Abstract
Secondary nucleation is an emerging approach for synthesizing higher-order supramolecular polymers with exciting topologies. However, a detailed understanding of growth processes and the synthesis of homochiral superstructures is yet to be demonstrated. Here, we report the non-covalent synthesis of dendritic homochiral superstructures using NIR triimide dyes as building blocks via a secondary nucleation elongation process. Detailed analysis of kinetics and temporal evolution of morphology indicates that the formation of dendritic homochiral superstructures proceeds via growth on the surface and growth from the surface of the seeds. The combination of these two processes leads to the formation of elegant homochiral superstructures with a size of ~0.4 mm2, having a superhelix at the center and helical fibres as branches. Moreover, these dendritic homochiral superstructures exhibit significantly high chiro-optical photoresponse with the magnitude of gfactor reaching a value as high as 0.55 - 0.6. Thus, our results provide insights into the growth process of homochiral superstructures with dendritic topology, which can be critically important for the design and optimization of chiral-selective optoelectronic devices leveraging controlled self-assembly.
Subject terms: Supramolecular polymers, Molecular self-assembly, Surface assembly, Dendrimers, Self-assembly
Secondary nucleation is an approach to synthesizing higher-order supramolecular polymers with exciting topologies, but a detailed understanding of growth processes is needed. Here, the authors report the non-covalent synthesis and high chiro-optical photoresponse of dendritic homochiral superstructures via a secondary nucleation elongation process.
Introduction
Supramolecular polymers (SPs)1,2 of π-systems find a wide range of applications in the fields of optoelectronics3–6, catalysis7,8 and energy storage9. SPs form mainly via isodesmic or cooperative (nucleation-elongation) mechanisms, resulting in one-dimensional (1D) chains with high internal order10–12. Recent advances in the primary nucleation-elongation process allowed us to synthesize homo13 and block SPs14,15 with controlled length and sequence via living supramolecular polymerization (LSP)16–18. Despite significant progress in the field of SPs, it is still challenging to synthesize higher-order SP structures19 using small molecules with precision like that of covalent polymers20,21. Controlling the topology of SPs is crucial to attain new properties and functions as seen in covalent polymers22,23. Moreover, many bio-systems possess unique topologies formed via hierarchical assembly24,25. Although Winnik and Manners et al. demonstrated the synthesis of various higher-order supramolecular assemblies with high precision26–28 via living crystallization-driven self-assembly of polymer building blocks, it is still challenging with small molecules. In the case of LSP via a primary nucleation event, elongation takes place at the active ends of the seeds and the resultant morphology is the same as the seeds but with increased dimensions16–18. Hence, LSP via primary nucleation could yield only 1D or 2D SPs with controlled dimensions18,29. In this context, surface-catalyzed secondary nucleation event, a mechanism that is well explored in protein assemblies plays a pivotal role in the synthesis of SPs with higher-order topologies30,31. The utilization of secondary nucleation mechanisms for synthesizing higher-order supramolecular assemblies using small molecules just began to appear32–34. For example, Yagai et al.33 demonstrated the synthesis of supramolecular nano-polycatenanes using hydrogen-bonded (H-bonded) rosette assemblies. In another report, Sugiyasu et al.34 achieved the synthesis of Archimedean spirals using H-bonded porphyrins. We have recently reported the synthesis of 3D spherical spherulites and scarf-like SP heterostructures using perylene diimides via a secondary nucleation-elongation process35.
Chirality is an important aspect of self-assembled structures such as SPs36,37 and polymer assemblies38–41. The study of chiral supramolecular assemblies helps us to understand the origin of homochirality in nature. Chiral assemblies are utilized for circularly polarized luminescence38–42, asymmetric catalysis37,43, biomedical applications44 and electron-spin filtering applications45. Several elegant molecular designs are reported for the synthesis of 1D chiral assemblies with single36 or double46 or superhelical structures47–49. However, rational design principles for the synthesis of chiral supramolecular assemblies with hierarchical topologies are seldom reported50,51. Although the synthesis of higher-ordered chiral assemblies is well executed in biological systems52, it is still a major challenge for synthetic systems. Recently, George et al. achieved stereoselective induction of secondary nucleation pathways with chiral monomers53. However, higher-ordered chiral assemblies could not be achieved due to the detachment of SPs from the surface of the seed. To overcome this, phenyl groups containing tripeptide side chains were introduced to stabilize the SP on the seed surface. However, the growth is restricted only across the length of the seed54. Hence, the non-covalent synthesis of higher-ordered chiral assemblies, reminiscent of biological systems, remains a grand challenge52. Thus, a deeper understanding of molecular designs and the growth process are indeed important for further development in this field and the controlled synthesis of higher-order chiral SPs as they are capable of showing outstanding chiroptical functions55–58.
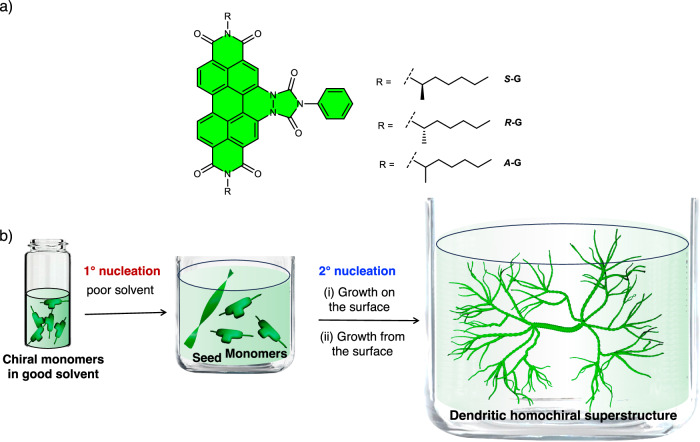
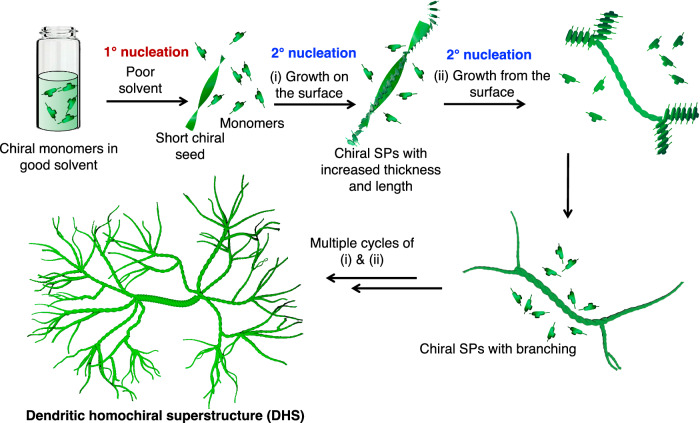
Herein, we present an important advancement in this field for the synthesis of higher-order chiral SP architectures of π-conjugated molecules having dendritic topology via secondary nucleation. This is demonstrated using NIR-absorbing triimide dyes substituted with chiral alkyl chains (S-G and R-G) (Fig. 1a). Although these NIR dyes have been known for a long time, they have not yet been explored for supramolecular polymerization59. The presence of donor groups and a phenyl ring at the bay position not only provides NIR absorption but also significantly enhances their stacking efficiency and order compared to the corresponding perylene diimides (PDIs)35,60. Interestingly, in 15 vol% CHCl3 (good solvent) in isopropanol (IPA, poor solvent), S-G and R-G display temporal evolution of morphology. With time, these molecules evolve into macroscopic dendritic homochiral superstructures (DHS) having a size of ~0.4 mm2 with an expression of macroscopic chirality (Fig. 1b). Detailed electron microscopy studies and kinetic analysis reveal that the formation of these higher-order chiral architectures take place via a secondary nucleation-elongation mechanism (Fig. 1b). Our investigations reveal that a few monomers are initially converted into short 1D chiral SPs through primary nucleation. Next, the remaining monomers in the solution nucleate and elongate ‘on the surface’ as well as ‘from the surface’ of the 1D chiral SP seeds (Fig. 1b). The repetition of these two processes results in the formation of DHS composed of a thick central superhelix and branches of thin 1D helical fibers. Under similar conditions, the corresponding PDIs do not self-assemble, and the achiral triimide dye (A-G) forms only 1D fibers, thus highlighting the importance of bay substitution and chiral side chains in the formation of DHS (Fig. 1). The temporal evolution of SPs into DHS and their interaction with circularly polarized light is also probed to understand the enhancement in the chirality with self-assembly. It was possible to follow the growth of the self-assembled structure with the variation in optoelectronic properties. Interestingly, the temporal evolution of DHS results in an enhancement of conductivity by at least two to three times. Moreover, the DHS exhibits a clean selective chiro-optical response in the solution phase with a magnitude of gfactor ~ 0.55–0.61 and responsivity as high as 2 mA/W, which is among the best for supramolecular semiconductors. Nevertheless, these measurements indicate that electrical measurements can be a way to probe supramolecular self-assembly while, at the same time, the differential photoresponse to left and right circularly polarized light highlights the potential of such programmable structures for applications in circularly polarized light detection and chiroptical devices.
Fig. 1. Molecular structures and summary of the formation of dendritic homochiral superstructures (DHS).
a Molecular structures of NIR triimides having chiral (S-G and R-G) and achiral alkyl (A-G) side chains. b schematic illustration of the formation of dendritic homochiral superstructures via ‘growth on the surface’ and ‘growth from the surface’ secondary nucleation-elongation process. The schematic has been made using Blender 3.5 software.
Results and discussion
The molecules under investigation, S-G, R-G and A-G were synthesized from respective PDIs (Fig. 1 and Supplementary Fig. 1). The detailed synthetic procedure is given in the supplementary information (Supplementary Note 2). From these PDIs (Supplementary Figs. 2–4), respective NIR dyes, S-G, R-G and A-G were synthesized in an efficient one-step oxidative Diels-Alder reaction59. The desired product was isolated, purified, and characterized using various analytic techniques (Supplementary Figs. 5–17). Thermogravimetric analysis reveals that all three triimide dyes are thermally stable up to 350 °C (Supplementary Fig. 17). The UV-Vis spectrum of S-G in CHCl3 (30 μM) solution at 25 °C shows absorption peaks ranging from 250 nm to 800 nm, with three distinct regions and absorption maxima at 300 nm (Supplementary Fig. 19a). The appearance of a broad peak from 500 nm to 800 nm is due to the introduction of bay substitution in PDI, which facilitates intramolecular charge transfer. Well-resolved vibronic features are observed between 390 nm and 460 nm59. These features indicate the molecularly dissolved state of S-G (30 μM) in CHCl3. We investigated the supramolecular polymerization behavior of a 30 μM solution of S-G by varying the solvent percentage of CHCl3 and IPA while probing its absorption and emission spectra. As the vol% of IPA increases in CHCl3, a decrease in the absorbance of all the peaks and broadening of the peak between 500 nm to 800 nm and the appearance of weak red-shifted absorbance around 800 nm was observed (Supplementary Fig. 19a). Moreover, the loss of vibronic features of the peaks from 390 nm to 460 nm was also clearly evident from the absorption spectra. Simultaneously, it has been noted that the molecularly dissolved state in CHCl3 is fluorescent, with a λmax ~768 nm, but upon an increase in vol% of IPA, the fluorescence emission is completely quenched (Supplementary Fig. 19b). These features can be attributed to the supramolecular polymerization of S-G with face-to-face stacking (vide infra). As expected, the molecularly dissolved solution of S-G (30 μM) is CD silent (Supplementary Fig. 19c). For the solutions having S-G (30 μM) in 85, 90 and 95 vol% of IPA in CHCl3, a strong CD signal is observed due to the transfer of chirality from the chiral alkyl side chains to the core of S-G upon supramolecular polymerization.
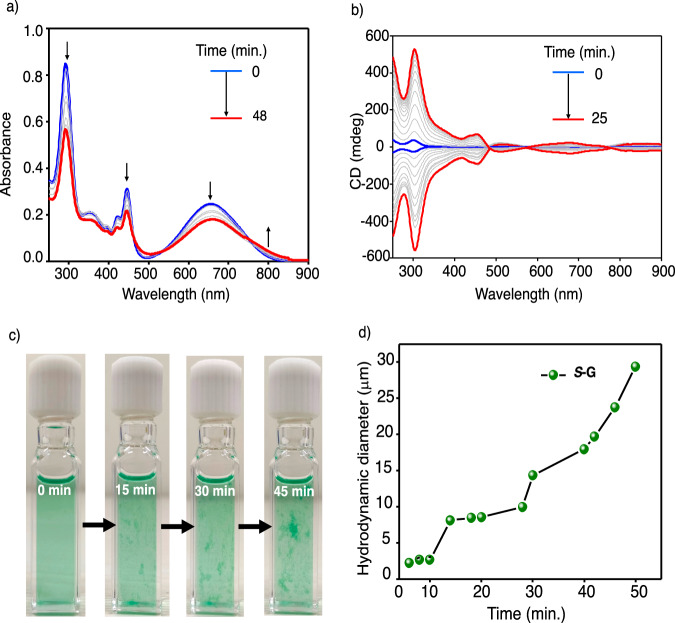
Interestingly, the freshly prepared solutions of S-G (30 μM) in 15% CHCl3 in IPA started to show further changes in the absorption spectra over a period of 48 min. at 25 °C (Fig. 2a). With time, a gradual decrease of all absorption bands, along with a further increase in absorbance around 800 nm was observed (Fig. 2a). Moreover, the peaks from 390 nm to 460 nm became further broad (Fig. 2a). These observations indicate that further assembly of S-G with time in solution. Along with the changes in the absorption spectra, the CD spectra also showed a further increase in the signal over time (Fig. 2b). In the case of S-G, a weak CD signal is obtained for the freshly prepared solution having a small positive cotton effect at 300 nm. The initially low-intense CD signals become more intense, with further progress in self-assembly peaking nearly at 25 min. (Fig. 2b and Supplementary Fig. 20). Similar behavior was obtained during the aggregation of R-G. Moreover, a mirror image relation in CD spectra is seen for the temporal aggregation of S-G and R-G (Fig. 2b). The freshly prepared solution of S-G (30 μM) in 15% CHCl3 in IPA is visibly clear but emerged as a green suspension in nearly 45 min. (Fig. 2c). As a result, a reduction in the CD signal after 25 min. was observed due to the crashing of self-assembled structures out of the solution. These observations suggest the formation of higher-ordered SPs of NIR dyes with time. We have also performed time-dependent linear dichroism (LD) measurements to understand its contribution to the CD signal and preferential orientation of SPs in solution. Although the LD signal is weak, we have seen a significant increase after 5 min. and saturation after 25 min. (Supplementary Fig. 21). Hence, the contribution of the LD signal to the CD signal is low, and the resultant SPs have some degree of preferential orientation in the solution. To validate this temporal hierarchical self-assembly process further, we have carried out a time-dependent DLS study by which we could monitor the growth in the size of aggregates (Fig. 2d). With time, an increase in hydrodynamic diameter is seen, establishing that a time-dependent self-assembly growth is underway. In 48 min., the hydrodynamic radius of the assemblies is increased from 2.0 μm to 30 μm.
Fig. 2. Probing the temporal evolution of supramolecular polymers.
a Time-dependent absorption spectra of 30 μM solution of S-G in 15% CHCl3 in IPA over a period of 48 min. representing its temporal growth. b Time-dependent CD spectra of a 30 μM solution of S-G and R-G in 15% CHCl3 in 85% IPA. c Photographs of solution of S-G (30 μM) in 15% CHCl3 in IPA at different time intervals. d Time-dependent dynamic light scattering (DLS) data of S-G (30 μM) in 15% CHCl3 in IPA.
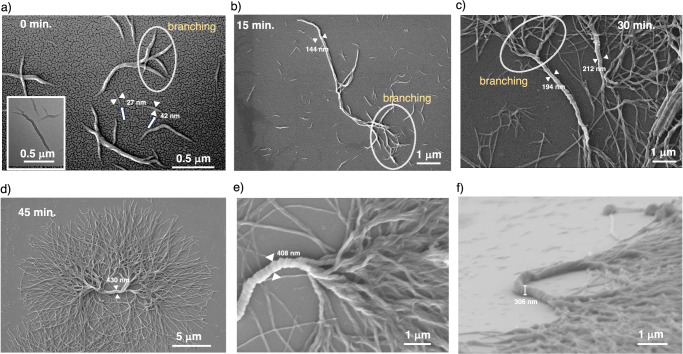
In order to gain insights into the morphological evolution of S-G with time, we performed field-emission scanning electron microscopy (FE-SEM) by spin-coating the solutions onto a silicon wafer substrate at different time intervals. It is observed that a freshly prepared solution of S-G in 15% CHCl3 in IPA showed the presence of small helical fibers and a few double helices of less than 1 μm in length and width ranging from 27 nm to 42 nm (Fig. 3a). This is further supported by transmission electron microscopy (TEM) images as well (Supplementary Fig. 22). Interestingly, we have also observed a few fibers having branches and higher thicknesses (Fig. 3a). After 15 min., the morphology grew into multiple intertwined helices along with increased branching to dimensions >5 μm in length and nearly 140 nm to 150 nm in width (Fig. 3b). After 30 min., the appearance of a thick superhelix, along with several small helical fiber branches, is seen. The width of the superhelix is between 190 nm to 220 nm, and the length of each branched structure is >10 μm (Fig. 3c). After 45 min., we found the formation of DHS with extensive branching covering an area of 0.4 mm2 bearing a central superhelix having a width greater than 400 nm (Fig. 3d). A closer look at the image of the central superhelix reveals that it is composed of multiple intertwined helices that segregate at the ends into smaller bunches of helices and ultimately into helical fibers (Fig. 3e). A 3D image of the DHS was obtained by tilting the sample at 72°, showing the superhelix’s height to be >300 nm (Fig. 3f). Further analysis of FE-SEM and atomic force microscopy (AFM) images revealed that the central superhelix and branched thin helical fibers formed from S-G have a right-handed helical orientation (Supplementary Fig. 23). Similarly, FE-SEM and AFM images revealed the left-handed helical orientation for DHS formed from of R-G (Supplementary Fig. 24). These observations not only confirm the opposite helicity of DHS formed from S-G and R-G but also their homochiral nature.
Fig. 3. Probing the evolution of supramolecular polymers into dendritic homochiral superstructures (DHS) using FE-SEM.
Field-emission scanning electron microscopy (FE-SEM) images of S-G (30 μM) in 15% CHCl3 in IPA spin-coated on a silicon wafer at various time intervals. a At 0 min., freshly prepared aggregates are smaller in size, consisting of single and double helices, white arrows show primary nucleation fibers, and circled areas indicate branching (inset: transmission electron microscopy (TEM) image showing a double helix formation). b After 15 min., intertwined helices grow bigger in length and width with increased branching. c After 30 min., the intertwined helices start forming a superhelix of widths between 190 nm to 220 nm and with further increased branching. d After 45 min., we observed dendritic homochiral superstructures (DHS) having a superhelix of width 430 nm with extensive branching. e A magnified FE-SEM image of the central superhelix from which smaller helices are originating shows that the superhelix is composed of multiple helices. f A 3D image of DHS showing a superhelix core with a height of ~305 nm.
Next, to understand the role of chiral side chains and bay substitution in the formation of DHS, we have investigated the supramolecular polymerization of A-G and S-PDI. First to understand the role of chiral side chains, we have synthesized A-G using commercially available racemic 2-heptylamine. Like S-G and R-G, a 30 μM solution A-G also showed temporal evolution of SPs in 15% CHCl3 in IPA (Supplementary Fig. 25a, b). However, the resultant morphology is 1D fibers, not DHS, as evidenced by FE-SEM images (Supplementary Fig. 25d, e). As expected, the CD signal of SPs of A-G is negligible due to the achiral nature of the resultant SPs (Supplementary Fig. 25c). This indicates that chirality in the side chain is crucial to form DHS. Next, to understand the role of bay substitution, we have investigated the supramolecular polymerization of S-PDI. We found that a 30 μM solution of S-PDI in 15% CHCl3 in IPA exists as a monomer even after keeping it at 25 °C for 14 h (Supplementary Fig. 26a). This indicates the weak stacking efficiency of S-PDI compared to S-G. At higher concentrations, such as 200 μM, S-PDI formed self-assembled structures (Supplementary Fig. 26b) with needles-like morphology after keeping for 3 h at 25 °C (Supplementary Fig. 26c). Hence, both chirality in the side chain and bay substitution are important for the formation of DHS.
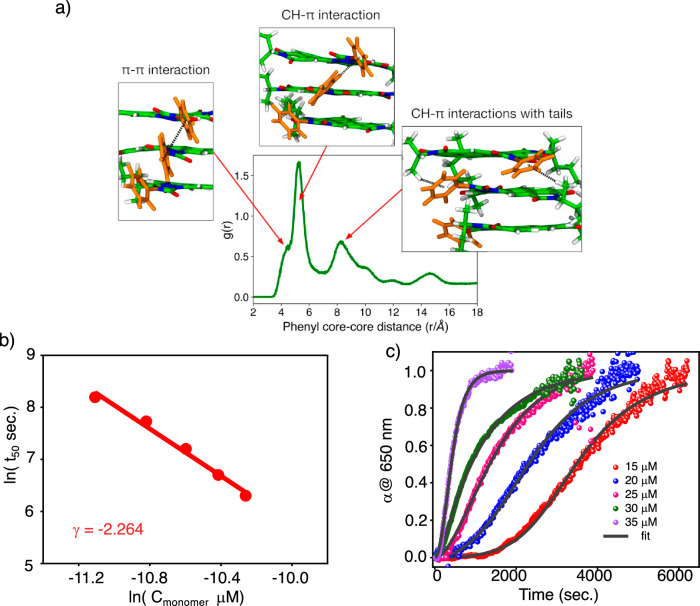
To further understand the role of the bay substitution in improving the stacking efficiency of NIR dyes (S-G and R-G), we have performed theoretical calculations on both dimers and a stack consisting of 24 monomers in 15% CHCl3 in IPA solvent mixture (Supplementary Note 4). Gas-phase quantum mechanical calculations revealed that the intermolecular interaction energy between S-G monomers is approximately 60 kJ/mol higher than that of the S-PDI monomers, demonstrating that the S-G dimer is more stable. Energy decomposition analysis indicated that the bay substitution enhances the stability of the S-G dimer by considerably strengthening the electrostatic, dispersion, and charge transfer interactions (Supplementary Fig. 27). In contrast, the absence of bay substitution in S-PDIs results in their self-assembly at relatively higher concentrations, primarily due to their weaker intermolecular interactions compared to those of S-G molecules. To further investigate the influence of solvent on the stacking propensity and orderliness in the extended fiber, we conducted all-atom classical molecular dynamics simulations on preformed stacks in an explicit solvent mixture of 15% CHCl3 in IPA. The presence of strong peaks in the radial distribution function (g(r)) of both systems indicates that both S-G and S-PDI stacks are stable in solution (Supplementary Fig. 28)61. Importantly, the intensity of the first peak is higher for S-G, suggesting a greater stacking propensity than S-PDI stacks. The g(r) calculated from the extended trajectory (200–300 ns), reveals the emergence of long-range order in the structures of S-G compared to S-PDI (Supplementary Fig. 28b). This is due to the phenyl rings exhibiting dynamic behavior, tending to adopt a slanted orientation with an average angle of approximately ±25° relative to the aromatic core (Supplementary Fig. 28e). As a result, several additional non-covalent interactions are favored in the S-G stack, such as π-π interactions between neighboring phenyl rings, CH-π interactions between adjacent phenyl rings and CH-π interactions between the branched methyl group of one molecule and the phenyl ring of a neighboring molecule (Fig. 4a). Thus, the bay substitution in the S-G enhances the ordering and stabilization of its SPs compared to the S-PDI, while the chiral side chains provide the bias for supramolecular chirality, resulting in the formation of DHS (See Supplementary Figs. 27 and 28 and further explanation there).
Fig. 4. Importance of bay substituted phenyl ring and kinetic analysis of self-assembly.
a The radial distribution functions (RDF), g(r), between geometric center of phenyl rings in S-G stack. The phenyl rings are observed to engage in three distinct types of interactions within the S-G stack: first, π-π interactions between neighboring phenyl rings, corresponding to the shoulder at 4.3 Å in the g(r); second, CH-π interactions between adjacent phenyl rings, leading to the peak at 5.2 Å; and third, CH-π interactions between the branched methyl group of one molecule and the phenyl ring of a neighboring molecule, resulting in the peak at 8.2 Å. b Double logarithmic plot of concentration and half-time of S-G at concentrations of 15 μM, 20 μM, 25 μM, 30 μM and 35 μM showing a linear fit. The slope gives the value of scaling exponent (γ) to be −2.264. c Kinetic profiles of aggregation of 15 μM, 20 μM, 25 μM, 30 μM and 35 μM solutions in 15% CHCl3 in IPA and corresponding fit in secondary nucleation dominated-unseeded, shows increasing lag time with decreasing concentration of S-G. Here α @650 nm represents the degree of supramolecular polymerization monitored at 650 nm.
A lag phase in the temporal aggregation indicates the presence of secondary nucleation events in the formation of DHS. To get further insights, we have analyzed the kinetic profiles of hierarchical assemblies at different monomer concentrations such as 15 μM, 20 μM, 25 μM, 30 μM and 35 μM (Fig. 4b, c). We found that 15 μM is the threshold concentration because, at 10 μM, no significant changes in the absorption spectra were observed even for 3 h at 25 °C (Supplementary Fig. 29). Above 35 μM, such as 40 μM, temporal self-assembly is rapid. Hence, we have analyzed the growth kinetics for five different concentrations (15 μM to 35 μM) by monitoring the absorbance at 650 nm wavelength. The decrease in absorbance at 650 nm indicates the supramolecular polymerization of S-G (Figs. 2a, 4c). Figure 4c shows the plot of the degree of supramolecular polymerization (α @650 nm) versus time at various concentrations of S-G. With the decrease in the concentration of S-G, an increase in half-time of supramolecular polymerization is observed. At 35 μM, the half-time is 550 sec. which increased to >3550 sec. by decreasing the concentration to 15 μM (Fig. 4c). The double logarithmic plot between the log of half-time and the log of concentration gives the value of slope (also known as γ value or scaling exponent value) as −2.264 (Fig. 4b). This value was obtained by using amylofit software (http://www.amylofit.ch.cam.ac.uk) developed by Knowles and group62. The scaling exponent value is indicative of the dependence of aggregation kinetics on the monomeric concentration. The absence of curvature in the linear fit suggests that it should align well with the secondary nucleation mechanism53,62. Given the relation between scaling exponent (γ) and secondary nucleation order n2, that comes out to be 4, and nc comes out to be 3. In this case, the best fit with MSE (mean square error) less than 0.00162 is obtained in secondary nucleation, dominated and unseeded (Fig. 4c and Supplementary Table 1). An ill-fit is obtained for the primary nucleation-elongation model with greater MSE (Supplementary Fig. 30 and Supplementary Table 1). Thus, we conclude that the formation of DHS is aided by a surface-assisted secondary nucleation phenomenon.
The detailed time-dependent microscopic and kinetic analysis revealed that the rapid addition of poor solvent (IPA) to the monomers in CHCl3 leads to the conversion of some of the monomers into short 1D helical SPs via a primary nucleation event63 (Figs. 3a, 5 and Supplementary Fig. 19). With time, we have seen a further decrease in monomer concentration and an increase in the width of fibers and branching. This indicates that the short 1D helical SPs formed via a primary nucleation event act as seeds for the secondary nucleation events. The increase in the width and length suggests that the remaining monomers in the solution nucleate and elongate on the surface of the short 1D helical SP seeds. This ‘growth on the surface’ first leads to the formation of a double helix (Figs. 3a, 5). The monomers also nucleate from the surface of the seeds to result in branching as evidenced by FE-SEM images (Figs. 3a, 5)64. The remnant monomers in the solution now assemble via ‘growth on the surface’ to contribute to the formation of a superhelix core and ‘growth from the surface’ leads to extensive branching (Figs. 3b–d and 5). The branched fibers also exhibit the formation of intertwined helices due to growth on their surface and further undergo branching from the surface to give extensive helical branched structures (Fig. 3e). This interplay of ‘growth on the surface’ and ‘growth from the surface,’ via secondary nucleation event eventually leads to the formation of final DHS (Figs. 3 and 5).
Fig. 5. Schematic illustration of DHS formation.
Schematic illustration of the formation of dendritic homochiral superstructures (DHS) via ‘growth on the surface’ and ‘growth from the surface’ of the seeds via secondary nucleation-elongation mechanism. The schematic has been made using Blender 3.5 software.
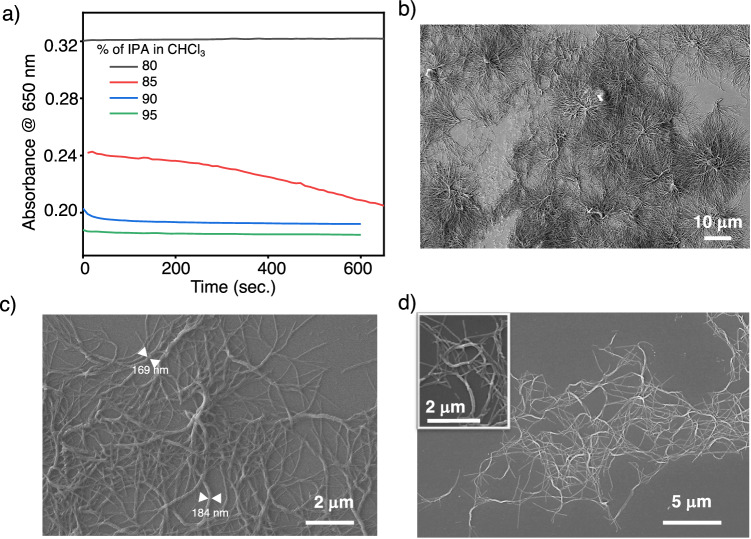
Next, we investigated the role of solvent percentage on the formation of DHS. At 20 vol% of CHCl3 in IPA, no significant change leading to the hierarchical self-assembly of S-G was observed, as evidenced by the absorbance versus time plot monitored at 650 nm wavelength (Fig. 6a). Upon increasing the poor solvent (IPA) to 85% (15% CHCl3), the self-assembly process is completed in 48 min., giving DHS (Figs. 3e, 4b, 6b and Supplementary Fig. 31a, b). The hierarchical self-assembly process was completed more quickly by further increasing the IPA vol% to 90 and 95 in CHCl3.When the IPA is 90%, we have observed the formation of superhelix with less branching and smaller widths (~170 nm to 185 nm) (Fig. 6c and Supplementary Fig. 31c, d). At 95% IPA (5% CHCl3), the self-assembly of S-G was completed in less than a min., but no evidence of DHS and mostly 1D superhelical fibers with very little branching was observed (Fig. 6d and Supplementary Fig. 31e, f). The increase in vol% of poor solvent (IPA) to 90 and 95, increases the number of seeds available for the growth of monomers compared to 85 vol% of IPA. This is further evidenced by the lower absorbance at 650 nm at zero min. (Fig. 6a). Due to the increased seed percentage with respect to monomers in the solution, the secondary nucleation and elongation process becomes rapid with shorter half-time for the solutions having 90 and 95 vol% IPA in CHCl3. As a result, the growth process is completed quickly before leading to the formation of DHS. The formation of mostly 1D superhelical fibers in 95 vol% IPA in CHCl3 indicate that the secondary nucleation and elongation process is mainly dominated by growth on the surface (Figs. 5 and 6d). These observations further prove that to achieve dendritic topology, secondary nucleation and elongation via growth from the surface are crucial.
Fig. 6. Effect of solvent composition on dendritic homochiral superstructures (DHS) formation.
a Time-dependent variation in the absorbance of S-G (30 μM) at various volume % of IPA in CHCl3 monitored at 650 nm. Field-emission scanning electron microscopy (FE-SEM) images of a 30 μM solution of S-G (b) in 15% CHCl3, (c) 10% CHCl3 and (d) 5% CHCl3 in IPA, spin-coated onto a silicon wafer. The inset of d shows the enlarged FE-SEM image of superhelices with very little branching.
We have also investigated the effect of temperature on DHS formation and depolymerization into monomers. At 25 °C, for a 30 μM solution of S-G in 15% CHCl3 in IPA, DHS were obtained in 48 min. When cooled to lower temperatures, such as 15 °C, the assembly became rapid and completed in <30 min. (Supplementary Fig. 32a, c). However, the DHS obtained were smaller in size (Supplementary Fig. 32c). At higher temperatures such as 30 °C, even after 3 h, no significant decrease in absorbance at 650 nm, indicating that DHS formation is hampered (Supplementary Fig. 32a, b). Even after 6000 sec., very little decrease in the absorbance is seen (Supplementary Fig. 32b). To understand whether S-G in 15% CHCl3 in IPA can be completely depolymerized into monomers, a freshly prepared 30 μM solution of S-G in 15% CHCl3 in IPA was heated to 65 °C. Although absorbance is increased compared to that of at 25 °C, but could not reach like monomers in pure CHCl3, indicating incomplete depolymerization into monomers even at 65 °C (Supplementary Fig. 33a, d). We could not go beyond 65 °C due to the evaporation of CHCl3. When this solution is cooled back to 25 °C, the absorption spectrum obtained is similar to the freshly prepared solution at 25 °C. After allowing it at 25 °C for 48 min., the formation of DHS was observed, as evidenced by the FE-SEM images (Supplementary Fig. 33a, e). We have also tried the 15 μM solution of S-G in 15% CHCl3 in IPA, but complete depolymerization into monomers is not observed at 65 °C (Supplementary Fig. 33b). Similarly, after the formation of DHS (30 μM), the solution was heated at 65 °C, but complete depolymerization was not observed (Supplementary Fig. 33c). When this hot solution was spin-coated on a silicon wafer and visualized using FE-SEM, short helical fibers were observed (Supplementary Fig. 33f). This indicates that in 15% CHCl3 in IPA at 65 °C, DHS can be dissociated into short helical fibers but not completely into monomers. When the DHS solution was sonicated for 15 min., they were also not fully depolymerized into monomers, as evidenced by the absorption spectra, but fragmented into superhelix and helical fibers (Supplementary Fig. 34).
To understand how the DHS formation will be affected by mixing different molar ratios of S-G and R-G, we have mixed them in their monomeric state in CHCl3 in 1:1, 1:2 and 1:5 molar ratios before adding IPA (Supplementary Fig. 35). The growth of the SPs has been monitored using CD spectroscopy and FE-SEM imaging. The equimolar mixture of S-G and R-G has negligible CD signal compared to homochiral aggregates, and it is similar to A-G (Supplementary Figs. 25c and 35a). FE-SEM images obtained after completion of the self-assembly process revealed the existence of 1D fibers similar to A-G but not DHS (Supplementary Fig. 35d, g). When the ratio of R-G to S-G is changed to 1:2 (10 μM of R-G and 20 μM of S-G) and 1:5 (5 μM of R-G and 25 μM of S-G), the CD signals obtained are slightly lower than pure S-G (Supplementary Fig. 35b, c). The FE-SEM analysis of these samples indicated the presence of chiral SPs with branches, and DHS with less branching, but not fully developed DHS as seen in the case of pure S-G (Supplementary Fig. 35e, f, h, i). Multiple areas were analyzed to identify homochirality, and it was concluded that these helices are right-handed similar to SPs obtained from S-G. Hence, we conclude that fully developed DHS can be synthesized via secondary nucleation using pure chiral isomers (S-G or R-G).
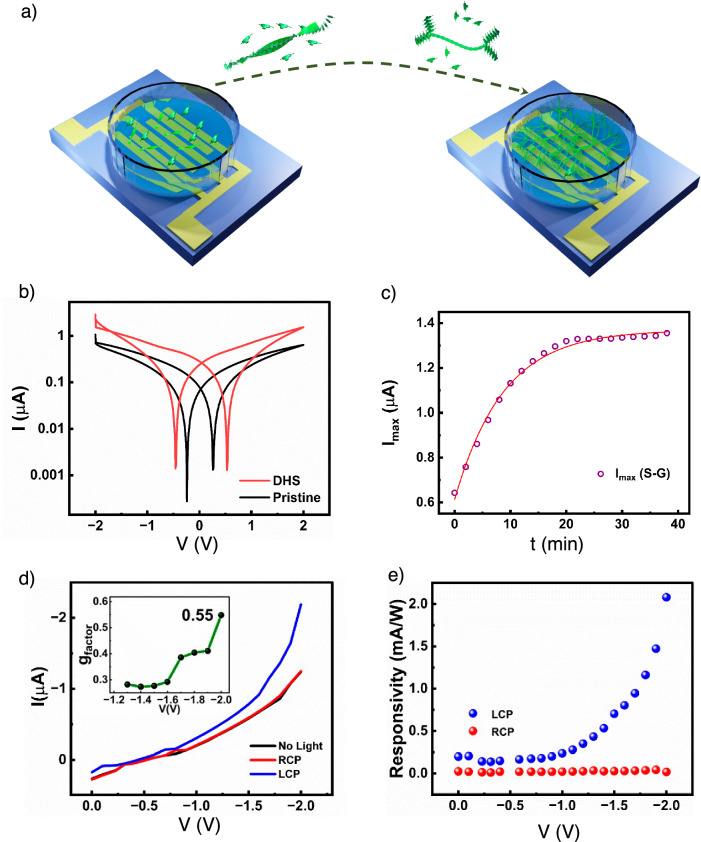
The temporal evolution of NIR-absorbing S-G and R-G into higher-ordered SP architectures such as DHS further motivated us to investigate the variation of electrical properties with the evolution in morphology. For this purpose, a custom-designed 2 mL hollow chamber was securely attached using silicone adhesive over a patterned Cr/Au electrode channel fabricated on glass substrates (Fig. 7a and Supplementary Fig. 36). A 30 μM solution of S-G in 15% CHCl3 in IPA is placed in the hollow chamber, and time-dependent I–V characteristics are recorded in the solution phase (See methods section). Typical time-dependent I–V characteristics are depicted in Supplementary Fig. 37. The self-assembly process begins from small helical fibers (pristine sample, 0 min.) and finally evolve into DHS over a period of 50 min. (Fig. 7a). Interestingly, the channel current increases monotonously from 0.6 μA to 1.4 μA for S-G samples (a similar level of enhancement in current was also observed in R-G samples) as the self-assembly proceeds from small helical fibers to DHS (Fig. 7b and Supplementary Fig. 37). This temporal evolution of Imax (maximum current at 2 V) followed an exponential growth (Fig. 7c and Supplementary Fig. 37a). These observations further indicate that DHS show more than two times enhancement in channel current than the initial short helical fibers. It is worth noting that the temporal evolution of the I–V characteristics is consistent with the temporal evolution observed from electronic absorption and CD measurements (Fig. 2a, b) in the solution phase, indicating a general internal consistency within the results.
Fig. 7. Chiro-optical response of dendritic homochiral superstructures (DHS) in solution.
a Schematic representation of device structure utilized for the time-dependent I–V measurements in solution. The schematic has been made using Blender 3.5 software. b I–V characteristics of S-G in 15% CHCl3 in IPA mixture at 0 min. (pristine) and after 50 min. (DHS). c Temporal evolution of Imax for the solution of S-G. d Photocurrent generated upon illumination of S-G with left circularly polarized (LCP) and right circularly polarized (RCP) light. The inset shows the variation of gfactor with voltage. e Estimation of photoresponsivity upon illumination of S-G solution with LCP and RCP.
Reports on the successful use of chiral π-conjugated molecules in chiroptical sensors55–58 have spurred interest in exploring the potential of our DHS for similar applications. These reports highlight the ability of π-conjugated molecules to exhibit unique chiro-optical properties, which can be harnessed for sensitive and selective detection of left and right circularly polarized light. Hence, we further explored the chiro-optical response of both S-G and R-G solutions while measuring the differential photocurrent response to left circularly and right circularly polarized light after the formation of the DHS (Supplementary Figs. 36 and 38a). Illumination of the solution chamber consisting of DHS of S-G with left circularly polarized light exhibits a differential channel current of up to 1 µA at 2 V when compared with the photocurrent obtained after illumination with right circularly polarized light (Fig. 7d, e). This differential photoresponse translates to dissymmetry factor, or gfactor value as high as 0.55 at 2 V. Note that the gfactor increased from 0.3 at 0 V to a value of 0.55 at 2 V for S-G samples. Similarly, photocurrent response with chiral light was also measured for R-G solution, interestingly however it exhibited higher photoresponsivity towards right circularly polarized light and the magnitude of gfactor was obtained to be ~0.61 at 2 V (Supplementary Fig. 38b, c). Previous reports of organic molecules have shown gfactor nearly −0.113 for polymer blends55, 0.054 for perylene diimide derivatives58 and 0.1 for polymer and small molecule hybrids57, all of which are lower than our measurement. Correspondingly, we observed a responsivity as high as 2 mA/W. More importantly, this enhanced selectivity towards chiral illumination brings out the potential utilization of these materials for chiral detection using programmable self-assembled structures.
In summary, we have demonstrated the non-covalent synthesis of higher-ordered chiral SP architectures having dendritic topology. We have observed that the secondary nucleation-elongation process involving ‘growth on the surface’ and ‘growth from the surface’ plays a crucial role in the formation of DHS. In the present case, we could synthesize DHS having a size of 0.4 mm2. Moreover, these DHS show the best chiro-optical response to date in the solution phase with a magnitude of gfactor ~ 0.55–0.61 and a responsivity as high as 2 mA/W. Our results further indicate that the combination of both ‘growth on the surface’ and ‘growth from the surface’ secondary nucleation-elongation process can be further explored to synthesize more complex topologies of SPs, which are difficult to achieve only by one of these processes. It was observed that the secondary nucleation events are the key sources for the generation of toxic oligomeric species in the context of diseases31,65. On the other hand, secondary nucleation events are potentially useful for the non-covalent synthesis of higher-ordered supramolecular architectures66, which are difficult to achieve solely via primary nucleation events. However, the same is challenging with small molecules as no rational design principles were available. Recent reports33–35 and the results reported here shed light on molecular design principles and growth process, which would serve as a handy tool for the non-covalent synthesis of higher-ordered complex supramolecular architectures with high precision and selectivity for utilization in material science and biology.
Methods
DHS preparation
2 mg of S-G/R-G was measured and transferred into a vial, and 2.634 mL of CHCl3 was added to it to obtain a concentration of 1 mM, which is the stock solution. Concentration-dependent experiments of S-G by varying the concentration from 100 μM to 300 μM performed in CHCl3 using UV-Vis absorption spectroscopy revealed a linear relation (Supplementary Fig. 39), indicating the absence of any preexisting aggregates of S-G/R-G in CHCl3 solution before the addition of IPA. From the 1 mM CHCl3 stock solution, 90 μL was transferred into a vial, and 360 μL of CHCl3 (good solvent) was added. To this, a further 2550 μL of IPA (poor solvent) was added and mixed well to make the whole volume 3 mL containing 30 μM of S-G/R-G in 15% of CHCl3 in IPA. These solutions were used for UV-Vis spectroscopy and CD measurements as such.
Sample preparation for FE-SEM and AFM measurements
FE-SEM images were collected using JEOL JIB4700F FIB-SEM, and AFM measurements were performed on Park Systems NX10 using NSG 30 tip with a scan rate of 0.3 Hz. Samples were prepared by spin-coating the solutions onto a silicon substrate at time intervals of 0 min., 15 min., 30 min. and 45 min. during evolution into DHS. Samples were dried by evaporation of solvent under vacuum at room temperature. For FE-SEM measurements, samples were sputtered with gold.
Device fabrication for electrical measurements
For the device fabrication, lateral Cr/Au electrodes were thermally evaporated (5/25 nm at 0.1 Å/s) onto a pre-cleaned glass substrate using the MiniLab 026 system by Moorfield Nanotechnology Limited, under a pressure of 10−6 mbar. A physical mask was employed during the evaporation process to create channels with lengths ranging from 1 μm to 3 μm and channel width of 0.7 to 0.78 cm on the substrate. Lithographically substrates with interdigitated electrodes were also utilized in some case. Notably both the device exhibit similar results. After that, a custom-designed 2 mL hollow chamber was securely attached over the channel using silicone adhesive as illustrated in Fig. 7a.
Time-dependent electrical current measurements and CPL detection
For each measurement, 1.5 mL of the prepared solution was transferred into the measurement chamber. Dual I–V sweeps were performed using a Keithley 2450 over a voltage range of −2 V to 2 V. The voltage value was chosen to ensure that it is smaller than the electrochemical window of the utilized solvents. To monitor the temporal evolution of the channel current the I–V sweeps were performed every 2 min. and continued for 50 min. For the chiro-optical measurement, circularly polarized light was generated using the set-up shown in Supplementary Fig. 36. After the formation of the DHS structure, I–V measurements were performed in the dark and then with left and right circularly polarized light illumination of λ = 454 nm. The gfactor was calculated using the expression: and responsivity was estimated using the expression R = ; where IPh is current under illumination, ID is current dark conditions and Pinput (457 µW/m2) is input power of the incident laser, IL and IR are the photocurrent upon illumination with left circularly and right circularly polarized light respectively.
Supplementary information
Acknowledgements
K.V.R. acknowledge the Core Research Grant (SERB-CRG) from the Science and Engineering Research Board (CRG/2023/001575) for financial support. S.K.R. and K.V.R. acknowledge Core Research Grant (SERB-CRG) from the Science and Engineering Research Board (Grant number CRG/2022/006457) for financial support. S.R.P. acknowledges the scholarship support from MOE, the government of India, India. D.S. acknowledges the scholarship support from MOE, the government of India, India. A.C.Y. thanks the MOE, India, for the Prime Minister research fellowship. We thank professor Subi J. George from JNCASR, Bangalore, for MALDI-TOF measurements. S.P.S. acknowledges funding support from Intensification of Research in High Priority Areas (IRHPA) funding from the Science and Engineering Research Board (IPA/2021/000096), funding from the Department of Atomic Energy (DAE), Government of India through RIN-4001. U.P. and D.I.S. acknowledge funding support from DAE through the Disha Fellowship. R.S. thanks UGC, India for the Senior Research Fellowship.
Author contributions
K.V.R. conceived the idea. S.R.P. performed all the experiments except electrical conductivity measurements and theoretical calculations. D.S. and A.C.Y. co-performed some of the experiments with S.R.P. U.P., D.I.S., and C.M. performed the electrical conductivity measurements of the self-assembled structures along with the chiro-optical measurements under the guidance of S.P.S. S.P.S. conceived the idea related to the electrical measurements and chiro-optical measurements. R.S. and S.K.R. carried out the MD simulations. K.V.R. and S.P.S. supervised the overall project. All the authors discussed the results and contributed to the manuscript writing. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks Jun Guan, Michel Rickhaus and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are available from the corresponding author upon request. The data generated in this study are provided in the Supplementary Information/Source Data file. Source data [10.5281/zenodo.14090280] are provided in this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Satyaprasad P. Senanayak, Email: satyaprasad@niser.ac.in
Kotagiri Venkata Rao, Email: kvrao@chy.iith.ac.in.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55107-y.
References
- 1.Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science335, 813–817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng, H. Q. et al. Supramolecular polymers: recent advances based on the types of underlying interactions. Prog. Polym. Sci.137, 101635 (2023). [Google Scholar]
- 3.Chen, H. & Stoddart, F. J. From molecular to supramolecular electronics. Nat. Rev. Mater.6, 804–828 (2021). [Google Scholar]
- 4.Jain, A. & George, S. J. New directions in supramolecular electronics. Mater. Today18, 206–214 (2015). [Google Scholar]
- 5.Moulin, E., Cid, J. J. & Giuseppone, N. Advances in supramolecular electronics – from randomly self-assembled nanostructures to addressable self-organized interconnects. Adv. Mater.25, 477–487 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Babu, S. S., Prasanthkumar, S. & Ajayaghosh, A. Self‐assembled gelators for organic electronics. Angew. Chem. Int. Ed.51, 1766–1776 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Kazantsev, R. V. et al. Molecular control of internal crystallization and photocatalytic function in supramolecular nanostructures. Chem4, 1596–1608 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosso, C., Filippini, G. & Prato, M. Use of perylene diimides in synthetic photochemistry. Eur. J. Org. Chem.8, 1193–1200 (2021). [Google Scholar]
- 9.Goudar, S. H. et al. Supramolecular engineering of Ti3C2Tx MXene -perylene diimide hybrid electrodes for the pseudocapacitive electrochemical storage of calcium ions. Small20, 2309905 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Greef, T. F. A. D. D. et al. Supramolecular polymerization. Chem. Rev.109, 5687–5754 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Smulders, M. M. J. et al. How to distinguish isodesmic from cooperative supramolecular polymerisation. Chem. Eur. J.16, 362–367 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Kotha, S. et al. Cooperative supramolecular polymerization guided by dispersive interactions. Chem. Asian J.17, e202200494 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Wehner, M. & Würthner, F. Supramolecular polymerization through kinetic pathway control and living chain growth. Nat. Rev. Chem.4, 38–53 (2020). [Google Scholar]
- 14.Wagner, W., Wehner, M., Stepanenko, V. & Würthner, F. Supramolecular block copolymers by seeded living polymerization of perylene bisimides. J. Am. Chem. Soc.141, 12044–12054 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Sarkar, A. et al. Tricomponent supramolecular multiblock copolymers with tunable composition via sequential seeded growth. Angew. Chem. Int. Ed.60, 18209–18216 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Jarrett-Wilkins, C. et al. Living supramolecular polymerisation of perylene diimide amphiphiles by seeded growth under kinetic control. Chem. Eur. J.24, 15556–15565 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Ogi, S. et al. Living supramolecular polymerization realized through a biomimetic approach. Nat. Chem.6, 188–195 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Fukui, T. et al. Control over differentiation of a metastable supramolecular assembly in one and two dimensions. Nat. Chem.9, 493–499 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Yagai, S., Kitamoto, Y., Datta, S. & Adhikari, B. Supramolecular polymers capable of controlling their topology. Acc. Chem. Res.52, 1325–1335 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Tezuka, Y. Topological polymer chemistry designing complex macromolecular graph constructions. Acc. Chem. Res.50, 2661–2672 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Wu, Q. et al. Poly[n]Catenanes: synthesis of molecular interlocked chains. Science358, 1434–1439 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Cook, A. B. & Perrier, S. Branched and dendritic polymer architectures: functional nanomaterials for therapeutic delivery. Adv. Funct. Mater.30, 1901001 (2020). [Google Scholar]
- 23.Schroffenegger, M. et al. Polymer topology determines the formation of protein corona on core–shell nanoparticles. ACS Nano14, 12708–12718 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miermans, C. A., Kusters, R. P., Hoogenraad, C. C. & Storm, C. Biophysical model of the role of actin remodeling on dendritic spine morphology. PLoS One12, e0170113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasai, M., Asakura, S. & Oosawa, F. The cooperative nature of G-F transformation of actin. Biochim. Biophys. Acta57, 22–31 (1962). [DOI] [PubMed] [Google Scholar]
- 26.MacFarlane, L. et al. Emerging applications for living crystallization-driven self-assembly. Chem. Sci.12, 4661–4682 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, X. et al. Cylindrical block copolymer micelles and co-micelles of controlled length and architecture. Science317, 644–647 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Gädt, T. et al. Complex and hierarchical micelle architectures from diblock copolymers using living, crystallization-driven polymerizations. Nat. Mater.8, 144–150 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Jin, Z. et al. Two‐dimensional living supramolecular polymerization: Improvement in edge roughness of supramolecular nanosheets by using a dummy monomer. Chem. Eur. J.29, e202302181 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Törnquist, M. et al. Secondary nucleation in amyloid formation. Chem. Commun.54, 8667–8684 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Cohen, S. I. A. et al. Proliferation of amyloid Aβ42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci.110, 9758–9763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laishram, R. et al. Secondary nucleation-triggered physical cross-links and tunable stiffness in seeded supramolecular hydrogels. J. Am. Chem. Soc.144, 11306–11315 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Datta, S. et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature583, 400–405 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Sasaki, N. et al. Supramolecular double-stranded archimedean spirals and concentric toroids. Nat. Commun.11, 3578 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotha, S. et al. Noncovalent synthesis of homo and heteroarchitectures of supramolecular polymers via secondary nucleation. Nat. Commun.15, 3672 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García, F., Gómez, R. & Sánchez, L. Chiral supramolecular polymers. Chem. Soc. Rev.52, 7524–7548 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Liu, M., Zhang, L. & Wang, T. Supramolecular chirality in self-assembled Systems. Chem. Rev.115, 7304–7397 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Xu, L. et al. Crystallization-driven asymmetric helical assembly of conjugated block copolymers and the aggregation induced white-light emission and circularly polarized luminescence. Angew. Chem. Int. Ed.59, 16675–16682 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Wang, C., Xu, L., Zhou, L., Liu, N. & Wu, Z. Q. Asymmetric living supramolecular polymerization: precise fabrication of one-handed helical supramolecular polymers. Angew. Chem. Int. Ed.61, e202207028 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Liu, N., Gao, R. T. & Wu, Z. Q. Helix-induced asymmetric self-assembly of π-conjugated block copolymers: from controlled syntheses to distinct properties. Acc. Chem. Res.56, 2954–2967 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Gao, R. T. et al. One-pot asymmetric living copolymerization-induced chiral self-assemblies and circularly polarized luminescence. Chem. Sci.15, 2946–2953 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sang, Y. et al. Circularly polarized luminescence in nanoassemblies: generation, amplification, and application. Adv. Mater.32, 1900110 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Zimbron, J. M. et al. Real-time control of the enantioselectivity of a supramolecular catalyst allows selecting the configuration of consecutively formed stereogenic centers. Angew. Chem. Int. Ed.56, 14016–14019 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Dou, X. et al. Supramolecular hydrogels with tunable chirality for promising biomedical applications. Acc. Chem. Res.53, 852–862 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Mondal, A. K. et al. Spin filtering in supramolecular polymers assembled from achiral monomers mediated by chiral solvents. J. Am. Chem. Soc.143, 7189–7195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lafleur, R. P. M. at al. Supramolecular double helices from small C3-symmetrical molecules aggregated in water. J. Am. Chem. Soc.142, 17644–17652 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hifsudheen, M. et al. The Helix to super-helix transition in the self-assembly of π-systems: superseding of molecular chirality at hierarchical level. Angew. Chem. Int. Ed.56, 12634–12638 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Li, H. & Chen, L. Three-level hierarchical self-assembly of azobenzene conjugated phenylalanines into superhelical nanostructures with light-switchable helicity. Org. Chem. Front.9, 6240–6246 (2022). [Google Scholar]
- 49.Liu, Y. et al. Emergent self-assembly pathways to multidimensional hierarchical assemblies using a hetero-seeding approach. Chem. Eur. J.25, 13484–13490 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Sun, B. et al. Homochiral porous nanosheets for enantiomer sieving. Nat. Mater.17, 599–604 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Du, C. et al. Hierarchically self-sssembled homochiral helical microtoroids. Nat. Nanotechnol.17, 1294–1302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, K. C. et al. Skip residues modulate the structural properties of the myosin rod and guide thick filament assembly. Proc. Natl. Acad. Sci.112, E3806–E3815 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarkar, S. et al. Stereoselective primary and secondary nucleation events in multicomponent seeded supramolecular polymerization. J. Am. Chem. Soc.143, 11777–11787 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Sarkar, S., Laishram, R., Deb, D. & George, S. J. Controlled noncovalent synthesis of secondary supramolecular polymers. J. Am. Chem. Soc.145, 22009–22018 (2023). [DOI] [PubMed] [Google Scholar]
- 55.Wan, L. et al. Sensitive near-infrared circularly polarized light detection via non-fullerene acceptor blends. Nat. Photonics17, 649–655 (2023). [Google Scholar]
- 56.Yang, Y., Correa da Costa, R., Fuchter, M. J. & Campbell, A. J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photonics7, 634–638 (2013). [Google Scholar]
- 57.Kim, N. Y. et al. Chiroptical-conjugated polymer/chiral small molecule hybrid thin films for circularly polarized light-detecting heterojunction devices. Adv. Funct. Mater.29, 1808668–1808678 (2019). [Google Scholar]
- 58.Zhang, L. et al. π-Extended perylene diimide double-heterohelicenes as ambipolar organic semiconductors for broadband circularly polarized light detection. Nat. Commun.12, 142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langhals, H. & Blanke, P. An Approach to novel NIR dyes utilising α-Effect donor groups. Dyes Pigm.59, 109–116 (2003). [Google Scholar]
- 60.Würthner, F. et al. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev.116, 962–1052 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Kotha, S. et al. Pathway selection in temporal evolution of supramolecular polymers of ionic π-systems: amphiphilic organic solvent dictates the fate of water. Chem. Eur. J.30, e202303813 (2024). [DOI] [PubMed] [Google Scholar]
- 62.Meisl, G. et al. Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat. Protoc.11, 252–272 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann, M. R. et al. Mechanism of secondary nucleation at the single fibril level from direct observations of Aβ42 aggregation. J. Am. Chem. Soc.143, 16621–16629 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Zhang, Y. et al. Dendritic micelles with controlled branching and sensor applications. J. Am. Chem. Soc.143, 5805–5814 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Gracia, P., Camino, J. D., Volpicelli-Daley, L. & Cremades, N. Multiplicity of a-synuclein aggregated species and their possible roles in disease. Int. J. Mol. Sci.21, 8043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Datta, S., Takahashi, S. & Yagai, S. Nanoengineering of curved supramolecular polymers: toward single-chain mesoscale materials. Acc. Mater. Res.3, 259–271 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon request. The data generated in this study are provided in the Supplementary Information/Source Data file. Source data [10.5281/zenodo.14090280] are provided in this paper.