Abstract
We have previously demonstrated that rat uterine epithelial cells (UEC) produce CCL20/macrophage inflammatory protein 3 alpha (MIP3α) and tumor necrosis factor alpha (TNF-α) in response to live and heat-killed Escherichia coli and to the pathogen-associated molecular patterns (PAMP) lipopolysaccharide (LPS) and Pam3Cys. To determine whether estradiol (E2) modulates PAMP-induced CCL20/MIP3α and TNF-α secretion, primary cultures of rat UEC were incubated with E2 for 24 h and then treated with LPS or Pam3Cys or not treated for an additional 12 h. E2 inhibited the constitutive secretion of TNF-α and CCL20/MIP3α into culture media. Interestingly, E2 pretreatment enhanced CCL20/MIP3α secretion due to LPS and Pam3Cys administration. In contrast, and at the same time, E2 lowered the TNF-α response to both PAMP. To determine whether estrogen receptors (ER) mediated the effects of E2, epithelial cells were incubated with E2 and/or ICI 182,780, a known ER antagonist. ICI 182,780 had no effect on E2 inhibition of constitutive TNF-α and CCL20/MIP3α secretion. In contrast, ICI 182,780 reversed the stimulatory effect of E2 on LPS- and/or Pam3Cys-induced CCL20/MIP3α secretion as well as partially reversed the inhibitory effect of E2 on TNF-α production by epithelial cells. Overall, these results indicate that E2 regulates the production of TNF-α and CCL20/MIP3α by UEC in the absence as well as presence of PAMP. Since CCL20/MIP3α has antimicrobial activity and is chemotactic for immune cells, these studies suggest that regulation of CCL20/MIP3α and TNF-α by E2 and PAMP may have profound effects on innate and adaptive immune responses to microbial challenge in the female reproductive tract.
Effective immune protection to microbial challenge in mammals is based on responses of both the innate and adaptive immune systems. Immune responses in the female reproductive tract pose a special problem. There must be effective immune protection against microbial infection but tolerance to the presence of allogeneic sperm and a fetal placental unit. Estrogen regulation of immune responses, both systemically and in the female reproductive tract, is an essential part of normal physiology (57, 58). It has been observed that, compared to men, the higher levels of estrogen in women lead to enhanced antibody and cell-mediated immune responses following infection or vaccination (4). Epithelial cells in the uterus and at other mucosal surfaces release cytokines that affect the migration and maturation of stromal immune cells (9, 11, 28, 42, 45). Estradiol (E2) has been shown to play an important role in modulating the release of cytokines by uterine tissues (24, 27, 29, 44, 47). However, little is known about the effects of E2 on uterine epithelial cell responses to potential pathogens. This emphasizes the need to more fully define the interactions between estradiol and epithelial cells that, in addition to being a physical barrier, provide protection by signaling to immune cells distant from the site(s) of infection.
Studies over the last decade demonstrating the rapid and continuous movement of vaginal contents into the lumen of the uterus and the Fallopian tubes (31-34) along with an increased appreciation for the relationship of perturbations of vaginal flora to maternal/fetal morbidity and mortality (5, 20, 55) have focused our attention on innate immune protection and the response of uterine epithelial cells (UEC) to bacteria and bacterial cell wall components (8, 9, 41, 49). Epithelial cells that form a physical barrier that lines the uterus, when cultured in vitro in cell culture inserts, reestablish a polarized monolayer similar to that seen in histological sections of uterine tissue.
Responses to estrogens are cell type and tissue specific in that E2 acting within the reproductive tract can have opposite effects. For example, in response to estradiol, antigen presentation by uterine epithelial cells increases but antigen presentation in the underlying stroma is inhibited (59). What is now widely accepted is that estrogen responses are mediated though several receptors. On the one hand, E2 exerts its effects though intracellular nuclear receptors that elicit genomic responses (56). The estrogen receptor (ER) consists of at least two isoforms (ERα and ERβ) which have overlapping but discrete functions (18). Estradiol may also act through membrane-associated receptors that utilize nongenomic pathways (38, 43). In other studies, E2 acts through soluble factors produced by underlying stromal cells that stimulate epithelial cell proliferation and tight junction formation (7, 10, 17). Indirect effects such as the influence of stromal cells on epithelial cell growth are known to require the presence of the ER in stromal cells (7).
Studies presented previously by our laboratory demonstrate that CCL20/macrophage inflammatory protein 3 alpha (MIP3α) and tumor necrosis factor alpha (TNF-α) are produced by uterine epithelial cells in response to exposure to Escherichia coli as well as to the specific toll-like receptor (TLR) 2 and TLR4 agonists (Pam3Cys and lipopolysaccharide [LPS]) (8, 9). While CCL20/MIP3α plays a role in the development and maintenance of normal immune cell populations in mucosal tissues (6, 26), it has also been found in association with autoimmune lesions, including histiocytosis and autoimmune encephalomyelitis (1, 3). E2 and progesterone regulate uterine epithelial cell expression of TNF-α mRNA and protein through the estrous cycle (11). In some studies E2 is inhibitory, while in others it appears to stimulate TNF-α production by uterine epithelial cells (23, 47). While no direct effects of E2 on CCL20/MIP3α have been reported, some studies indicate that TNF-α up-regulates CCL20/MIP3α gene expression (14, 19, 37, 52, 53).
The overall goal of the studies presented in this paper was to test the hypothesis that E2 regulates the stimulation by pathogen-associated molecular patterns (PAMP) of CCL20/MIP3α and TNF-α secretion by polarized rat UEC in culture. Our objectives were the following: (i) to identify cell culture conditions that would optimize the detection of responsiveness of epithelial cells to the presence of E2; (ii) to determine if E2 influences the constitutive release of CCL20/MIP3α and TNF-α by uterine cells; and (iii) to examine the role of E2 in regulating PAMP-stimulated release of CCL20/MIP3α and TNF-α in UEC.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free Lewis rats weighing from 125 to 175 g (Charles River Breeding Laboratories, Kingston, NY) were used in all studies. Animals were maintained with alternating 12-h dark/light cycles and given free access to food and water. All procedures involving these rats were conducted following protocols approved by the Dartmouth College Institutional Animal Care and Use Committee.
Preparation of epithelial cell cultures.
All cell cultures were established by pooling the uteri of five or more animals at various stages of the estrous cycle (7). Briefly, rat uteri were removed, rinsed in sterile ice-cold Hank's balanced salt solution (Gibco, Grand Island, NY), weighed, and then transferred to a pancreatin (Gibco), trypsin (Sigma, St. Louis, MO), DNase (Worthington, Lakewood, NJ) digest (400 U of DNase/ml of pancreatin, 46,500 U of trypsin/ml of pancreatin, 19.5 ml of pancreatin/gram of uterine tissue). Uteri were minced and incubated for 1 h at 4°C on a rotating platform at 60 rpm. The tissues were then moved to room temperature for an additional 60 min of incubation. Tissues were vortexed vigorously for 10 to 15 s prior to being poured through a sterile 250-μm nylon mesh screen to remove tissue fragments from suspension of cells prior to recovering epithelial cell sheets. Rinses of the uterine tissues with culture media (described below) produced a cell suspension. Epithelial cell sheets were recovered by pouring the resulting suspension onto a 20-μm mesh capture screen and then collected and suspended in medium and plated on growth factor-reduced Matrigel-coated 10-mm by 0.4-μm polycarbonate membrane inserts (Nalgene Nunc International) at a density of one rat uterus per three to four cell culture insert wells. In experiments using NUNC cell culture inserts, cells were plated in 500 μl of medium in the apical compartment, and 500 μl of medium was placed in the basolateral compartment.
Uterine epithelial cells were incubated at 37°C in 5% CO2 and allowed to develop into polarized confluent monolayer cultures. Treatments of polarized cultures described for individual experiments took place between days 4 and 7 from the day that cultures were established. The presence of a confluent monolayer of epithelial cells that has formed tight junctions (16) was determined by measuring transepithelial resistance (TER) on an EVOM Voltohmmeter (World Precision Instruments). Measurements were taken using a sterile chopstick type probe preconditioned in medium prior to each session. This device conducts an electrical current across the epithelial cell culture being measured, and resistance to the current is displayed on the EVOM Voltohmmeter. A Matrigel-coated control well containing medium was measured at each session to determine background resistance.
Culture media.
In preliminary studies (data not included), a variety of media as well as cell culture inserts were tested for the ability to support rat uterine epithelial cell growth in culture. To optimize conditions for rat uterine epithelial cell culture, we used F12K medium (American Type Culture Collection, Rockville, MD) plus 10% defined fetal bovine serum (F12K-DS) supplemented with 100 μg/ml streptomycin and 100 U/ml penicillin to initiate cell cultures. When average TER exceeded 1,000 Ω/well (generally on day 4), media were replaced with F12K medium plus 10% charcoal-dextran-stripped fetal bovine serum (F12K-SS), also supplemented with 100 μg/ml streptomycin and 100 U/ml penicillin. After 24 h of incubation with F12K-SS medium and continued monitoring of TER, cultures were exposed to F12K-SS medium containing E2 or ICI 182,780. In experiments with LPS or Pam3Cys and E2, cell cultures were exposed to the hormone in F12K-SS medium for 12 to 24 h prior to treatment with PAMP.
Treatment of epithelial cells with PAMP.
PAMP, including Pam3Cys, a synthetic analog of bacterial lipopeptides (L2000 EMC Microcollections, Tuebingen, Germany), and repurified (ultrapure) lipopolysaccharides (R595, Salmonella minnesota; List Biological Laboratories, Campbell, CA), were reconstituted in sterile endotoxin-free irrigation water under sterile conditions. Cell cultures were treated with PAMP in the apical compartment at a dose of 1 μg/ml in all experiments. Medium was harvested at the end of the experimental period(s) for analysis. PAMP were tested by Limulus assay (Limulus Amebocyte Lysate QCL-1000; Biowhittaker) for the presence of endotoxin. Pam3Cys (1 μg/ml) contained <0.018 endotoxin U/ml.
Hormone treatment.
To prepare hormone for cell incubation, E2 (Calbiochem, La Jolla, CA) was dissolved in 100% ethanol, evaporated to dryness, and then resuspended in F12K-SS medium. The estrogen receptor antagonist ICI 182,780 (Tocris, Ellisville, MO) was prepared in the same manner. To control for any residue from the ethanol, an equivalent amount of ethanol was evaporated in another container and used to prepare control medium used in the experiments. After epithelial cell cultures had grown to confluence as determined by TER, F12K-DS was replaced with F12K-SS medium as described above. UEC cultures were incubated in F12K-SS medium for 24 h prior to transfer to new plates containing medium and treated with E2 and/or ICI 182,780 in F12K-SS at concentrations indicated in figure legends and Results.
Measurement of CCL20/MIP3α and TNF-α.
CCL20/MIP3α and TNF-α were measured using an enzyme-linked immunosorbent assay (ELISA) kit for rat MIP3α or rat TNF-α (DuoSet ELISA Development System; R&D Systems, Minneapolis, MN). Directions and materials supplied with these commercial kits were followed exactly for CCL20/MIP3α assays. However, in order to increase the sensitivity of the TNF-α ELISA, the standard protocol was modified by increasing the amount of capture antibody and detection antibody to 120% of normal concentration. Also, the concentration of streptavidin-horseradish peroxidase was increased to 125% of that called for in the standard protocol. These modifications permitted consistent measurement of TNF-α of ≥5 pg/ml.
Statistics.
Error bars in all figures refer to variations between wells (four to six wells/treatment). Data were compared by one-way analysis of variance (ANOVA) followed by a Tukey multiple comparison posttest. Student's t test was used only as indicted in figure legends. Differences with P values of <0.05 were considered significant.
RESULTS
Effect of fetal bovine serum and E2 on epithelial cell TER.
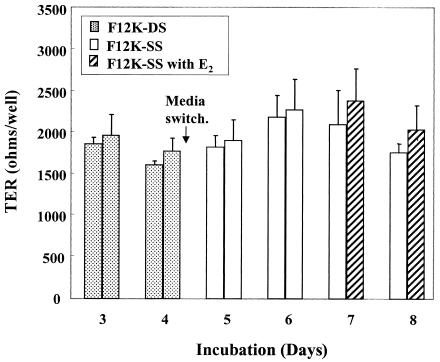
Since mouse uterine epithelial cells respond to E2 in culture with lower TER (17) and because TER measures the electrical integrity of polarized epithelial cells (16), a study was undertaken to determine the effect of E2 on rat UEC incubated initially in F12K-DS medium and then switched to F12K medium containing stripped serum (F12K-SS). This switch was made because defined fetal bovine serum contains estrogens, while the levels in stripped serum are below the level of detection (Hyclone Technical Support, personal communication). Following culture in F12K-SS for 48 h, some wells were treated with F12K-SS with E2 (10−7 M). As seen in Fig. 1, treatment with E2 had no effect on epithelial TER measured at 24 and 48 h (days 7 and 8) after treatment. Moreover, this study, which is representative of five experiments, indicated that transition from F12K-DS to F12K-SS had no effect on TER.
FIG. 1.
Development of TER of uterine epithelial cells switched to F12K-SS and treated with E2. On day 4 of culture, UEC cultures were switched to F12K media with stripped serum. One group was then treated with E2 (10−7 M). Values shown are mean TER ± standard error (SE) per group of four to five wells each. This figure is representative of methods used and results of five experiments.
Effect of E2 on constitutive and PAMP-induced release of CCL20/MIP3α and TNF-α by uterine epithelial cells.
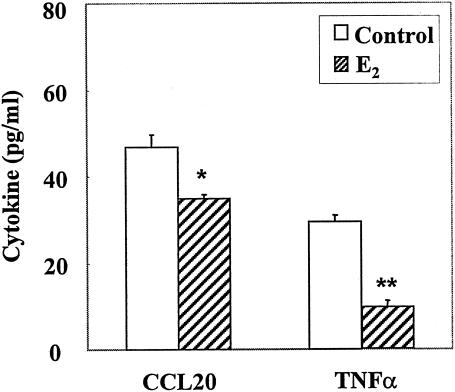
To determine the effect of E2 on the constitutive release of CCL20/MIP3α and TNF-α, UEC were grown in F12K-DS for 3 to 4 days prior to transfer into F12K-SS. Following 24 h in F12K-SS, media were changed and cells were incubated in the presence or absence of E2 (10−8 M) for an additional 24 h. Cells were incubated in fresh medium (with E2 or control) for an additional 12 h, after which basolateral medium was collected for ELISA analysis. Others have shown that the dissociation constants (Kds) for ERα and ERβ in the rodent uterus are 0.1 and 0.4 nM, respectively (30). To ensure saturation of receptors, which occurs between 10−9 and 10−8 M, as well as to account for the loss of free estradiol in culture owing to adherence to plastic and association with proteins in stripped serum, estradiol was added to the incubation media at concentrations ranging from 10−8 to 10−7 M. As seen in Fig. 2, E 2 added to the incubation medium at 10−8 M as well as 10−7 M (not shown) significantly inhibited the constitutive release of CCL20/MIP3α and TNF-α by uterine epithelial cells.
FIG. 2.
Effect of E2 on constitutive basolateral release of cytokines. For uterine epithelial cells cultured as described in Materials and Methods, following a change to fresh medium and a 12-h incubation in fresh medium or medium with E2 (10−8 M), basolateral media were harvested and analyzed by ELISA. Values shown are means of cytokine levels ± SE of five wells per group. Results for each cytokine are representative of three or more separate experiments. *, significantly (P < 0.05) different from cytokine measured in control cultures; **, significantly (P < 0.01) different from cytokine measured in control cultures.
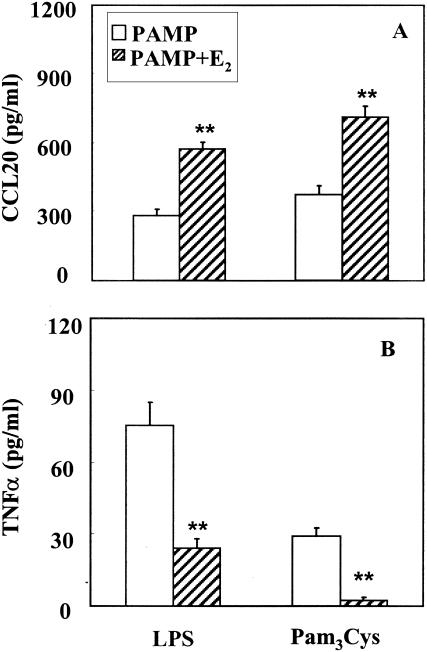
In contrast, with cultures pretreated with E2 (10−8 M) for 24 h prior to addition of either LPS or Pam3Cys for 12 h, E2 doubled secretion of CCL20/MIP3α into the basolateral compartment compared to either PAMP alone (Fig. 3A). Unexpectedly, E2 had the opposite effect on the TNF-α response to LPS and Pam3Cys, inhibiting TNF-α secretion (Fig. 3B). These findings indicate that in response to LPS and Pam3Cys, estradiol exerts opposite effects on CCL20/MIP3α and TNF-α release.
FIG. 3.
Effect of E2 on PAMP-stimulated release of (A) CCL20/MIP3α and (B) TNF-α. Rat uterine epithelial cell cultures grown as described in Materials and Methods were treated with ultrapure LPS (1 μg/ml) or Pam3Cys (1 μg/ml) with or without E2 (10−8 M). Basolateral media were harvested after a 12-h incubation, and CCL20/MIP3α and TNF-α levels were determined by ELISA. Values shown are means of cytokine levels ± SE for five wells per group. Results are representative of four experiments for LPS treatment and two experiments for Pam3Cys. **, significantly (P < 0.01) different from cytokine measured in cultures treated with PAMP alone.
Response of uterine epithelial cells in culture to treatment with the estrogen receptor antagonist ICI 182,780.
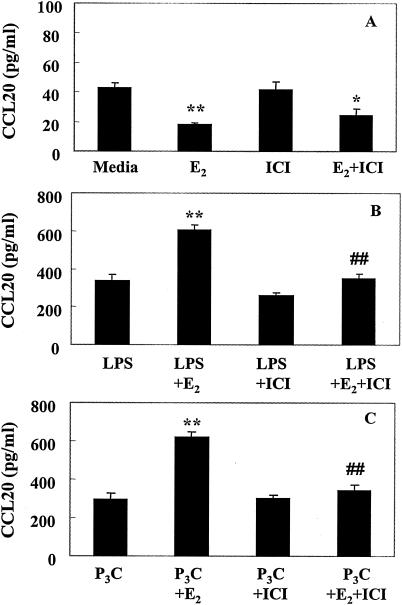
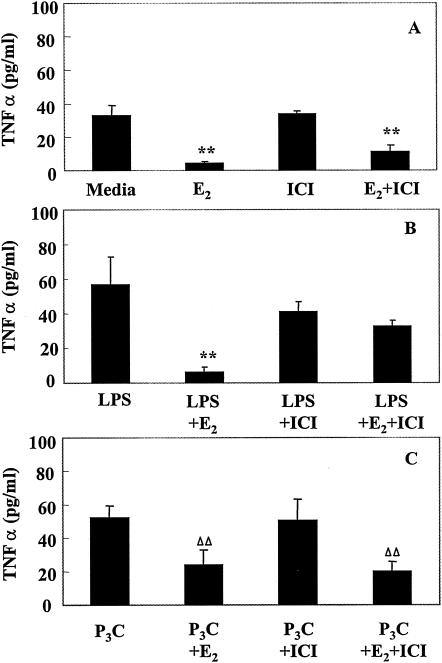
To determine whether the effects of E2 on uterine epithelial cell secretion of CCL20/MIP3α and TNF-α were mediated through the estrogen receptor, cell cultures were grown as described above for 24 h in F12K-SS medium prior to a 24-h period of treatment with medium containing E2 (10−8 M), ICI 182,780 (10−6 M), or both, after which cells were treated apically with LPS or Pam3Cys. Following a 12-h incubation, basolateral media were analyzed for the presence of CCL20/MIP3α or TNF-α. ICI 182,780, a known antagonist of E2 binding to ER, hardly reversed the inhibitory effect of E2 on CCL20/MIP3α constitutive release (Fig. 4A). In contrast, when ICI 182,780 was added along with E2 to PAMP-treated cells, the stimulatory effect of E2 alone on CCL20/MIP3α release was reversed (Fig. 4B and C). A similar pattern of ICI 182,780 activity was observed when TNF-α was analyzed (Fig. 5). ICI 182,780 had little effect on E2 inhibition of constitutive release of TNF-α (Fig. 5A) but almost completely reversed the inhibitory effect of E2 on TNF-α release in response to LPS (Fig. 5B). When cells were treated with Pam3Cys, ICI 182,780 also did not reverse the inhibitory effect of E2 on Pam3Cys-stimulated release of TNF-α (Fig. 5C). Overall, these studies indicate that ICI 182,780 reversal of estradiol effects may or may not occur, depending on the PAMP involved and the conditions under which CCL20/MIP3α and TNF-α are secreted (constitutively or stimulated).
FIG. 4.
Effect of ICI 182,780 and E2 treatment on CCL20/MIP3α. Rat uterine epithelial cell cultures as described in Materials and Methods were treated with E2 (10−8 M), ICI 182,780 (10−6 M), or both, prepared in F12K stripped medium. Twelve hours after hormone treatment, all cell cultures received fresh hormone-treated medium, and some were also treated with ultrapure LPS (1 μg/ml) (B) or Pam3Cys (1 μg/ml) (C). Basolateral media were harvested after a 12-h incubation, and the level of CCL20/MIP3α was determined by ELISA. Values shown are means of cytokine levels ± SE of four wells per group. Results are representative of four experiments for LPS treatment and two experiments for Pam3Cys. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) from cytokine measured in (A) medium, (B) medium plus LPS, or (C) medium plus Pam3Cys control cultures. ##, significantly (P < 0.01) different from (B) LPS plus E2 or (C) Pam3Cys plus E2 cytokine measured in E2-treated cultures.
FIG. 5.
Influence of ICI 182,780 and E2 treatment on TNF-α. Rat uterine epithelial cell cultures as described in Materials and Methods were treated with E2 (10−8 M), ICI 182,780 (10−6 M), or both, prepared in F12K stripped medium. Twelve hours after hormone treatment, all cell cultures received fresh hormone-treated medium, and some were also treated with ultrapure LPS (1 μg/ml) (B) or Pam3Cys (1 μg/ml) (C). Basolateral media were harvested after a 12-h incubation, and TNF-α level was determined by ELISA. Values shown are means of cytokine levels ± SE of four wells per group. Results are representative of four experiments for LPS treatment and two experiments for Pam3Cys. **, significantly (P < 0.01) different from cytokine measured in (A) medium, (B) medium plus LPS, or (C) medium plus Pam3Cys control cultures by ANOVA. ΔΔ, significantly (P < 0.05) different from Pam3Cys control by t test but not by ANOVA.
DISCUSSION
The research presented here demonstrates that the secretion of CCL20/MIP3α and TNF-α by rat uterine epithelial cells is influenced by estradiol. We show that E2 significantly inhibits the constitutive release of both CCL20/MIP3α and TNF-α. In contrast, when both E2 and PAMP are present, E2 increases CCL20/MIP3α production beyond that seen with PAMP stimulation alone. These studies indicate that E2 has an inhibitory effect on the basolateral release of TNF-α in the presence as well as the absence of PAMP. Moreover, this work demonstrates that ICI 182,780, which has little or no effect on reversing the inhibitory action of E2 treatment on the constitutive release of TNF-α or CCL20/MIP3α, reverses the stimulatory effect of E2 on CCL20/MIP3α release in response to LPS and Pam3Cys as well as partially reverses the inhibitory effect of E2 in the presence of LPS.
Production of CCL20/MIP3α at other mucosal surfaces, including the lungs and gastrointestinal tract, is enhanced by pathogenic challenge (36, 51). Previously, we demonstrated that polarized uterine epithelial cells in culture produce CCL20/MIP3α in response to live and heat-killed E. coli as well as selected PAMP (8, 9). To the best of our knowledge, our finding that estradiol exerts a stimulatory effect on CCL20/MIP3α is the first demonstration that this chemokine is under hormonal control. Moreover, these studies demonstrate a unique role of estradiol, in that constitutive release of CCL20/MIP3α is inhibited by estradiol, whereas the release of CCL20/MIP3α in response to PAMP is stimulated by estradiol. That release of CCL20/MIP3α in response to PAMP is ER mediated is suggested by our finding that when added along with estradiol, ICI 182,780, a receptor antagonist of estradiol, reverses the stimulatory effects of estradiol on CCL20/MIP3α secretion. In contrast, when estradiol and ICI 182,780 were added to cell cultures releasing CCL20/MIP3α under constitutive release conditions, the effect of estradiol was not reversed.
The complexities of regulation observed in these studies suggest that estradiol acts though different pathways to differentially control CCL20/MIP3α and TNF-α production. This may explain the apparent contradiction of our findings with those of others who have shown that TNF-α plays an important role in stimulating CCL20/MIP3α production (14, 19, 37, 52, 53). For example, estradiol is able to bind to different isoforms of the same receptor and may either increase or decrease mRNA expression, the net result being either enhanced or suppressed cytokine secretion. Alternatively, since cytokines such as TNF-α are known to exist as procytokines, estradiol may act on matrix metalloproteinase to either enhance or suppress processing and/or release from epithelial membranes (15, 21).
Our findings that the constitutive release of TNF-α and CCL20/MIP3α is inhibited by E2 suggest that in the absence of potential pathogens, E2, produced during the reproductive cycle, acts in the uterus to suppress these two proinflammatory molecules. Unexpected was our finding that under conditions of PAMP stimulation, E2 increased CCL20/MIP3α release while continuing to inhibit TNF-α secretion. Estradiol levels in blood vary with the stage of the reproductive cycle, with highest levels measured just prior to ovulation and mating (50). Our finding that CCL20/MIP3α is differentially regulated by estradiol suggests that CCL20/MIP3α production in the uterus may vary with the stage of the cycle. If transferable to in vivo situations, CCL20/MIP3α production may be inhibited in the uterus by estradiol if mating does not occur, while under conditions of mating and the presence of bacteria, estradiol may stimulate the release of CCL20/MIP3α, thus affording protection against infection. The observed influx of leukocytes that occurs in the uterus following mating (46, 54) may in part be attributable to putative increases in CCL20/MIP3α. Since an additional dimension of CCL20/MIP3α immune protection is its ability to act as a microbicide (22), elevated levels of CCL20/MIP3α stimulated by estradiol at mating might protect by limiting bacterial growth until such time that recruitment of immune cells can occur.
Estradiol influences on CCL20/MIP3α and TNF-α are complex and may be associated with genomic and/or nongenomic effects of E2. Estradiol is known to influence genes when the liganded ERα binds to an Sp1 promoter site (12, 48). In other studies, it has been demonstrated that the promoter region of the CCL20/MIP3α gene contains an Sp1 promoter sequence (35). This offers an explanation for a mechanism whereby estradiol could influence the transcription of CCL20/MIP3α. In contrast to the stimulatory role of estrogen on the transcription of CCL20/MIP3α, the TNF-α promoter contains an estrogen inhibitory element (2). Nongenomic effects of E2 in the endometrium have also been reported. For example, treatment of endometrial cells in culture with E2 results in Ca2+ influx within 10 min, and treatment of ovariectomized rats with E2 results in changes in the morphology of uterine epithelial cells within 1 min (13, 39, 40). Some of the nongenomic effects of E2 are reported not to be antagonized by the ER antagonist ICI 182,780 (13). For example, in a study of neurite growth, the effects of E2 could be inhibited by an inhibitor of cyclic AMP/PKA and Ca2+ signaling pathways but not by ICI 182,780. This and other studies have shown that ICI 182,780 antagonism of the effects of E2 is complex. Further studies are needed to define the mechanism(s) whereby E2 exerts its effects on TNF-α and CCL20/MIP3α production by uterine epithelial cells.
In conclusion, estradiol influences the production of CCL20/MIP3α and TNF-α by uterine epithelial cells both constitutively and in response to PAMP. Estradiol regulation of the release of CCL20/MIP3α and TNF-α suggests that the mucosal immune cell response to the presence of bacteria in the reproductive tract is precisely regulated and coordinated with the reproductive cycle to optimize the potential for successful mammalian reproduction.
Acknowledgments
We gratefully thank Richard Rossoll for technical assistance that led to the completion of these studies. We also express our appreciation to James Leiter for his help in statistical analysis and to Allan Munck and John Fahey for assistance in preparing the manuscript.
This work was supported by research grants AI-13541 and AI-51877 from NIH.
Editor: J. D. Clements
REFERENCES
- 1.Ambrosini, E., S. Columba-Cabezas, B. Serafini, A. Muscella, and F. Aloisi. 2003. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia 41:290-300. [DOI] [PubMed] [Google Scholar]
- 2.An, J., R. C. Ribeiro, P. Webb, J. A. Gustafsson, P. J. Kushner, J. D. Baxter, and D. C. Leitman. 1999. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc. Natl. Acad. Sci. USA 96:15161-15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annels, N. E., C. E. Da Costa, F. A. Prins, A. Willemze, P. C. Hogendoorn, and R. M. Egeler. 2003. Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis. J. Exp. Med. 197:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beagley, K. W., and C. M. Gockel. 2003. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38:13-22. [DOI] [PubMed] [Google Scholar]
- 5.Cherpes, T. L., L. A. Meyn, M. A. Krohn, and S. L. Hillier. 2003. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex. Transm. Dis. 30:405-410. [DOI] [PubMed] [Google Scholar]
- 6.Cook, D. N., D. M. Prosser, R. Forster, J. Zhang, N. A. Kuklin, S. J. Abbondanzo, X. D. Niu, S. C. Chen, D. J. Manfra, M. T. Wiekowski, L. M. Sullivan, S. R. Smith, H. B. Greenberg, S. K. Narula, M. Lipp, and S. A. Lira. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495-503. [DOI] [PubMed] [Google Scholar]
- 7.Cooke, P. S., D. L. Buchanan, P. Young, T. Setiawan, J. Brody, K. S. Korach, J. Taylor, D. B. Lubahn, and G. R. Cunha. 1997. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc. Natl. Acad. Sci. USA 94:6535-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane-Godreau, M. A., and C. R. Wira. 2005. CCL20/macrophage inflammatory protein 3α and tumor necrosis factor alpha production by primary uterine epithelial cells in response to treatment with lipopolysaccharide or Pam3Cys. Infect. Immun. 73:476-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane-Godreau, M. A., and C. R. Wira. 2004. Effect of Escherichia coli and Lactobacillus rhamnosus on macrophage inflammatory protein 3 alpha, tumor necrosis factor alpha, and transforming growth factor beta release by polarized rat uterine epithelial cells in culture. Infect. Immun. 72:1866-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunha, G. R., and P. Young. 1992. Role of stroma in oestrogen-induced epithelial proliferation. Epithelial Cell Biol. 1:18-31. [PubMed] [Google Scholar]
- 11.De, M., T. R. Sanford, and G. W. Wood. 1992. Interleukin-1, interleukin-6, and tumor necrosis factor alpha are produced in the mouse uterus during the estrous cycle and are induced by estrogen and progesterone. Dev. Biol. 151:297-305. [DOI] [PubMed] [Google Scholar]
- 12.deGraffenried, L. A., S. G. Hilsenbeck, and S. A. Fuqua. 2002. Sp1 is essential for estrogen receptor alpha gene transcription. J. Steroid Biochem. Mol. Biol. 82:7-18. [DOI] [PubMed] [Google Scholar]
- 13.Falkenstein, E., H. C. Tillmann, M. Christ, M. Feuring, and M. Wehling. 2000. Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol. Rev. 52:513-556. [PubMed] [Google Scholar]
- 14.Fujiie, S., K. Hieshima, D. Izawa, T. Nakayama, R. Fujisawa, H. Ohyanagi, and O. Yoshie. 2001. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3alpha/CCL20 in mucosal epithelial cells through NF-kappaB. Int. Immunol. 13:1255-1263. [DOI] [PubMed] [Google Scholar]
- 15.Gearing, A. J., P. Beckett, M. Christodoulou, M. Churchill, J. M. Clements, M. Crimmin, A. H. Davidson, A. H. Drummond, W. A. Galloway, R. Gilbert, et al. 1995. Matrix metalloproteinases and processing of pro-TNF-alpha. J. Leukoc. Biol. 57:774-777. [DOI] [PubMed] [Google Scholar]
- 16.Glasser, S. R., J. Julian, G. L. Decker, J. P. Tang, and D. D. Carson. 1988. Development of morphological and functional polarity in primary cultures of immature rat uterine epithelial cells. J. Cell Biol. 107:2409-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant, K. S., and C. R. Wira. 2004. Effect of estradiol on mouse uterine epithelial cell transepithelial resistance (TER). Am. J. Reprod. Immunol. 52:252-262. [DOI] [PubMed]
- 18.Gustafsson, J. A. 2000. An update on estrogen receptors. Semin. Perinatol. 24:66-69. [DOI] [PubMed] [Google Scholar]
- 19.Harant, H., S. A. Eldershaw, and I. J. Lindley. 2001. Human macrophage inflammatory protein-3alpha/CCL20/LARC/Exodus/SCYA20 is transcriptionally upregulated by tumor necrosis factor-alpha via a non-standard NF-kappaB site. FEBS Lett. 509:439-445. [DOI] [PubMed] [Google Scholar]
- 20.Hauth, J. C., C. Macpherson, J. C. Carey, M. A. Klebanoff, S. L. Hillier, J. M. Ernest, K. J. Leveno, R. Wapner, M. Varner, W. Trout, A. Moawad, and B. Sibai. 2003. Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am. J. Obstet. Gynecol. 188:831-835. [DOI] [PubMed] [Google Scholar]
- 21.Hickey, M., J. Higham, M. Sullivan, L. Miles, and I. S. Fraser. 2001. Endometrial bleeding in hormone replacement therapy users: preliminary findings regarding the role of matrix metalloproteinase 9 (MMP-9) and tissue inhibitors of MMPs. Fertil. Steril. 75:288-296. [DOI] [PubMed] [Google Scholar]
- 22.Hoover, D. M., C. Boulegue, D. Yang, J. J. Oppenheim, K. Tucker, W. Lu, and J. Lubkowski. 2002. The structure of human macrophage inflammatory protein-3alpha /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J. Biol. Chem. 277:37647-37654. [DOI] [PubMed] [Google Scholar]
- 23.Hunt, J. S., H. L. Chen, X. L. Hu, and S. Tabibzadeh. 1992. Tumor necrosis factor-alpha messenger ribonucleic acid and protein in human endometrium. Biol. Reprod. 47:141-147. [DOI] [PubMed] [Google Scholar]
- 24.Hunt, J. S., L. Miller, K. F. Roby, J. Huang, J. S. Platt, and B. L. DeBrot. 1997. Female steroid hormones regulate production of pro-inflammatory molecules in uterine leukocytes. J. Reprod. Immunol. 35:87-99. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Iwasaki, A., and B. L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs, A. L., P. B. Sehgal, J. Julian, and D. D. Carson. 1992. Secretion and hormonal regulation of interleukin-6 production by mouse uterine stromal and polarized epithelial cells cultured in vitro. Endocrinology 131:1037-1046. [DOI] [PubMed] [Google Scholar]
- 28.Kaushic, C., K. Grant, M. Crane, and C. R. Wira. 2000. Infection of polarized primary epithelial cells from rat uterus with Chlamydia trachomatis: cell-cell interaction and cytokine secretion. Am. J. Reprod. Immunol. 44:73-79. [DOI] [PubMed] [Google Scholar]
- 29.Kover, K., L. Liang, G. K. Andrews, and S. K. Dey. 1995. Differential expression and regulation of cytokine genes in the mouse uterus. Endocrinology 136:1666-1673. [DOI] [PubMed] [Google Scholar]
- 30.Kuiper, G. G., B. Carlsson, K. Grandien, E. Enmark, J. Haggblad, S. Nilsson, and J. A. Gustafsson. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863-870. [DOI] [PubMed] [Google Scholar]
- 31.Kunz, G., D. Beil, H. Deiniger, A. Einspanier, G. Mall, and G. Leyendecker. 1997. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Adv. Exp. Med. Biol. 424:267-277. [PubMed] [Google Scholar]
- 32.Kunz, G., D. Beil, P. Huppert, and G. Leyendecker. 2000. Structural abnormalities of the uterine wall in women with endometriosis and infertility visualized by vaginal sonography and magnetic resonance imaging. Hum. Reprod. 15:76-82. [DOI] [PubMed] [Google Scholar]
- 33.Kunz, G., M. Herbertz, M. Noe, and G. Leyendecker. 1998. Sonographic evidence for the involvement of the utero-ovarian counter-current system in the ovarian control of directed uterine sperm transport. Hum. Reprod. Update 4:667-672. [DOI] [PubMed] [Google Scholar]
- 34.Kunz, G., and G. Leyendecker. 2002. Uterine peristaltic activity during the menstrual cycle: characterization, regulation, function and dysfunction. Reprod. Biomed. Online 4(Suppl. 3):5-9. [DOI] [PubMed] [Google Scholar]
- 35.Kwon, J. H., S. Keates, S. Simeonidis, F. Grall, T. A. Libermann, and A. C. Keates. 2003. ESE-1, an enterocyte-specific Ets transcription factor, regulates MIP-3alpha gene expression in Caco-2 human colonic epithelial cells. J. Biol. Chem. 278:875-884. [DOI] [PubMed] [Google Scholar]
- 36.Lin, T.-J., L. H. Maher, K. Gomi, J. D. McCurdy, R. Garduno, and J. S. Marshall. 2003. Selective early production of CCL20, or macrophage inflammatory protein 3α, by human mast cells in response to Pseudomonas aeruginosa. Infect. Immun. 71:365-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama, T., R. Fujisawa, H. Yamada, T. Horikawa, H. Kawasaki, K. Hieshima, D. Izawa, S. Fujiie, T. Tezuka, and O. Yoshie. 2001. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int. Immunol. 13:95-103. [DOI] [PubMed] [Google Scholar]
- 38.Pedram, A., M. Razandi, M. Aitkenhead, C. C. Hughes, and E. R. Levin. 2002. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J. Biol. Chem. 277:50768-50775. [DOI] [PubMed] [Google Scholar]
- 39.Pietras, R. J., and C. M. Szego. 1975. Endometrial cell calcium and oestrogen action. Nature 253:357-359. [DOI] [PubMed] [Google Scholar]
- 40.Pietras, R. J., and C. M. Szego. 1975. Steroid hormone-responsive, isolated endometrial cells. Endocrinology 96:946-954. [DOI] [PubMed] [Google Scholar]
- 41.Pioli, P. A., E. Amiel, T. M. Schaefer, J. E. Connolly, C. R. Wira, and P. M. Guyre. 2004. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect. Immun. 72:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prabhala, R. H., and C. R. Wira. 1991. Cytokine regulation of the mucosal immune system: in vivo stimulation by interferon-gamma of secretory component and immunoglobulin A in uterine secretions and proliferation of lymphocytes from spleen. Endocrinology 129:2915-2923. [DOI] [PubMed] [Google Scholar]
- 43.Razandi, M., A. Pedram, S. T. Park, and E. R. Levin. 2003. Proximal events in signaling by plasma membrane estrogen receptors. J. Biol. Chem. 278:2701-2712. [DOI] [PubMed] [Google Scholar]
- 44.Robertson, S. A., G. Mayrhofer, and R. F. Seamark. 1996. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol. Reprod. 54:183-196. [DOI] [PubMed] [Google Scholar]
- 45.Robertson, S. A., G. Mayrhofer, and R. F. Seamark. 1992. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol. Reprod. 46:1069-1079. [DOI] [PubMed] [Google Scholar]
- 46.Robertson, S. A., A. C. O'Connell, S. N. Hudson, and R. F. Seamark. 2000. Granulocyte-macrophage colony-stimulating factor (GM-CSF) targets myeloid leukocytes in the uterus during the post-mating inflammatory response in mice. J. Reprod. Immunol. 46:131-154. [DOI] [PubMed] [Google Scholar]
- 47.Roby, K. F., and J. S. Hunt. 1994. Mouse endometrial tumor necrosis factor-alpha messenger ribonucleic acid and protein: localization and regulation by estradiol and progesterone. Endocrinology 135:2780-2789. [DOI] [PubMed] [Google Scholar]
- 48.Saville, B., M. Wormke, F. Wang, T. Nguyen, E. Enmark, G. Kuiper, J. A. Gustafsson, and S. Safe. 2000. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J. Biol. Chem. 275:5379-5387. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer, T. M., K. DeSouza, J. V. Fahey, K. W. Beagley, and C. R. Wira. 2004. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 112:428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaikh, A. A. 1971. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol. Reprod. 5:297-307. [DOI] [PubMed] [Google Scholar]
- 51.Sierro, F., B. Dubois, A. Coste, D. Kaiserlian, J. P. Kraehenbuhl, and J. C. Sirard. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 98:13722-13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugita, S., T. Kohno, K. Yamamoto, Y. Imaizumi, H. Nakajima, T. Ishimaru, and T. Matsuyama. 2002. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappaB activation. J. Immunol. 168:5621-5628. [DOI] [PubMed] [Google Scholar]
- 53.Tohyama, M., Y. Shirakara, K. Yamasaki, K. Sayama, and K. Hashimoto. 2001. Differentiated keratinocytes are responsible for TNF-alpha regulated production of macrophage inflammatory protein 3alpha/CCL20, a potent chemokine for Langerhans cells. J. Dermatol. Sci. 27:130-139. [DOI] [PubMed] [Google Scholar]
- 54.Tremellen, K. P., R. F. Seamark, and S. A. Robertson. 1998. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol. Reprod. 58:1217-1225. [DOI] [PubMed] [Google Scholar]
- 55.Wiesenfeld, H. C., S. L. Hillier, M. A. Krohn, A. J. Amortegui, R. P. Heine, D. V. Landers, and R. L. Sweet. 2002. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet. Gynecol. 100:456-463. [DOI] [PubMed] [Google Scholar]
- 56.Williams, R. H., and J. D. Wilson. 1998. Williams textbook of endocrinology, 9th ed. W. B. Saunders, Philadelphia, Pa.
- 57.Wira, C. 2003. Female reproductive physiology, p. 78-89. In V. A. Galton (ed.), Endocrinology. Department of Physiology, Dartmouth Medical School, Lebanon, N.H.
- 58.Wira, C., M. A. Crane-Godreau, and K. Grant. 2004. Endocrine regulation of the mucosal immune system in the female reproductive tract, p. 1661-1676. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, J. R. McGhee, and H. W. Strobel (ed.), Mucosal immunology, 3rd ed. Elsevier Inc., San Diego, Calif.
- 59.Wira, C. R., and R. M. Rossoll. 1995. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology 136:4526-4534. [DOI] [PubMed] [Google Scholar]