Abstract
A murine model of endotoxin-induced lethal liver injury induced by Mycobacterium bovis BCG plus lipopolysaccharide (LPS) has been widely accepted and used. It has been reported that T cells play an important role in the pathogenesis of liver damage in this model. However, the precise mechanisms involved in regulation of the trafficking of effector T cells need to be elucidated. In the present study, we first reported that CXCL16/SR-PSOX (CXC chemokine ligand 16/scavenger receptor that binds phosphatidylserine and oxidized lipoprotein), a chemokine containing both membrane-anchored and soluble forms, was strongly up-regulated and predominantly distributed in the vascular endothelium in the injured liver tissue in the model. The secretory and membrane-anchored CXCL16/SR-PSOX functioned as a chemokine and an adhesive molecule, respectively, to attract T cells to a tumor necrosis factor alpha-activated endothelial cell line (SVEC) in vitro. To further identify the pathophysiological roles of CXCL16/SR-PSOX in the liver injury, the anti-CXCL16 antibody was administered to the BCG-primed mice before LPS challenge in vivo. Significant protection effects were observed with 70% of mice regarding lethality, the massive necrosis in the liver was reduced, and the intrahepatic infiltrating T cells were significantly inhibited. Taken together, these findings strongly suggest that functional CXCL16/SR-PSOX, as both a chemokine and an adhesion molecule, may be involved in the pathogenesis of the endotoxin-induced lethal liver injury via recruitment and adhesion of activated T cells to the vascular endothelium.
Endotoxin syndrome (or septic shock) is a systemic inflammatory response caused by lipopolysaccharide (LPS), a major component of the cell wall of gram-negative bacteria, which leads to the activation of various inflammatory cells, including macrophages, monocytes, and leukocytes. These activated cells release inflammatory mediators such as tumor necrosis factor alpha (TNF-α), nitric oxide, superoxide anions, and lipid mediators, leading to leukocyte infiltration, shock, and multiple organ failure (6, 38). It has been reported that injection of a small dose of LPS into mice pretreated with heat-killed Propionibacterium acnes or viable Mycobacterium bovis BCG causes liver injury and lethality, which has been widely used as an experimental model mimicking hepatic failure associated with endotoxin (13, 22, 31, 42, 51). This liver injury model can be pathophysiologically classified into two phases. At the priming phase induced by BCG, mononuclear cells infiltrate into the liver lobes, leading to granuloma formation. At the eliciting phase induced by LPS, inflammatory infiltrates are further increased, resulting in massive hepatocellular necrosis and apoptosis. Some molecules, including the various cytokines such as tumor necrosis factor alpha (33, 50, 52), interleukin-1 (10, 14), gamma interferon (30, 51), adhesion molecules (24, 32), and chemokines such as gamma interferon-inducible protein 10 and migration inhibitory factor (22, 60), were reported to be closely associated with the pathogenesis of this experimental endotoxin-induced lethal liver injury. It has been reported that T-cell-deficient ICR nude mice were resistant to P. acnes and LPS-induced liver injury (47). Although activated T cells are also presumed to be one of the effector lymphocytes in this model (36, 44, 48, 57), the mechanism of recruitment and infiltration of T cells, to be essential for endotoxin-induced liver injury and lethality, has not yet been clarified.
CXC chemokine ligand 16 (CXCL16)/SR-PSOX (scavenger receptor that binds phosphatidylserine and oxidized lipoprotein) is a chemokine and exists in a membrane-anchored form, similar to the structure of fractalkine/FKN/CX3C chemokine ligand 1 (3, 18, 29, 56). CXCL16/SR-PSOX is expressed on antigen-presenting cells and selectively attracts CXCR6-expressing T cells such as natural killer T (NKT) cells, and type 1-polarized, activated CD4+ and CD8+ T cells. Notably, the membrane-anchored CXCL16 was recently reported to support T cell and NKT cell firm adhesion to the dendritic cells, indicating its possible involvement in antigen-presenting cell-T cell interaction for immune responses (29, 43, 56). Up-regulation of CXCL16/SR-PSOX was also proposed to be involved in recruitment of effector T cells in inflammatory valvular heart disease (58). On the other hand, recent investigations have revealed the existence of a large number of infiltrating T cells in inflamed liver injury (30, 50, 57), and CXCR6-expressing effector T cells were also found to be abundant in inflamed liver lesions (16, 21). Although it seems that CXCL16/SR-PSOX may play a key role in efficient recruitment of effector T cells during the inflammatory lesions, the precise mechanisms of CXCL16/SR-PSOX in endotoxin-induced lethal liver injury are unknown. In this study, we found that CXCL16/SR-PSOX was markedly up-regulated and distributed in the vascular endothelium in injured liver tissue of LPS-induced mice pretreated with BCG and showed that CXCL16/SR-PSOX might play an important role in infiltration of T cells in LPS-induced lethal liver injury in BCG-primed mice, as both a chemokine and an adhesion molecule.
MATERIALS AND METHODS
Mice.
Male BALB/c (6 to 7 weeks old, 20 ± 2 g) mice obtained from the animal center of the Chinese Academy of Science (Shanghai, China) were bred in specific-pathogen-free conditions and allowed free access to food and water.
Microorganisms and cells.
The M. bovis BCG strain was from the Institute of Biological Products (Shanghai, China). The mouse vascular endothelial cell line (SVEC) was kindly provided by Qian Huang (Shanghai No. 1 hospital, China). The SVEC is an endothelial cell line derived by simian virus 40 (strain 4A) transformation of murine small vessel endothelial cells, originally isolated from the axillary lymph node vessels of an adult male C3H/HeJ mouse.
Antibodies and reagents.
Recombinant mouse interleukin-2 (IL-2), mouse TNF-α, and rat anti-mouse CXCL16 monoclonal antibody (142417, rat immunoglobulin G2a [IgG2a]) were purchased from R&D Systems (Minneapolis, MN). The SYBR Green I kit was from Roche Molecular Biochemicals (Mannheim, Germany). Rat immunoglobulin (R35-95, rat IgG2a), anti-CD3ɛ antibody (145-2C11), phycoerythrin-labeled anti-CD4 monoclonal antibody (MAb) (L3T4 RM4-5), fluorescein isothiocyanate (FITC)-labeled anti-CD8α MAb (Ly-2 53-6.7), anti-CD28 antibody (37.51), horseradish peroxidase (HRP)-conjugated anti-rat IgG, and FITC-conjugated rabbit anti-rat IgG were purchased from PharMingen (San Diego, CA). The CD8α+ T-cell isolation kit was from Miltenyi Biotec (Bergisch Gladbach, Germany). Recombinant CXCL16 protein was prepared in our lab. The fluorescence dye calcein acetoxymethyl ester was from Molecular Probes (Eugene, OR). LPS (Escherichia coli O55:B5) was from Sigma (St. Louis, MO). Trizol was purchased from Invitrogen (Carlsbad, CA). Primers were as follows: CXCL16 forward (+), 5′-AAC CAG GGC AGT GTC GC-3′; CXCL16 reverse (−), 5′-AGG CAA ATG TTT TTG GTG G-3′; TNF-α +, 5′-TCT CAT TCC TGC TTG TGG C-3′; TNF-α −, 5′-GCT GGC ACC ACT AGT TGG TT-3′; FasL +, 5′-GGA TAC TTA GAG TTC CTC AT-3′; FasL −, 5′-CCT CTG GAA TGG GAA GAC AC-3′; β-actin +, 5′-GCT ACA GCT TCA CCA CCA CAG-3′; β-actin −, 5′-GGT CTT TAC GGA TGT CAA CGT C-3′.
Induction of liver injury.
The liver injury was induced as described previously with minor modifications (22). In brief, mice were primed by intravenous injection of BCG (5 × 107 viable bacilli) via tail vein injection. Twelve days later, they were given an intravenous injection of LPS (7.5 μg/mouse) suspended in pyrogen-free phosphate-buffered saline. To examine the effect of anti-CXCL16 antibodies on BCG-LPS-induced liver injury, the monoclonal anti-CXCL16 antibody (0.2 mg/mouse) or nonimmune rat IgG (0.2 mg/mouse) was administered intravenously 1 h before LPS challenge. Mice were sacrificed to collect sera and liver specimens at 0, 6, 12, 18, 24, and 36 h after LPS injection (n = 5 mice per time point).
Serum ALT measurement.
Serum was collected for the measurement of serum alanine transaminase (ALT) levels at various times after LPS challenge using standardized techniques.
Histological examination.
The liver specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Deparaffinized thin sections (6 μm) from each paraffin block were stained with hematoxylin and eosin for histological examination by light microscopy.
Immunohistochemistry.
Immunohistochemistry experiments were conducted on frozen sections as described in detail elsewhere (28). Sections were incubated with anti-CXCL16 antibody and, after being washed, incubated sequentially with HRP-conjugated anti-rat IgG antibodies. After visualization using 3,3′-diaminobenzidine containing 0.01% hydrogen peroxide and counterstaining with hematoxylin, sections were microscopically examined. Control sections were treated with omission of the primary antibody.
Preparation of LIL.
Liver-infiltrating lymphocytes (LIL) were prepared as described by Watanabe et al. (55). In brief, at the indicated time interval, mice were sacrificed to collect liver specimens. Entire livers taken from 4 mice were minced, pressed through the nylon mesh, and suspended in RPMI 1640 supplemented with 2% fetal calf serum (FCS). The cell suspension was treated with Percoll density (1.09) gradient centrifugation to remove liver parenchymal cells. The hepatic LIL that had been washed three times were resuspended in phosphate-buffered saline (PBS) and counted with a hemacytometer.
Cell culture.
The mouse vascular endothelial cells (SVEC) were maintained in RPMI 1640 supplemented with 10% fetal calf serum. Monolayers of SVEC were exposed for 24 h to TNF-α (20 ng/ml), and then the culture supernatants and cells were collected. Total RNA was isolated from cultured SVEC using Trizol according to the manufacturer's instruction.
Flow cytometry.
Flow cytometric immunofluorescence analyses were performed with a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA). To analyze expression of CXCL16/SR-PSOX on SVEC, after saturation of nonspecific binding sites with nonimmune rat IgG, 4 × 105 SVEC were incubated with rat anti-mouse CXCL16 or rat isotype IgG (as a control) at 4°C for 30 min and then washed two times with PBS solution. Cell suspensions were then incubated with FITC-conjugated anti-rat IgG and washed again. A total of 104 events for samples were acquired. The T cells of LIL were also analyzed with phycoerythrin-labeled anti-CD4 MAb and FITC-conjugated anti-CD8 MAb.
Analysis of CXCL16/SR-PSOX mRNA level by real-time quantitative PCR.
The specific mRNA level was quantified by real-time quantitative PCR using a LightCycler Instrument (Roche Molecular Biochemicals, Germany) as described previously, with minor modifications (9). The amplification conditions were as follows: initial denaturation at 95°C for 30 s and 40 cycles of 95°C for 0 s, 60°C for 3 s, and 72°C for 12 s. In the present study, the acquisition temperature for the fluorescence signal was adjusted to 84°C. PCR products were placed on 2% agarose gels by electrophoresis, and specificity and sensitivity were identified under UV.
Chemotaxis assay.
In vitro activation of T cells from mouse spleen was done as described previously, with minor modifications (15, 29). T cells were first isolated from single-spleen-cell suspensions by using nylon wool, and then CD8+ T cells were further prepared from nylon wool-enriched T cells using magnitude analysis cell sorting (MACS) immunobeads (1). For nylon wool enrichment, in brief, the spleen was pressed through sterile 70-μm nylon mesh and the pellet was treated with red blood cell lysis solution and washed twice with PBS. The nylon wool was teased into fine threads, rolled into a loose ball, and stuffed into a fresh 20-ml syringe. The weight of nylon wool for T-cell enrichment is 4 × 108 spleen cells/g. The nylon wool cushion was pressed to the mark of 12 ml, packed in a sterilization bag together with a piece of aluminum foil, and steam sterilized before use. Before T-cell preparation, a sterilized nylon wool column was gently filled with 20 ml RPMI 1640 supplemented with 2% FCS, covered with the aluminum foil, and incubated at 37°C, 5% CO2 for 1 h. After RPMI 1640 supplemented with 2% FCS was allowed to drip out, the cell suspension was filled into the column and incubated for 1 h at 37°C, 5% CO2. A fresh 50-ml collection tube in ice was put under the needle after incubation. The column was filled up completely with the prewarmed RPMI 1640 supplemented with 2% FCS, and the eluate cells (∼40 ml) were collected, counted, and stored on ice for later use (2). The magnetic enrichment of CD8+ T cells was performed according to the manufacturer's instructions. T cells enriched by nylon wool were spun down (5 min, 300 × g), resuspended at 108 cells/ml in labeling buffer, and filled into a 1.5-ml Eppendorf tube. The cell suspension was added to a cocktail of biotin-conjugated antibodies, mixed, incubated at 4°C for 10 min, washed (centrifugation at 450 × g for 3 min) with labeling buffer, and resuspended in MACS buffer. The MACS microbeads were added. After incubation for 15 min at 4°C, cells were washed twice in MACS buffer (450 × g for 3 min) and resuspended in 500 μl of buffer per 108 total cells. After the column was placed in the magnetic field of the MACS separator, the cell suspension was allowed to pass through the column and the effluent was collected as a fraction of the enriched CD8+ T cells. Both isolated T cells and CD8+ T cells were incubated at 106 cells/ml in anti-CD3- and anti-CD28-coated plates in RPMI 1640 medium supplemented with 10% fetal calf serum and mouse IL-2 (4 ng/ml) for 5 days, respectively. The activated T cells were then rested in the same medium supplemented with 2 ng/ml IL-2 for 4 days and were used as CXCR6-expressing cells. Chemotaxis assays for activated CD8+ T cells were conducted using transwell plates with 5-μm-pore-size membranes (Corning Costar, Corning, NY) as described previously, with minor modifications (35). The supernatants were added to control antibody (Ab) (100 μg/ml) or anti-CXCL16 Ab (final concentration was 30 μg/ml). The results were expressed as the chemotaxis index, which is the ratio of migration cells in experiment solution to those in medium solution.
Adhesion assay.
Leukocyte-endothelial cell adhesion was analyzed by fluorescent labeling of leukocytes according to the method of De Clerck et al., with minor modifications (7). In brief, activated T cells isolated by nylon wool were labeled with the dye calcein acetoxymethyl ester (10 pM, 30 min, 37°C). Cells were washed twice, resuspended in RPMI containing 2% FCS at 5 × 106 cells/ml, and added (5 × 105/ml, 100 μl/well) to confluent monolayers of SVEC (with or without TNF-α stimulation) in 24-well trays for 1 h. Total numbers of labeled test cells were assessed by recording the fluorescence intensity. Nonadherent calcein-labeled cells were removed by careful washing, and the adherent cells were measured for fluorescence intensity. To determine the role of CXCL16/SR-PSOX as adhesion molecules in leukocyte binding, confluent SVEC were incubated with anti-CXCL16 Ab (blocking Ab, 60 μg/well) for 30 min; nonimmune rat IgG (100 μg/well) served as a control before the addition of labeled cells. The percentage of test cells adhering to SVEC was calculated by the following formula: % adherence = (adherent intensity/total intensity) × 100. The results were expressed as follows: % binding = (% adherence/% adherence of test cells to activated SVEC) × 100.
Statistical analysis.
Data were expressed as means ± standard deviations (SD). The survival rates were analyzed using the generalized Wilcoxon test. Other data were determined by Student's t test, unless otherwise indicated, and by two-way analysis of variance and multiple comparison methods by Scheffe. P values of <0.05 were considered statistically significant.
RESULTS
Expression pattern and distribution of CXCL16/SR-PSOX in liver injury in mice.
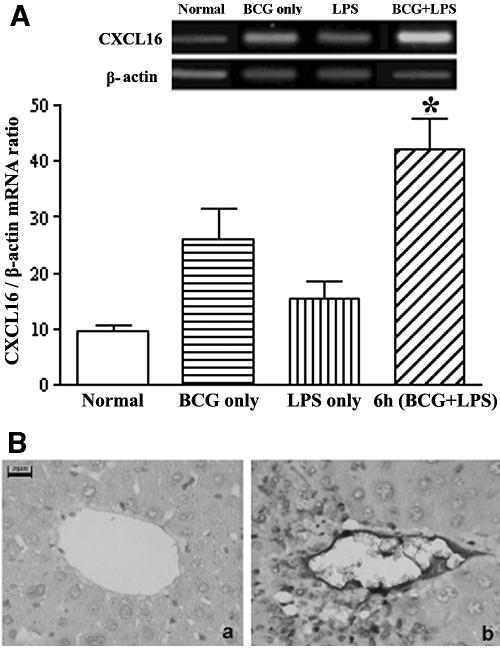
By real-time quantitative reverse transcription (RT)-PCR, the results showed that CXCL16/SR-PSOX mRNA transcripts were significantly up-regulated in the liver tissues of BCG-primed mice sequentially after LPS injection at 6 h (P < 0.05) and markedly higher than treatment with BCG alone or LPS alone, but expression of CXCL16/SR-PSOX was weak in normal liver tissue (Fig. 1A).
FIG. 1.
CXCL16/SR-PSOX expression in injured liver tissue examined by real-time quantitative RT-PCR and immunohistochemical examination. (A) Detection of CXCL16/SR-PSOX mRNA level. Total RNA was isolated from liver tissues as follows: normal (open bars); 12 days after BCG-only treatment (horizontally striped bars); 6 h after LPS-only injection (vertically striped bars); 6 h after sequential LPS challenge with BCG priming (diagonally striped bars). Total RNA was reverse transcribed with or without reverse transcriptase and detected by real-time quantitative PCR for CXCL16/SR-PSOX and β-actin. The amount of CXCL16/SR-PSOX was normalized to the level of β-actin. Data are means ± SD of the results from a representative of three separate experiments. *, P < 0.05 versus normal control. (B) Immunohistochemical examination of CXCL16/SR-PSOX expression. Sections of liver tissues obtained from normal mice (a) and BCG-primed mice sequentially injected with LPS at 6 h (b) are shown. Sections were incubated with anti-CXCL16 antibody and then with HRP-conjugated anti-rat IgG antibodies. After staining with 3,3′-diaminobenzidine and hematoxylin, sections were microscopically examined. CXCL16/SR-PSOX-positive cells are shown both in the endothelium and periportal area (b). Bars, 20 μm.
Immunohistochemical analysis further confirmed that expression of CXCL16/SR-PSOX significantly increased at 6 h in the liver tissue after LPS challenge in BCG-primed mice compared with the untreated mice, which was in agreement with the transcriptional level of CXCL16/SR-PSOX (Fig. 1A). Notably, up-regulated expression of CXCL16/SR-PSOX was predominantly distributed in the endothelium and periportal area (Fig. 1B).
CXCL16/SR-PSOX was expressed in resting vascular endothelial cells and could be up-regulated by TNF-α.
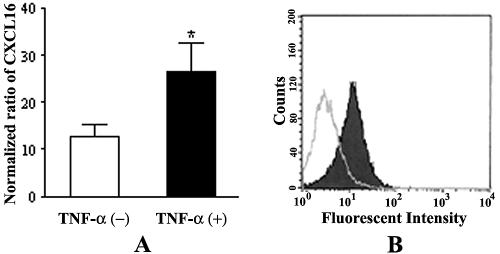
Based on up-regulation and distribution of CXCL16/SR-PSOX expression in the hepatic vascular endothelium in injured liver tissues, expression of CXCL16 regulated by TNF-α was further investigated in the SVEC cell line in vitro. The results showed that the total transcriptional level of CXCL16/SR-PSOX mRNA in SVEC markedly increased in response to TNF-α stimulation compared with the absence of TNF-α treatment (Fig. 2A) (P < 0.05). Moreover, membrane-anchored CXCL16/SR-PSOX on the SVEC surface was also up-regulated as determined by fluorescence-activated cell sorter analysis (Fig. 2B).
FIG. 2.
Analysis of CXCL16 expression in SVEC stimulated by TNF-α. Monolayers of SVEC were exposed to TNF-α (20 ng/ml) for 24 h. (A) Transcriptional levels of specific CXCL16/SR-PSOX mRNA in SVEC were measured by real-time quantitative RT-PCR and normalized to those of β-actin. The expression of CXCL16 in SVEC was up-regulated by TNF-α stimulation (*, P < 0.05 compared with nonstimulated cells). Results were obtained from three independent experiments. +, TNF-α stimulation; −, no TNF-α stimulation. (B) Membrane-anchored CXCL16 expression on endothelial cells was measured by flow cytometry. Black and white areas represent CXCL16 expression on SVEC with and without TNF-α stimulation, respectively.
Soluble CXCL16/SR-PSOX secreted by SVEC induced chemotaxis of activated CD8+ T cells.
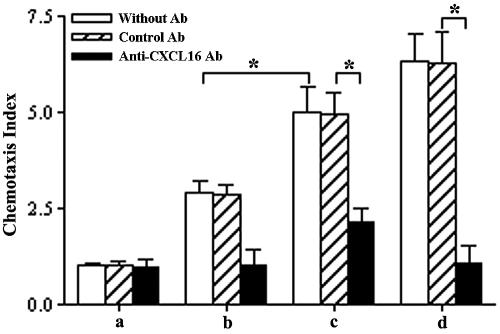
To detect the secreted CXCL16/SR-PSOX activity and expression in TNF-α-activated SVEC, a chemotactic activity assay was performed. The results showed that supernatants of SVEC induced a chemotactic response of activated CD8+ T cells, which further increased after TNF-α stimulation, whereas Dulbecco's modified Eagle's medium (control) failed to induce chemoattraction. On the other hand, the neutralizing anti-CXCL16 Ab added to supernatants significantly reduced chemotaxis of the activated CD8+ T cells, but the control Ab had no effect (Fig. 3) (P < 0.01).
FIG. 3.
Chemotaxis of activated CD8+ T cells to supernatants of TNF-α-activated vascular endothelial cells. a, Dulbecco's modified Eagle's medium; b, supernatant of cultured SVEC; c, supernatant of cultured SVEC stimulated with TNF-α for 24 h; d, recombinant CXCL16 (100 ng/ml). Chemotaxis was measured in the medium and supernatant without Ab (white bars) and with addition of control rat IgG Ab (striped bars) (100 μg/ml) or anti-CXCL16 Ab (black bars) (30 μg/ml). Three independent experiments were performed. The chemotaxis of CD8+ T cells to cell supernatants significantly increased without TNF-α treatment and was suppressed by specific Ab compared with control Ab. *, P < 0.01.
Membrane-anchored CXCL16/SR-PSOX-mediated adhesion of activated T cells to vascular endothelial cells.
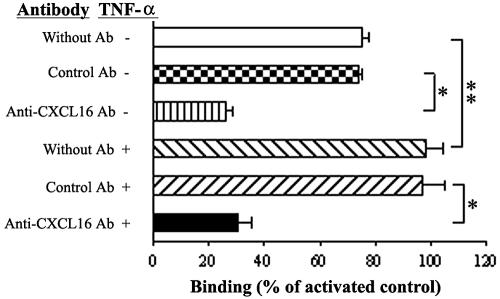
A leukocyte adhesion assay was performed to investigate the adhesion function of membrane-anchored CXCL16/SR-PSOX on SVEC. The results showed that activated T cells could adhere to SVEC in the absence of TNF-α stimulation to some extent but further increased about 30% after activation by TNF-α. This increase was 70% reduced by a blocking Ab to CXCL16/SR-PSOX, whereas the control antibody had no effect (Fig. 4).
FIG. 4.
Cell binding assay with SVEC. Increased binding of activated T cells to SVEC stimulated with TNF-α (20 ng/ml, 24 h) was inhibited by antibody to CXCL16 (142417, rat IgG2a). The binding of activated T cells is expressed as the percentage of binding of each group compared with a group of TNF-α-stimulated SVEC without blocking antibody. Results represent those from at least three independent experiments. The binding of T cells to TNF-α-activated (+) SVEC significantly increased compared to no TNF-α stimulation (−) and was markedly inhibited by specific Ab compared to control Ab. * and **, P < 0.01.
Survival of mice in endotoxin-induced lethal liver injury was rescued by anti-CXCL16 antibody administration in vivo.
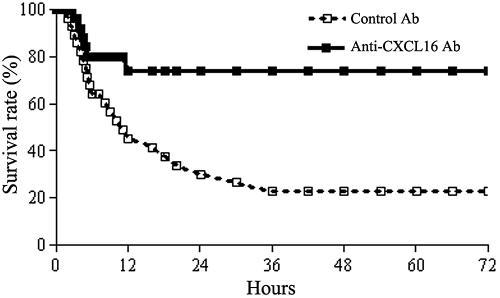
To investigate the biological role of CXCL16 in this murine model, mice were treated with neutralizing anti-CXCL16 antibody 1 h before LPS injection. The results showed that anti-CXCL16 antibody administration could significantly protect mice from lethal liver injury induced by BCG and LPS treatment (P < 0.01). Approximately 70% of the mice (n = 10) survived for 72 h, whereas 40% of the mice treated with control Ab died within 6 h after LPS injection and 60% died within 12 h (Fig. 5).
FIG. 5.
Survival of mice with endotoxin-induced lethal liver injury administered by anti-CXCL16 antibody. The endotoxin-induced lethal liver injury was induced by intravenous injection of 7.5 μg of LPS 12 days after priming with 5 × 107 BCG. The mice were intravenously injected with 0.2 mg/mouse anti-CXCL16 Ab (▪, solid line, n = 10) or 0.2 mg/mouse control rat IgG (□, dashed line, n = 10) 1 h before LPS challenge. The survival rates were analyzed statistically using the generalized Wilcoxon test. The anti-CXCL16 antibody significantly protected the mice from lethality (P < 0.01, compared with control Ab).
Endotoxin-induced lethal liver injury was reduced in the murine model by treatment with anti-CXCL16 antibody.
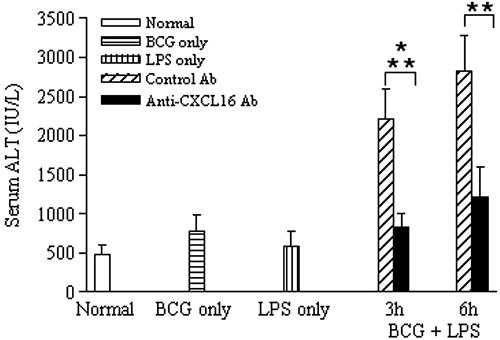
It is well known that viable BCG priming followed by LPS injection induces a widely utilized murine endotoxin-induced lethal liver injury (22, 42, 57). The serum ALT level moderately increased in mice induced by BCG priming alone or LPS injection alone but was elevated dramatically at 3 h to 6 h (n = 5) after subsequent LPS challenge (Fig. 6) (P < 0.05). By examining the effect of anti-CXCL16 antibody on ALT activity, as shown in Fig. 6, it was determined that administration of the anti-CXCL16 antibody significantly decreased the ALT level (P < 0.01) but control Ab did not.
FIG. 6.
Effect of anti-CXCL16 Ab on serum ALT levels. The serum samples (n = 5 at each time point) were obtained from mice as follows: normal (open bars), 12 days after BCG-only treatment (horizontally striped bars), 6 h after LPS-only injection (vertically striped bars), and 3 h and 6 h after sequential LPS injection (with control Ab [diagonally striped bars] or anti-CXCL16 Ab [black bars]). Data represent means ± SD. Differences in serum ALT levels between LPS challenge and normal mice are significant. *, P < 0.05. Specific Ab treatment significantly suppressed the elevation of ALT compared to control Ab. **, P < 0.05.
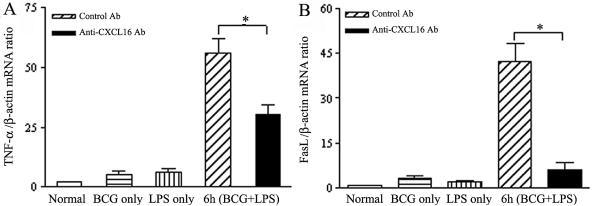
TNF-α and FasL have been reported to contribute to liver injury in various animal models, including an animal model of endotoxin-induced hepatitis (33, 50, 51, 52). Both TNF-α and FasL were weakly expressed in the normal liver, and a little increase was observed at 12 days after treatment with BCG alone or at 6 h after injection of LPS alone, but their expression in the injured liver tissue was highly induced after 6 h of sequential LPS injection with BCG priming. Administration of anti-CXL16 Ab markedly reduced both TNF-α and FasL mRNA expression in the liver after LPS challenge compared to control Ab (Fig. 7A and B) (P < 0.01).
FIG. 7.
Effect of anti-CXCL16 antibody on expression of TNF-α and FasL in liver tissues. Expression levels of TNF-α (A) and FasL (B) mRNA in the liver tissue are shown. Total RNA was isolated from liver tissues as follows: normal (open bars), 12 days after BCG-only treatment (horizontally striped bars), 6 h after LPS-only injection (vertically striped bars), and 6 h after sequential LPS injection to BCG-primed mice (with control Ab [diagonally striped bars] or anti-CXCL16 Ab [black bars]). Total RNA was reverse transcribed with or without reverse transcriptase and detected by real-time quantitative PCR for CXCL16/SR-PSOX and β-actin. The amount of CXCL16/SR-PSOX was normalized to the level of β-actin. Data represent the means ± SD of four measurements. Differences between anti-CXCL16 Ab- and control Ab-treated mice are significant. *, P < 0.05.
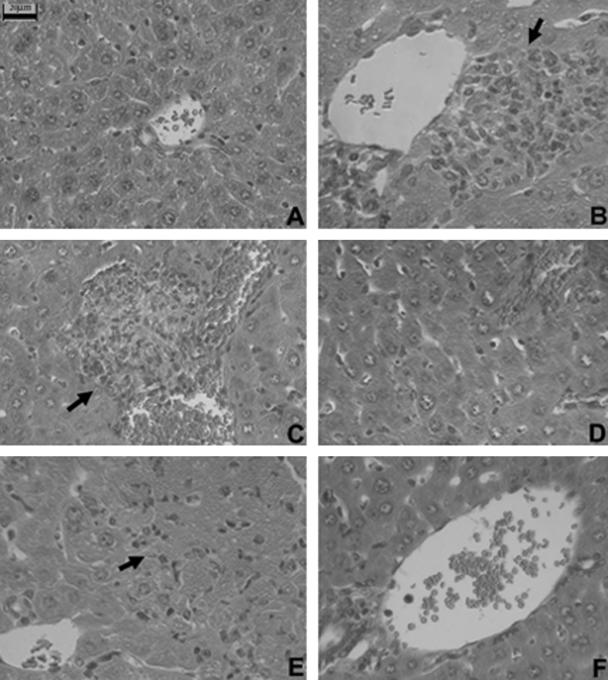
Pathological examination also supported the protective role of anti-CXCL16 antibody treatment in vivo. Mononuclear cells were shown to infiltrate into liver lobes through the vascular endothelium and form granulomas in the liver at 12 days after BCG treatment compared to the untreated mice (Fig. 8A and B). Diffuse infiltration of mononuclear cells and multilobular hepatocellular necrosis appeared but not in BCG-primed mice that received anti-CXCL16 treatment at 6 h and that were sequentially challenged by LPS (Fig. 8C and D). Massive necrosis with diffuse infiltration of mononuclear cells was observed in the liver of BCG-primed mice after LPS challenge at 24 h with control Ab treatment (Fig. 8E). In contrast, liver necrosis was significantly reduced by anti-CXCL16 Ab treatment, and the specific Ab blocked the increase of mononuclear cell infiltration in lobes and portal areas (Fig. 8F). Polymorphonuclear cells were scarcely found in histological study.
FIG. 8.
Effect of anti-CXCL16 antibody on multilobular necrosis in liver injury induced by BCG and LPS. (A) Normal liver tissue without any treatment. (B) Liver tissue of BCG-primed mice at 12 days (control) without LPS challenge. An arrow shows granulomas. (C) Liver tissue of BCG-primed mice sequentially injected with LPS at 6 h. An arrow shows hepatocellular necrosis and liver-infiltrating lymphocytes. (D) Liver tissue of mice primed with BCG treated with anti-CXCL16 Ab followed by LPS challenge at 6 h. (E) Liver tissue of mice primed with BCG treated with nonimmune rat IgG followed by LPS challenge at 24 h. An arrow shows a necrotic lesion. (F) Liver tissue of mice primed with BCG treated with the anti-CXCL16 Ab followed by LPS challenge at 24 h. Tissues were stained with hematoxylin and eosin and examined histologically. Bars, 20 μm.
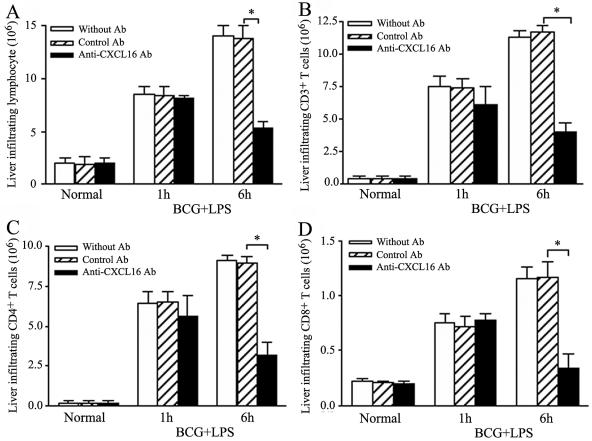
Recruitment of liver-infiltrating lymphocytes and T cells into the liver was inhibited with anti-CXCL16 antibody administration.
To identify the infiltration of lymphocytes, LIL were isolated and counted. The results showed that numbers of LIL increased from 1.98 × 106/liver to 8.92 × 106/liver at 1 h and further increased to 13.8 × 106/liver at 6 h after subsequent LPS challenge to the BCG-primed mice. In contrast, the number of LIL significantly diminished from 13.8 × 106/liver to 5.3 × 106/liver at 6 h after subsequent LPS challenge by anti-CXCL16 Ab administration (P < 0.01) compared with control Ab treatment (Fig. 9A).
FIG. 9.
Effects of anti-CXCL16 antibody on infiltration of LIL in injured liver tissues. (A) Effect of anti-CXCL16 on total number of LIL. (B) Effect of anti-CXCL16 on liver-infiltrating CD3+ T lymphocytes. (C, D) Intrahepatic infiltration of CD4+ (C) and CD8+ (D) T cells in the mice treated with anti-CXCL16 antibody. Mice were primed with BCG and sequentially challenged by LPS 12 days after priming. Anti-CXCL16 antibody and control antibody were administrated to the mice 1 h before LPS injection, respectively. The LIL were prepared from normal, BCG-primed mice sequentially injected with LPS at 1 h and 6 h (without any treatment [open bars], with control Ab [striped bars], or with anti-CXCL16 Ab [black bars]). The absolute numbers of CD3+, CD4+, and CD8+ T cells were determined by multiplying the total LIL number by the fractions of the CD3+, CD4+, and CD8+ T-cell populations. Results were obtained from three independent experiments. Statistically significant differences between specific Ab and control Ab are labeled. *, P < 0.05.
To confirm the anti-CXCL16 antibody could block infiltration of T cells during the liver damage, cell surface expression of CD3, CD4, and CD8 in the LIL was analyzed using a flow cytometer. The results revealed that administration of anti-CXCL16 antibody in vivo significantly decreased the number of CD3+ T cells (from 11.75 × 106 to 3.955 × 106/liver), CD4+ T cells (from 8.942 × 106 to 3.185 × 106/liver), and CD8+ T cells (from 1.162 × 106 to 0.339 × 106/liver) in the liver in BCG-primed mice injected with LPS at 6 h (Fig. 9B, C, and D) compared with control antibody treatment (P < 0.05).
DISCUSSION
In this study, we reported that expression of CXCL16/SR-PSOX was up-regulated and predominantly distributed in the endothelium in injured liver tissues. Functional CXCL16/SR-PSOX in vascular endothelial cells was up-regulated by TNF-α stimulation and involved in chemotaxis and adhesion of activated T cells with secretory and membrane-anchored forms. Administration of anti-CXCL16 antibody markedly blocked the infiltration of T cells and protected the mice from endotoxin-induced lethal liver injury. These findings indicate that CXCL16/SR-PSOX, as both a chemokine and as an adhesion molecule, might contribute to the recruitment of T cells from the subendothelial space into liver tissue and play an important role in endotoxin-induced lethal liver injury.
Little or no expression of CXCL16/SR-PSOX could be detected in the normal liver tissue. However, CXCL16/SR-PSOX expression significantly increased in our previous study using the suppression subtractive hybridization, consistent with a previous study using cDNA microarray analysis (8). Immunohistochemical analysis further showed that CXCL16/SR-PSOX predominantly distributed in the periportal area and endothelium (including the sinusoidal and vascular endothelia) in the liver tissue, and its roles expressed inside the liver and endothelium are still not clear. CXCL16/SR-PSOX is a chemokine with both soluble and membrane-anchored forms and is predominantly expressed in antigen-presenting cells. Its counterreceptor, CXCR6, is mainly distributed on activated T cells (29, 34, 56) but not on B cells, macrophages, or neutrophils. Endotoxin-induced liver injury in mice pretreated with heat-killed Propionibacterium acnes or viable BCG has been widely used as a model of acute liver failure. Evidence has revealed that T cells might play an important role in endotoxin-induced shock/liver injury (20, 47, 50). However, the mechanisms involved in recruitment of T cells remain unclear. Besides T cells, macrophages, polymorphonuclear neutrophils and NK cells, etc., might be the most important cell types. Systemic high-dose LPS exposure induces an inflammatory response: neutrophils infiltrate and adhere to parenchymal cells to cause hepatocellular injury in LPS-induced shock and even provoke the liver injury by recruitment of other leukocytes (17, 40). However, available evidence suggested that small doses of LPS that do not cause overt organ injury may be most effective at inhibiting extravascular recruitment of neutrophils, and susceptibility of mice to low-dose LPS was virtually unchanged by in vivo depletion of granulocytes (11, 19, 54). Likewise, polymorphonuclear cells were scarcely found in histological study in the present experimental liver injury. IFN-γ produced by liver extrathymic T cells might induce FasL in liver NK cells, and these liver NK cells could drive Fas-FasL-mediated liver apoptosis, leading to massive live injury (51a). The natural killer 1.1+ (NK1.1+) T-cell population is a T-cell subset that expresses NK markers and expresses the canonical Vα14 Jα281. However, it was reported that administration of P. acnes diminished CD4+ NK1.1+ T cells, and a numerical reduction of Vα14+ NK T cells in the liver is not responsible for the low-dose-LPS-induced shock/liver injury in mice (11, 30). In our experiments, we also examined the expression of Vα14 Jα281 mRNA, and the results showed that expression level of Vα14 Jα281 did not significantly change in the different time points in BCG-LPS-induced liver injury (data not shown). Hence, it could be speculated that CD4+ NK1.1+ T cells, NK cells, and granulocytes are not responsible for endotoxic shock/liver injury.
The up-regulated CXCL16/SR-PSOX was mostly distributed in periportal regions, which consist of the interlobular vein, interlobular arteries, and interlobular bile duct and are rich in vascular endothelial cells. It is extensively reported that TNF-α is involved in endotoxin-induced shock/liver injury and potently increases the expression of many cell adhesion molecules, thus increasing the adhesion between leukocytes and the endothelium (5, 10, 27). In our study, it seemed that TNF-α expression was closely correlated with the expression of CXCL16 in the model. Therefore, the inflammatory mediator, TNF-α, at least in part, could mimic the milieu of the inflamed liver and could be employed as a typical inflammatory stimulant. Moreover, the membrane-anchored CXCL16/SR-PSOX, as an adhesion molecule, may also function to recruit and retain T cells on the endothelium during inflamed liver injury. These prompted us to further examine the expression and function of CXCL16/SR-PSOX in SVEC. The results indicated that both secretory and membrane-anchored CXCL16 were up-regulated by TNF-α stimulation and involved in the chemotactic response and direct adhesion of activated T cells to the endothelial cells, respectively. In contrast, its corresponding functions were significantly inhibited with a neutralizing anti-CXCL16 Ab blockade. However, specific anti-CXCL16 antibody couldn't abrogate the chemotaxis and adhesion of activated T cells and even showed a slight increase compared with non-TNF-α stimulation; these may be associated with the production of other chemokines, such as gamma interferon-inducible protein 10 and monokine induced by gamma interferon (1, 24), and other adhesion molecules, such as vascular adhesion protein 1 and intercellular adhesion molecule 1 (4, 61). The CXCL16/SR-PSOX has been reported to mediate adhesion of activated CD8+ T cells to vascular cell adhesion molecule 1 through activation of very late antigen 4 (58). TNF-α (20 ng/ml) could up-regulate expression of CXCL16/SR-PSOX; however, a higher dose of TNF-α obviously induced SVEC apoptosis (data not shown). The blockade of adhesion between activated T cells and SVEC was shown to be anti-CXCL16 Ab concentration dependent; a higher specific antibody concentration (80 μg/ml) had no further effect on adhesion (data not shown). Combined, these findings indicated that CXCL16/SR-PSOX expressed in the vascular endothelium, as both a chemokine and an adhesion molecule, might contribute to the recruitment and adhesion of activated T cells in the inflammation milieu.
To elucidate the involvement of CXCL16/SR-PSOX in T cell trafficking in endotoxin-induced lethal liver injury in mice, a neutralizing anti-CXCL16 Ab was further administrated to BCG-primed mice before LPS challenge in vivo. The results showed that both CD4+ and CD8+ T cells significantly increased at the time point of 6 h after sequential LPS challenge in BCG-primed mice and that administration of anti-CXCL16 antibody significantly inhibited the recruitment of CD4+ and CD8+ T cells and protected mice from multilobular necrosis and lethality. These could explain, at least in part, the report in which T cells were considered to participate in endotoxic shock/live injury as one of the cytotoxic effectors (36, 44, 47, 48, 53, 57, 60). These findings indicated that T cells, which are CXCR6-expressing lymphocytes, perhaps accompanying other leukocytes if T cells are not the one and only, are involved in the pathogenesis of acute liver injury in this model. Furthermore, several reports have shown that some molecules, such as chemokines and adhesion molecules, also contributed to the pathogenesis of endotoxin-induced liver damage (2, 3, 22, 24, 32, 59), and these might explain why anti-CXCL16 antibody administration could not completely protect the mice from lethality. The present paradigm of leukocytes recruited to the inflamed liver is a multiple-step model which describes the sequence of tethering/rolling, firm adhesion, and diapedesis/extravasation. Expression of CXCL16/SR-PSOX in the endothelium and inside the liver might be involved in T cell accumulation through adhesion and chemotaxis in the pathogenesis of endotoxin-induced liver injury.
Anti-CXCL16 antibody significantly protected the mice from lethality, accompanied by reduction in serum ALT levels and decreased TNF-α and FasL expression. From this study, it seemed that TNF-α could up-regulate CXCL16 expression in the endothelium, which in turn recruited T cells. It is possible that the TNF-α produced is from the CXCL16-recruited T cells so that inhibition of CXCL16 results in decreased T-cell accumulation and, thus, TNF-α secretion. The FasL-Fas interaction plays an important role in T-cell-mediated cytotoxicity and liver injury (26, 37, 39, 41, 46, 52). The FasL is expressed in activated T effector cells and induces apoptosis in Fas-bearing hepatocytes (23, 25, 45). Moreover, Fas may also trigger hepatic inflammatory responses by inducing the expression of hepatic chemokines that recruit and activate immune cells, leading to liver injury (12). CXCL16/SR-PSOX might affect infiltration of activated T cells, resulting in hepatocyte apoptosis, but the precise mechanism by which the anti-CXCL16 antibody decreased FasL expression needs to be further investigated. Combined, our experimental data suggest that CXCL16/SR-PSOX might play a crucial role in endotoxin-induced liver injury by mediating infiltration of activated T cells.
In summary, the CXCL16/SR-PSOX, as a dual-function molecule, was involved in recruitment and adhesion of activated T cells to the vascular endothelium in endotoxin-induced lethal liver injury. Notably, blockade of CXCL16/SR-PSOX protected the mice from endotoxin-induced liver injury. These results demonstrated a critical function of CXCL16/SR-PSOX in inflammatory responses to bacteria and implicated CXCL16 as a potential therapeutic target for such diseases.
Acknowledgments
We thank R. S. Veazey (Tulane National primate research center, New Orleans, LA) for helpful comments.
This work was supported by National Natural Science Fund of China (30230320, 39925031), National High Technology Research and Development Program of China (2004AA215242), Major State Basic Research Development Program of China (2001CB510005), and the Program of STCSM (04DZ14902).
Editor: J. L. Flynn
REFERENCES
- 1.Adams, D. H., P. Burra, S. G. Hubscher, E. Elias, and W. Newman. 1994. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology 19:588-594. [DOI] [PubMed] [Google Scholar]
- 2.Agace, W. W., J. M. Higgins, B. Sadasivan, M. B. Brenner, and C. M. Parker. 2000. T-lymphocyte-epithelial-cell interactions: integrin α(E)(CD103)β(7), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 12:563-568. [DOI] [PubMed] [Google Scholar]
- 3.Bazan, J. F., K. B. Bacon, G. Hardiman, W. Wang, K. Soo, D. Rossi, D. R. Greaves, A. Zlotnik, and T. J. Schall. 1997. A new class of chemokine with a CX3C motif. Nature 385:640-644. [DOI] [PubMed] [Google Scholar]
- 4.Bonder, C. S., M. N. Ajuebor, L. D. Zbytnuik, P. Kubes, and M. G. Swain. 2004. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 172:45-53. [DOI] [PubMed] [Google Scholar]
- 5.Cines, D. B., E. S. Pollak, C. A. Buck, J. Loscalzo, G. A. Zimmerman, R. P. McEver, J. S. Pober, T. M. Wick, B. A. Konkle, B. S. Schwartz, E. S. Barnathan, K. R. McCrae, B. A. Hug, A. M. Schmidt, and D. M. Stern. 1998. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91:3527-3561. [PubMed] [Google Scholar]
- 6.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 7.De Clerck, L. S., C. H. Bridts, A. M. Mertens, M. M. Moens, and W. J. Stevens. 1994. Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J. Immunol. Methods 172:115-124. [DOI] [PubMed] [Google Scholar]
- 8.Dong, H. Y., N. Toyoda, H. Yoneyama, M. Kurachi, T. Kasahara, Y. Kobayashi, H. Inadera, S. Hashimoto, and K. Matsushima. 2002. Gene expression profile analysis of the mouse liver during bacteria-induced fulminant hepatitis by a cDNA microarray system. Biochem. Biophys. Res. Commun. 298:675-686. [DOI] [PubMed] [Google Scholar]
- 9.Dumoulin, F. L., H. D. Nischalke, L. Leifeld, A. V. dem Bussche, J. K. Rochstroh, T. Sauerbruch, and U. Spengler. 2000. Semi-quantitation of human C-C chemokine mRNA with reverse transcription/real-time PCR using multi-specific standards. J. Immunol. Methods 241:109-119. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, J. L., R. G. Collins, A. L. Beaudet, C. M. Ballantyne, and K. Ley. 2003. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-α-induced inflammation. J. Immunol. 171:6105-6111. [DOI] [PubMed] [Google Scholar]
- 11.Emoto, M., Y. Emoto, V. Brinkmann, M. Miyamoto, I. Yoshizawa, M. Stäber, N. van Rooijen, A. Hamann, and S. Kaufmann. 2003. Increased resistance of LFA-1-deficient mice to lipopolysaccharide-induced shock/liver injury in the presence of TNF-α and IL-12 is mediated by IL-10: a novel role for LFA-1 in the regulation of the proinflammatory and anti-inflammatory cytokine balance. J. Immunol. 171:584-593. [DOI] [PubMed] [Google Scholar]
- 12.Faouzi, S., B. E. Burckhardt, J. C. Hanson, C. B. Campe, L. W. Schrum, R. A. Rippe, and J. J. Maher. 2001. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-κB-independent, caspase-3-dependent pathway. J. Biol. Chem. 276:49077-49082. [DOI] [PubMed] [Google Scholar]
- 13.Ferluga, J. 1981. Tuberculin hypersensitivity hepatitis in mice infected with Mycobacterium bovis (BCG). Am. J. Pathol. 105:82-90. [PMC free article] [PubMed] [Google Scholar]
- 14.Fujioka, N., N. Mukaida, A. Harada, M. Akiyama, T. Kasahara, K. Kuno, A. Ooi, M. Mai, and K. Matsushima. 1995. Preparation of specific antibodies against murine IL-1ra and the establishment of IL-1rα as an endogenous regulator of bacteria-induced fulminant hepatitis in mice. J. Leukoc. Biol. 58:90-98. [DOI] [PubMed] [Google Scholar]
- 15.Gunzer, M., C. Weishaupt, L. Planelles, and S. Grabbe. 2001. Two-step negative enrichment of CD4q and CD8q T cells from murine spleen via nylon wool adherence and an optimized antibody cocktail. J. Immunol. Methods 258:55-63. [DOI] [PubMed] [Google Scholar]
- 16.Heydtmann, M., and D. H. Adams. 2002. Understanding selective trafficking of lymphocyte subsets. Gut 50:102-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeschke, H., A. Farhood, and C. W. Smith. 1991. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am. J. Physiol. 261:G1051-G1056. [DOI] [PubMed] [Google Scholar]
- 18.Johnston, B., C. H. Kim, D. Soler, M. Emoto, and E. C. Butcher. 2003. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J. Immunol. 171:2960-2969. [DOI] [PubMed] [Google Scholar]
- 19.Kajdacsy-Balla, A., E. M. Doi, M. R. Lerner, W. D. Bales, L. T. Archer, P. R. Wunder, M. F. Wilson, and D. J. Brackett. 1996. Dose-response effect of in vivo administration of endotoxin on polymorphonuclear leukocytes oxidative burst. Shock 5:357-361. [DOI] [PubMed] [Google Scholar]
- 20.Kamiyasu, M., Y. Watanabe, T. Miura, K. Masuda, T. Nakanishi, G. Kajiyama, and M. E. Gershwin. 1997. Experimental hepatitis in neonatally thymectomized mice: transfer of disease and the role of T cells. Clin. Immunol. Immunopathol. 83:302-309. [DOI] [PubMed] [Google Scholar]
- 21.Kim, C. H., E. J. Kunkel, J. Boisvert, B. Johnston, J. J. Campbell, M. C. Genovese, H. B. Greenberg, and E. C. Butcher. 2001. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Investig. 107:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, S., J. Nishihira, S. Watanabe, and S. Todo. 1999. Prevention of lethal acute hepatic failure by antimacrophage migration inhibitory factor antibody in mice treated with Bacille Calmette-Guerin and lipopolysaccharide. Hepatology 29:1752-1759. [DOI] [PubMed] [Google Scholar]
- 23.Kondo, T., T. Suda, H. Fukuyama, M. Adachi, and S. Nagata. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3:409-413. [DOI] [PubMed] [Google Scholar]
- 24.Lalor, P. F., P. Shields, A. J. Grant, and D. H. Adams. 2002. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 80:52-64. [DOI] [PubMed] [Google Scholar]
- 25.Li, X. K., M. Fujino, A. Sugioka, M. Morita, T. Okuyama, L. Guo, N. Funeshima, H. Kimura, S. Enosawa, H. Amemiya, and S. Suzuki. 2001. Fulminant hepatitis by Fas-ligand expression in MRL-lpr/lpr mice grafted with Fas-positive livers and wild-type mice with Fas-mutant livers. Transplantation 71:503-508. [DOI] [PubMed] [Google Scholar]
- 26.Lowin, B., M. Hahne, C. Mattmann, and J. Tschopp. 1994. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathway. Nature 370:650-652. [DOI] [PubMed] [Google Scholar]
- 27.Luscinskas, F. W., and M. A. Gimbrone, Jr. 1996. Endothelial-dependent mechanisms in chronic inflammatory leukocyte recruitment. Annu. Rev. Med. 47:413-421. [DOI] [PubMed] [Google Scholar]
- 28.Marra, F., R. DeFranco, C. Grappone, S. Milani, S. Pastacaldi, M. Pinzani, R. G. Romanelli, G. Laffi, and P. Gentilini. 1998. Increased expression of monocytes chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am. J. Pathol. 152:423-430. [PMC free article] [PubMed] [Google Scholar]
- 29.Matloubian, M., A. David, S. Engel, J. E. Ryan, and J. G. Cyster. 2000. A membrane-anchored CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 1:298-304. [DOI] [PubMed] [Google Scholar]
- 30.Matsui, K., T. Yoshimoto, H. Tsutsui, Y. Hyodo, N. Hayashi, K. Hiroishi, N. Kawada, H. Okamura, K. Nakanishi, and K. Higashino. 1997. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J. Immunol. 159:97-106. [PubMed] [Google Scholar]
- 31.Mizoguchi, Y., Y. Sakagami, H. Kuboi, K. Kobayashi, and I. Yano. 1988. Effects of the polysaccharide chain of lipopolysaccharide in an experimental massive hepatic cell necrosis model. Biochem. Biophys. Res. Commun. 155:1305-1310. [DOI] [PubMed] [Google Scholar]
- 32.Mochida, S., A. Ohno, M. Arai, T. Tamatani, M. Miyasaka, and K. Fujiwara. 1996. Role of adhesion molecules in the development of massive hepatic necrosis in rats. Hepatology 23:320-328. [DOI] [PubMed] [Google Scholar]
- 33.Nagakawa, J., I. Hishinuma, K. Hirota, K. Miyamoto, T. Yamanaka, K. Tsukidate, K. Katayama, and I. Yamatsu. 1990. Involvement of tumor necrosis factor-alpha in the pathogenesis of activated macrophage-mediated hepatitis in mice. Gastroenterology 99:758-765. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama, T., K. Hieshima, D. Izawa, Y. Tatsumi, A. Kanamaru, and O. Yoshie. 2003. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J. Immunol. 170:1136-1140. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama, T., R. Fujisawa, D. Izawa, K. Hieshima, K. Takada, and L. Yoshie. 2002. Human B cells immortalized with Epstein-Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J. Virol. 76:3072-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura, T., and A. Ohta. 1999. A critical role for antigen-specific Th1 cells in acute liver injury in mice. J. Immunol. 162:6503-6509. [PubMed] [Google Scholar]
- 37.Ogasawara, J., R. Watanabe-Fukunaga, M. Adachi, A. Matsuzawa, T. Kasugai, Y. Kitamura, N. Itoh, T. Suda, and S. Nagata. 1993. Lethal effect of the anti-Fas antibody in mice. Nature 364:806-809. [DOI] [PubMed] [Google Scholar]
- 38.Raetz, C. R. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59:129-170. [DOI] [PubMed] [Google Scholar]
- 39.Ryo, K., Y. Kamogawa, I. Ikeda, K. Yamauchi, S. Yonehara, S. Nagata, and N. Hayashi. 2000. Significance of Fas antigen-mediated apoptosis in human fulminant hepatic failure. Am. J. Gastroenterol. 95:2047-2055. [DOI] [PubMed] [Google Scholar]
- 40.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 41.Seino, K., N. Kayagaki, K. Takeda, K. Fukao, K. Okumura, and H. Yagita. 1997. Contribution of Fas ligand to T cell-mediated hepatic injury in mice. Gastroenterology 113:1315-1322. [DOI] [PubMed] [Google Scholar]
- 42.Shands, J. W., and V. C. Senterfitt. 1972. Endotoxin-induced hepatic damage in BCG-infected mice. Am. J. Pathol. 67:23-40. [PMC free article] [PubMed] [Google Scholar]
- 43.Shimaoka, T., T. Nakayama, N. Fukumoto, N. Kume, S. Takahashi, J. Yamaguchi, M. Minami, K. Hayashida, T. Kita, J. Ohsumi, O. Yoshie, and S. Yonehara. 2004. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J. Leukoc. Biol. 75:267-274. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu, Y., J. A. Margenthaler, K. Landeros, N. Otomo, G. Doherty, and M. W. Flye. 2002. The resistance of P. acnes-primed interferon gamma deficient mice to low-dose lipopolysaccharide-induced acute liver injury. Hepatology 35:805-814. [DOI] [PubMed] [Google Scholar]
- 45.Song, E., S. K. Lee, J. Wang, N. Ince, N. T. Ouyang, J. Min, J. Chen, P. Shankar, and J. Lieberman. 2003. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 9:347-351. [DOI] [PubMed] [Google Scholar]
- 46.Takeda, K., Y. Hayakawa, L. Van Kaer, H. Matsuda, H. Yagita, and K. Okumura. 2000. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. USA 97:5498-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka, Y., A. Takahashi, K. Kobayashi, I. Arai, S. Higuchi, S. Otomo, K. Watanabe, S. Habu, and T. Nishimura. 1995. Establishment of a T cell-dependent nude mouse liver injury model induced by Propionibacterium acnes and LPS. J. Immunol. Methods 182:21-28. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, Y., A. Takahashi, K. Watanabe, Y. Takayama, T. Yahata, S. Habu, and T. Nishimura. 1996. A pivotal role of IL-12 in Th1-dependent mouse liver injury. Int. Immunol. 8:569-576. [DOI] [PubMed] [Google Scholar]
- 49.Reference deleted.
- 50.Tsuji, H., A. Harada, N. Mukaida, Y. Nakanuma, H. Bluethmann, S. Kaneko, K. Yamakawa, S. I. Nakamura, K. I. Kobayashi, and K. Matsushima. 1997. Tumor necrosis factor receptor p55 is essential for intrahepatic granuloma formation and hepatocellular apoptosis in a murine model of bacterium-induced fulminant hepatitis. Infect. Immun. 65:1892-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuji, H., N. Mukaida, A. Harada, S. Kaneko, E. Matsushita, Y. Nakanuma, H. Tsutsui, H. Okamura, K. Nakanishi, Y. Tagawa, Y. Iwakura, K. Kobayashi, and K. Matsushima. 1999. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-γ-deficient mice by a concomitant reduction of TNF-α, IL-12, and IL-18 production. J. Immunol. 162:1049-1055. [PubMed] [Google Scholar]
- 51a.Tsutsui, H., K. Nakanishi, K. Matsui, K. Higashino, H. Okamura, Y. Miyazawa, and K. Kaneda. 1996. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J. Immunol. 157:3967-3973. [PubMed] [Google Scholar]
- 52.Tsutsui, H., K. Matsui, N. Kawada, Y. Hyodo, N. Hayashi, H. Okamura, K. Higashino, and K. Nakanishi. 1997. IL-18 accounts for both TNF-alpha and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol. 159:3961-3967. [PubMed] [Google Scholar]
- 53.Ulmer, A. J., H. D. Flad, T. Rietschel, and T. Mattern. 2000. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology 152:37-45. [DOI] [PubMed] [Google Scholar]
- 54.Wagner, J. G., and R. A. Roth. 1999. Neutrophil migration during endotoxemia. J. Leukoc. Biol. 66:10-24. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe, H., K. Ohtsuka, M. Kimura, Y. Ikarashi, K. Ohmori, A. Kusumi, T. Ohteki, S. Seki, and T. Abo. 1992. Details of an isolation method for hepatic lymphocytes in mice. J. Immunol. Methods 146:145-154. [DOI] [PubMed] [Google Scholar]
- 56.Wilbanks, A., S. C. Zondlo, K. Murphy, S. Mak, D. Soler, P. Langdon, D. P. Andrew, L. Wu, and M. Briskin. 2001. Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC, CXC, and CX3C chemokines. J. Immunol. 166:5145-5154. [DOI] [PubMed] [Google Scholar]
- 57.Yajima, T., H. Nishimura, K. Saito, H. Kuwano, and Y. Yoshikai. 2004. Overexpression of interleukin-15 increases susceptibility to lipopolysaccharide-induced liver injury in mice primed with Mycobacterium bovis bacillus Calmette-Guerin. Infect. Immun. 72:3855-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamauchi, R., M. Tanaka, N. Kume, M. Minami, T. Kawamoto, K. Togi, T. Shimaoka, S. Takahashi, J. Yamaguchi, T. Nishina, M. Kitaichi, M. Komeda, T. Manabe, S. Yonehara, and T. Kita. 2004. Upregulation of SR-PSOX/CXCL16 and recruitment of CD8+ T cells in cardiac valves during inflammatory valvular heart disease. Arterioscler. Thromb. Vasc. Biol. 24:282-287. [DOI] [PubMed] [Google Scholar]
- 59.Yoneyama, H., A. Harada, T. Imai, B. Masataka, Y. Osamu, Y. Zhang, H. Higashi, M. Murai, H. Asakura, and K. Matsushima. 1998. Pivotal role of TARC, a CC chemokine, in bacteria-induced fulminant hepatic failure in mice. J. Clin. Investig. 102:1933-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneyama, H., S. Narumi, Y. Zhang, M. Murai, M. Baggiolini, A. Lanzavecchia, T. Ichida, H. Asakura, and K. Matsushima. 2002. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J. Exp. Med. 195:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoong, K. F., G. McNab, S. G. Hubscher, and D. H. Adams. 1998. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J. Immunol. 160:3978-3988. [PubMed] [Google Scholar]