain and P. gingivalis FLL93 grown to stationary phase. Specific oligonucleotide primers as described in Table 2 for rgpB, rgpA, and kgp were used in RT-PCR to amplify a predicted region of the transcripts. When the reverse transcriptase was present in the reaction, amplified products of the predicted size (0.8 kb for rgpB and 0.9 kb for rgpA and kgp) were observed for all three gingipain gene transcripts in both strains (Fig. 6). This suggests that the gingipains were being expressed in P. gingivalis FLL95 in comparison to the wild type.
FIG. 6.
Expression of the gingipains in the vimF-defective mutant. DNase-treated total RNA for P. gingivalis W83, FLL93, and FLL95 was grown to stationary phase and subjected to reverse transcriptase PCR. (A) Performed in the presence of reverse transcriptase. (B) Performed in the absence of reverse transcriptase.
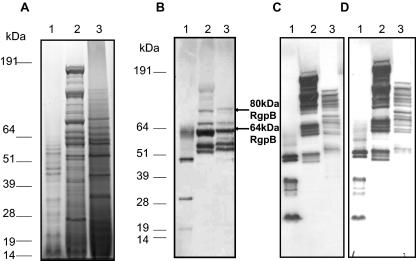
Extracellular proteins from P. gingivalis FLL95.
Even though there was a dramatic reduction in proteolytic activity in P. gingivalis FLL95, the gingipains appeared to be expressed. This may suggest a posttranscriptional regulation of gingipain maturation/activation. To examine any changes in extracellular proteins from P. gingivalis FLL95 in comparison to the wild-type W83 and vimE-defective mutant FLL93, extracellular fractions from stationary growth phase of all three strains were analyzed by SDS-PAGE. As shown in Fig. 7A, there were multiple high-molecular-weight protein bands in P. gingivalis FLL95 that were absent in the wild type. Specific antibodies against RgpB, RgpA, and Kgp were used to determine the presence of the gingipain immunoreactive proteins in P. gingivalis FLL95. Similar to the vimE-defective mutant, the extracellular proteins from P. gingivalis FLL95 showed multiple unique immunoreactive bands to RgpB (Fig. 7B), RgpA (Fig. 7C), and Kgp (Fig. 7D) in contrast to the wild-type strain. A 48-kDa band representing the catalytic domain of RgpB was detected in the wild-type strain but was absent in P. gingivalis FLL95 (Fig. 7B). It is noteworthy that other immunoreactive bands representing the processed mature gingipains were absent in P. gingivalis FLL93 unlike the vimA-defective mutant. Similar to the vimE-defective mutant, when the vimF-defective mutant was probed with antibodies against RgpA, high-molecular-weight protein bands were seen. A 185-kDa band that was absent in the vimF mutant was present in the vimE mutant (Fig. 7C). Also, immunoblot analysis using anti-RgpB antibodies showed an 80-kDa immunoreacting band consistent with the size of the RgpB proenzyme (Fig. 7B).
FIG. 7.
Extracellular protease profile of P. gingivalis FLL93 and FLL95 from stationary phase. Acetone-precipitated proteins isolated from culture supernatants grown to an OD600 of 1.4 to 1.5 were analyzed by SDS-PAGE and stained with Simply Blue safe stain (A) or analyzed by immunoblotting using gingipain-specific antibodies (B to D). Approximately 20 to 25 μg of protein was loaded in each lane. Lanes 1, P. gingivalis W83; lanes 2, P. gingivalis FLL93; lanes 3, P. gingivalis FLL95. Antibodies: panel B, rabbit anti-RgpB; panel C, chicken anti-RgpA; panel D, chicken anti-Kgp. Arrows indicate the unprocessed and partially processed RgpB; molecular mass markers are shown at left.
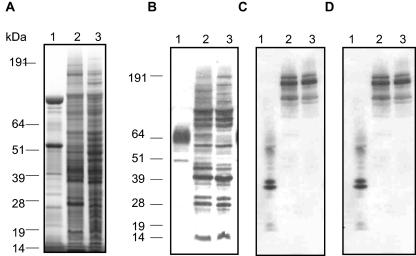
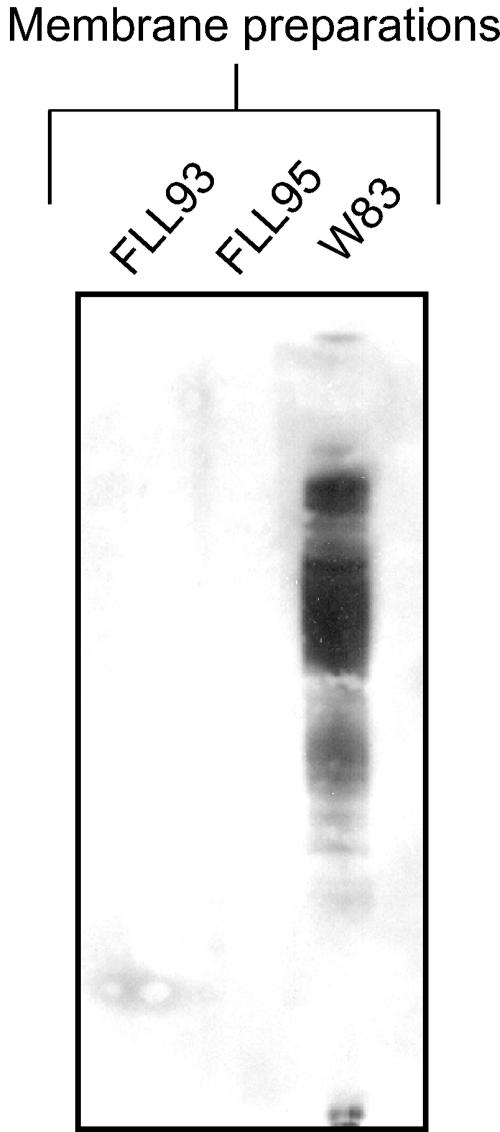
Membrane protein profile of P. gingivalis FLL95.
Gingipain-dependent protein processing is documented in P. gingivalis (22). Thus, a reduction in functional or active gingipains may result in unprocessed proteins on the membrane of P. gingivalis. As shown in Fig. 8A, SDS-PAGE analysis of membrane preparations from P. gingivalis strains W83, FLL93, and FLL95 shows that there are multiple high-molecular-weight protein bands that are present in the membrane preparation of P. gingivalis FLL93 and FLL95 that are missing in the wild type. To determine the presence of the inactive cell-associated gingipain species in P. gingivalis FLL95, membrane preparations of P. gingivalis W83, FLL93, and FLL95 were immunoreacted with antibodies against RgpA, RgpB, and Kgp. As shown in Fig. 8, there were high-molecular-weight immunoreactive bands in the membrane preparations from P. gingivalis FLL93 and FLL95 when antibodies against RgpB (Fig. 8B), RgpA (Fig. 8C), or Kgp (Fig. 8D) were used. It is noteworthy that, in contrast to the wild-type strain, there were unique high-molecular-weight bands in P. gingivalis FLL95 that immunoreacted with antibodies against RgpA, RgpB, and Kgp. Taken together, these data may suggest altered processing of the gingipains on the membrane of the vimE-defective mutant P. gingivalis FLL93 or the vimF-defective mutant FLL95. In contrast to the wild type, all the gingipains, although inactive, were present in the membrane fraction of the vimE-defective mutant P. gingivalis FLL93 and vimF-defective mutant FLL95.
FIG. 8.
Membrane protease profile of P. gingivalis FLL93 and FLL95. Membrane preparations taken from P. gingivalis strains W83, FLL93, and FLL95 taken from stationary phase were analyzed by SDS-PAGE and stained with Simply Blue Safe stain (A) or analyzed by immunoblotting using gingipain-specific antibodies (B to D). Approximately 20 to 25 μg of protein was loaded in each lane. Lanes 1, P. gingivalis W83; lanes 2, P. gingivalis FLL93; lanes 3, P. gingivalis FLL95. Antibodies: panel B, rabbit anti-RgpB; panel C, chicken anti-RgpA; panel D, chicken anti-Kgp. Molecular mass markers are shown at left of panels A and B.
P. gingivalis FLL95 has reduced ability to cleave fibronectin.
The gingipains of P. gingivalis have been shown to cleave extracellular matrix components, such as fibronectin, in vitro (39). A defect in gingipain maturation should abolish or reduce the potential of the organism to cleave fibronectin. To test this hypothesis, 75 μg of fibronectin was incubated for 30 min at 37°C with P. gingivalis W83 or isogenic mutant FLL92, FLL93, or FLL95 cells washed with and resuspended in PBS. Incubation of fibronectin with the vimA-defective mutant of P. gingivalis demonstrated no visible cleavage bands (Fig. 9); however, incubation with the vimE-defective mutant resulted in a slight cleavage band at 50 kDa, and incubation with the vimF-defective mutant demonstrated slight cleavage bands ranging between 39 to 52 kDa (Fig. 9). After incubation with P. gingivalis W83, the fibronectin was completely degraded in contrast to results in the isogenic mutants of P. gingivalis (Fig. 9).
FIG. 9.
Reduced ability to cleave fibronectin in isogenic mutants of P. gingivalis. Fibronectin was incubated for 30 min with P. gingivalis W83 and isogenic mutants FLL92, FLL93, and FLL95 grown of to early stationary phase (OD600 of 1.1), harvested, washed, and resuspended in 1× PBS to a final OD600 of 1.5. Cell-free supernatant from samples was analyzed by SDS-PAGE and stained with Simply Blue Safe Stain. Molecular mass markers are shown at left.
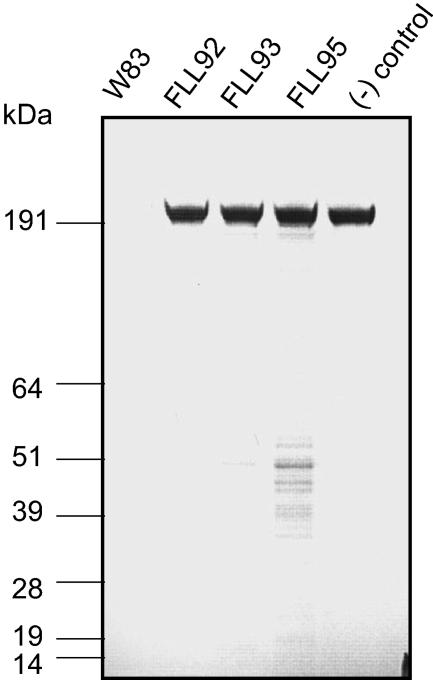
Immunoblot analysis using MAb 1B5.
Monoclonal antibody (Mab) 1B5 has been demonstrated to immunoreact with membrane-associated Rgp (mt-Rgp) and certain LPS modifications in P. gingivalis (13, 16). The polysaccharide profiles of the wild-type and the vimF-defective mutant are similar (data not shown). However, as shown in Fig. 10, there was no detectable immunoreactivity in membrane preparations from P. gingivalis FLL93 or FLL95 compared to the wild type.
FIG. 10.
Immunoblot analysis of membrane using MAb 1B5. Membrane preparations from P. gingivalis W83 and isogenic mutants FLL93 and FLL95 were separated by SDS-PAGE and analyzed for certain carbohydrate modifications of mt-Rgps and/or LPS modifications of membrane proteins using MAb 1B5. All lanes contained 20 μg of protein.
DISCUSSION
We have used a genetic approach in this study to further assess the role of specific host factors in protease regulation/activation. Several recent studies (1, 19, 46; reviewed in reference 34) have identified nongingipain genes that are involved in the modulation of gingipain activity and other virulence factors in P. gingivalis. A comparison of several of the P. gingivalis mutants from these studies has raised the possibility of multiple mechanisms for gingipain activation. Mutation in the vimA, porR, or gppX gene has shown delayed activation of proteolytic activity that is mostly soluble (1, 19, 46). Currently, a mechanism of activation by these genes is not understood; furthermore, it is unclear if they are part of a common pathway for gingipain activation. Although these mutants had similar proteolytic profiles, inactivation of each gene did not affect the expression of the others. This raises the possibility that regulation of virulence factors of P. gingivalis W83 may occur through the function of gene products that may affect carbohydrate biogenesis.
The vimF gene, which is downstream of vimE, does appear to show similarity with glycosyltransferase 1 genes from several organisms (www.oralgen.lanl.gov). P. gingivalis FLL95, the isogenic mutant defective in this gene, showed reduced proteolytic activity and was non-black-pigmented. This is also similar to a mutant of P. gingivalis that was defective in the glycosyl (rhamnosyl) transferase gene (10). Other glycosyltransferases in P. gingivalis including NahA and GtfA have been identified (29, 30, 35). They were demonstrated to be involved in the maturation of fimbriae and the attachment of the bacteria to epithelial cells. While the effect of NahA on protease activation was not evaluated (30), the gtfA-defective mutant was pigmented, which may suggest that the mutation had no effect on gingipain activity (35). Collectively, these observations would support an important role for specific glycosyltransferases in the expression of gingipain activity in P. gingivalis.
Because the posttranslational addition of carbohydrates to the gingipains is highly variable, it may implicate a role for multiple genes in this process. In P. gingivalis FLL95, the vimF-defective mutant, membrane-associated RgpA, RgpB, and Kgp were detected. Based on the high molecular weight of immunoreacting bands to antibodies against these gingipains, the membrane-associated forms could represent the unprocessed or partially processed gingipains. Further, no immunoreactivity was detected using MAb 1B5, which is specific for carbohydrate modifications of membrane-associated Rgp and LPS. While we cannot rule out an alteration in the carbohydrate composition of the LPS in P. gingivalis FLL95, its mobility profile on SDS-PAGE appeared to be similar to the wild-type strain. This may correlate with the anchorage of the gingipains in the membrane of FLL95 and may suggest the ability of VimF to affect the gingipains more directly. This is in contrast to a mutation in the porR locus, which is associated with the synthesis of the O-antigen side chains of the LPS (16, 46). In the porR-defective mutant, there was reduced membrane-associated gingipain activity and a late onset of protease activity. The cell surface polysaccharide profile of this mutant was different from the wild-type strain. This defective polysaccharide may have prevented the correct distribution and activation of the gingipain. If the inactive gingipains are cell-associated as observed in P. gingivalis FLL95, it may be possible that VimF could function independently of PorR and that it is essential for gingipain activation. A specific mechanism for the role of the vimF gene in protease activation is currently being investigated in the laboratory.
The phenotype of P. gingivalis FLL95 could be related to the reduced gingipain activity and, in particular, reduced membrane-associated activity. This would be similar to P. gingivalis FLL32, a recA-defective mutant (2), P. gingivalis FLL92, a vimA-defective mutant (1), and vimE-defective mutant FLL93 (51). These results would also be consistent with other reports (14, 28, 38, 47) on the involvement of gingipains, especially Kgp, with hemoglobin binding, absorption, and heme accumulation. Hemolysin activity could also be modulated by the vimF gene in P. gingivalis. In this study, P. gingivalis FLL95, the vimF-defective mutant, showed no hemolytic activity when grown on blood plates. Because the gingipain Kgp has been shown to have hemoglobinase activity (28) and plays a role in erythrocyte degradation (44), our results are not surprising due to the reduced proteolytic activity in P. gingivalis FLL95. In addition to the effect of proteolytic activity on the hemolysin potential, several hemolysin-like genes have been identified from the P. gingivalis genome project (36; www.oralgen.lanl.gov). Furthermore, there is genetic evidence for the involvement of two distinct hemolysins in the hemolysin activity of P. gingivalis (23). Taken together, these observations may suggest regulation of these genes or gene products by the vimF gene although we cannot rule out the effect of the gingipains on their expression. There is evidence that the gingipains are involved in the processing of other proteins (22, 52).
The vimF gene in this study appears to also affect hemagglutination in P. gingivalis. Hemagglutination of sheep erythrocytes was reduced in P. gingivalis FLL93, the vimE-defective mutant and the vimF-deficient mutant FLL95. Again, these observations are not unexpected due to the reduced proteolytic activity in P. gingivalis FLL95. The gingipains RgpA and Kgp have hemagglutinating activity (45). Further, a monoclonal antibody (MAb 61BG1.3) that inhibited the hemagglutination and selectively prevented the recolonization of P. gingivalis in periodontal patients was found to recognize a peptide within the adhesin domain encoded by rgpA, kgp, and hagA (7, 24). In addition to the association of the gingipains with hemagglutination in P. gingivalis, the presence of several genetically distinct genes including the hemagglutinin genes hagB and hagC has been reported (27, 42). In this study, the effects of the vimF gene product on hemagglutination in P. gingivalis may also implicate the regulation of these genes or gene products by this gene. The HagA protein appears to range in size from 210 to 283 kDa (210 kDa [www.tigr.org], 233 kDa [www.oralgen.lanl.gov], or 283 kDa [18]). Analysis of the hemagglutinin protein HagA from the vimE- and vimF-defective mutants demonstrated multiple high-molecular-weight bands in both the membrane and extracellular fractions that were absent in the wild type. The high-molecular-weight band greater than 191 kDa in the membrane of the vimE- and the vimF-defective mutants, which are absent from the wild type, may represent the unprocessed or partially processed HagA protein. The other, multiple immunoreactive bands that were present in the isogenic mutants may represent the multiple intermediate HagA species. Collectively, these data suggest that vimF is needed for the proper processing of the hemagglutinin protein HagA.
The formation of zymogens is an important strategy that organisms use to regulate the activation of several enzymes. Thus, the gingipain zymogen may be crucial in the timely activation of the gingipains. Consistent with previous reports on two isogenic mutants (P. gingivalis FLL92, a vimA-defective mutant, and P. gingivalis FLL93, a vimE-defective mutant) with reduced proteolytic activity, there was expression of the gingipain genes in P. gingivalis FLL95 (1, 50). The gingipain RgpB was secreted in an inactive form in the vimA- and vimE-defective mutants, suggesting a role of the vimA and vimE genes in the posttranslational regulation of protease activity in P. gingivalis (37). A comparison of the extracellular proteins using the RgpB proenzyme-specific antibodies revealed a 64-kDa and an 80-kDa immunoreactive band in P. gingivalis FLL95, which is similar in size to the RgpB proenzyme previously reported (37). Consistent with the lack of protease activation in this isogenic mutant, this protein was present in stationary phase culture medium, unlike the vimA-defective mutant that demonstrated a late onset of proteolytic activity (1). In contrast to the wild type, high-molecular-weight immunoreactive protein bands that could represent the unprocessed gingipains were also secreted in P. gingivalis FLL95. In addition, there appeared to be RgpB-immunoreactive bands between 80 and 54 kDa, which may represent an intermediate, processed species. Furthermore, the multiple immunoreactive bands to RgpA and Kgp may represent the gingipain precursors and other inactive gingipain intermediates. Collectively, these data have confirmed a defect in the maturation of the gingipains in P. gingivalis FLL95.
Fibronectin plays important roles in cellular signal transduction by its interaction with the integrins (3). It has been documented that the incubation of culture supernatants of P. gingivalis with fibroblasts resulted in the disappearance of fibronectin and the integrin subunits (4); however, incubation with culture supernatants of the Rgp-deficient mutant showed very little change in the adhesion molecules (4). In this study, incubation of P. gingivalis W83 cells with fibronectin resulted in complete degradation of the fibronectin. As expected, due to reduced gingipain activity in the vimA-, vimE-, or vimF-defective mutants, there was a significant reduction in cleavage of the fibronectin when these isogenic strains were incubated with fibronectin. The absence of fibronectin cleavage when the vimA-defective mutant was incubated with fibronectin could be explained by the absence of Kgp and RgpA on the surface of the cell (51). The vimE- and vimF-defective mutants have low cell-associated gingipain activity. We cannot rule out the possibility that other cell-associated protease(s) involved in fibronectin degradation might be present in the vimE- and vimF-defective mutants. This is under further investigation in the laboratory.
It is possible that a defect in the proper glycosylation of proteins, including the gingipains, may contribute to the abnormal maturation of the gingipains. In the vimA-defective mutant FLL92, the 64-kDa RgpB (37, 51) may be partially processed, resulting in an inactive intermediate of the full-length 80-kDa RgpB proenzyme. The initial cleavage of the 80-kDa RgpB and 185-kDa RgpA proenzyme probably occurs at a normal rate. However, in the vimE and vimF mutants, the presence of the 80-kDa RgpB and/or the 185-kDa RgpA (51) and the 64-kDa partially processed RgpB proenzyme intermediate (51) suggests that there may be a slow processing of the full-length RgpB and RgpA, possibly due to aberrant modification of the proteins. VimA, VimE, and/or VimF are possible factors needed for carbohydrate biogenesis involved in the anchorage and/or activation of the gingipains. In contrast, the absence of the 80-kDa RgpB in P. gingivalis FLL92 suggests that vimA may not be needed for the initial step of activation (51). Further, in the vimA mutant, the normal activation mechanism may be disrupted, resulting in a alternate pathway of activation, similar to that described by Mikolajczyk et al. (33). Since purified RgpB has been shown to activate the Rgps but not Kgp in vitro (51), we can envision a scenario where, in the vimA mutant, the concentration of the secreted 64-kDa RgpB reaches a high, critical concentration in stationary phase, such that RgpB is activated by proximity-induced activation and subsequently activates RgpA. The activated Rgps may then be able to process other proteins or factors needed for Kgp activation. In contrast to the wild type, the drastically reduced proteolytic activities of the vimE and vimF mutants may be the result of a defect in the initial activation step of the RgpB proenzyme. Taken together, these data suggest that the VimE and VimF proteins in P. gingivalis may be involved in protease activation upstream of VimA.
The modulation of virulence in P. gingivalis may be coordinated via an ability to modulate proteolytic activity (reviewed in references 12, 21, 25, and 39). Although not directly tested in this study, the vimF gene may be an important virulence gene. Because inactivation of the vimF gene resulted in a reduction in proteolytic activity and had a pleiotropic effect on other important virulence factors, P. gingivalis FLL95 would be expected to have a reduced pathogenic potential in contrast to the wild-type strain. This would be consistent with the vimA-defective mutant that had a similar phenotype as P. gingivalis FLL93 and FLL95 and was dramatically less virulent that the wild-type strain in the mouse model (1).
In summary, we have constructed an isogenic mutant of P. gingivalis that is defective in a putative glycosyltransferase gene. While this mutant had reduced proteolytic activity, there was expression of the gingipain genes. Further, this mutant in contrast to the wild-type strain showed reduced hemolytic and hemagglutinating activities. Because glycosyltransferases are important in carbohydrate biosynthesis and posttranslational glycosylation, further study of this process in bacteria, in addition to its ability to modulate virulence factors in pathogenic bacteria, is of significance. Identification of the vimF gene represents a potentially new mechanism for regulating proteolytic activity and virulence in P. gingivalis and could possibly be an important therapeutic target.
Acknowledgments
This work was supported by Loma Linda University School of Dentistry and by Public Health Service grants DE13664 and DE13664-S1 from the National Institute of Dental and Craniofacial Research (to H.M.F.) and grant GM60507, a minority training grant, from the National Institute of General Medicine.
We thank Jan Potempa for gingipain antibodies, Ann Progulske-Fox for her donation of HagA antibodies, and Michael Curtis for MAb 1B5.
Editor: A. D. O'Brien
REFERENCES
- 1.Abaibou, H., Z. Chen, G. J. Olango, Y. Liu, J. Edwards, and H. M. Fletcher. 2001. vimA Gene downstream of recA is involved in virulence modulation in Porphyromonas gingivalis W83. Infect. Immun. 69:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abaibou, H., Q. Ma, G. J. Olango, J. Potempa, J. Travis, and H. M. Fletcher. 2000. Unaltered expression of the major protease genes in a non-virulent recA-defective mutant of Porphyromonas gingivalis W83. Oral Microbiol. Immunol. 15:40-47. [DOI] [PubMed] [Google Scholar]
- 3.Amano, A. 2003. Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease. J. Periodontol. 74:90-96. [DOI] [PubMed] [Google Scholar]
- 4.Baba, A., N. Abe, T. Kadowaki, H. Nakanishi, M. Ohishi, T. Asao, and K. Yamamoto. 2001. Arg-gingipain is responsible for the degradation of cell adhesion molecules of human gingival fibroblasts and their death induced by Porphyromonas gingivalis. Biol. Chem. 382:817-824. [DOI] [PubMed] [Google Scholar]
- 5.Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. [DOI] [PubMed] [Google Scholar]
- 6.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth, V., and T. Lehner. 1997. Characterization of the Porphyromonas gingivalis antigen recognized by a monoclonal antibody which prevents colonization by the organism. J. Periodontal Res. 32:54-60. [DOI] [PubMed] [Google Scholar]
- 8.Bosques, C. J., S. M. Tschampel, R. J. Woods, and B. Imperiali. 2004. Effects of glycosylation on peptide conformation: a synergistic experimental and computational study. J. Am. Chem. Soc. 126:8421-8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, J. A., G. J. Davies, V. Bulone, V., and B. Henrissat. 1998. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 329:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, T., H. Dong, R. Yong, and M. J. Duncan. 2000. Pleiotropic pigmentation mutants of Porphyromonas gingivalis. Microb. Pathog. 28:235-247. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran, M. L., D. E. Kleiner, Jr., and W. G. Stetler-Stevenson. 1995. Regulation of matrix metalloproteinases during extracellular matrix turnover. Adv. Exp. Med. Biol. 385:151-159. [DOI] [PubMed] [Google Scholar]
- 12.Curtis, M. A., J. Aduse-Opoku, and M. Rangarajan. 2001. Cysteine proteases of Porphyromonas gingivalis. Crit. Rev. Oral Biol. Med. 12:192-216. [DOI] [PubMed] [Google Scholar]
- 13.Curtis, M. A., A. Thickett, J. M. Slaney, M. Rangarajan, J. Aduse-Opoku, P. Shepherd, N. Paramonov, and E. F. Hounsell. 1999. Variable carbohydrate modifications to the catalytic chains of the RgpA and RgpB proteases of Porphyromonas gingivalis W50. Infect. Immun. 67:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dashper, S. G., K. J. Cross, N. Slakeski, P. Lissel, P. Aulakh, C. Moore, and E. C. Reynolds. 2004. Hemoglobin hydrolysis and heme acquisition by Porphyromonas gingivalis. Oral Microbiol. Immunol. 19:50-56. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of a mutant of Porphyromonas gingivalis W83 that is defective in the prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher, A., J. Aduse-Opoku, M. Rangarajan, J. M. Slaney, and M. A. Curtis. 2003. Glycosylation of the Arg-gingipains of Porphyromonas gingivalis and comparison with glycoconjugate structure and synthesis in other bacteria. Curr. Protein Pept. Sci. 4:427-441. [DOI] [PubMed] [Google Scholar]
- 17.Grenier, D., S. Roy, F. Chandad, P. Plamondon, M. Yoshioka, K. Nakayama, and D. Mayrand. 2003. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect. Immun. 71:4742-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han, N. M., J. Whitlock, and A. Progulske-Fox. 1996. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect. Immun. 64:4000-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa, Y., S. Nishiyama, K. Nishikawa, T. Kadowaki, K. Yamamoto, T. Noguchi, and F. Yoshimura. 2003. A novel type of two-component regulatory system affecting gingipains in Porphyromonas gingivalis. Microbiol. Immunol. 47:849-858. [DOI] [PubMed] [Google Scholar]
- 20.Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73:1019-1049. [DOI] [PubMed] [Google Scholar]
- 21.Imamura, T. 2003. The role of gingipains in the pathogenesis of periodontal disease. J. Periodontol. 74:111-118. [DOI] [PubMed] [Google Scholar]
- 22.Kadowaki, T., K. Nakayama, F. Yoshimura, K. Okamoto, N. Abe, and K. Yamamoto. 1998. Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J. Biol. Chem. 273:29072-29076. [DOI] [PubMed] [Google Scholar]
- 23.Karunakaran, T., and S. C. Holt. 1993. Cloning of two distinct hemolysin genes from Porphyromonas (Bacteroides) gingivalis in Escherichia coli. Microb. Pathog. 15:37-49. [DOI] [PubMed] [Google Scholar]
- 24.Kelly, C. G., V. Booth, H. Kendal, J. M. Slaney, M. A. Curtis, and T. Lehner. 1997. The relationship between colonization and haemagglutination inhibiting and B cell epitopes of Porphyromonas gingivalis. Clin. Exp. Immunol. 110:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lantz, M. S. 1996. New insights into mechanisms of bacterial pathogenesis in periodontitis. Curr. Opin. Periodontol. 3:10-18. [PubMed] [Google Scholar]
- 27.Lepine, G., and A. Progulske-Fox. 1996. Duplication and differential expression of hemagglutinin genes in Porphyromonas gingivalis. Oral Microbiol. Immunol. 11:65-78. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, J. P., J. A. Dawson, J. C. Hannis, D. Muddiman, and F. L. Macrina. 1999. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J. Bacteriol. 181:4905-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovatt, A., and I. Roberts. 1992. Cloning of the β-N-acetyl-d-hexosaminidase gene from P. gingivalis. J. Dent. Res. 71:293. [Google Scholar]
- 30.Lovatt, A., and I. S. Roberts. 1994. Cloning and expression in Escherichia coli of the nahA gene from Porphyromonas gingivalis indicates that β-N-acetylhexosaminidase is an outer-membrane-associated lipoprotein. Microbiology 140:3399-3406. [DOI] [PubMed] [Google Scholar]
- 31.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 32.Mayrand, D., and S. C. Holt. 1988. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol. Rev. 52:134-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikolajczyk, J., K. M. Boatright, H. R. Stennicke, T. Nazif, J. Potempa, M. Bogyo, and G. S. Salvesen. 2003. Sequential autolytic processing activates the zymogen of Arg-gingipain. J. Biol. Chem. 278:10458-10464. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama, K. 2003. Molecular genetics of Porphyromonas gingivalis: gingipains and other virulence factors. Curr. Protein Pept. Sci. 4:389-395. [DOI] [PubMed] [Google Scholar]
- 35.Narimatsu, M., Y. Noiri, S. Itoh, N. Noguchi, T. Kawahara, and S. Ebisu. 2004. Essential role for the gtfA gene encoding a putative glycosyltransferase in the adherence of Porphyromonas gingivalis. Infect. Immun. 72:2698-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson, K. E., R. D. Fleischmann, R. T. DeBoy, I. T. Paulsen, D. E. Fouts, J. A. Eisen, S. C. Daugherty, R. J. Dodson, A. S. Durkin, M. Gwinn, D. H. Haft, J. F. Kolonay, W. C. Nelson, T. Mason, L. Tallon, J. Gray, D. Granger, H. Tettelin, H. Dong, J. L. Galvin, M. J. Duncan, F. E. Dewhirst, and C. M. Fraser. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591-5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olango, G. J., F. Roy, S. M. Sheets, M. K. Young, and H. M. Fletcher. 2003. Gingipain RgpB is excreted as a proenzyme in the vimA-defective mutant Porphyromonas gingivalis FLL92. Infect. Immun. 71:3740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olczak, T., D. W. Dixon, and C. A. Genco. 2001. Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J. Bacteriol. 183:5599-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potempa, J., A. Banbula, and J. Travis. 2000. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol. 2000 24:153-192. [DOI] [PubMed] [Google Scholar]
- 40.Potempa, J., J. Mikolajczyk-Pawlinska, D. Brassell, D. Nelson, I. B. Thogersen, J. J. Enghild, and J. Travis. 1998. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J. Biol. Chem. 273:21648-21657. [DOI] [PubMed] [Google Scholar]
- 41.Potempa, J., A. Sroka, T. Imamura, and J. Travis. 2003. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr. Protein Pept. Sci. 4:397-407. [DOI] [PubMed] [Google Scholar]
- 42.Progulske-Fox, A., S. Tumwasorn, G. Lepine, J. Whitlock, D. Savett, J. J. Ferretti, and J. A. Banas. 1995. The cloning, expression and sequence analysis of a second Porphyromonas gingivalis gene that codes for a protein involved in hemagglutination. Oral Microbiol. Immunol. 10:311-318. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, M. A., L. W. Riley, and I. Benz. 2003. Sweet new world: glycoproteins in bacterial pathogens. Trends Microbiol. 11:554-561. [DOI] [PubMed] [Google Scholar]
- 44.Shah, H. N., and S. E. Gharbia. 1989. Lysis of erythrocytes by the secreted cysteine protease of Porphyromonas gingivalis W83. FEMS Microbiol. Lett. 61:213-218. [DOI] [PubMed] [Google Scholar]
- 45.Shi, Y., D. B. Ratnayake, K. Okamoto, N. Abe, K. Yamamoto, and K. Nakayama. 1999. Genetic analyses of proteolysis, hemoglobin finding, and hemagglutination of Porphyromonas gingivalis: construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 274:17955-17960. [DOI] [PubMed] [Google Scholar]
- 46.Shoji, M., D. B. Ratnayake, Y. Shi, T. Kadowaki, K. Yamamoto, F. Yoshimura, A. Akamine, M. A. Curtis, and K. Nakayama. 2002. Construction and characterization of a nonpigmented mutant of Porphyromonas gingivalis: cell surface polysaccharide as an anchorage for gingipains. Microbiology 148:1183-1191. [DOI] [PubMed] [Google Scholar]
- 47.Sroka, A., M. Sztukowska, J. Potempa, J. Travis, and C. A. Genco. 2001. Degradation of host heme proteins by lysine- and arginine-specific cysteine proteinases (gingipains) of Porphyromonas gingivalis. J. Bacteriol. 183:5609-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundqvist, G. 1993. Pathogenicity and virulence of black-pigmented gram-negative anaerobes. FEMS Immunol. Med. Microbiol. 6:125-138. [DOI] [PubMed] [Google Scholar]
- 49.Upreti, R. K., M. Kumar, and V. Shankar. 2003. Bacterial glycoproteins: functions, biosynthesis and applications. Proteomics 3:363-379. [DOI] [PubMed] [Google Scholar]
- 50.Vanterpool, E., F. Roy, and H. M. Fletcher. 2004. The vimE gene downstream of vimA is independently expressed and is involved in modulating proteolytic activity in Porphyromonas gingivalis W83. Infect. Immun. 72:5555-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanterpool, E., F. Roy, L. Sandberg, and H. M. Fletcher. 2005. Altered gingipain maturation in vimA- and vimE-defective isogenic mutants of Porphyromonas gingivalis. Infect. Immun. 73:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veith, P. D., G. H. Talbo, N. Slakeski, S. G. Dashper, C. Moore, R. A. Paolini, and E. C. Reynolds. 2002. Major outer membrane proteins and proteolytic processing of RgpA and Kgp of Porphyromonas gingivalis W50. Biochem. J. 363:105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wandersman, C. 1989. Secretion, processing and activation of bacterial extracellular proteases. Mol. Microbiol. 3:1825-1831. [DOI] [PubMed] [Google Scholar]