Abstract
This study aimed to investigate alterations in a multilayer network combining structural and functional layers in patients with end-stage kidney disease (ESKD) compared with healthy controls. In all, 38 ESKD patients and 43 healthy participants were prospectively enrolled. They exhibited normal brain magnetic resonance imaging (MRI) without any structural lesions. All participants, both ESRD patients and healthy controls, underwent T1-weighted imaging, diffusion tensor imaging (DTI), and resting-state functional MRI (rs-fMRI) using the same three-tesla MRI scanner. A structural connectivity matrix was generated using the DTI and DSI programs, and a functional connectivity matrix was created using the rs-fMRI and SPM programs in the CONN toolbox. Multilayer network analysis was conducted based on structural and functional connectivity matrices using BRAPH. Significant differences were observed at the global level in the multilayer network between patients with ESKD and healthy controls. The weighted multiplex participation was lower in patients with ESKD than in healthy controls (0.6454 vs. 0.7212, adjusted p = 0.049). However, other multilayer network measures did not differ. The weighted multiplex participation in the right subcentral gyrus, right opercular part of the inferior frontal gyrus, right occipitotemporal medial lingual gyrus, and right postcentral gyrus in patients with ESKD was lower than that in the corresponding regions in healthy controls (0.6704 vs. 0.8562, 0.8593 vs. 0.9388, 0.7778 vs. 0.8849, and 0.6825 vs. 0.8112; adjusted p < 0.05, respectively).This study demonstrated that the multilayer network combining structural and functional layers in patients with ESKD was different from that in healthy controls. The specific differences in weighted multiplex participation suggest potential disruptions in the integrated communication between different brain regions in these patients.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80645-2.
Keywords: Neural networks, Connectome, Magnetic resonance imaging
Subject terms: Neuroscience, Nephrology
Introduction
End-stage kidney disease (ESKD) is of paramount importance to global public health owing to its diverse complications. ESKD is denoted by a glomerular filtration rate (GFR) persistently below 15 ml/min/1.73 m² for a duration exceeding three months1,2. Neurological complications of ESKD are widely recognized as common concerns, and they encompass conditions such as insomnia, depression, encephalopathy, stroke, and restless leg syndrome. These complications manifest as a diverse range of symptoms and can vary significantly in severity3,4. These complications can arise as a result of several factors, including uremic toxins, electrolyte imbalances (such as hyperkalemia), metabolic disturbances, vascular changes, and effects of dialysis4,5.
Numerous trials have been conducted to elucidate the underlying pathophysiology of neurological complications in patients with ESKD. Significant advancements in brain imaging modalities have complemented these efforts. Several studies have concluded that there is general cerebral atrophy and prominent lesions in the frontal lobes, based on brain imaging, including magnetic resonance imaging (MRI) and computed tomography (CT) scans6,7. Some studies have demonstrated white matter lesions, such as decreased deep white matter volume and hyperintensities, which are indicative of small vessel disease, as observed on brain MRI8,9.
Recently, there has been a notable surge in interest in graph theory-based research on brain connectivity. This approach offers a comprehensive framework for analyzing the intricate networks formed by both structural and functional connectivity in the brain10. Consequently, there has been a growing effort to clarify the neurological complications in patients with ESKD. In one study, neural tract lesions in patients with ESKD were identified based on diffusion tensor imaging (DTI) findings11. Some studies have reported alterations in white matter integrity and connectivity among patients with ESKD, as evidenced by lower fractional anisotropy (FA) and higher mean diffusivity (MD)12–15. A previous study showed significantly lower functional connectivity in hemodialysis-dependent patients with ESKD, especially in the frontal lobe, which is strongly associated with neurocognitive dysfunction16. Several studies using resting-state functional MRI (rs-fMRI) have provided evidence indicating a disturbance of typical global integration within brain networks, characterized by a diminished clustering coefficient and global efficiency, alterations in small-worldness, and a heightened characteristic path length in patients with ESKD15–18.
Graph theory has ignited a surge of interest in multilayer networks in the domain of brain neuroscience by incorporating various layers such as structural and functional layers within the brain19–21. The brain’s network can be better understood and explained by performing multilayer analysis rather than a simple unilayer analysis, and the structural and functional networks are not separated but closely related to each other. Furthermore, by integrating multimodal data, a comprehensive understanding of the brain can be achieved, while also capturing temporal changes in brain dynamics22,23. Recently, brain multilayer network analysis has been conducted to understand the pathophysiology of various neurological disorders. Using multilayer network analysis, one study demonstrated a diminished loss of inter-frequency centrality in memory-related association areas in patients with Alzheimer’s disease2. Other studies have shown significant changes between the multilayer networks of patients with migraine and those of healthy controls24. Multilayer network analysis in patients with post-traumatic stress syndrome show reduced switching rates in brain functional network modules at global, sub-network, and nodal levels25.
However, studies on multilayered networks in patients with ESKD are currently scarce. This study aimed to investigate alterations in a multilayer network combining structural and functional layers in patients with ESKD compared with healthy controls.
Methods
Participants
This prospective study was approved by the Institutional Review Board (IRB) of Haeundae Paik Hospital, and all methods were performed in accordance with the guidelines and regulations (IRB number: 2018-09-015-003). Informed consent was obtained from all participants before participation. In all, 38 patients with ESKD and a glomerular filtration rate < 15 mL/min/1.73 m2 were prospectively enrolled. All patients with ERKD were undergoing dialysis. All the enrolled patients exhibited normal brain MRI findings without any structural lesions at the time of inclusion. We also enrolled 43 age- and sex-matched healthy participants with no previous history of any medical or neurological disease as a control group. Furthermore, the brain MRIs of these participants revealed no structural lesions.
MRI acquisition
All participants, both ESKD patients and healthy controls, underwent T1-weighted imaging, DTI, and rs-fMRI using the same three-tesla MRI scanner (Achieva Tx; Phillips Healthcare). DTI was performed using spin-echo single-shot echo-planar pulse sequences with 32 diffusion directions (repetition time/time to echo = 8620/85 ms, flip angle = 90°, slice thickness = 2.25 mm, acquisition matrix = 120 × 120, field of view = 240 × 240 mm2, and b-value = 1,000 s/mm2). The rs-fMRI was performed using multi-slice echo-planar imaging sequences (Repetition time/Time to echo = 3000/30 ms, Flip angle = 65°, slice thickness = 4.4 mm, acquisition matrix = 128 × 128, Field of view = 220 × 220 mm2, scan time = 7 min 30 s).
Multilayer network analysis
First, a structural connectivity matrix was generated for individual participants using the DSI program. Raw DTI data were preprocessed in the DSI program, with the help of image distortion and artifact correction, as well as image intensity normalization. Diffusion tensors were computed using a generalized Q-sampling imaging method at each voxel in the brain, and the fiber orientation distribution function was employed to estimate white matter fiber orientation. A deterministic tractography algorithm was applied to reconstruct white matter fiber tracts between different brain regions, resulting in a structural connectivity matrix. A total of 150 nodes in this matrix represented the regions of interest (Suppl. 1) and the edges represented the number of fiber tracts connecting each region. Second, a functional connectivity matrix was created using the SPM program (version 12) and the CONN toolbox (version 22a). Raw fMRI data were preprocessed using the SPM program. The detailed process was described in our previous work26. Regions of interest were defined in a manner similar to that used when creating the structural matrix, and a functional connectivity matrix was in individual participants.
Third, a multilayer network analysis was conducted based on structural and functional connectivity matrices using the BRAPH program (version 2). The detailed process was also described in our previous work26. The analysis involved a comparison of the two participant groups using connectivity-functional multiplex data and weighted undirected graphs. Various network measures were calculated at the global level, including weighted multiplex participation, persistence, average overlapping strength, average multiplex participation, average multilayer clustering, multilayer modularity, and average flexibility. Weighted multiplex participation was the nodal homogeneity of the number of neighbors across layers. Persistence was calculated as the normalized sum of the number of nodes that did not change their community assignments. The average overlapping strength was the average sum of the strengths of a node in all layers. The average multiplex participation indicated the nodal homogeneity of the number of neighbors of a node across layers. Average multilayer clustering was the average of the two-layer clustering coefficients for all nodes. The clustering coefficient was the fraction of the triangles around a node. Multilayer modularity was the quality of the resulting partitions in a multilayer network. Average flexibility is the average flexibility of all the nodes in a multilayer network. The flexibility of each node was calculated as the number of times it changed the community assignment, normalized by the total possible number of changes.
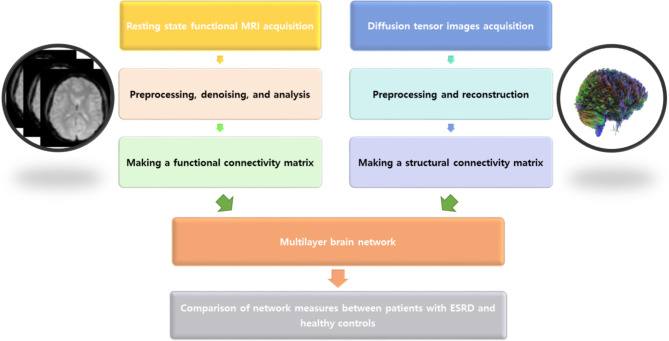
Finally, the network measures were compared between patients with ESKD and healthy controls. In case of significant differences at the global level in multilayer network analysis, the nodal level of the multilayer network was also analyzed. The multilayer network process is illustrated in Fig. 1.
Fig. 1.
Process of multilayer network analysis. A functional connectivity matrix was created using the SPM program and CONN toolbox based on resting-state functional MRI, and a structural connectivity matrix was generated using a DSI program based on diffusion tensor imaging. Subsequently, a multilayer network analysis was conducted based on the structural and functional connectivity matrices using the BRAPH program. Finally, multilayer network measures were compared between patients with ESRD and healthy controls.
Statistical analysis
The demographic characteristics of patients with ESKD and healthy controls were assessed using an independent t-test and chi-square test using MedCalc Statistical Software version 20.014 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021). Network measures in the multilayer network analysis were compared between groups using a permutation test integrated into the BRAPH program. Statistical significance was set at p-value < 0.05. For comparisons at both global and nodal levels in the multilayer network analysis, an adjusted p-value was calculated by applying multiple corrections using the false discovery rate (Benjamini-Hochberg procedure)27–30.
Results
Participants
Table 1 shows the demographic characteristics of patients with ESKD and healthy controls. There were no differences in the demographic characteristics between patients with ESKD and healthy controls. The ages in the two groups were 61.7 ± 6.8 vs. 60.0 ± 7.8, and no statistically significant difference was found (p = 0.286). Additionally, the male-to-female ratios were 45% vs. 42%, with no difference between the two groups (p = 0.794).
Table 1.
Demographic and clinical characteristics of patients with ESKD and heathy controls.
| Variables | Patients with ESKD (N = 38) |
Healthy controls (N = 43) |
p-value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 61.7 ± 6.8 | 60.0 ± 7.8 | 0.286 | |
| Sex, male | 17 (45) | 18 (42) | 0.794 | |
| Hemodialysis | 19 (50) | |||
| Peritoneal dialysis | 19 (50) | |||
| Kt/V | 1.86 ± 0.5 | |||
| Dialysis duration, month | 47.9 ± 50.6 | |||
| Laboratory data | ||||
| Hemoglobin, g/dL | 10.5 ± 1.1 | |||
| Hematocrit, % | 32.2 ± 3.6 | |||
| Protein, g/dL | 6.5 ± 0.6 | |||
| Albumin, d/dL | 3.8 ± 0.4 | |||
| BUN, mg/dL | 57.3 ± 17.2 | |||
| Creatinine, mg/dL | 9.0 ± 2.6 | |||
| Sodium, mmol/L | 139.0 ± 3.3 | |||
| Potassium, mmol/L | 4.7 ± 0.7 | |||
| Chloride, mmol/L | 99.6 ± 4.1 | |||
| Calcium, mg/dL | 8.5 ± 0.8 | |||
| Phosphate, mg/dL | 4.5 ± 0.9 | |||
| Parathyroid hormone, pg/mL | 268.4 ± 210.5 | |||
| Total CO2 contents, mmol/L | 24.2 ± 4.7 | |||
Data are presented as number (%) or mean ± standard deviation.
ESKD end-stage kidney disease, Kt/V dialyzer clearance; time/distribution volume of urea, BUN blood urea nitrogen.
Global level of multilayer network analysis
Table 2 shows the results of the global level of multilayer network analysis. There were significant differences at the global level in the multilayer network between patients with ESKD and healthy controls. The weighted multiplex participation was lower in patients with ESKD than in healthy controls (0.6454 vs. 0.7212, adjusted p = 0.049). However, other multilayer network measures, including persistence, average overlapping strength, average multiplex participation, average multilayer clustering, multilayer modularity, and average flexibility, did not differ.
Table 2.
Results of the multilayer network analysis at the global level.
| Patients with ESRD | Controls | Difference | Lower value of the 95% confidence interval | Upper value of the 95% confidence interval | p-value | adjusted p-value | |
|---|---|---|---|---|---|---|---|
| Weighted multiplex participation | 0.6454 | 0.7212 | 0.0758 | -0.0128 | 0.0112 | 0.013 | *0.048 |
| Persistence | 0.4425 | 0.4526 | 0.0101 | -0.0348 | 0.0311 | 0.199 | 0.337 |
| Average overlapping strength | 34.2239 | 34.5994 | 0.3755 | -1.6153 | 1.5247 | 0.241 | 0.337 |
| Average multiplex participation | 0.7454 | 0.7245 | -0.021 | -0.0128 | 0.0112 | 0.016 | 0.056 |
| Average multiplex clustering | 0.1547 | 0.1551 | 0.0004 | -0.0095 | 0.0091 | 0.353 | 0.353 |
| Multilayer modularity | 0.3961 | 0.3804 | -0.0157 | -0.0147 | 0.0141 | 0.036 | 0.084 |
| Average flexibility | 0.5516 | 0.555 | 0.0035 | -0.0382 | 0.0353 | 0.347 | 0.353 |
*Indicates statistical significance (p < 0.05).
Nodal level of multilayer network analysis
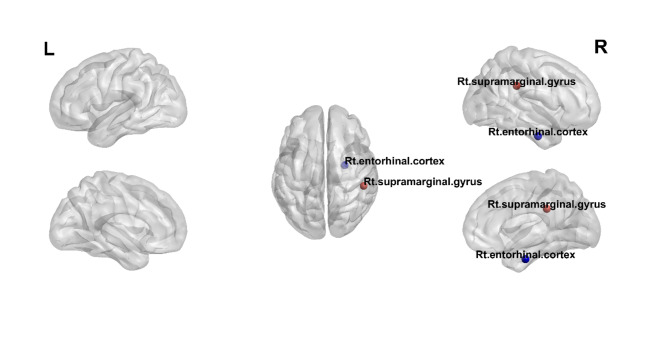
Figure 2 shows the nodes demonstrating significant differences in the weighted multiplex participation between patients with ESKD and healthy controls. The weighted multiplex participation in the right subcentral gyrus, right opercular part of the inferior frontal gyrus, right occipitotemporal medial lingual gyrus, and right postcentral gyrus among patients with ESKD was lower than that in the corresponding regions among healthy controls (right subcentral gyrus, 0.6704 vs. 0.8562; right opercular part of the inferior frontal gyrus, 0.8593 vs. 0.9388; right occipitotemporal medial lingual gyrus, 0.7778 vs. 0.8849; and right postcentral gyrus, 0.6825 vs. 0.8112; adjusted p < 0.05, respectively).
Fig. 2.
Nodes demonstrating significant differences in the weighted multiplex participation between patients with ESRD and healthy controls. The red circles show regions with decreased weighted multiplex participation in patients with ESRD compared with healthy controls; these regions include the right subcentral gyrus, right opercular part of the inferior frontal gyrus, right occipitotemporal medial lingual gyrus, and right postcentral gyrus.
Discussion
The present study aimed to investigate alterations in the multilayer network combining structural and functional layers between patients with ESKD and healthy controls. Numerous attempts have been made to explain the use of multilayer network analysis in many neurological disorders; however, there have been no approaches using multilayer networks in patients with ESKD2,24,25. Our study revealed a significant reduction in weighted multiplex participation at both the global and nodal levels among patients with ESKD compared with healthy controls. Furthermore, examination at the nodal level identified several specific brain regions characterized by decreased weighted multiplex participation, highlighting localized deficits in the interlayer interactions within the brain network of patients with ESKD.
At the global level, weighted multiplex participation was lower in patients with ESKD than in healthy controls. Weighted multiplex participation is a quantitative measure of a node’s involvement across multiple network layers determined by evaluating the weighted interactions within each layer31–35. Therefore, decreased weighted multiplex participation suggests a diminished level of integration or engagement of brain regions across the structural and functional layers of connectivity in patients with ESKD. This result corresponds to the finding of a deficit in integration within multilayer networks among patients with Alzheimer’s disease and mild cognitive disorders35.
Additional analysis was conducted at the nodal level of the multilayer network, revealing a decrease in weighted multiplex participation within specific regions, such as the right subcentral gyrus, right opercular part of the inferior frontal gyrus, right occipito-temporal medial lingual gyrus, and right postcentral gyrus, in patients with ESKD compared with healthy controls. The right subcentral gyrus plays a crucial role in sensorimotor integration; somatosensory processing; language and speech; and motor coordination36,37. The right postcentral gyrus, located on the lateral surface of the anterior parietal lobe, plays a role in somatosensory processing and sensorimotor integration37,38. The right opercular part of the inferior frontal gyrus is important for language production, executive functioning, and motor control37,39. The right occipitotemporal medial lingual gyrus plays a role in visual processing and word and face recognition37,40. These findings suggest the possibility that the complementary relationship between structural and functional networks in these nodes had decreased.
Previously, the dominant cerebral hemisphere, which mainly refers to the left side of a left-handed person, was known to play a crucial role in brain function. However, recent research has revealed the importance of the non-dominant cerebral hemisphere, primarily referring to the right side, in brain function. The non-dominant hemisphere plays a significant role in primary cognitive functions, such as visuospatial and social cognition41. The white matter fiber tract, known as the superior longitudinal fasciculus in the perisylvian area of the right hemisphere, plays a crucial role in cognitive function. The subcentral, inferior frontal, and postcentral gyri, which showed significant changes in this study, correspond to these regions. Accordingly, damage to the multilayer network in these areas may be related to neurological impairments in patients with ESKD. These regions are distinct areas with diverse functions; however, they have interconnected neural networks and functional relationships. Further research utilizing multilayer network analysis may provide valuable insights into the pathophysiology of neurological complications in patients with ESKD, and inform targeted interventions to mitigate these adverse outcomes.
Our study represents the first attempt to conduct a multilayer network analysis in patients with ESKD; however, it has several limitations. First, this study was planned at a single center, and the relatively small sample size is a limitation. This may have led to a selection bias, and we believe that larger-scale studies are necessary in the future. Second, we conducted a multilayer network analysis of patients with ESKD at a group level rather than at an individual level. Therefore, we could not perform a correlation analysis between clinical data and the multilayer network. Third, cognitive function tests were not performed. This represents a limitation in understanding the relationship between cognitive function impairment in patients with ESKD and variables within the multilayer network. Despite these limitations, this study demonstrates the value of multilayer network analysis in understanding the pathophysiology of neurological complications in patients with ESKD and its potential for diverse future applications.
Conclusion
This study demonstrated that the multilayer network combining structural and functional layers differs between patients with ESKD and healthy controls. The specific differences in weighted multiplex participation suggest potential disruptions in integrated communication between different brain regions in patients with ESKD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the participants who generously volunteered their time and effort to take part in our study.
Author contributions
Conceptualization, methodology and original draft preparation: KMP, BSP.Data collecting: SHP, CMH.Statistical analysis: YJL, DAL.Writing manuscript: YJY, CMHRevising manuscript: YWK, JSK, JHK.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiyae Yi and Chang Min Heo.
References
- 1.Levey, A. S. et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int.67(6), 2089–2100 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Guillon, J. et al. Loss of brain inter-frequency hubs in Alzheimer’s disease. Sci. Rep.7(1), 10879 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouns, R. & De Deyn, P. P. Neurological complications in renal failure: a review. Clin. Neurol. Neurosurg.107(1), 1–16 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Bronas, U. G., Puzantian, H. & Hannan, M. Cognitive impairment in chronic kidney disease: vascular milieu and the potential therapeutic role of Exercise. Biomed. Res. Int.2017, 2726369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liabeuf, S. et al. Chronic kidney disease and neurological disorders: are uraemic toxins the missing piece of the puzzle? Nephrol. Dial Transpl.37(Suppl 2), ii33–ii44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passer, J. A. Cerebral atrophy in end-stage uremia. Proc. Clin. Dial Transpl. Forum7, 91–94 (1977). [PubMed] [Google Scholar]
- 7.Kamata, T. et al. Morphologic abnormalities in the brain of chronically hemodialyzed patients without cerebrovascular disease. Am. J. Nephrol.20(1), 27–31 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Cho, A. H. et al. Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology73(20), 1645–1648 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Ikram, M. A. et al. Kidney function is related to cerebral small vessel disease. Stroke39(1), 55–61 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Hallquist, M. N. & Hillary, F. G. Graph theory approaches to functional network organization in brain disorders: a critique for a brave new small-world. Netw. Neurosci.3(1), 1–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, H. S. et al. Diffusion tensor imaging findings in neurologically asymptomatic patients with end stage renal disease. NeuroRehabilitation29(1), 111–116 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Chen, H. J., Zhang, L. J. & Lu, G. M. Multimodality MRI findings in patients with end-stage renal disease. Biomed. Res. Int.2015, 697402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou, M. C. et al. Widespread white matter alterations in patients with end-stage renal disease: a voxelwise diffusion tensor imaging study. AJNR Am. J. Neuroradiol.34(10), 1945–1951 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, R. et al. Reduced white matter integrity and cognitive deficits in maintenance hemodialysis ESRD patients: a diffusion-tensor study. Eur. Radiol.25(3), 661–668 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Park, B. S. et al. Alterations in structural and functional connectivities in patients with end-stage renal disease. J. Clin. Neurol.16(3), 390–400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng, G. et al. Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functionalMR imaging study. Metab. Brain Dis.29(3), 777–786 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Lee, Y. J. et al. Alteration of brain connectivity in neurologically asymptomatic patients with chronic kidney disease. Med. (Baltim).100(16), e25633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, M. et al. Altered resting-state functional networks in patients with hemodialysis: a graph-theoretical based study. Brain Imaging Behav.15(2), 833–845 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci.10(3), 186–198 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Sporns, O. Contributions and challenges for network models in cognitive neuroscience. Nat. Neurosci.17(5), 652–660 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Park, K. M. et al. Alterations of functional connectivity in patients with restless legs syndrome. J. Clin. Neurol.18(3), 290–297 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fornito, A., Zalesky, A. & Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci.16(3), 159–172 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Bassett, D. S. & Sporns, O. Network neuroscience. Nat. Neurosci.20(3), 353–364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, J., Lee, D. A., Lee, H. J. & Park, K. M. Multilayer network changes in patients with migraine. Brain Behav.13(12), e3316 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suo, X. et al. Multilayer Network Analysis of Dynamic Network Reconfiguration in Adults With Posttraumatic Stress Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. ;8(4):452 – 61. (2023). [DOI] [PubMed]
- 26.Park, K. M., Kim, K. T., Lee, D. A., Motamedi, G. K. & Cho, Y. W. Structural and functional multilayer network analysis in restless legs syndrome patients. J. Sleep. Res. e14104. (2023). [DOI] [PubMed]
- 27.Yu, H., Si, G. & Si, F. Mendelian randomization validates the Immune Landscape mediated by Aggrephagy in Esophageal squamous cell carcinoma patients from the perspectives of multi-omics. J. Cancer15(7), 1940–1953 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu, H., Ji, X. & Ouyang, Y. Unfolded protein response pathways in stroke patients: a comprehensive landscape assessed through machine learning algorithms and experimental verification. J. Transl Med.21(1), 759 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu, H. et al. Utilising Network Pharmacology to explore underlying mechanism of Astragalus membranaceus in improving Sepsis-Induced Inflammatory response by regulating the balance of IκBα and NF-κB in rats. Evid. Based Complement. Alternat Med.2022, 7141767 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu, H. & Song, X. The relationship between Alzheimer disease and thyroiditis: a two-sample mendelian randomization study. Med. (Baltim).102(44), e35712 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, D. A., Lee, H-J. & Park, K. M. Involvement of the default mode network in patients with transient global amnesia: multilayer network. Neuroradiology65(12), 1729–1736 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Puxeddu, M. G., Petti, M. & Astolfi, L. A comprehensive analysis of Multilayer Community Detection algorithms for application to EEG-Based brain networks. Front. Syst. Neurosci.15, 624183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahabi, H., Nair, D. R. & Leahy, R. M. Multilayer brain networks can identify the epileptogenic zone and seizure dynamics. eLife12, e68531 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casas-Roma, J. et al. Applying multilayer analysis to morphological, structural, and functional brain networks to identify relevant dysfunction patterns. Netw. Neurosci.6 (3), 916–933 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, X. et al. Deficit of cross-frequency integration in mild cognitive impairment and Alzheimer’s Disease: a Multilayer Network Approach. J. Magn. Reson. Imaging53(5), 1387–1398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGlone, F. et al. Functional neuroimaging studies of human somatosensory cortex. Behav. Brain. Res.135(1), 147–158 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Ribas, G. C. The cerebral sulci and gyri. Neurosurgical Focus FOC. 28(2), E2 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Kropf, E., Syan, S. K., Minuzzi, L. & Frey, B. N. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J. Psychiatry41(3), 261–269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampshire, A., Chamberlain, S. R., Monti, M. M., Duncan, J. & Owen, A. M. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage50(3), 1313–1319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palejwala, A. H. et al. Anatomy and White Matter connections of the Lingual Gyrus and Cuneus. World Neurosurg.151, e426–e37 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Bernard, F., Lemée, J. M., Ter Minassian, A. & Menei, P. Right hemisphere cognitive functions: from clinical and anatomic bases to Brain Mapping during Awake Craniotomy Part I: clinical and functional anatomy. World Neurosurg.118, 348–359 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.