Abstract
This study aimed to establish and validate a multiparameter prediction model for Ki67 expression in hepatocellular carcinoma (HCC) patients while also exploring its potential to predict the one-year recurrence risk. The clinical, pathological, and imaging data of 83 patients with HCC confirmed by postoperative pathology were analyzed, and the patients were randomly divided into a training set (n = 58) and a validation set (n = 25) at a ratio of 7:3. All patients underwent a magnetic resonance imaging (MRI) scan that included multi-b value diffusion-weighted scanning before surgery, and quantitative parameters were obtained via intravoxel incoherent motion (IVIM) and diffusion kurtosis (DKI) models. Univariate and multivariate logistic regression analyses were conducted using the training set data to construct a model, which was internally validated. The area under the curve (AUC) of the receiver operating characteristics (ROC), a decision curve analysis (DCA), and a calibration analysis were used to evaluate the model’s performance. Additionally, for patients with available follow-up data, the combined model was evaluated for its potential utility in predicting the one-year recurrence risk by analyzing the area under the curve (AUC) of the receiver operating characteristic (ROC) curve.The combined model outperformed the clinicaland parametric models in predicting high Ki67 expression. The nomograms based on the combined model included the neutrophil-to-lymphocyte ratio (NLR), ADCslow_Aver. The model showed strong discrimination in the training set, with an AUC of 0.836 (95% CI: 0.729–0.942) and acceptable calibration (Hosmer–Lemeshow p = 0.109). In the validation set, the model maintained moderate discrimination (AUC 0.806, 95% CI: 0.621–0.990) with good calibration (p = 0.663). DCA revealed that the combined model provided good clinical value and correction effects. Additionally, when used to predict the one-year recurrence risk, the combined model achieved moderate accuracy (AUC = 0.747), highlighting its potential utility in identifying patients at a higher risk of recurrence. A nomogram incorporating the NLR and quantitative MR diffusion parameters effectively predicts Ki67 expression in HCC patients before surgery. The model also shows promise in predicting recurrence risk, which may aid in postoperative risk stratification and patient management.
Clinical Relevance Statement We established a model that incorporated the NLR and quantitative magnetic resonance diffusion parameters, which demonstrated robust performance in predicting both high Ki67 expression and the one-year recurrence risk in HCC patients. This model shows potential clinical value in guiding postoperative risk stratification and personalized treatment planning.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82333-7.
Keywords: Hepatocellular carcinoma, Neutrophil-to-lymphocyte ratio, Ki67 expression, Nomogram, Predictive model
Subject terms: Cancer imaging, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor with the sixth-highest incidence and the third-highest mortality rate worldwide1, and the prognosis for patients with advanced HCC remains poor. The early and accurate diagnosis of HCC is thus crucial for facilitating personalized treatment, improving the prognosis of patients, and reducing mortality rates2. Identifying reliable biological markers can contribute significantly to achieving these goals.
Ki67, a monoclonal antibody expressed mainly in the nucleus, has emerged as a promising prognostic marker for HCC. It serves as an important indicator for measuring the degree of cell proliferation and the biological behavior of cells within the tumor microenvironment3,4. High Ki67 expression often indicates that HCC has greater malignant potential, serves as an independent risk factor for disease-free and overall survival outcomes of HCC patients, and functions as a reliable prognostic indicator for HCC5,6. Pathological analysis via needle biopsy or surgical resection provides evidence of Ki67 expression; however, few preoperative noninvasive assessments of Ki67 expression have been performed.
Noninvasive imaging techniques, especially magnetic resonance imaging (MRI), offer potential alternatives. MRI multiparameter diffusion-weighted imaging is an effective noninvasive tool for diagnosing HCC and evaluating its prognosis and treatment efficacy. Some studies have demonstrated that preoperative MRI parameters can predict high and low Ki67 expression in HCC7. In the evaluation of the liver cancer microenvironment, the intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) models derived from DWI have demonstrated effectiveness8,9, and they can also be used to evaluate biological behaviors, such as microvascular infiltration in HCC tumors10–12.
Given the close relationship between inflammation and tumor progression13,14, thet inflammatory response is another important factor requiring consideration. The neutrophil-to-lymphocyte ratio (NLR), a marker of systemic inflammatory response, has shown potential as a predictor of immune status and survival outcomes in cancer patients15. The preoperative peripheral blood NLR has been reported to predict the recurrence and survival rates of patients with HCC15–17. Further research is needed to confirm whether there is a correlation between the NLR, an indicator of inflammatory status, and Ki67, an indicator of tumor cell proliferation.
In this study, we aimed to explore whether imaging and clinical parameters can serve as biological indicators for the preoperative prediction of Ki67 expression in HCC patients. We analyzed the correlation between Ki67 expression and preoperative diffusion-weighted MR imaging parameters and MRI features, as well as the peripheral blood NLR. Additionally, we developed multiple preoperative prediction models for Ki67 expression and evaluated their predictive performance.
Materials and methods
Patients
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval Number: 2024-E432-01). We systematically collected data from 354 patients with primary liver cancer who underwent liver MRI scans within 1 month before surgery at the First Affiliated Hospital of Guangxi Medical University from March 2021 to December 2023. All patients signed provided their written informed consent.
The inclusion criteria were as follows: (1) patients who underwent contrast-enhanced MRI of the liver, including IVIM-DKI sequences, within 1 month before surgery and were diagnosed with primary liver cancer; (2) patients with a pathologically confirmed diagnosis of HCC after surgical resection; and (3) patients with a solitary liver tumor with a diameter greater than 2 cm.
The exclusion criteria were as follows: (1) history of preoperative surgical resection, radiotherapy, or chemotherapy; (2) unsatisfactory imaging data; or (3) incomplete clinical or pathological data.
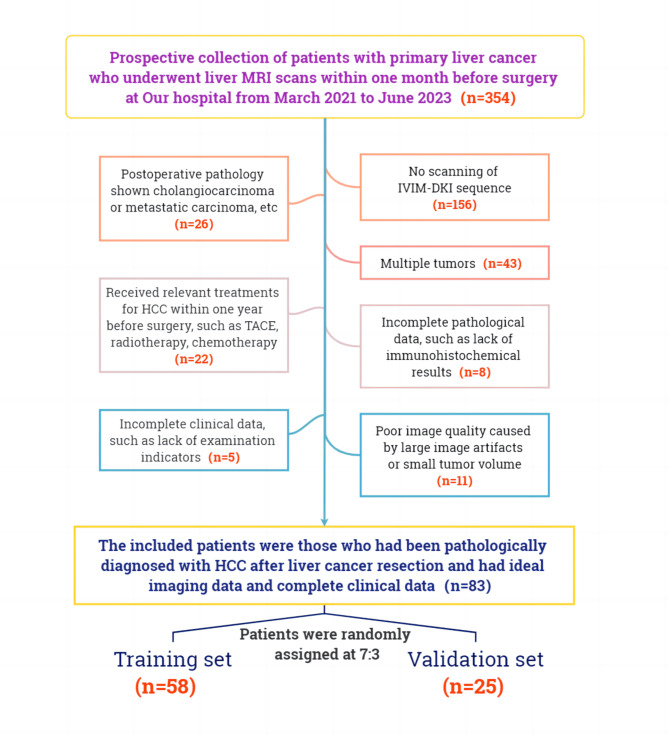
The flowchart illustrating the inclusion and exclusion criteria for this study is shown in Fig. 1. The patients were randomly divided into training and validation sets at a ratio of 7:3 via the R function “createDataPartition” to ensure a balanced distribution of outcome events between these two sets.
Fig. 1.
Flowchart of the inclusion and exclusion criteria of this study.
Clinical Data Collection
The clinical features included age, sex, weight, neutrophil count, absolute lymphocyte count, alpha-fetoprotein (AFP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and hepatitis B virus (HBV) infection. The pathological features included the degree of tumor differentiation and the percentage of Ki67 expression.
MRI technique
MRI scans were performed on a 3.0T MRI scanner (MAGNETOM Prisma, Siemens Healthineers, Germany) with a 16-channel body coil, and were performed alongside respiratory gating. This technique synchronized image acquisition with the patient’s breathing cycle, helping to minimize motion artifacts that could obscure image quality, especially in areas that moved when the patient breathed, such as the abdomen.
All patients underwent multiphase gadoxetate-ethoxybenzyl-diethylen etriamine pentanoate (Gd-EOB-DTPA) enhanced scanning, and the IVIM-DKI scan sequence was added. The b values used for the IVIM-DKI sequence were 0, 20, 50, 100, 150, 200, 600, 1000, 2000, and 3000 s/mm2. The scanning parameters are shown in Table 1.
Table 1.
MRI scanning parameters.
| TR(ms) | TE(ms) | slice thickness (mm) | slice gap (mm) | FOV(mm) | Matrix | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1WI | 3.97 | 129 | 5.0 | 2.0 | 380 × 380 | 177 × 320 | ||||
| T2WI | 1000.0 | 95.0 | 5.0 | 2.0 | 380 × 380 | 192 × 192 | ||||
| Hepatobiliary phase | 4.48 | 1.33 | 3.0 | 2.0 | 380 × 380 | 163 × 288 | ||||
| IVIM-DKI | 4900 | 57 | 5.0 | 6.4 | 380 × 261 | 88 × 128 | ||||
Note: TR: Repetition Time; TE: Echo Time; FOV: Field of View; T1WI: T1-weighted image; T2WI: T2-weighted image; IVIM-DKI: intravoxel incoherent motion‒diffusion kurtosis imaging.
Image analysis
Two radiologists with 3 and 5 years of experience in abdominal imaging diagnosis, who were blinded to the clinical and histopathological information, independently analyzed the patients’ MR images and postprocessed the IVIM-DKI images using MR software (Body Diffusion 1.4.0, Siemens Healthineers, Germany). In instances of disagreement, resolution was achieved through consultation with a senior radiologist with 10 years of experience in abdominal imaging diagnosis.
The following MRI findings were assessed as previously described18: (1) background of liver cirrhosis; (2) maximum tumor diameter; (3) tumor location; (4) tumor margin: observation of whether there are nodular protrusions on the tumor edge; (5) tumor pseudocapsule: thin annular high signal at the edge during the venous phase; (6) peritumoral enhancement: irregular enhancement area of the peritumoral liver parenchyma during the arterial phase; (7) peritumoral hypointensity: irregular low signal area of the peritumoral liver parenchyma during the hepatobiliary phase; and (8) necrosis, cyst, or hemorrhage.
In the DKI model, the b values were 0, 200, 600, 1000, 2000, and 3000 s/mm2; in the IVIM model, they were 0, 20, 50, 100, 150, 200, 600, and 1000 s/mm2; in the conventional single-exponential model, they were 0, 500, and 1000 s/mm2. In the ADC model, with ultrahigh b values, they were 2000 and 3000 s/mm2.
Three ROIs were drawn along the tumor edge at b = 0 s/mm2. IVIM-DKI images, including the maximum cross-sectional position of the tumor and its upper and lower levels, were collected, with blood vessels, the bile duct, hemorrhage, and imaging artifacts avoided by referring to T1WI and T2WI sequences. These images were then copied into different parameter maps. For each ROI fraction, the average value was recorded as MK_Aver, MD_Aver, D_Aver, Dp_Aver, f_Aver, ADCslow_Aver, and ADCuh_Aver.
The mean kurtosis (MK) and mean diffusivity (MD) parameters from the DKI model; the true diffusion coefficient (D), perfusion-dependent diffusion coefficient (Dp), and perfusion fraction (f) parameters from the IVIM model; the ADC value (ADCslow) from the conventional single-exponential model; and the ADC value (ADCuh) from the ultrahigh b value were all obtained.
Follow-up data Collection
Follow-up data were collected for at least one year post-surgery to monitor very early recurrence or death. Very early recurrence was defined as the presence of intrahepatic and/or extrahepatic recurrence within one year following HCC resection19. The follow-up included imaging examinations (such as CT, MRI, and ultrasound) and serum tumor marker evaluation (such as AFP). These data were obtained from the patients’ routine follow-up visits at the hospital. While every effort was made to gather follow-up data from all patients, some were lost to follow-up, and as a result, complete follow-up data were not available for all individuals. The cutoff date for follow-up was March 31, 2024.
Statistical analysis
All statistical analyses were performed using SPSS (RRID: SCR_002865, version 26.0) and the R Project for Statistical Computing (RRID: SCR_001905, version 4.4.0). The intraclass correlation coefficient (ICC) was used to evaluate the consistency of the two radiologists in observing the same imaging data. When the ICC > 0.75, the consistency was considered to be strong.
The Shapiro‒Wilk test was used to determine whether the measurement data conformed to a normal distribution. Then, Pearson and Spearman rank correlation analyses were performed for normally and nonnormally distributed data, respectively. For the Ki67 low- and high-expression groups, normally distributed data were expressed as the means ± standard deviations (SDs), and they were compared via independent sample t-tests. Nonnormally distributed measurement data were represented by M (P25, P75) and compared via the Mann‒Whitney U test. The chi-square test or Fisher’s exact test was used to compare the differences in enumeration data.
The ROC curve was constructed using the “roc_curve” package, which was used to analyze the predictive performance. The confidence interval for the mean was set to 95%, and when the P value was less than 0.05, the difference was considered significant. The “rms” package was used to plot the calibration curve to evaluate the model’s goodness of fit, and the Hosmer–Lemeshow test was performed.
Results
Patient characteristics
A total of 83 patients, including 65 males and 18 females, were enrolled in this study. In the entire population, as well as in the training and validation sets, 46 (55.4%), 30 (51.7%), and 16 (64%) patients presented with high Ki67 expression (> 10%), respectively. The average ages were 54.39 ± 10.94, 54.52 ± 11.60, and 54.08 ± 9.45 years, respectively, and the average weights were 63.35 ± 10.14, 62.71 ± 11.09, and 64.84 ± 7.49 kg, respectively.
In the assessment of the diffusion-weighted MR imaging parameters, both radiologists demonstrated strong interobserver agreement. The interobserver ICC values of MK_Aver, MD_Aver, f_Aver, D_Aver, Dp_Aver, ADCslow_Aver, and ADCuh_Aver were 0.904, 0.924, 0.908, 0.931, 0.914, 0.893, and 0.906 respectively.
The clinical, pathological, and MRI features, as well as the MRI diffusion-weighted imaging parameters of the enrolled patients, are summarized in Table 2. These indicators were comparable between the training and validation sets (P > 0.05).
Table 2.
Comparison of patient characteristics and parameters.
| Total(n = 83) | Training set(n = 58) | |||||
|---|---|---|---|---|---|---|
| Validation (n = 25) |
Training (n = 58) |
Pα | Low KI67 expression group (n = 28) |
High KI67 expression group (n = 30) |
Pβ | |
| Clinical data | ||||||
| Age(years) | 54.08 ± 9.45 | 54.52 ± 11.60 | 0.869 | 56.43 ± 11.10 | 52.73 ± 11.96 | 0.229 |
| Sex, n(%) | 0.160 | 0.179 | ||||
| Male | 22(88.00%) | 43(74.14%) | 23(82.14%) | 20(66.67%) | ||
| Female | 3(12.00%) | 15(25.86%) | 5(17.86%) | 10(33.33%) | ||
| Weight(kg) | 64.84 ± 7.49 | 62.71 ± 11.09 | 0.311 | 65.16 ± 12.00 | 60.42 ± 9.81 | 0.104 |
| HBV, n(%) | 0.257 | 0.670 | ||||
| No | 4(16.00%) | 16(27.59%) | 7(25.00%) | 9(30.00%) | ||
| Yes | 21(84.00%) | 42(72.41%) | 21(75.00%) | 21(70.00%) | ||
| AFP |
20.73 (7.08, 381.88) |
55.90 (4.29, 1071.90) |
0.972 |
5.76 (2.54, 87.26) |
503.39 (20.11, 16484.02) |
< 0.001* |
| ALT |
41.00 (24.00, 53.00) |
28.00 (20.00, 41.75) |
0.101 |
30.00 (21.75, 48.25) |
23.50 (16.25, 38.75) |
0.196 |
| AST |
39.00 (29.00, 50.00) |
32.00 (26.00, 54.50) |
0.659 |
29.50 (24.75, 47.75) |
37.50 (27.25, 64.75) |
0.114 |
| NLR |
2.16 (1.55, 2.64) |
2.11 (1.77, 2.86) |
0.497 |
1.97 (1.68, 2.20) |
2.72 (2.02, 3.91) |
< 0.001* |
| Differentiation grade, n(%) | 0.149 | < 0.001* | ||||
| High | 6(24.00%) | 5(8.62%) | 5(17.86%) | 0(0.00%) | ||
| Middle | 16(64.00%) | 41(70.69%) | 22(78.57%) | 19(63.33%) | ||
| Low | 3(12.00%) | 12(20.69%) | 1(3.57%) | 11(36.67%) | ||
| MRI imaging features | ||||||
| Background of liver cirrhosis, n(%) | 0.276 | 0.444 | ||||
| No | 8(32.00%) | 26(44.83%) | 14(50.00%) | 12(40.00%) | ||
| Yes | 17(68.00%) | 32(55.17%) | 14(50.00%) | 18(60.00%) | ||
| Tumor maximum diameter(cm) | 5.66 ± 3.64 | 5.31 ± 2.87 | 0.643 | 4.22 ± 2.38 | 6.32 ± 2.96 | 0.005* |
| Tumor location, n(%) | 0.602 | 0.543 | ||||
| Left lobe | 8(32.00%) | 14(24.14%) | 5(17.86) | 9(30.00) | ||
| Right lobe | 13(52.00%) | 37(63.79%) | 20(71.43) | 17(56.67) | ||
| Left and right lobes | 4(16.00%) | 7(12.07%) | 3(10.71) | 4(13.33) | ||
| Tumor margin, n(%) | 0.830 | 0.037* | ||||
| Smooth | 11(44.00%) | 27(46.55%) | 17(60.71) | 10(33.33) | ||
| Rough | 14(56.00%) | 31(53.45%) | 11(39.29) | 20(66.67) | ||
| Tumor pseudocapsule, n(%) | 0.195 | 0.002* | ||||
| Complete | 8(32.00%) | 11(18.97%) | 10(35.71) | 1(3.33) | ||
| No/incomplete | 17(68.00%) | 47(81.03%) | 18(64.29) | 29(96.67) | ||
| Peritumoral enhancement, n(%) | 0.205 | 0.019* | ||||
| No | 15(60.00%) | 26(44.83%) | 17(60.71) | 9(30.00) | ||
| Yes | 10(40.00%) | 32(55.17%) | 11(39.29) | 21(70.00) | ||
| Peritumoral hypointensity, n(%) | 0.276 | 0.003* | ||||
| No | 17(68.00%) | 32(55.17%) | 21(75.00) | 11(36.67) | ||
| Yes | 8(32.00%) | 26(44.83%) | 7(25.00) | 19(63.33) | ||
| Necrosis, cyst, or hemorrhage, n(%) | 0.170 | 0.036* | ||||
| No | 6(24.00%) | 23(39.66%) | 15(53.57) | 8(26.67) | ||
| Yes | 19(76.00%) | 35(60.34%) | 13(46.43) | 22(73.33) | ||
| MRI quantitative parameters | ||||||
|
MK_Aver (×10−6 mm2/s) |
690.94 ± 101.55 | 669.70 ± 91.51 | 0.351 | 633.89 ± 79.58 | 703.13 ± 90.38 | 0.003* |
|
MD_Aver (×10−6 mm2/s) |
1643.33 (1438.59,2006.15) |
1594.99 (1419.16, 1899.26) |
0.747 |
1769.11 (1504.32, 2282.08) |
1521.22 (1275.33, 1753.47) |
0.026* |
|
D_Aver (×10−6 mm2/s) |
903.95 ± 253.18 | 858.21 ± 212.68 | 0.399 | 912.32 ± 231.61 | 807.71 ± 183.02 | 0.061 |
|
f_Aver (×10−3) |
236.21 (157.73, 305.95) |
206.70 (169.01, 312.56) |
0.886 |
220.25 (179.85, 332.17) |
200.51 (160.98, 260.53) |
0.318 |
|
Dp_Aver (×10−6 mm2/s) |
153.77 (110.69, 224.91) |
183.28 (152.47, 232.41) |
0.133 |
179.01 (153.40, 228.37) |
190.19 (155.39, 242.93) |
0.521 |
|
ADCslow_Aver (×10−6 mm2/s) |
1139.46 (953.21, 1330.27) |
1156.25 (1027.16, 1305.79) |
0.516 |
1277.74 (1198.13, 1365.38) |
1038.21 (938.93, 1150.22) |
< 0.001* |
|
ADCuh_Aver (×10−6 mm2/s) |
478.18 (422.81,551.49) |
478.83 (443.29, 528.75) |
0.897 |
478.83 (455.33, 529.64) |
477.95 (436.88, 525.38) |
0.799 |
HBV: Hepatitis B virus; AFP: Alpha-fetoprotein; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; NLR: Neutrophil-to-lymphocyte ratio; MRI: Magnetic resonance imaging; MK: Mean kurtosis; MD: Mean diffusivity; D: True diffusion coefficient; f: Perfusion fraction; Dp: Perfusion-related diffusion coefficient; ADCslow: Slow apparent diffusion coefficient; ADCuh: Apparent diffusion coefficient under ultra-high b values; t: t test; Z: Mann‒Whitney test; χ²: chi-square test; Pα: comparison between the training group and the validation group; Pβ: comparison between the Ki67 high- and low-expression groups in the training group. *indicates P < 0.05.
Model development
>A total of 24 variables were analyzed. As shown in Tables 2 and 12 indicators significantly differed between the Ki67 high- and low-expression groups in the training set (P < 0.05). Given the significant difference in proportion between different degrees of pathological differentiation, we included only the remaining 11 indicators in the univariate logistic regression.
As shown in Table 3, nine important variables related to high Ki67 expression in HCC were identified, including the NLR (P < 0.01), the maximum tumor diameter (P < 0.01), rough margin (P = 0.04), absent or incomplete tumor pseudocapsule (P = 0.01), peritumoral enhancement (P = 0.02), peritumoral hypointensity (P < 0.01), necrosis, cyst, or hemorrhage (P = 0.04), MK average (P < 0.01), and ADCslow_Aver (P < 0.01). Incorporating these indicators into multivariate logistic regression analysis revealed that the NLR and ADCslow_Aver were independent risk factors for high Ki67 expression in HCC patients. The NLR was used to construct a clinical model for high Ki67 expression in HCC, while ADCslow_Aver was used to construct the parametric model.The NLR and ADCslow_Aver were used to develop the combined model.
Table 3.
Results of the univariate and multivariate logistic regression analyses of each variable in the training set note: AFP: Alpha-fetoprotein; NLR: neutrophil-to-lymphocyte ratio; MRI: magnetic resonance imaging; MK: Mean kurtosis; MD: Mean diffusivity; ADCslow: slow apparent diffusion coefficient; OR: odds ratio; CI: confidence interval; * indicates P < 0.05.
| Univariate logistic regression | Multivariate logistic regression | |||
|---|---|---|---|---|
| P | OR (95%CI) | P | OR (95%CI) | |
| Clinical data | ||||
| AFP | 0.10 | 1.00 (1.00–1.00) | ||
| NLR | < 0.01* | 3.72 (1.59–8.70) | 0.03* | 3.84 (1.12–13.14) |
| Imaging features of MRI | ||||
| Tumor maximum diameter | < 0.01* | 1.36 (1.08–1.71) | 0.96 | 0.99 (0.63–1.56) |
| Tumor margin | 0.04* | 3.09 (1.06–9.04) | 0.19 | 3.17 (0.57–17.61) |
| Tumor pseudocapsule | 0.01* | 16.11 (1.90–136.62) | 0.29 | 4.11 (0.31–55.34) |
| Peritumoral enhancement | 0.02* | 3.61 (1.21–10.71) | 0.44 | 0.41 (0.04–4.02) |
| Peritumoral hypointensity | < 0.01* | 5.18 (1.67–16.09) | 0.12 | 5.93 (0.63–56.10) |
| Necrosis, cyst, or hemorrhage | 0.04* | 3.17 (1.06–9.52) | 0.75 | 0.73 (0.11–5.05) |
| MRI quantitative parameters | ||||
| MK Aver | < 0.01* | 1.01 (1.01–1.02) | 0.30 | 0.99 (0.98–1.01) |
| MD Aver | 0.06 | 1.00 (1.00–1.00) | ||
| ADCslow_Aver | < 0.01* | 0.99 (0.99–0.99) | 0.04* | 0.99 (0.99–0.99) |
Comparison of the predictive performance of different models
The effectiveness of the three models in predicting high Ki67 expression is presented in Table 4; Fig. 2. The combined model outperformed the standalone clinical and parametric in predicting high Ki67 expression, with higher AUCs observed in the training and validation sets (0.836 and 0.806, respectively).
Table 4.
ROC curve analysis for prediction of high and low Ki67 expression using different combined models.
| Training set | Validation set | |||||
|---|---|---|---|---|---|---|
| AUC (95%CI) | Sensitivity(%) | Specificity(%) | AUC (95%CI) | Sensitivity(%) | Specificity(%) | |
| Clinical model | 0.751 (0.623–0.880) | 82.1% | 63.3% | 0.771(0.556–0.986) | 77.8% | 43.8% |
| Parametric model | 0.817 (0.704–0.929) | 85.7% | 70.0% | 0.764(0.562–0.966) | 88.9% | 68.8% |
| Combined model | 0.836(0.729–0.942) | 96.4% | 63.3% | 0.806(0.621–0.990) | 77.8% | 81.2% |
Magnetic resonance imaging; AUC: area under the curve; CI: confidence interval.
Fig. 2.
ROC curves of each model in the training (A) and validation (B) sets.
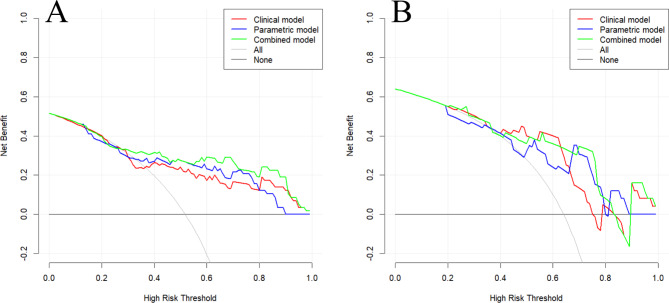
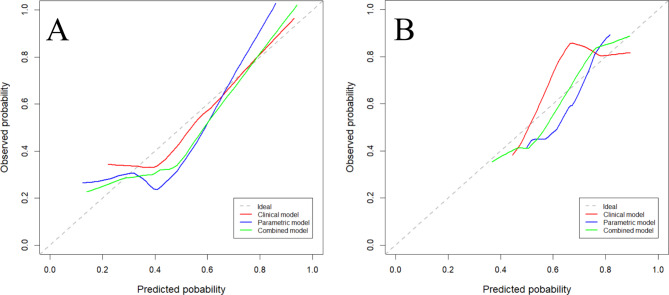
As shown in Fig. 3, the combination model exhibited a high net gain in clinical decision-making. The calibration curve of each model demonstrated that the predicted probability was almost identical to the actual probability in the training and validation sets (Fig. 4).
Fig. 3.
Clinical decision analysis of each model in the training (A) and validation (B) sets. The abscissa is the threshold probability and the ordinate is the net benefit. The gray curve indicates that all patients received the intervention, while the black horizontal line indicates that if the patients receive no intervention, the net benefit would be zero.
Fig. 4.
Calibration curves of the models in the training (A) and validation (B) sets. The dashed line represents the ideal predictive performance, while the solid line represents the model’s predictive performance. The closer the solid line is to the dotted line, the better the model’s prediction accuracy.
Nomogram establishment
A risk prediction model was constructed using a nomogram based on the combined model (Fig. 5). The points of each variable were added to obtain the total points. The total points correspond to the probability of high Ki67 expression. A Hosmer–Lemeshow test was conducted on the model, and the P values for the training and validation sets were 0.109 and 0.663, respectively, both of which are > 0.05. This finding further indicates that the model exhibits good fit.
Fig. 5.
Construction of a nomogram based on the combined model. Note: MK: Mean kurtosis; ADCslow: Slow apparent diffusion coefficient; NLR: Neutrophil-to-lymphocyte ratio.
One-year recurrence analysis
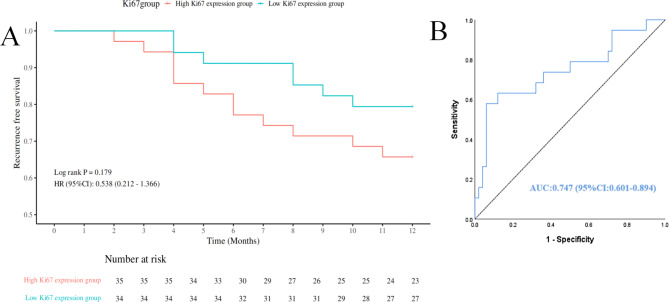
Ultimately, one-year follow-up data were collected for 69 patients.Among them, 35 patients presented with high Ki67 expression(12 cases experienced recurrence), while 34 patients presented with low Ki67 expression(7 cases experienced recurrence). As shown in Fig. 6A, the one-year recurrence-free survival rate was higher in the low Ki67 expression group (79.4%) than in the high Ki67 expression group (65.7%). However, the Log-rank test indicated that this difference was not statistically significant (P = 0.179), possibly due to the small sample size, which could have limited the ability to detect significant differences between the groups.
Fig. 6.
(A) One-year recurrence-free survival curves for patients with high and low Ki67 expression. (B) ROC curve of the combined model for predicting one-year recurrence.
Figure 6B showed the ROC curve of the combined model for predicting one-year recurrence, with an AUC of 0.747 (95% CI: 0.601–0.894), indicating meaningful predictive capability. This model not only predicted high Ki67 expression but also effectively forecasted one-year recurrence, highlighting its clinical utility for early identification and intervention of high-risk patients.
Typical case image
Figure 7 shows the typical MR and pathological images of a patient.
Fig. 7.
A 42-year-old male patient with poorly differentiated hepatocellular carcinoma with high Ki67 expression. (A) Transverse T2WI of the tumor, with high signal intensity and intratumoral patchy and spotty higher signal foci. (B–E) At the arterial phase (B), the tumor exhibited nonuniform enhancement with intratumoral patches of nonenhanced necrosis, whereas the peripheral liver parenchyma showed marked patchy enhancement. During the portal venous (C) and transitional phases (D), the tumor demonstrated diminished enhancement; in contrast, during the hepatic parenchymal phase (E), it exhibited low signal intensity. The peripheral tumor exhibited slightly lower patchy signal intensity. Additionally, there is an incomplete pseudocapsule around the tumor with irregular nodular edges. (F–N) Original intravoxel incoherent motion‒diffusion kurtosis imaging (IVIM-DKI) images at b = 0 s/mm2, the MK, MD, f, D, Dp, conventional ADC, and high b value ADC maps, and the histological examination via light microscopy (HE×100). Note: T2WI: T2 weighted image; MK: Mean kurtosis; MD: Mean diffusivity; f: Perfusion fraction; D: True diffusion coefficient; Dp: Perfusion-related diffusion coefficient; ADC: Apparent diffusion coefficient; HE: Hematoxylin-eosin.
Discussion
We established a model incorporating the NLR and ADCslow_Aver that demonstrated good performance in predicting high Ki67 expression. Additionally, this model showed the ability to predict recurrence within one year, further indicating its potential utility in the clinic. By providing valuable insights into both proliferation and recurrence risks, this model may serve as a useful tool for identifying high-risk HCC patients and guiding clinical decision-making.
The NLR reflects the immune status of cancer patients20, with elevated NLR values significantly associated with risk factors for tumor malignancy. Our study revealed that a high NLR was associated with increased Ki67 expression in tumors, indicating poor patient prognosis. Our findings align with previously reported findings21–23. Ki67 is related to tumor cell proliferation, and the associations of the NLR with tumor angiogenesis, immune evasion, and metastatic diseases are linked to poor prognosis in HCC patients24,25, which may explain why the NLR and Ki67 expression are highly correlated. This association can be attributed to the proinflammatory environment produced by chronic liver disease, which leads to inflammatory neutrophilism that inhibits the cytolytic activity of other immune cells, such as lymphocytes and activated T cells, thereby suppressing tumor cell apoptosis26. Additionally, neutrophilia can stimulate tumor neovascularization through the secretion of vascular endothelial growth factor (VEGF)27,28.
We also analyzed the role of other clinical and pathological factors. The pathological grade of HCC can reflect the biological behavior of the tumor and aid in assessing postoperative recurrence29. A previous study30 revealed that the higher the expression of Ki67 is, the greater the degree of tumor invasion and heterogeneity, and the lower the degree of pathological differentiation. Our results were similar; however, we need to increase the sample size for each degree of differentiation for further confirmation. AFP is an independent risk factor for HCC tumor differentiation and patient survival31. Wu et al.32 demonstrated that AFP correlated with Ki67 expression in HCC, which aligned with our findings. However, the AFP level was not significant in the univariate logistic regression, which warrants further investigation. ALT and AST are important liver serum enzymes, and they serve as sensitive indicators for assessing impaired liver function33. This study revealed no significant difference in ALT or AST between the high- and low-expression groups of Ki67, possibly attributable to the lack of significant disparity in HBV infection and cirrhosis cases between the two groups.
Certain imaging features showed P values < 0.05 in univariate logistic regression, indicating a potential association with Ki67 expression, such as tumor pseudocapsule, tumor margin, and peritumoral hypointensity.The dense tumor pseudocapsule restricts tumor cells to the tumor boundary, and its narrow vessels prevent cancer cells from passing through34. When the pseudocapsule is incomplete or absent, HCC tends to exhibit infiltrative growth. Nonsmooth tumor margins35 and peritumoral hypointensity36 in the hepatobiliary phase are independent predictors of microvascular invasion (MVI) in HCC patients and have a significant impact on tumor dissemination and aggressive tumor behavior34. These imaging features correlate with the Ki67 index, possibly because they facilitate tumor growth and invasion.
Diverse perspectives exist regarding the correlation between multiple-parameter diffusion-weighted MR imaging parameters and Ki67 expression. The MK and MD values were not correlated with Ki67 expression in a study conducted by Yuan37. However, Zhang38 reported that the Ki67 high-expression group had lower standard ADC, D, and MD values and high MK values, which aligned with our results. This effect may be attributed to the fact that tumors in the high Ki67 expression group possess a more intricate tissue architecture and exhibit a richer blood supply than those in the low Ki67 expression group. Therefore, the degree to which water molecules inside the tumor deviate from the Gaussian distribution and move in a disordered manner, as well as the degree of tumor blood flow, are greater39. The MK value is an indicator of organizational complexity, whereas the MD value is the average diffusion degree of water molecules in a diffusion gradient field, which can reflect the overall diffusion situation and is independent of direction11,40. Our results were consistent with those of previous studies, which showed that ADCslow eliminated the effect of microcirculation and could better reflect water restriction due to tumor density and changes in extracellular volume or deposition during tumor proliferation39, which was highly correlated with Ki67 expression, indicating a tumor cell proliferation-related diffusion restriction effect41.
To the best of our knowledge, this study is the first to systematically combine MRI diffusion parameters and NLR to predict Ki67 expression, offering a valuable contribution to current noninvasive predictive methods for HCC. Previous studies have often been limited to MRI features or parameters alone7,42, which can be subjective and lack a comprehensive biological context.By combining MRI diffusion parameters with the inflammatory marker NLR, this approach not only improves the accuracy of tumor proliferation prediction, but also provides insights into the inflammatory tumor microenvironment, offering a novel perspective on tumor behavior in HCC.
This study has several limitations. As a prospective study; although we scanned many patients, only a small number of enrolled patients met the inclusion and exclusion criteria, resulting in a relatively modest sample size that could have affected the model’s generalizability. Additionally, as a single-center study, it lacked external validation; therefore, further studies in diverse clinical settings would be beneficial. The study also included a limited number of variables, which may have restricted the model’s predictive power and comprehensive analysis. We also currently lack long-term follow-up data that would allow us to fully assess the model’s prognostic value over time. Future efforts will focus on increasing the sample size and incorporating more comprehensive clinical and imaging features to develop a more robust prediction model and to comprehensively evaluate its long-term clinical value.
Conclusion
The nomogram model we constructed uses two selected factors, including the NLR and MR diffusion quantitative parameters. This model demonstrates robust predictive power and serves as a valuable clinical decision-making tool with favorable net benefits and calibration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Abbreviations
- ADC
Apparent diffusion coefficient
- ADCslow
Slow apparent diffusion coefficient
- ADCuh
Apparent diffusion coefficient under ultra-high b values
- AFP
Alpha-fetoprotein
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AUC
Area under the curve
- CI
Confidence interval
- D
True diffusion coefficient
- DCA
Decision curve analysis
- Dp
Perfusion-related diffusion coefficient
- DKI
Diffusion kurtosis imaging
- DWI
Diffusion weighted imaging
- f
Perfusion fraction
- Gd-EOB-DTPA
Gadoxetate-ethoxybenzyl-diethylen etriamine pentaace
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- ICC
Intraclass correlation coefficient
- IVIM
Intravoxel incoherent motion
- MVI
Microvascular invasion
- MK
Mean kurtosis
- MD
Mean diffusivity
- MRI
Magnetic resonance imaging
- NLR
Neutrophil-to-lymphocyte ratio
- ROI
Region of interest
- ROC
Receiver operating characteristic
- RRID
Research resource identifier
Author contributions
Yu-chen Wei and Liang yun: Performed the experiments, Contributed to the design of the studyAnalyzed the data , Wrote the paper.Yan-ling Liang: Collected and assembled data, Contributed to the writing of the manuscript.Contributed to the design of the study.Robert Grimm, PhD done software.Chongze Yang and Yuan-fang Tao: Performed data analysis.Provided critical feedback and helped shape the research.Sheng-chen Jiang: Performed data analysis, Contributed to the writing of the manuscript.Jin-yuan Liao: Corresponding author, Provided funding, Supervised the research, Final approval of the version to be published.
Funding
This work was supported by the Guangxi Natural Science Foundation Project (2023GXNSFAA026053); the Development, Popularization, and Application of Appropriate Medical and Health Technology in Guangxi (S2021090); and the National Natural Science Foundation of China (NSFC81360220, 81260214).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research involved human participants. All data were obtained from routine clinical tests, and there was no clinical intervention for the participants in the study. This was a prospective study conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Guangxi Medical (Approval Number: 2024-E432-01).
Informed consent
All patients provided their written informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu-chen Wei and Liang yun contributed equally.
References
- 1.Sung, H., Ferlay, J., Siegel, R. L. & Global Cancer Statistics. : GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J].CA: A Cancer Journal for Clinicians, 2021,71(3):209–249. (2020). [DOI] [PubMed]
- 2.Singal, A. G., Kanwal, F. & Llovet, J. M. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol.20 (12), 864–884 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Yerushalmi, R. et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. vol. 11 (2), 174–183 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Meng, Y. et al. Prognostic Value of Ki-67 index in patients with endometrial stromal Sarcoma[J]. Front. Med.8, 823506 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, Y. et al. DNA topoisomerase IIα and Ki67 are prognostic factors in patients with hepatocellular carcinoma[J]. Oncol. Lett.13 (6), 4109–4116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo, Y. H. et al. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysis[J]. Int. J. Clin. Exp. Med.8 (7), 10235–10247 (2015). [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y. D. et al. Diagnostic value of Gd-EOB‐DTPA‐Enhanced MRI for the expression of Ki67 and microvascular density in Hepatocellular Carcinoma[J]. J. Magn. Reson. Imaging. 51 (6), 1755–1763 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Tramontano, L., Cavaliere, C., Salvatore, M. & Brancato, V. The role of non-gaussian models of Diffusion Weighted MRI in Hepatocellular Carcinoma: a systematic review. J. Clin. Med.10 (12), 2641 (2021). Published 2021 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, H. W. et al. Quantitative analysis for detection and grading of hepatocellular carcinoma: comparison of diffusion kurtosis imaging, intravoxel incoherent motion and conventional diffusion-weighted imaging[J]. Oncol. Lett.24 (5), 403 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, F. et al. The roles of Diffusion Kurtosis Imaging and Intravoxel Incoherent Motion Diffusion-Weighted Imaging Parameters in Preoperative Evaluation of Pathological Grades and Microvascular Invasion in Hepatocellular Carcinoma[J]. Front. Oncol.12, 884854 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao, L. K. et al. Diffusion kurtosis imaging (DKI) of hepatocellular carcinoma: correlation with microvascular invasion and histologic grade[J]. Quant. Imaging Med. Surg.9 (4), 590–602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, W. T. et al. Assessment of microvascular invasion of hepatocellular carcinoma with Diffusion Kurtosis imaging[J]. Radiology286, 571–580 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Greten, F. R. & Sergei, I. G. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity 51,1 : 27–41. doi: (2019). 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed]
- 14.Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer13(11), 759–771 (2013). [DOI] [PubMed]
- 15.Wen, S. et al. Combination of Tertiary Lymphoid structure and neutrophil-to-lymphocyte ratio predicts survival in patients with Hepatocellular Carcinoma[J]. Front. Immunol.12, 788640 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng, X. et al. Neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers to predict relapse and survival in posthepatectomy HBV-related hepatocellular carcinoma: a meta-analysis and preliminary immune perspective[J]. Translational Cancer Res.10 (3), 1261–1272 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minici, R. et al. Prognostic role of Neutrophil-to-lymphocyte ratio (NLR), Lymphocyte-to-Monocyte Ratio (LMR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-C reactive protein ratio (LCR) in patients with Hepatocellular Carcinoma (HCC) undergoing chemoembolizations (TACE) of the liver: the unexplored corner linking Tumor Microenvironment. Biomarkers Interventional Radiology[J] Cancers. 15 (1), 257 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo, Z. Y. et al. Pre-operative MRI features predict early post-operative recurrence of hepatocellular carcinoma with different degrees of pathological differentiation[J]. Radiol. Med.128 (3), 261–273 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, L. et al. Development and comprehensive validation of a predictive prognosis model for very early HCC recurrence within one year after curative resection: a multicenter cohort study. Int. J. Surg.110 (6), 3401–3411 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, W. T. et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy[J]. Annals Translational Med.7 (18), 431 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv, Y. J. et al. Prognostic value of preoperative neutrophil to lymphocyte ratio is superior to systemic immune inflammation index for survival in patients with Glioblastoma[J]. Clin. Neurol. Neurosurg.181, 24–27 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Xu, G. D. et al. Correlation between preoperative inflammatory markers, Ki-67 and the pathological grade of glioma[J]. Medicine100 (36), e26750 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun, Z. H. et al. Clinical implications of pretreatment inflammatory biomarkers as independent prognostic indicators in prostate cancer[J]. J. Clin. Lab. Anal.32 (3), e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng, J. et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for Hepatocellular Carcinoma patients with various treatments: a Meta-analysis and systematic Review[J]. Cell. Physiol. Biochem.44 (3), 967–981 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Zhou, D. S. et al. Inflammation scores predict survival for hepatitis B virus-related hepatocellular carcinoma patients after transarterial chemoembolization[J]. World J. Gastroenterol.21 (18), 5582–5590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nat. vol. 612 (7938), 141–147 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Zeng, F. R. et al. Preoperative neutrophil-lymphocyte ratio predicts the risk of microvascular invasion in hepatocellular carcinoma: a meta-analysis[J]. Int. J. Biol. Mark.34 (3), 213–220 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Kuang, D. M. et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma[J]. J. Hepatol.54 (5), 948–955 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Martins-Filho, S. N. et al. Histological grading of Hepatocellular Carcinoma—A. Syst. Rev. Literature[J] Front. Med.4, 193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi, W. et al. Expression of MTA2 and Ki-67 in hepatocellular carcinoma and their correlation with prognosis[J]. Int. J. Clin. Exp. Pathol.8 (10), 13083–13089 (2015). [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao, T. et al. Heterogeneities of site-specific N-Glycosylation in HCC Tumors with Low and High AFP Concentrations[J]. Front. Oncol.10, 496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, C. Y. et al. Nomogram based on CT Radiomics features combined with clinical factors to Predict Ki-67 expression in Hepatocellular Carcinoma[J]. Front. Oncol.12, 943942 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trefts, E., Gannon, M. & Wasserman, D. H. The liver. Curr. Biol.27 (21), R1147–R1151. 10.1016/j.cub.2017.09.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song, L., Li, J. & Luo, Y. The importance of a nonsmooth tumor margin and incomplete tumor capsule in predicting HCC microvascular invasion on preoperative imaging examination: a systematic review and meta-analysis. Clin. Imaging. 76, 77–82 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Choi, Y. S. et al. Histological characteristics of small hepatocellular carcinomas showing atypical enhancement patterns on gadoxetic acid-enhanced MR imaging. J. Magn. Reson. Imaging: JMRI vol. 37 (6), 1384–1391 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Min, J. H. et al. Interobserver Variability and Diagnostic Performance of Gadoxetic Acid-enhanced MRI for Predicting Microvascular Invasion in Hepatocellular Carcinoma. Radiology.297(3), 573–581 (2020). [DOI] [PubMed]
- 37.Yuan, J. et al. Correlation between diffusion kurtosis and intravoxel incoherent motion derived (IVIM) parameters and tumor tissue composition in rectal cancer: a pilot study[J]. Abdom. Radiol.47 (4), 1223–1231 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Zhang, K. et al. Soft tissue sarcoma: IVIM and DKI parameters correlate with Ki-67 labeling index on direct comparison of MRI and histopathological slices[J]. Eur. Radiol.32 (8), 5659–5668 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Wang, C. & Dong, H. Ki-67 labeling index and the grading of cerebral gliomas by using Intravoxel Incoherent Motion Diffusion-Weighted Imaging and three-dimensional arterial spin labeling magnetic resonance imaging. Acta Radiol.61 (8), 1057–1063 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Filli, L. et al. Whole-body diffusion kurtosis imaging: initial experience on non-gaussian diffusion in various Organs[J]. Invest. Radiol.49 (12), 773–778 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Bai, Y. et al. Study of Diffusion Weighted Imaging Derived Diffusion parameters as biomarkers for the Microenvironment in Gliomas. Front. Oncol.11, 672265 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, Y. et al. The value of multiple diffusion metrics based on whole-lesion histogram analysis in evaluating the subtypes and proliferation status of non-small cell lung cancer. Front. Oncol.14, 1434326 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.