Abstract
Erectile dysfunction(ED), a prevalent condition within the male genitourinary system, significantly impairs the quality of life for affected men. Although certain inflammatory indicators, such as the neutrophil-to-lymphocyte ratio(NLR), systemic inflammatory response index (SIRI), and systemic immune-inflammation index(SII), have been linked to ED, the correlation with other markers and their impact on survival outcomes in ED patients remain largely unexplored. This research aims to investigate the correlation between inflammatory biomarkers derived from a complete blood cell count(CBC) and the occurrence of ED. Data regarding ED were extracted from the 2001–2004 National Health and Nutrition Examination Survey(NHANES), and mortality events were ascertained through the National Death Index up to December 2019. The CBC-derived inflammatory indicators assessed in this study included the NLR, derived neutrophil-to-lymphocyte ratio(dNLR), monocyte-to-lymphocyte ratio(MLR), neutrophil-monocyte to lymphocyte ratio (NMLR), SIRI, and SII. The prognostic significance of these CBC-derived inflammatory indicators was evaluated using random survival forests(RSF) analysis. Our study encompassed a cohort of 3,639 individuals, among whom 1,031 were diagnosed with ED. Among individuals with ED, 610 experienced all-cause mortality. Following adjustment for all confounding variables, it was observed that elevated levels of NLR(OR = 1.09, 95%CI 1.00–1.19, p = 0.021), MLR (OR = 2.97, 95% CI 1.18–7.50, p = 0.01), NMLR(OR = 1.10, 95% CI 1.01–1.11, p = 0.006), and SIRI(OR = 1.11, 95% CI 1.01–1.22, p = 0.017) were associated with an increased prevalence of ED. Among participants with ED, those in the highest quartile of NLR(HR = 1.06, 95% CI 1.00–1.11, p = 0.032), MLR(HR = 2.00, 95% CI 1.33–3.01, p < 0.001), NMLR (HR = 1.06, 95% CI 1.01–1.11, p = 0.024), and SII(HR = 1.00, 95% CI 1.00–1.00, p = 0.015) exhibited an elevated risk of all-cause mortality compared to those in the lower levels of inflammation-derived indicators. Our research suggests that, compared with other inflammatory markers derived from complete blood cell count, MLR has the highest predictive power for the prevalence of ED and all-cause mortality in these populations.
Keywords: Inflammatory markers, Complete blood cell, Erectile dysfunction, NHANES, Cross-sectional study
Subject terms: Biomarkers, Diseases, Medical research, Urology
Introduction
Erectile Dysfunction(ED) is defined as the persistent inability to attain and maintain an erection sufficient to permit satisfactory sexual performance1. A recent study has shown that the overall prevalence of ED among adult men in the United States is 24.2%, and this rate increases significantly with age, with a prevalence of 48.0% in men aged 65 to 74 years and as high as 52.2% in men aged 75 years or older2. As the most common type of male sexual dysfunction, ED not only seriously affects patients’ quality of life, but may also be an early sign and risk signal for cardiovascular disease3. The etiology and predisposing factors of ED are complex, including ageing, poor lifestyle choices (such as smoking, alcoholism, and obesity), psychosomatic factors (such as depression and anxiety) and chronic medical conditions (such as cardiovascular disease, diabetes, gastrointestinal diseases and obstructive sleep apnoea)4–8. Particularly, systemic inflammatory response has been widely recognized as an important factor leading to vascular endothelial dysfunction, which in turn triggers ED9. Therefore, individualised assessment of biomarkers that can accurately reflect the state of systemic inflammation can not only serve as an indicator for assessing the severity of the condition, but may also be a potential target in future therapeutic strategies.
The complete blood count (CBC) is a routine and cost-effective laboratory test that provides information on a variety of blood components, including white blood cells, red blood cells, platelets, and so on. Notably, CBC-derived inflammation indicators provide a more comprehensive response to the systemic inflammatory state of the body than single indicators10. Among them, Neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio(dNLR), monocyte-to-lymphocyte ratio (MLR), and neutrophil-monocyte to lymphocyte ratio(NMLR), systemic inflammatory response index (SIRI), and systemic immune-inflammation index (SII), have been shown to be of significant value in the diagnosis, management and mortality risks assessment in a variety of diseases such as prostatic hyperplasia, bladder cancer, and sarcopenia11–14. Furthermore, it has been observed that there is a positive correlation between NLR, SIRI, SII and the prevalence of ED15–17. Nevertheless, the relationship between other CBC-derived inflammatory biomarkers and the incidence of ED, as well as the role of these markers in the survival prognosis of ED patients remains currently unknown.
Therefore, the aim of our study was to investigate the relationship between CBC-derived inflammatory biomarkers and the incidence of ED, and the prognostic value of these biomarkers, in order to elucidate the potential link between inflammation levels and ED and to provide new guidelines for the prevention and treatment of ED patients.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive, cross-sectional survey conducted in the United States. The ethical approval for NHANES was granted by the Research Ethics Review Board of the National Center for Health Statistics, and each participant provided a signed informed consent form. The dataset, as well as complete documentation and protocols, is publicly accessible through the designated website (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Assessment of CBC-derived inflammatory indicators
Fasting venous blood samples were collected from all study participants to ascertain leukocyte, neutrophil, lymphocyte, monocyte, and platelet counts. The CBC-derived inflammatory indicators were calculated using the following formulas: Neutrophil-to-Lymphocyte Ratio (NLR) = neutrophil counts / lymphocyte counts, Derived Neutrophil-to-Lymphocyte Ratio (dNLR) = neutrophil counts / (white blood cell counts - lymphocyte counts), Monocyte-to-Lymphocyte Ratio (MLR) = monocyte counts / lymphocyte counts, Neutrophil-to-Monocyte-to-Lymphocyte Ratio (NMLR) = (monocyte counts + neutrophil counts) / lymphocyte counts, Systemic Immune-Inflammation Index (SIRI) = neutrophil counts × monocyte counts / lymphocyte counts, and Platelet-to-Lymphocyte Ratio (SII) = platelet counts × neutrophil counts / lymphocyte counts18.
Assessment of erectile dysfunction
Participants were queried regarding erectile dysfunction (ED) with the question: “How would you describe your ability to develop and maintain an erection sufficient for sexual intercourse?” Responses were categorized into “never,” “sometimes,” “usually,” or “almost always or nearly always.” Individuals who selected “never” or “sometimes” were classified as having ED.
Assessment of mortality
We ascertained the vital status of participants by referencing the National Death Index (NDI). Data on all-cause mortality were collected through the end of December 2019, utilizing the 2019 Linked Mortality File (LMF). This file represents the most recent amalgamation of specific National Center for Health Statistics (NCHS) surveys with the NDI.
Covariates
In line with previous studies, we identified several potential covariates that may influence the outcomes, including age, race, body mass index (BMI), marital status (categorized as living with a partner or single), poverty-to-income ratio (PIR), education level, exercise status, alcohol consumption (binary: yes or no), smoking status (categorized as current, former, or never), and the presence of hypertension, diabetes, hypercholesterolemia, and cardiovascular disease. The PIR was stratified into three groups: ≤1.5, 1.5 to 3.5, and > 3.5. Data on the prevalence of diabetes, hypertension, hypercholesterolemia, and cardiovascular disease were obtained through self-reported questionnaires.
Statistical analysis
The Mann-Whitney U test was utilized to evaluate differences in continuous variables, with results presented as medians and their corresponding interquartile ranges (IQR). For categorical variables, comparisons were made using the chi-square test, and results were expressed as frequencies and percentages. Multiple logistic regression analyses were conducted to calculate adjusted odds ratios (OR) and their respective 95% confidence intervals (CI), assessing the association between CBC-derived inflammatory markers and erectile dysfunction (ED) occurrence. Furthermore, multiple Cox regression analyses were employed to determine adjusted hazard ratios (HR) and 95% CI for all-cause mortality among individuals with ED. The association between CBC-derived inflammatory markers and the prevalence and mortality of ED was further investigated using dose-response curves with restricted cubic splines (RCS). Spearman’s rank correlation coefficient was applied to assess the correlation between CBC-derived inflammatory markers and CBC parameters. The predictive value of CBC-derived inflammatory markers for all-cause mortality in ED patients was compared using the random survival forest method. All statistical analyses were conducted using R software, version 4.3.2, with statistical significance defined as a P-value less than 0.05.
Results
Characteristics of the study participants
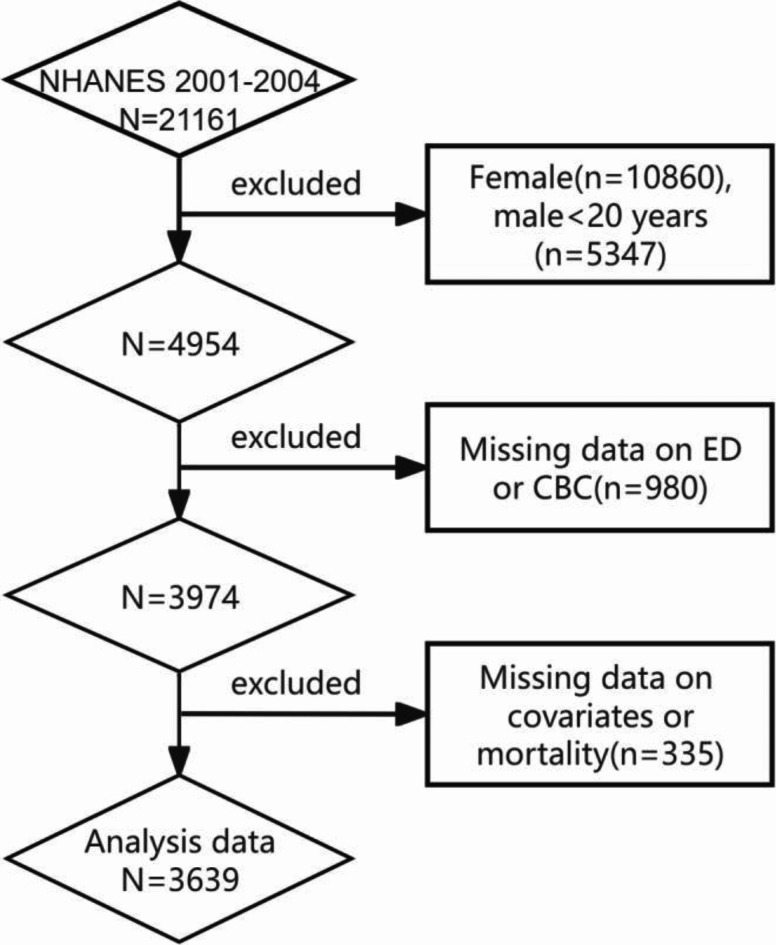
For our analysis, we selected two cycles of the National Health and Nutrition Examination Survey (NHANES) from 2001 to 2002 and 2003–2004, as these were the only cycles providing comprehensive data on erectile dysfunction (ED) and complete blood count (CBC). A total of 21,161 individuals participated in NHANES from 2001 to 2004. The following exclusions were applied: (1) females (n = 16,207) and males under 20 years of age (n = 16,207); (2) participants with missing data on ED (n = 838), CBC (n = 142), and survival status (n = 6); (3) those with incomplete information on covariates, including BMI (n = 112), PIR (n = 52), education level (n = 1), marital status (n = 4), hypertension (n = 4), hypercholesterolemia (n = 68), cardiovascular disease (n = 6), diabetes (n = 1), smoking (n = 4), alcohol consumption (n = 2), and exercise status (n = 1). Ultimately, 3,639 participants were included in our study; of these, 1,031 had a self-reported history of ED, and 610 experienced all-cause mortality (Fig. 1).
Fig. 1.
Flow chart of the study.
Individuals with ED were more likely to be older males (over 59 years), non-Hispanic whites, with lower levels of education and income, married or cohabiting, former smokers, non-drinkers, physically inactive, and without hypertension but with comorbidities such as diabetes, hypercholesterolemia, and cardiovascular disease (Table 1).
Table 1.
Baseline characteristics of adults with CBC-derived inflammatory biomarkers.
| Characteristic | Overall n = 78,785,355 N = 36391 |
Erectile dysfunction | P Value2 | |
|---|---|---|---|---|
| No n = 63,793,918 N = 26081 |
Yes n = 14,991,437 N = 10311 |
|||
| Age (years) | 45.1 ± 15.8 | 41.3 ± 13.4 | 61.1 ± 15.3 | < 0.001 |
| Race | 0.2 | |||
| Mexican American | 753(7.8%) | 538(8.1%) | 215(6.9%) | |
| Other Hispanic | 121(4.2%) | 85(4.0%) | 36(5.0%) | |
| Non-Hispanic White | 1,998(74.6%) | 1,387(74.0%) | 611(77.1%) | |
| Non-Hispanic Black | 658(9.3%) | 508(9.6%) | 150(8.1%) | |
| Other Race | 109(4.1%) | 90(4.3%) | 19(2.9%) | |
| BMI(kg/m2Superscript>) | 28.1 ± 5.5 | 27.9 ± 5.3 | 29.1 ± 6.2 | < 0.001 |
| Education level | < 0.001 | |||
| < High school | 1,018(16.8%) | 602(13.8%) | 416(29.3%) | |
| High school | 893(27.1%) | 681(27.9%) | 212(23.5%) | |
| > High school | 1,728(56.2%) | 1,325(58.3%) | 403(47.2%) | |
| Marital status | < 0.001 | |||
|
Married or living with partner |
2,538(70.7%) | 1,768(69.1%) | 770(77.6%) | |
| Living alone | 1,101(29.3%) | 840(30.9%) | 261(22.4%) | |
| Poverty-income ratio | < 0.001 | |||
| < 1.5 | 1,032(19.2%) | 699(18.5%) | 333(22.5%) | |
| 1.5–3.5 | 1,255(32.7%) | 859(31.4%) | 396(37.9%) | |
| > 3.5 | 1,352(48.1%) | 1,050(50.1%) | 302(39.6%) | |
| Smoking | < 0.001 | |||
| Nonsmoker | 1,466(42.6%) | 1,157(45.5%) | 309(30.3%) | |
| Former smoker | 1,190(29.2%) | 676(25.0%) | 514(46.8%) | |
| Current smoker | 983(28.3%) | 775(29.5%) | 208(22.9%) | |
| Alcohol intake | 3,017(83.6%) | 2,187(84.4%) | 830(80.1%) | 0.004 |
| Physical Activity | < 0.001 | |||
| Inactive | 1,371(31.1%) | 871(28.3%) | 500(42.8%) | |
| Moderate | 1,032(29.1%) | 676(27.2%) | 356(37.3%) | |
| Vigorous | 1,236(39.8%) | 1,061(44.4%) | 175(19.9%) | |
| Hypertension | 2,159(65.5%) | 1,780(71.2%) | 379(41.4%) | < 0.001 |
| Diabetes | < 0.001 | |||
| No | 3,220(92.1%) | 2,448(95.6%) | 772(77.4%) | |
| Borderline | 49(1.0%) | 30(1.0%) | 19(1.3%) | |
| Diabetes | 370(6.8%) | 130(3.4%) | 240(21.2%) | |
| History of CVD | 403(8.0%) | 154(4.6%) | 249(22.6%) | < 0.001 |
| High cholesterol | 1,334(36.3%) | 847(33.0%) | 487(50.7%) | < 0.001 |
| CBC count, 103/µL | ||||
| White blood cell | 7.2 ± 2.5 | 7.2 ± 2.3 | 7.3 ± 3.0 | 0.5 |
| Neutrophils | 4.3 ± 1.6 | 4.2 ± 1.6 | 4.3 ± 1.5 | 0.004 |
| Monocyte | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.008 |
| Platelet | 254.4 ± 61.2 | 257.2 ± 59.8 | 242.5 ± 65.8 | < 0.001 |
| Lymphocyte | 2.1 ± 1.5 | 2.1 ± 1.2 | 2.0 ± 2.4 | < 0.001 |
| CBC-derived indicators | ||||
| NLR | 2.3 ± 1.2 | 2.2 ± 1.1 | 2.6 ± 1.4 | < 0.001 |
| dNLR | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 |
| MLR | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.2 | < 0.001 |
| NMLR | 2.6 ± 1.2 | 2.5 ± 1.2 | 2.9 ± 1.5 | < 0.001 |
| SIRI | 1.3 ± 1.0 | 1.3 ± 0.9 | 1.6 ± 1.1 | < 0.001 |
| SII | 575.7 ± 354.3 | 565.0 ± 324.4 | 621.4 ± 457.8 | 0.003 |
1.Sample sizes presented as n = weighted estimates/N = raw number; 2.Wilcoxon rank-sum test for complex survey samples; chi-squared test with Rao & Scott’s second-order correction. Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as numbers (percentages). 3.Abbreviations: PIR, poverty income ratio; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; NMLR, neutrophil-monocyte to lymphocyte ratio; SIRI, systemic inflammatory response index; SII, systemic immune-inflammation index; CBC, complete blood cell.
Associations between CBC-derived indicators and ED prevalence
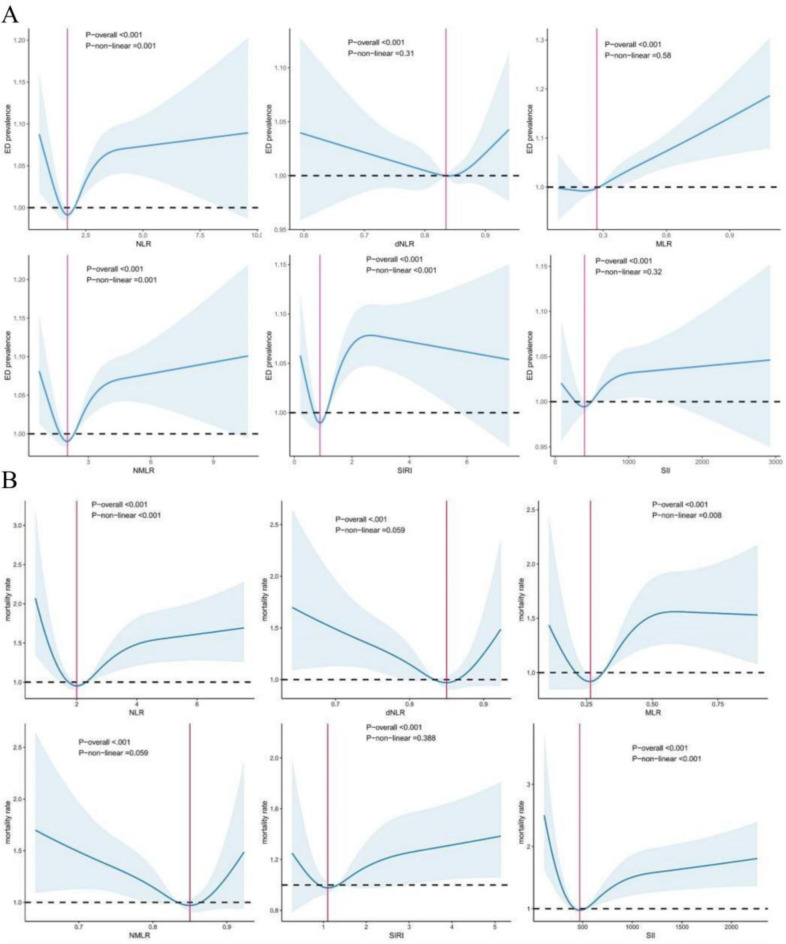
In patients with ED, neutrophil levels and CBC-derived markers were significantly elevated, whereas platelet and lymphocyte counts were significantly reduced (Table 1). The crude model indicated a positive association between CBC-derived inflammatory indicators and the prevalence of ED, with the exception of the dNLR (Table 2). After adjusting for confounding variables, this association remained significant for all markers except dNLR and the SII. In the crude model, individuals in the higher quartiles of CBC-derived inflammatory indicators, excluding dNLR, exhibited a higher prevalence of ED compared to those in the lower quartile. However, this association was not maintained in the fully adjusted Model 3. To further elucidate the relationship between CBC-derived indicators and ED prevalence, restricted cubic spline (RCS) curves were plotted (Fig. 2A), suggesting a nonlinear association between ED prevalence and the levels of NLR, NMLR and SIRI.
Table 2.
The relationship between the prevalence of ED and complete blood cell-derived inflammatory biomarkers.
| continuous | Quartiles of CBC-derived inflammatory biomarkers levels | P trend | |||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | Q1 | Q2 | Q3 | Q4 | ||
| NLR | |||||||
| Crude | 1.27(1.18,1.36) | < 0.001 | - | 0.92(0.67,1.25) | 1.23(0.96,1.57) | 2.16(1.70,2.73) | < 0.001 |
| Model 1 | 1.09(1.01,1.19) | 0.028 | - | 0.68(0.49,0.95) | 0.97(0.71,1.24) | 1.17(0.88,1.56) | 0.091 |
| Model 2 | 1.10(1.01,1.20) | 0.018 | - | 0.74(0.52,1.06) | 1.03(0.76,1.41) | 1.25(0.91,1.71) | 0.052 |
| Model 3 | 1.09(1.00,1.19) | 0.021 | - | 0.75(0.50,1.11) | 1.01(0.73,1.39) | 1.25(0.91,1.73) | 0.061 |
| dNLR | |||||||
| Crude | 0.64(0.12,3.42) | 0.6 | - | 0.87(0.69,1.10) | 0.76(0.60,0.94) | 1.05(0.81,1.38) | > 0.9 |
| Model 1 | 1.11(0.12,10.1) | > 0.9 | - | 0.85(0.63,1.14) | 0.70(0.53,0.94) | 1.19(0.84,1.70) | 0.5 |
| Model 2 | 1.26(0.09,16.9) | 0.9 | - | 0.89(0.63,1.24) | 0.75(0.55,1.03) | 1.23(0.82,1.83) | 0.5 |
| Model 3 | 0.92(0.07,12.6) | > 0.9 | - | 0.89(0.61,1.30) | 0.74(0.54,1.02) | 1.19(0.80,1.79) | 0.6 |
| MLR | |||||||
| Crude | 13.8(6.88,27.6) | < 0.001 | - | 1.04(0.71,1.50) | 1.46(1.09,1.94) | 2.32(1.71,3.15) | < 0.001 |
| Model 1 | 2.50(1.04,6.02) | 0.032 | - | 0.80(0.53,1.22) | 0.96(0.70,1.32) | 1.11(0.75,1.64) | 0.4 |
| Model 2 | 2.94(1.15,7.51) | 0.015 | - | 0.89(0.58,1.38) | 1.10(0.77,1.57) | 1.24(0.81,1.91) | 0.2 |
| Model 3 | 2.97(1.18,7.50) | 0.01 | - | 0.88(0.54,1.41) | 1.12(0.76,1.66) | 1.25(0.79,1.97) | 0.2 |
| NMLR | |||||||
| Crude | 1.26(1.18,1.35) | < 0.001 | - | 0.86(0.66,1.12) | 1.17(0.92,1.49) | 2.16(1.70,2.75) | < 0.001 |
| Model 1 | 1.09(1.01,1.18) | 0.014 | - | 0.65(0.49,0.86) | 0.87(0.67,1.15) | 1.15(0.86,1.54) | 0.12 |
| Model 2 | 1.10(1.02,1.19) | 0.007 | - | 0.70(0.53,0.93) | 0.96(0.70,1.31) | 1.23(0.90,1.69) | 0.064 |
| Model 3 | 1.10(1.01,1.11) | 0.006 | - | 0.71(0.52,0.98) | 0.93(0.67,1.28) | 1.25(0.90,1.73) | 0.073 |
| SIRI | |||||||
| Crude | 1.27(1.17,1.39) | < 0.001 | - | 1.07(0.85,1.35) | 1.43(1.08,1.88) | 2.25(1.85,2.74) | < 0.001 |
| Model 1 | 1.15(1.05,1.26) | 0.002 | - | 0.89(0.69,1.14) | 1.05(0.76,1.45) | 1.45(1.09,1.93) | 0.009 |
| Model 2 | 1.11(1.01,1.22) | 0.019 | - | 0.88(0.65,1.19) | 1.04(0.71,1.51) | 1.35(0.98,1.87) | 0.055 |
| Model 3 | 1.11(1.01,1.22) | 0.017 | - | 0.82(0.60,1.13) | 1.02(0.67,1.54) | 1.28(0.91,1.81) | 0.1 |
| SII | |||||||
| Crude | 1.00(1.00,1.00) | < 0.001 | - | 0.99(0.76,1.29) | 1.06(0.81,1.39) | 1.44(1.16,1.80) | 0.003 |
| Model 1 | 1.00(1.00,1.01) | 0.2 | - | 0.92(0.66,1.29) | 1.03(0.74,1.43) | 1.23(0.89,1.70) | 0.14 |
| Model 2 | 1.00(1.00,1.02) | 0.2 | - | 0.99(0.70,1.42) | 1.14(0.81,1.61) | 1.28(0.91,1.80) | 0.1 |
| Model 3 | 1.00(1.00,1.03) | 0.2 | - | 0.98(0.68,1.42) | 1.18(0.79,1.74) | 1.25(0.86,1.82) | 0.13 |
Crude Model: Unadjusted for covariates; Model 1: Adjusted for age and race; Model 2: Adjusted for age, race, marital status, BMI, PIR, education, smoking, alcohol consumption, and physical activity; Model 3: Fully adjusted model including all covariates.
Fig. 2A.
Utilization of Restricted Cubic Spline (RCS) Regression Analysis to Assess the Relationship between CBC-derived Indicators and ED prevalence in adult males; Fig. 2B: Application of RCS Regression Analysis to Investigate the Correlation between CBC-derived Indicators and All-Cause Mortality in Adults with ED.
Associations between CBC-derived indicators and all-cause mortality among adults with ED
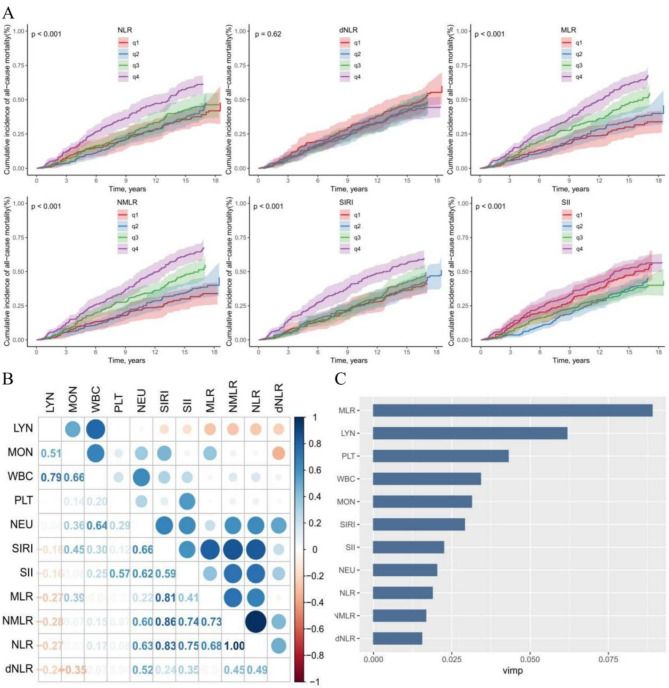
During the subsequent observation period, of the 1,031 adults diagnosed with ED, 610 individuals, accounting for 59.17%, experienced all-cause mortality (Table 3). The decedent group demonstrated significantly higher levels of NLR(HR = 1.06[1.0–1.11], P < 0.05), MLR (HR = 2.0[1.33–3.01], P < 0.001), NMLR(HR = 1.06[1.01–1.11], P < 0.05), and SII(HR = 1.00 [1.0–1.0], P < 0.05) compared to survivors in the fully adjusted model. Except for SII and dNLR, individuals with ED in the higher quartiles of CBC-derived markers exhibited an increased risk of all-cause mortality in the crude model relative to those in the lower quartile. In the fully adjusted model, this association was significant only for MLR (P for trend < 0.001). Kaplan-Meier survival curves (Fig. 3) showed a positive association between CBC-derived markers (excluding dNLR) and all-cause mortality among participants with ED. Additionally, the restricted cubic spline analysis (Fig. 2B) indicated a nonlinear association between all-cause mortality and the levels of NLR, MLR, and SII among ED participants, with notable inflection points at 2.03, 0.26, and 471.21, respectively.
Table 3.
HRs (95% CIs) of all-cause mortality according CBC-derived inflammatory biomarkers among adults with ED.
| continuous | Quartiles of CBC-derived inflammatory biomarkers levels | P trend | |||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | Q1 | Q2 | Q3 | Q4 | ||
| NLR | |||||||
| Crude | 1.15(1.08,1.22) | < 0.001 | - | 1.08(0.80,1.46) | 1.17(0.83,1.64) | 1.85(1.34,2.55) | < 0.001 |
| Model 1 | 1.06(1.00,1.11) | 0.041 | - | 0.76(0.59,0.98) | 0.86(0.65,1.14) | 1.15(0.88,1.52) | 0.2 |
| Model 2 | 1.06(1.00,1.12) | 0.036 | - | 0.74(0.57,0.95) | 0.92(0.70,1.20) | 1.18(0.90,1.54) | 0.08 |
| Model 3 | 1.06(1.00,1.11) | 0.032 | - | 0.68(0.51,0.92) | 0.83(0.63,1.09) | 1.09(0.84,1.44) | 0.2 |
| dNLR | |||||||
| Crude | 0.14(0.01,1.22) | 0.087 | - | 0.83(0.59,1.16) | 0.84(0.62,1.13) | 0.79(0.55,1.14) | 0.2 |
| Model 1 | 0.29(0.04,2.12) | 0.2 | - | 0.81(0.61,1.06) | 0.75(0.59,0.97) | 0.93(0.67,1.28) | 0.6 |
| Model 2 | 0.44(0.06,3.30) | 0.4 | - | 0.83(0.61,1.13) | 0.76(0.59,0.98) | 0.95(0.69,1.31) | 0.6 |
| Model 3 | 0.22(0.03,1.58) | 0.13 | - | 0.80(0.59,1.07) | 0.72(0.57,0.91) | 0.86(0.61,1.21) | 0.3 |
| MLR | |||||||
| Crude | 4.46(2.85,6.98) | < 0.001 | - | 1.23(0.82,1.85) | 1.82(1.25,2.66) | 2.64(1.92,3.62) | < 0.001 |
| Model 1 | 1.67(1.14,2.45) | 0.009 | - | 0.93(0.62,1.38) | 1.18(0.83,1.69) | 1.31(0.96,1.78) | 0.033 |
| Model 2 | 1.90(1.23,2.92) | 0.004 | - | 1.01(0.69,1.50) | 1.28(0.91,1.81) | 1.45(1.07,1.95) | 0.005 |
| Model 3 | 2.00(1.33,3.01) | < 0.001 | - | 1.01(0.70,1.45) | 1.29(0.92,1.82) | 1.44(1.09,1.90) | 0.002 |
| NMLR | |||||||
| Crude | 1.14(1.08,1.21) | < 0.001 | - | 1.12(0.85,1.47) | 1.17(0.80,1.70) | 2.01(1.48,2.71) | < 0.001 |
| Model 1 | 1.05(1.00,1.00) | 0.033 | - | 0.84(0.65,1.09) | 0.87(0.63,1.19) | 1.23(0.94,1.62) | 0.2 |
| Model 2 | 1.06(1.01,1.12) | 0.029 | - | 0.84(0.64,1.09) | 0.95(0.70,1.29) | 1.29(0.99,1.68) | 0.04 |
| Model 3 | 1.06(1.01,1.11) | 0.024 | - | 0.78(0.58,1.06) | 0.86(0.63,1.16) | 1.21(0.92,1.59) | 0.14 |
| SIRI | |||||||
| Crude | 1.17(1.08,1.26) | < 0.001 | - | 1.13(0.84,1.51) | 1.22(0.88,1.69) | 1.83(1.36,2.47) | < 0.001 |
| Model 1 | 1.07(1.00,1.14) | 0.04 | - | 0.93(0.72,1.21) | 0.99(0.73,1.33) | 1.23(0.97,1.57) | 0.1 |
| Model 2 | 1.06(0.99,1.13) | 0.1 | - | 0.99(0.77,1.28) | 0.98(0.72,1.34) | 1.21(0.92,1.59) | 0.2 |
| Model 3 | 1.06(0.99,1.14) | 0.078 | - | 0.97(0.74,1.27) | 0.93(0.69,1.25) | 1.19(0.91,1.55) | 0.3 |
| SII | |||||||
| Crude | 1.00(1.00,1.00) | < 0.001 | - | 0.72(0.52,0.99) | 0.67(0.47,0.97) | 1.10(0.81,1.51) | 0.7 |
| Model 1 | 1.00(1.00,1.00) | 0.006 | - | 0.68(0.50,0.93) | 0.70(0.50,0.99) | 1.04(0.82,1.31) | 0.7 |
| Model 2 | 1.00(1.00,1.02) | 0.007 | - | 0.70(0.51,0.96) | 0.76(0.54,1.07) | 1.05(0.83,1.31) | 0.6 |
| Model 3 | 1.00(1.00,1.00) | 0.015 | - | 0.69(0.50,0.95) | 0.75(0.54,1.04) | 1.03(0.84,1.27) | 0.6 |
Crude Model: Unadjusted for covariates; Model 1: Adjusted for age and race; Model 2: Adjusted for age, race, marital status, BMI, PIR, education, smoking, alcohol consumption, and physical activity; Model 3: Fully adjusted model including all covariates.
Fig. 3A.
Kaplan-Meier Survival Analysis of the relationship between CBC-derived Indicators and All-cause mortality in adult men with ED(adjusted by Model 3); Fig. 3B: Assessment of prognostic Significance of CBC-derived Indicators: Spearman Correlation Analysis of CBC parameters and inflammatory markers; Fig. 3C: Comparative Evaluation of CBC Parameters and CBC-derived Indicators in predicting All-cause mortality using Random Survival Forests (RSF) method.
Prognostic value of CBC-Derived indicators
The correlation among CBC-derived inflammatory markers is depicted in Fig. 3B. A remarkably strong positive correlation is observed between the NLR and NMLR, with a correlation coefficient of 1.00. In contrast, a significant negative correlation is present between the monocyte count and dNLR, with a coefficient of −0.35. Furthermore, among these markers, MLR exhibits the strongest predictive power for all-cause mortality in adults with ED, as shown in Fig. 3C.
Discussion
In this study, we investigated the relationship between six CBC-derived inflammatory markers—NLR, dNLR, MLR, NMLR, SIRI, and SII—and erectile dysfunction (ED) among adult US men using data from the 2001–2004 NHANES. Our findings revealed that elevated neutrophil counts and reduced lymphocyte counts were significantly associated with a higher prevalence of ED, aligning with the outcomes of prior research19. Furthermore, four markers (NLR, MLR, NMLR, and SIRI) exhibited a positive correlation with the prevalence of ED, which corroborates earlier findings that NLR, SIRI, and SII were significantly linked to ED17,20,21. After stratifying the markers into quartiles and setting the lowest quartile (Q1) as the reference, our analysis indicated that, contrary to studies proposing NLR as a rapid, straightforward, and cost-effective measure for assessing ED severity with high sensitivity and specificity22, the fully adjusted model demonstrated no significant correlation between higher quartile fractions of CBC-based inflammatory biomarkers and ED incidence compared to the lower fractions. Discrepancies in the results of previous studies may be attributed to variations in study populations or methodologies.
We also investigated the relationship between CBC-derived metrics and all-cause mortality in patients with ED. Again, these metrics were stratified into quartiles, with the lowest quartile (Q1) as the reference category. We were particularly interested in the fact that among the four metrics associated with all-cause mortality (NLR, MLR, NMLR, and SII), MLR had the highest predictive value for all-cause mortality in ED patients. In summary, our results suggest that CBC-based inflammatory biomarkers have potential prognostic value in predicting all-cause mortality in ED patients. These findings provide new perspectives for future studies and may contribute to the development of new diagnostic tools and therapeutic strategies.
The role of inflammation in ED is currently a focus of research. Some researchers have suggested that ED is an immune-mediated inflammatory state, with significantly elevated levels of inflammatory factors in ED patients compared to controls23, and that some inflammation-associated disorders appear to contribute to the increased prevalence of ED24,25. However, the molecular mechanisms by which chronic inflammatory signalling is involved in the pathogenesis of ED are still poorly understood. Theoretically, stimuli from exogenous or endogenous antigens may induce an inflammatory response in the vascular endothelium, leading inflammatory cells to produce inflammatory and growth factors, activate the immune response of T cells and macrophages, and release additional inflammatory factors. At the same time, increased oxygen consumption due to cell proliferation creates a relatively hypoxic environment. The infiltration of inflammatory cells and the relatively hypoxic environment exacerbate tissue damage and promote vascular endothelial damage and thus ED26. In contrast, biological therapies targeting inflammatory cytokines are effective in reducing the risk of CVD and ED27.
CBC-derived indicators are commonly used as markers of the body’s immune and inflammatory status and have gained interest in clinical practice due to their simplicity and cost-effectiveness. These indicators reflect more than two immune pathways, are less affected by confounding conditions than assessing monocytes, lymphocytes, or neutrophils alone, and may be more predictive in assessing inflammation28. Studies have shown that NLR, NMLR, PLR, SIRI, and SII are significantly associated with prognosis in cancer patients, and each parameter has a good predictive value29,30, while MLR, PLR, and NLR are valid indicators for assessing cardiovascular disease (CVD) risk and predicting mortality31. Furthermore, the predictive performance of several CBC-derived indicators has been demonstrated for the incidence and all-cause mortality of a number of diseases, such as adult sarcopenia, psoriasis, cardiovascular disease, and prostate hyperplasia11,14,32,33. CBCs have been found to be important components of the immune system and are involved in the regulation of inflammatory responses. Moreover, monocytes play a more critical role in the chronic inflammatory process, whereas neutrophils are primarily involved in the acute inflammatory response34,35. Therefore, MLR demonstrates a closer correlation with ED associated with chronic inflammation than other biomarkers. Their immunoreactivity may lead to ED by increasing oxidative stress, enhancing cytokine release, and causing tissue damage through the production of free radicals and reactive oxygen species36. Subsequently, it increases the risk of developing ED and mortality, especially from cardiovascular causes37.
Among these indicators, we found that MLR had the greatest predictive power for the incidence of ED and all-cause mortality in the ED population compared to others. Monocytes are recognized as important effector cells in inflammatory processes and play a crucial role in microbial clearance and in the pathogenesis of inflammatory and degenerative diseases38. The heterogeneous subpopulation composition of monocytes in different environments determines the progression of systemic inflammation by linking innate and acquired immunity through phagocytosis, cytokine and chemokine release, antigen presentation, and lymphocyte activation39. During initial inflammation, monocytes are recruited from the bone marrow into the circulation, leading to an increase in the absolute and relative number of monocytes in the blood. Conversely, the inflammatory response can suppress the immune system by inhibiting the cytolytic activity of immune cells such as lymphocytes. One possible explanation is that lymphocytopenia is due to the direct effects of elevated serum cortisol and catecholamines, which contribute to increased apoptosis and downregulation of lymphocyte proliferation and differentiation40,41. In contrast, combining monocytes and lymphocytes into a single measure (MLR) is theorized to be a more reliable indicator of systemic inflammatory status than a single parameter, especially when monocyte and lymphocyte counts are close to the upper or lower limits of normal42.
Our study provides insights into the relationship between blood-derived inflammatory markers and ED. These markers are clinically accessible and cost-effective, allowing for convenient assessment by clinicians. They may also offer valuable perspective into the immuno-inflammatory mechanisms underlying ED. Our study has several advantages over previous studies. Firstly, it is based on NHANES data, which provides a more representative and larger sample, enabling the discovery of significant associations between the independent and dependent variables. Secondly, we adjusted for confounding covariates to ensure the validity of the current findings. Lastly, CBC-derived indicators, consisting of multiple CBC parameters, may provide more comprehensive information than a single indicator.
It should be noted that the present study is not without limitations. The cross-sectional nature of the study precludes the establishment of definitive causal relationships. Furthermore, the diagnosis of ED was based on participants’ self-reports, which may have introduced recall bias. However, previous studies have confirmed the reliability of self-reports43. Additionally, although exclusion criteria were employed to minimize the potential for confounding effects and a variety of confounders were adjusted for, it is possible that unknown or unmeasured confounders not included in the NHANES database may have influenced the analysis. Furthermore, hematological parameters may be influenced by numerous other factors, such as active infection or inflammation.
Conclusions
In conclusion, our study demonstrates that there is a significant association between CBC-derived inflammatory biomarkers and the prevalence of ED, as well as survival prognosis in participants with ED, and MLR exhibited the most pronounced predictive capacity. Therefore, CBC-derived markers, especially MLR, have promising applications in the initial assessment of ED patients and in the prognostic assessment of their survival. In the future, we plan to conduct prospective studies in a wider population and include more potential confounders to deeply explore the role of these biomarkers in the ED population.
Ethics statement
The studies involving humans were approved by the NCHS Research Ethics Review Board (ERB) Protocol #98 − 12. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The article does not contain any identifying images or data.
Acknowledgements
We are grateful to all the participants involved in the present study for their enthusiasm and commitment.
Author contributions
ZY: Data curation, Supervision, Validation; LT: Validation, Resources; CQ: Investigation, Methodology, Formal analysis; SM: Visualization, Project administration; FX: Funding acquisition, Writing—review & editing; LC: Conceptualization, Software, Writing-original draft. All authors reviewed the manuscript.
Funding
The research did not receive specific funding.
Data availability
The data covered in this study are freely available in the NHANES database (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. Jama. ;270(1):83–90. (1993). [PubMed]
- 2.Mark, K. P. et al. Erectile dysfunction prevalence in the United States: report from the 2021 National Survey of sexual wellbeing. J. Sex. Med.21 (4), 296–303 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Randrup, E., Baum, N. & Feibus, A. Erectile dysfunction and cardiovascular disease. Postgrad. Med.127 (2), 166–172 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Westheide, J. et al. Sexuality in male psychiatric inpatients. A descriptive comparison of psychiatric patients, patients with epilepsy and healthy volunteers. Pharmacopsychiatry40 (5), 183–190 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Zhao, B. et al. Erectile Dysfunction predicts Cardiovascular events as an independent risk factor: a systematic review and Meta-analysis. J. Sex. Med.16 (7), 1005–1017 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Salonia, A. et al. European Association of Urology Guidelines on sexual and Reproductive Health-2021 update: male sexual dysfunction. Eur. Urol.80 (3), 333–357 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Romano, L. et al. Sexual dysfunction in gastroenterological patients: do gastroenterologists care enough? A nationwide survey from the Italian Society of Gastroenterology (SIGE). Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the study of the liver. ;54(11):1494–1501. (2022). [DOI] [PubMed]
- 8.Cantone, E. et al. The relationship between obstructive sleep apnoea and erectile dysfunction: an underdiagnosed link? A prospective cross-sectional study. Andrologia54 (9), e14504 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlachopoulos, C., Rokkas, K., Ioakeimidis, N. & Stefanadis, C. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. Eur. Urol.52 (6), 1590–1600 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Xing, Y. et al. A practical nomogram based on systemic inflammatory markers for predicting portal vein thrombosis in patients with liver cirrhosis. Ann. Med.54 (1), 302–309 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi, C., Cao, H., Zeng, G., Yang, L. & Wang, Y. The relationship between complete blood cell count-derived inflammatory biomarkers and benign prostatic hyperplasia in middle-aged and elderly individuals in the United States: evidence from NHANES 2001–2008. PloS One. 19 (7), e0306860 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferro, M. et al. Lymphocyte to monocyte ratio: a New Independent Prognostic factor in bladder Cancer progression? Front. Oncol.11, 754649 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone, B. et al. Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study. Medicina (Kaunas, Lithuania). ;58(10). (2022). [DOI] [PMC free article] [PubMed]
- 14.Guo, B. et al. Associations of CBC-Derived inflammatory indicators with Sarcopenia and mortality in adults: evidence from Nhanes 1999 ∼ 2006. BMC Geriatr.24 (1), 432 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, X., Mei, Y., Wang, X., Cui, L. & Xu, R. Association between neutrophil to lymphocyte ratio and erectile dysfunction among US males: a population-based cross-sectional study. Front. Endocrinol.14, 1192113 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong, L., Zhan, X. & Luo, X. Higher systemic immune-inflammation index is associated with increased risk of erectile dysfunction: result from NHANES 2001–2004. Med. (Baltim).102 (45), e35724 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, W., Wang, H. & Lin, M. E. Relationship between systemic inflammatory response index and Erectile Dysfunction: a cross-sectional study. Urology181, 69–75 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Zhou, D. et al. Calculated inflammatory markers derived from complete blood count results, along with routine laboratory and clinical data, predict treatment failure of acute peritonitis in chronic peritoneal dialysis patients. Ren. Fail.45 (1), 2179856 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao, Z., Tang, Y., Li, X. & Li, D. The relationship between hematologic parameters and Erectile Dysfunction. Sex. Med.9 (4), 100401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Y. et al. A systematic review and meta-analysis of the relationship between erectile dysfunction and the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. Andrologia54 (3), e14337 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Chen, D. et al. Association between the systemic immune-inflammation index and erectile dysfunction: a cross-sectional study. Immun. Inflamm. Dis.12 (8), e1363 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, W. et al. An objective correlation of specific haematological parameters with ED severity. Andrologia54 (9), e14448 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Liu, D. et al. Inflammatory cytokine profiles in erectile dysfunction: a bidirectional mendelian randomization. Front. Immunol.15, 1342658 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, C., Lei, Q., Li, J. & Liu, W. Arthritis increases the risk of erectile dysfunction: results from the NHANES 2001–2004. Front. Endocrinol.15, 1390691 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, T. et al. Association between Psoriasis and Erectile Dysfunction: a Meta-analysis. J. Sex. Med.15 (6), 839–847 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Konstantinovsky, A. et al. Erectile Dysfunction, Sleep disorders, and endothelial function. Isr. Med. Association Journal: IMAJ. 21 (6), 408–411 (2019). [PubMed] [Google Scholar]
- 27.Lutgens, E. et al. Immunotherapy for cardiovascular disease. Eur. Heart J.40 (48), 3937–3946 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy. 122 (7), 474–488 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Xu, F. et al. Combined inflammatory parameters and tertiary lymphoid structure predict prognosis in patients with resectable non-small cell lung cancer treated with neoadjuvant chemoimmunotherapy. Front. Immunol.14, 1244256 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar, A. et al. Correlation of hematological parameters with clinical outcomes in cervical Cancer patients treated with Radical Radio(chemo)therapy: a retrospective study. Int. J. Radiat. Oncol. Biol. Phys.118 (1), 182–191 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Zhang, Y., Feng, L., Zhu, Z., He, Y. & Li, X. Association between blood inflammatory indicators and heart failure: a cross-sectional study of NHANES 2009–2018. Acta Cardiol.79 (4), 473–485 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Zhao, Y., Yang, X. T., Bai, Y. P. & Li, L. F. Association of Complete Blood Cell Count-Derived inflammatory biomarkers with psoriasis and mortality. Clinical, cosmetic and investigational dermatology. ;16:3267–3278. (2023). [DOI] [PMC free article] [PubMed]
- 33.Yang, Y. et al. Prognostic value of multiple complete blood count-derived indicators in Intermediate Coronary lesions. Angiology :33197231198678. (2023). [DOI] [PubMed]
- 34.Wen, J. H. et al. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front. Immunol.13, 946832 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol.13 (3), 159–175 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Calmasini, F. B., Klee, N., Webb, R. C. & Priviero, F. Impact of Immune System activation and vascular impairment on male and female sexual dysfunction. Sex. Med. Reviews. 7 (4), 604–613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araujo, A. B. et al. Erectile dysfunction and mortality. J. Sex. Med.6 (9), 2445–2454 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol.11 (11), 762–774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guilliams, M., Mildner, A. & Yona, S. Developmental and Functional Heterogeneity of monocytes. Immunity49 (4), 595–613 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Krüger, K. et al. Apoptosis of T-Cell subsets after Acute High-Intensity interval Exercise. Med. Sci. Sports. Exerc.48 (10), 2021–2029 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Aslan, A. et al. Neutrophil-lymphocyte ratio could be a marker for Erectile Dysfunction. Urol. J.16 (2), 216–220 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Cai, C., Zeng, W., Wang, H. & Ren, S. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and monocyte-to-lymphocyte ratio (MLR) as biomarkers in diagnosis evaluation of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: a retrospective, observational study. Int. J. Chronic Obstr. Pulm. Dis.19, 933–943 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Donnell, A. B., Araujo, A. B., Goldstein, I. & McKinlay, J. B. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J. Gen. Intern. Med.20 (6), 515–519 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data covered in this study are freely available in the NHANES database (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).