Abstract
Streptococcus agalactiae is a frequent cause of bacterial sepsis and meningitis in neonates. During the course of infection, S. agalactiae colonizes and invades a number of host compartments, thereby interacting with different host tissues. Deletion of the fbsA gene, encoding the fibrinogen protein FbsA, significantly impaired the adherence and invasion of human brain microvascular endothelial cells (HBMEC) by S. agalactiae. The adherence and invasiveness of an fbsA deletion mutant were restored by reintroducing the fbsA gene on an expression vector. Heterologous expression of fbsA in Lactococcus lactis enabled this bacterium to adhere to but not to invade HBMEC, suggesting that FbsA is a streptococcal adhesin. Finally, host cell adherence and invasion were significantly blocked in competition experiments with either purified FbsA fusion protein or a monoclonal antibody directed against the fibrinogen-binding epitope of FbsA. The S. agalactiae fbsA mutant induced a release of the neutrophil chemoattractant interleukin-8 (IL-8) equal to that induced by the wild type. Taken together, our studies demonstrate that FbsA promotes the adherence of S. agalactiae to HBMEC but that FbsA neither mediates the bacterial invasion into host cells nor plays a role in IL-8 release for HBMEC.
Streptococcus agalactiae, also called group B streptococcus (GBS), is the leading cause of bacterial sepsis and meningitis in neonates in many industrialized countries (2). In addition, it causes substantial pregnancy-related morbidity and has emerged as an increasingly common cause of invasive disease in the elderly and in immunocompromised persons (35). In the pathogenesis of meningitis, blood-borne pathogens must interact with cerebral endothelial cells which constitute the blood-brain barrier (BBB); subsequent bacterial replication within the central nervous system (CNS) provokes the host inflammatory response, resulting in meningitis.
While in vitro and in vivo models have been informative about the pathogenesis of bacterial meningitis and CNS factors that contribute to inflammation and brain injury, little is known about the contribution of specific virulence factors of the invading pathogens at the BBB endothelium. Like many other pathogens, GBS can attach to host cell surfaces by binding to different host cell proteins. Binding of GBS to human laminin is mediated by the lipoprotein Lmb (laminin-binding protein), which has been studied at the molecular level (28). Although GBS does not bind soluble fibronectin on its surface (4), adherence of the bacterium to immobilized fibronectin has been demonstrated (32). In a recent study, Beckmann et al. (3) identified C5a peptidase (from GBS) as a mediator of bacterial binding to fibronectin. In addition, fibronectin binding of GBS has been shown to mediate bacterial invasion into host epithelial cells (5). Fibrinogen binding in GBS is mediated by the proteins FbsA and FbsB (14, 26). On the amino acid level, the fibrinogen-binding proteins FbsA and FbsB are unrelated, but both have surface-exposed localization in the cell wall of the bacterium. The FbsB protein was shown to bind to human fibrinogen by its N-terminal 388 amino acids (14), whereas the FbsA protein interacts with fibrinogen by repetitive units, each 16 amino acids in length. Even a single repeat of FbsA was demonstrated to bind to human fibrinogen. Epidemiological studies revealed significant variation in the number of repeats in the FbsA protein between various S. agalactiae strains (35).
Human brain microvascular endothelial cells (HBMEC) have been used previously to study the pathogenesis of CNS infections by meningitis pathogens such as Escherichia coli, Citrobacter spp., Streptococcus pneumoniae, Staphylococcus aureus, and GBS (1, 19, 21, 25, 31, 36). The HBMEC used in this study were isolated from a brain biopsy specimen from an adult female with epilepsy by methods previously described (29, 30). HBMEC were thawed and cultured in medium supplemented with 10% heat-inactivated fetal calf serum and 10% NuSerum (Becton Dickinson, Bedford, Mass.). Thereafter, cells were cultured in RPMI medium supplemented with 5% fetal calf serum in culture flasks (Costar, Cambridge, Mass.) and split weekly in 1:10 ratios by using trypsin-EDTA (Gibco, Grand Island, N.Y).
Adherence of GBS to HBMEC and their invasion into HBMEC were assayed essentially as described previously, with minor modifications (19, 22). In brief, for adherence and invasion assays, HBMEC were transferred to 12-well tissue culture plates at a seeding density of 5 × 105 cells per well and cultivated in RPMI tissue culture medium until confluence was reached. The medium was subsequently replaced with 2 ml of fresh RPMI medium each day. For all infection assays, we used S. agalactiae wild-type strain 6313, a serotype III strain, which is a clinical isolate from an infected neonate (34). The fbsA gene was deleted from the chromosome of S. agalactiae strain 6313 as previously described by Schubert et al. (26). S. agalactiae strains were cultivated at 37°C in Todd-Hewitt yeast broth containing 1% yeast extract. The recombinant S. agalactiae clone carrying plasmid pOri23 was selected with erythromycin (5 μg/ml). The construction of plasmid pOrifbsA has been described previously (27). Lactococcus lactis strain MG1363 was used for heterologous fbsA gene expression as described by Schubert et al. (26) and was grown at 30°C in M17 medium (Oxoid, Basingstoke, United Kingdom) supplemented with 0.5% glucose. The L. lactis strain carrying the pOri23 derivative was selected with erythromycin (5 μg/ml). For adherence assays, the HBMEC were infected with log-phase GBS at multiplicities of infection (MOIs) of 1:1, 10:1, and 100:1 (∼5 × 105, 5 × 106, and 5 × 107 bacteria; 5 × 105 cells per well) in RPMI tissue culture medium for 2 h at 37°C in 5% CO2. The HBMEC were washed three times with phosphate-buffered saline (PBS) and subsequently detached from the well by sonication. The number of cell-adherent bacteria was determined by plating appropriate dilutions of the lysate onto blood agar plates. Due to the lysis of the eukaryotic cells in this process, the calculation of cell-adherent bacteria also included bacteria that had invaded the host cells. Therefore, the number of invaded bacteria was subtracted from the apparent number of cell-adherent bacteria to calculate the actual number of adherent bacteria. For invasion assays, the HBMEC were also infected with log-phase GBS at MOIs of 1:1, 10:1, and 100:1. Streptococci were incubated for 2 h at 37°C in 5% CO2 and washed three times with PBS. Subsequently, the infected cells were incubated for 2 h in tissue culture medium supplemented with penicillin G (5 μg/ml) and gentamicin (100 μg/ml) to kill extracellular bacteria. After three washes with PBS, the HBMEC were detached by sonication. The number of invasive bacteria was quantified by plating serial dilutions of the lysate onto blood agar plates. Each experiment was performed at least three separate times, each time in triplicate. The fbsA mutant grew as well as the wild type in tissue culture medium and showed no increased susceptibility to the antibiotics gentamicin and penicillin. The fbsA mutant also revealed no difference from the wild type in its sensitivity to sonication or in its buoyancy or chain length.
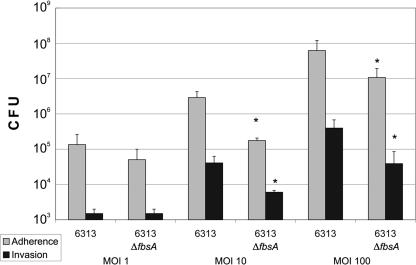
As depicted in Fig. 1, the tested strains revealed a concentration-dependent adherence to and invasion of HBMEC. The adherence to as well as the invasion of HBMEC by strain 6313 ΔfbsA was approximately 90% decreased (at MOIs 10 and 100), indicating that the fibrinogen-binding protein FbsA is involved in the adherence to and invasion of endothelial cells by S. agalactiae. The invasion index (determined by dividing the number of internalized bacteria by the total number of adherent bacteria and internalized bacteria) of GBS strain 6313 was not significantly different from that of its ΔfbsA mutant (at an MOI of 10, strains 6313 and 6313 ΔfbsA had indexes of 0.052 and 0.062, respectively; at an MOI of 100, strains 6313 and 6313 ΔfbsA had indexes of 0.006 and 0.004, respectively).
FIG. 1.
Adherence to and invasion of HBMEC by S. agalactiae strains 6313 and 6313 ΔfbsA (at MOIs of 1, 10, and 100). Data shown are means + standard errors of the means of three independent experiments, each performed in triplicate. An asterisk indicates that the adherence and invasion values of the ΔfbsA mutant were significantly lower than the values of the parental strain, at a P value of <0.05.
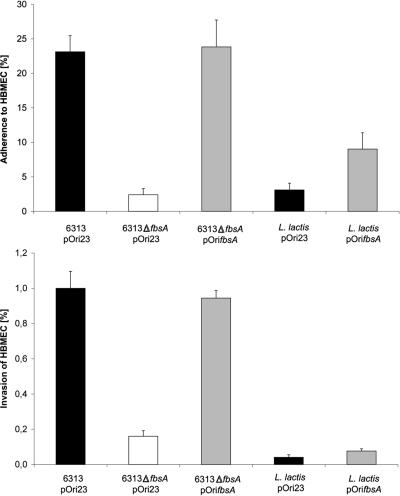
S. agalactiae strains 6313(pOri23) and 6313 ΔfbsA(pOri23) showed adherence and invasion rates comparable to those of their plasmid-free parental strains, demonstrating that the vector pOri23 does not influence the adherence and invasion properties of these strains. In contrast, plasmid-mediated expression of fbsA in strain 6313 ΔfbsA(pOrifbsA) significantly increased its adherence to and invasion of HBMEC compared to that of strain 6313 ΔfbsA(pOri23). Our findings therefore demonstrate that the reduced adherence to and invasion of HBMEC by the 6313 ΔfbsA mutant is due to its fbsA deficiency and not to unrelated mutations in its chromosome. The adhesive and invasive efficiencies of 6313 ΔfbsA(pOrifbsA) were almost equal to those of 6313(pOri23) (Fig. 2).
FIG. 2.
Adherence to and invasion of HBMEC by S. agalactiae strains 6313(pOri23), 6313 ΔfbsA(pOri23), and 6313 ΔfbsA(pOrifbsA), L. lactis(pOri23), and L. lactis(pOrifbsA). HBMEC were infected with equal amounts of each strain (MOI, 10), and the numbers of cell-adherent and internalized bacteria were related to the number of input bacteria. Each experiment was performed at least four separate times, each time in triplicate. Error bars indicate standard errors of the means.
To investigate whether S. agalactiae factors other than FbsA are required for the bacterial adherence to and invasion of host cells, plasmids pOri23 and pOrifbsA were introduced in L. lactis, a nonpathogenic gram-positive bacterium. L. lactis(pOri23) exhibited little adherence to and no invasion of HBMEC, whereas L. lactis(pOrifbsA) showed significant adherence to HBMEC cells but no invasion into this cell line (Fig. 2). Our results therefore suggest that FbsA promotes bacterial adherence to but not invasion of host cells.
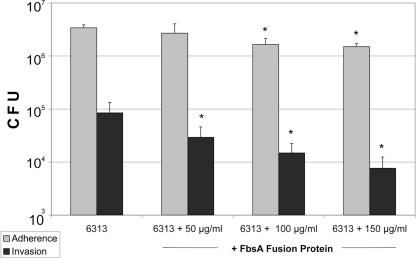
To further analyze whether the fibrinogen-binding protein FbsA is directly involved in the adherence and invasion of HBMEC by S. agalactiae, competitive inhibition experiments with purified FbsA fusion protein were performed. Endothelial cells were incubated for 15 min in 10 μl of PBS with different amounts of purified proteins as described previously (26). Bacterial cells were then added in tissue culture medium, and the cells were then incubated at 37°C for 2 h in 5% CO2. The remainder of the experiments were carried out as described above. As shown in Fig. 3, the FbsA fusion protein significantly interfered in a concentration-dependent manner with the adherence to and invasion of HBMEC by S. agalactiae strain 6313.
FIG. 3.
Adherence to and invasion of HBMEC by S. agalactiae strain 6313 (MOI, 10) in the presence of different concentrations of FbsA fusion protein. Data shown are means + standard errors of the means of three independent experiments, each performed in triplicate. An asterisk indicates that the adherence and invasion values of the ΔfbsA mutant were significantly lower than the values of the parental strain, at a P value of <0.05.
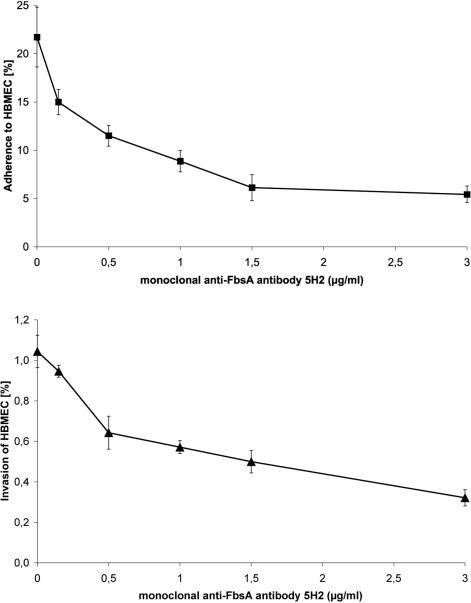
To better understand the interaction of FbsA with the host cell surface at the molecular level, we used monoclonal antibodies (MAbs) directed against different epitopes of the FbsA protein (20). MAb 5H2 binds to the repeat region of FbsA, thereby blocking the fibrinogen binding of the FbsA protein. In contrast, MAb 2B1 binds to the repeat region of FbsA without interfering with the binding of FbsA to human fibrinogen. After preincubation of S. agalactiae 6313 with either of the two MAbs, the streptococcal host cell adherence and invasion were quantified in tissue culture experiments. As depicted in Fig. 4, increasing concentrations of MAb 5H2 caused a dose-dependent inhibition of bacterial adherence and invasiveness. Preincubation of strain 6313 with 3.0 μg of MAb 5H2 per ml strongly blocked the streptococcal adherence to and invasion of HBMEC. In contrast, preincubation of strain 6313 with up to 10 μg of MAb 2B1 per ml did not influence its host cell adherence or invasion (data not shown). These findings indicate that direct interaction of FbsA with host cell structures is crucial for the efficient adhesion and entry of S. agalactiae into host cells.
FIG. 4.
Competitive inhibition of streptococcal adherence (top) and invasion (bottom) by MAb 5H2, which specifically blocks the binding of FbsA to human fibrinogen. Tissue culture experiments were performed after pretreatment of S. agalactiae 6313 (MOI, 10) with different concentrations of MAb 5H2. Each experiment was performed at least four separate times, each time in triplicate. Error bars indicate standard errors of the means.
Because neutrophilic infiltration is a hallmark of GBS meningitis, we hypothesized that exposure to GBS would trigger brain endothelial cells to release the neutrophil chemoattractant interleukin-8 (IL-8), which might differ between GBS strain 6313 and its ΔfbsA mutant. For the IL-8 enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN), log-phase GBS (MOI, 10) were added to HBMEC monolayers in six-well plates, which were then incubated for 2 and 4 h at 37°C in 5% CO2. Following the 2- or 4-h incubation period, the tissue culture medium was supplemented with penicillin G (5 μg/ml) and gentamicin (100 μg/ml) to kill extracellular bacteria. Monolayers were then incubated for a total period of 6 or 24 h. At the end of the exposure period, cell culture supernatants were collected, and bacteria were removed by centrifugation and stored at 4°C until IL-8 measurement. IL-8 from cell culture supernatants was measured by enzyme-linked immunosorbent assay, as described previously by Eckmann et al. (9). IL-8 assays were performed three separate times, each time in triplicate.
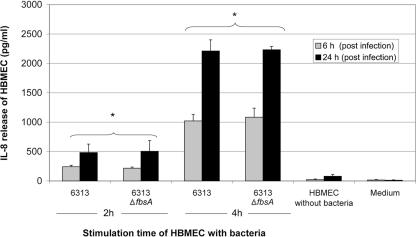
Time-dependent IL-8 release by HBMEC could be detected by stimulation with both 6313 strains. However, no significant difference in IL-8 production could be detected between the 6313 strain and its ΔfbsA mutant. Maximal IL-8 release was noted after an incubation period of 24 h (Fig. 5).
FIG. 5.
Changes in HBMEC IL-8 secretion after infection with wild-type GBS strain 6313 and its fbsA mutant (MOI, 10). Cell culture supernatants were assayed after 6 and 24 h, after initial incubation with GBS for 2 and 4 h. Data shown are means + standard errors of the means of three independent experiments, each performed in triplicate. *, significantly different from value for uninfected HBMEC (P < 0.05) at each respective time.
In none of the experiments (adherence/invasion/cytokine assay) was any relevant cytotoxicity detected (data not shown). Cytotoxicity to HBMEC by GBS strains was determined by measuring the release of the intracellular enzyme lactate dehydrogenase (Roche, Mannheim, Germany) from HBMEC monolayers in a 96-well microtiter plate assay, as described previously (18).
The pathogenesis of S. agalactiae meningitis involves the binding of the bacteria to pulmonary epithelium, the penetration of the lung mucosa, hematogenous spread, and entry into the cerebrospinal fluid by crossing the BBB. The exact route of entry into the cerebrospinal fluid and the molecular mechanisms of bacterial attachment to and invasion of the BBB are poorly understood. The BBB is located in the vessel walls of cerebral capillaries that are lined with highly specialized endothelial cells. The interaction of S. agalactiae with the BBB is a central event in the pathogenesis of streptococcal meningitis. Several studies of experimental animal models of hematogenous meningitis have shown the distribution of S. agalactiae bacteria in the perivasculature of the subarachnoid space, suggesting that the site of bacterial entry into the CNS is the cerebral vasculature (11). In the present study, we used endothelial cells from human brain capillaries, which have already been used for the study of different meningitis pathogens, including S. agalactiae (1, 12, 19, 22, 29-31).
Attachment of GBS to host cell receptors represents an important initial step in colonization and subsequent infection. However, the adhesins contributing to the binding of host cell and/or host extracellular-matrix molecules are poorly understood. Numerous studies have described the ability of clinical S. agalactiae isolates to interact with human fibrinogen (24). In various in vitro models, S. agalactiae was shown to adhere to different epithelial and endothelial cells (5, 8, 19, 22), but the molecular basis of this interaction is currently only poorly understood. Schubert et al. (26) identified in S. agalactiae a fibrinogen-binding protein, which was termed FbsA. In S. agalactiae, deletion of the fbsA gene results in the complete loss of fibrinogen-binding activity (26). The transcriptional regulator RogB and the oligopeptide permease Opp from S. agalactiae were recently shown to control both the expression of the fbsA gene and the bacterial adherence to epithelial cells (13, 23). These findings indicate a link between the fibrinogen-binding protein FbsA and the adherence of S. agalactiae to epithelial cells and endothelia. Recently, Schubert et al. demonstrated that FbsA promotes the adherence of S. agalactiae to A549 lung epithelial cells but that FbsA does not mediate the bacterial invasion into host cells (27). The FbsA protein was therefore suggested to be the major fibrinogen-binding protein in S. agalactiae. Previous findings by Pietrocola et al. also indicate that FbsA is a crucial factor in S. agalactiae-induced platelet aggregation and may therefore play an important role in S. agalactiae-induced endocarditis (20).
In the present study, different experimental approaches demonstrate that the fibrinogen-binding protein FbsA is able to promote the adherence to and invasion of HBMEC by S. agalactiae. Adherence of S. agalactiae to endothelial cells is an important step in its spread into different host compartments. Competition experiments and the analysis of fbsA deletion mutants indicate that FbsA might also play a role in the invasion of endothelial cells by S. agalactiae. However, adherence is often a prerequisite for the successful invasion of host cells (10). In line with this, the adherence of the fbsA deletion mutants was reduced by the same order of magnitude as was the level of their host cell invasion. Similar results were obtained in the competition experiments with purified FbsA protein or MAb 5H2. Finally, plasmid-mediated fbsA expression allowed L. lactis to adhere to but not to enter endothelial cells. Thus, our findings suggest that FbsA does not promote the invasion of S. agalactiae into HBMEC. Therefore, FbsA is suggested to be the first adhesin identified in these bacteria (27). Interestingly, the fibrinogen-binding protein FbsB was recently shown to mediate the invasion of S. agalactiae into epithelial cells (14). Thus, fibrinogen-binding proteins obviously play a prominent role in both host cell adherence and invasion by S. agalactiae. S. pyogenes and S. aureus have also been shown to harbor several fibrinogen-binding proteins (6, 16, 17). In S. pyogenes, M proteins are postulated to be the major fibrinogen-binding proteins (15, 33), and in S. aureus, fibrinogen binding is mediated, dependent on the growth phase, by either ClfA or ClfB (17).
In addition, we have demonstrated a GBS-dependent IL-8 activation in HBMEC. This signaling appears to be mediated largely by the bacterial β-hemolysin/cytolysin (β-h/c) of GBS. Doran et al. (7) recently showed that GBS β-h/c promoted the release of IL-8 from human lung epithelial cells. Furthermore, infection with a GBS strain lacking β-h/c resulted in a marked reduction in expression of genes involved in the immune response (including the IL-8 gene) of HBMEC (8). Since GBS strain 6313 is strongly hemolytic, our data also suggest that hemolysin contributes to the development and severity of the GBS-induced inflammatory response in HBMEC, but the FbsA protein seems to play no role in this respect. Thus, we speculate that the net-effect proinflammatory properties of hemolysin work to the detriment of the host.
Based on our observations, we believe that FbsA of S. agalactiae may promote adherence leading to entry of the bacterium into the CNS across the BBB, a prerequisite for the development of meningitis. Our data support a crucial role for the fibrinogen-binding protein FbsA in the ability of S. agalactiae to colonize and infect humans.
Acknowledgments
We thank K. S. Kim for kindly providing the HBMEC cell line, P. Speziale for kindly providing the anti-FbsA monoclonal antibodies, and U. Friedrichs and our chief technicians M. L. Mölleken and A. Seibt for expert assistance.
This work was supported by DFG SPP 1130.
Editor: V. J. DiRita
REFERENCES
- 1.Badger, J. L., M. F. Stins, and K. S. Kim. 1999. Citrobacter freundii invades and replicates in human brain microvascular endothelial cells. Infect. Immun. 67:4208-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, C. J., and M. S. Edwards. 1995. Group B streptococcal infections, p. 980-1054. In J. S. Remington and J. O. Klein (ed.), Infectious disease of the fetus and newborn infant. The W. B. Saunders Co., Philadelphia, Pa.
- 3.Beckmann, C., J. D. Waggoner, T. O. Harris, G. S. Tamura, and C. E. Rubens. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 70:2869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, K. M., C. J. Baker, and M. S. Edwards. 1987. Interaction of soluble fibronectin with group B streptococci. Infect. Immun. 55:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Q., D. Stafslien, S. S. Purushothaman, and P. Cleary. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 70:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney, H. S., Y. Li, J. B. Dale, and D. L. Hasty. 1994. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect. Immun. 62:3937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doran, K. S., J. C. Chang, V. M. Benoit, L. Eckmann, and V. Nizet. 2002. Group B streptococcal β-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185:196-203. [DOI] [PubMed] [Google Scholar]
- 8.Doran, K. S., G. Y. Liu, and V. Nizet. 2003. Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Investig. 112:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckmann, L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 105:1689-1697. [DOI] [PubMed] [Google Scholar]
- 10.Elsinghorst, E. A. 1994. Measurement of invasion by gentamycin resistance, p. 405-419. In J. N. Abelson and M. I. Simon (ed.), Bacterial pathogenesis, part B. Academic Press, New York, N.Y.
- 11.Ferrieri, P., B. Burke, and J. Nelson. 1980. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect. Immun. 27:1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greiffenberg, L., W. Goebel, K. S Kim, I. Weiglein, A. Bubert, F. Engelbrecht, M. Stins, and M. Kuhn. 1998. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 66:5260-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2003. Analysis of RogB-controlled virulence mechanisms and gene expression in Streptococcus agalactiae. Infect. Immun. 71:5056-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2004. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 72:3495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotarsky, H., A. Thern, G. Lindahl, and U. Sjöbring. 2000. Strain-specific restriction of the antiphagocytic property of group A streptococcal M proteins. Infect. Immun. 68:107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 17.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 18.Nizet, V., R. L. Gibson, E. Y. Chi, P. E. Framson, M. Hulse, and C. E. Rubens. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64:3818-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrocola, G., A. Schubert, L. Visai, M. Torti, J. R. Fitzgerald, T. J. Foster, D. J. Reinscheid, and P. Speziale. 2005. FbsA, a fibrinogen-binding protein from Streptococcus agalactiae, mediates platelet aggregation. Blood 105:1052-1059. [DOI] [PubMed] [Google Scholar]
- 21.Prasadarao, N. V., C. A. Wass, J. N. Weiser, M. F. Stins, S.-H. Huang, and K. S. Kim. 1996. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect. Immun. 64:146-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubens, C. E., S. Smith, M. Hulse, E. Y. Chi, and G. van Belle. 1992. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 60:5157-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samen, U., B. Gottschalk, B. J. Eikmanns, and D. J. Reinscheid. 2004. Relevance of peptide uptake systems to the physiology and virulence of Streptococcus agalactiae. J. Bacteriol. 186:1398-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönbeck, C., L. Björck, and G. Kronvall. 1981. Receptors for fibrinogen and aggregated β2-microglobulin detected in strains of group B streptococci. Infect. Immun. 31:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroten, H., B. Spors, C. Hucke, M. Stins, K. S. Kim, R. Adam, and W. Daubener. 2001. Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3-dioxygenase. Neuropediatrics 32:206-210. [DOI] [PubMed] [Google Scholar]
- 26.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. J. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46:557-569. [DOI] [PubMed] [Google Scholar]
- 27.Schubert, A., K. Zakikhany, G. Pietrocola, A. Meinke, P. Speziale, B. J. Eikmanns, and D. J. Reinscheid. 2004. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect. Immun. 72:6197-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lütticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stins, M., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 30.Stins, M. F., N. V. Prasadarao, J. Zhou, M. Arditi, and K. S. Kim. 1997. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell. Dev. Biol. Anim. 33:243-247. [DOI] [PubMed] [Google Scholar]
- 31.Stins, M. F., J. Badger, and K. Sik Kim. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30:19-28. [DOI] [PubMed] [Google Scholar]
- 32.Tamura, G. S., and C. E. Rubens. 1995. Group B streptococci adhere to a variant of fibronectin attached to a solid phase. Mol. Microbiol. 15:581-589. [DOI] [PubMed] [Google Scholar]
- 33.Thern, A., M. Wastfelt, and G. Lindahl. 1998. Expression of two different antiphagocytic M proteins by Streptococcus pyogenes of the OF+ lineage. J. Immunol. 160:860-869. [PubMed] [Google Scholar]
- 34.Valenti-Weigand, P., P. Benkel, M. Rohde, and G. S. Chhatwal. 1996. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect. Immun. 64:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waite, D. C., E. J. Alper, and B. J. Mady. 1996. Adult group B streptococcal disease. Ann. Intern. Med. 125:152-153. [DOI] [PubMed] [Google Scholar]
- 36.Zysk, G., B. K. Schneider-Wald, J. H. Hwang, L. Bejo, K. S. Kim, T. J. Mitchell, R. Hakenbeck, and H.-P. Heinz. 2001. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect. Immun. 69:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]