Abstract
Jharkhand is a minerally prosperous state with geogenic and industrial origins of metals. This study assesses the seasonal variation of pseudo-total metal contents (Cr, Ni, Pb, Zn, Mn, Cu, Fe, Mg, Al) and related contamination and risks in indoor dust, street dust, and soils of four major cities of Jharkhand. Across cities and seasons, Zn, Cu, and Pb were the most common pollutants. Indoor dust showed higher metal concentrations than street dust and soil, suggesting their indoor origins. Geo-accumulation indices indicated significant Cu contamination, followed by Pb and Zn. Street dust exhibited notable enrichment in Zn and Pb in all cities except Dhanbad, where Cu contamination was substantial. Ecological risk indices peaked during summer in street dusts of Ranchi and Bokaro (for Pb) and during monsoons in soils of Jamshedpur and Dhanbad (for Cu). Based on chemical sequential extractions, the mobilities of Mg, Mn, Zn, and Cu were high, while Pb had moderate mobility. The probable sources of immediate concern were vehicles and paints, wire, electroplating, metal casting, and steel manufacturing industries. The findings emphasize the urgent need for implementing stringent regulations to mitigate metal emissions and ensure compliance with environmental standards.
Keywords: Indoor dust, Street dust, Soil, Metals, Enrichment factor, Ecological risks, Bioavailability, Mobility
Subject terms: Ecology, Environmental sciences
Introduction
The elevated concentrations of heavy metals in pollutants pose significant risks to plants and animals through their uptake. A primary source of these metals in soil is atmospheric deposition, contributing to the widespread environmental impact of heavy metal contamination1. Atmospheric particulates in dust are re-suspended by wind, adversely affecting the environment2. Various human activities, such as industrial emissions, traffic, construction, heating, and the disposal or combustion of waste, coal, and fuel, have become significant sources of toxic heavy metals in dust. These pollutants originate from numerous anthropogenic activities and contaminate the environment3. Heavy metals in dust pose severe threats to ecosystems due to their toxicity, persistence, bioaccumulation, and biomagnification properties4. Consequently, there is an urgent need for a deeper understanding of the physicochemical characteristics of these heavy metals, as well as their mobilities and bioavailabilities.
Internationally, numerous studies have examined the presence of metals and their associated risks in street dust5. However, there is insufficient information regarding the presence of metal(loid) in small towns and rapidly industrialized areas. Statistics from 1999 indicate that the average person spends 95% of their time indoors, primarily in homes, making prolonged exposure to metals a significant concern. The indoor environment poses exposure risks to contaminants for all age groups, with toddlers being especially susceptible due to increased hand-to-mouth activity6. Indoors, chemical degradation occurs more slowly than outdoors due to the lack of sunlight and microbial activity7. Despite the majority of time being spent indoors, including in homes and businesses, studies on indoor chemical exposure are relatively rare8. Small cities lacking adequate pollution control technologies are at higher risk from pollution.
Ranchi, the capital city of Jharkhand, is a commercial hub with potential socioeconomic and political growth, rapid urbanization, and pollution. On the other hand, Jamshedpur is a minerally rich industrial city, well known for minerals like iron ore, manganese, bauxite, lime, iron steel, and industries manufacturing trucks, tinplate, and cement. Even the economy of Bokaro is mainly governed by the Steel Authority of India Limited (SAIL) and Oil and Natural Gas Corporation Limited (ONGC). Dhanbad has the oldest and largest coal mines9, and is surrounded by coal mines10. These cities were chosen because they have distinct sources of metal pollution from industries, metal and coal mining, traffic emissions, and various commercial activities. Although few studies have been conducted on metal pollution at the Jharia coalfields of Dhanbad, none have been conducted in other urban cities of Jharkhand.
Moreover, indoor dust has always been neglected/overlooked in these studies. Although the total metal concentration in dust samples helps predict the overall contamination potential, the mobility depends on their partitioning among different chemical species. Sequential chemical extraction processes help discover the potential remobilization of metals from the dust and soil. It is also quintessential to evaluate the total organic carbon content, which influences mobility, bioavailability, and contamination of heavy metals. Organic matter can reduce the mobility of heavy metals by forming stable metal–organic complexes. High organic content ensures enhanced microbial activity, influencing their transformation through processes like methylation and immobilization. As far as we know, no other study has reported seasonal variations of metals in dust and soil samples, along with their metal speciation and mobilities.
Focusing on several critical aspects of heavy metal contamination in urban environments, the objectives of this study include (i) evaluation of heavy metal content and various pollution indices in indoor dust, street dust, and soil across Ranchi, Jamshedpur, Bokaro, and Dhanbad during the summer, monsoon, and winters, (ii) seasonal analysis of ecological risks and ecological risk indices, and (iii) determination of the bioavailability and mobility of metals using to understand their potential impact. Finally, identifying highly mobile metals and their bioavailabilities will be instrumental in seeking sustainable practices and policies to ensure a healthier and more resilient urban environment.
Materials and methodology
Study area
This study focuses on the state of Jharkhand, mainly four significant cities: Ranchi, Jamshedpur, Bokaro, and Dhanbad (Fig. 1). Arc Map 10.8 and Google Earth Pro were used for making the map. Ranchi, the capital of Jharkhand, has witnessed substantial socioeconomic and political development since its designation as the capital in 2000. However, rapid urbanization and population growth have led to unplanned development and increased air pollution, exacerbated by the burning of solid waste and escalating vehicular numbers. Jamshedpur, renowned for its steel production, is the most populous and first planned city in Jharkhand. The city’s economic landscape is dominated by industries such as iron steel, bauxite, lime, cement, truck manufacturing, and medium- and small-sized enterprises.
Fig. 1.
Study area map (yellow pins indicate the sampling points).
Similarly, Bokaro is a planned urban industrial hub driven primarily by the Steel Authority of India Limited and Oil and Natural Gas Corporation Limited. Besides industrial activities, shopping centers and malls significantly contribute to the city’s economic growth. Moreover, the city is a central hub for mining, industries, wholesale trade, and commerce, further enhancing its economic vitality. Dhanbad, known as the Coal Capital of India, hosts the oldest and largest coal mines, surrounded by coal transport routes, mine overburden, dust, smoke, and power plant ash9. Consequently, the city has been designated a "Critically Polluted Area" due to severe environmental degradation, particularly from coal-related activities11.
Jharkhand’s mineral-rich landscape predisposes it to heavy metal contamination, heightening environmental risks12. Air quality monitoring data from recent years reveal alarming levels of pollutants in the atmosphere, exceeding World Health Organization (WHO) recommendations in several instances. During 2017–2019, SO2, NO2, and PM10 concentrations in Ranchi were 18.33, 34.67, and 124.33 (µg/m3), respectively, while in Jamshedpur, were 37.16, 46.33, and 132.5 (µg/m3) respectively13. The 18-year mean PM2.5 concentration was 40 in Ranchi and 43.30 (µg/m3) in Jamshedpur, exceeding WHO recommended limits of 10 μg/m314. In Bokaro, the concentrations of PM10, PM2.5, and NO2 (in μg/m3) were 93.9215, 40016, and 1617 (µg/m3) respectively. Meanwhile, in Dhanbad, the concentrations of PM10, PM2.5, SO2, and NOX were 234, 140, 20, and 55 µg/m3), respectively18. The number of registered vehicles in Ranchi was 0.35 million (1997–2010)19, Jamshedpur was 0.14 million (2001–2012)20, Bokaro was 0.25 million21, and Dhanbad was 0.41 million11.
The Birla Institute of Technology, Mesra, located 16 km from Ranchi’s central business district, is a control location with no commercial, transportation, mining, or manufacturing operations. Surrounded by expansive greenery and government forests, this site presents an optimal contrast to evaluate environmental contamination in the studied cities. Dust and soil samples were systematically collected across all four cities from roadside locations to facilitate thorough analysis.
Sampling
The high density of vehicles, extensive industrial operations, coal mining activities, and bustling commercial enterprises in Ranchi, Jamshedpur, Bokaro, and Dhanbad have been widely acknowledged22 Samples, comprising indoor dust, street dust, and soil samples, were collected, accurately labeled with GPS coordinates, and stored in zip bags to evaluate the pollution status of the cities. Typical indoor dust, street dust, and soil samples are given in S1.
Indoor dust
Sampling locations were strategically chosen to encompass diverse industrial sectors, residential neighborhoods, and varying traffic densities. The snowball sampling method was adapted for visiting residences. Indoor dust was collected using the dust-pan method, ensuring comprehensive coverage across floor area23. Composite floor dust samples were meticulously gathered from each household and securely stored in zip-top bags with corresponding coordinates for accurate documentation and analysis. The indoor dust samples were named RSID (n = 11), RMID (n = 21), and RWID (n = 8) for the summer, monsoon, and winter seasons. Similarly, other samples for summer, monsoon, and winter months were named JSID (n = 23), JMID (n = 7), and JWID (n = 15) for Jamshedpur, BSID (n = 29), BMID (n = 4), BWID (n = 24) for Bokaro, and DSID (n = 50), DMID (n = 14), and DWID (n = 20) for Dhanbad respectively.
Street dust
Roadside street dust from Ranchi, Jamshedpur, Bokaro, and Dhanbad was collected using the conventional dustpan and brush technique. The samples were carefully stored and labeled in zip-lock bags, ensuring the exclusion of dry leaves, oil stains, cigarette butts, and other unwanted debris to maintain sample integrity. Special attention was paid to minimizing loss through resuspension, and thorough brush cleaning was conducted to prevent cross-contamination24. Subsequently, the dust samples were sieved using a 2 mm mesh size sieve before digestion, following standard procedures outlined by the USEPA25. The sample was prepared using the coning and quartering methods. The metal content was analyzed using ICP-OES (Make-Perkin Elmer, USA; Optical 2100DV) after centrifugation and filtration, adhering to established protocols. The street dust of the cities was named RSSD (n = 10), RMSD (n = 31), and RWSD (n = 27) for summer, monsoon, and winter, respectively. Similarly, the samples of other cities were also named likewise for the three seasons (JSSD = 10, JMSD = 23, JWSD = 35, BSSD = 30, BMSD = 18, BWSD = 16, DSSD = 10, DMSD = 13, DWSD = 10).
Soil
Soil samples were collected at a depth of 10 cm using an auger, considering root biomass as a critical factor affecting harvesting efficiency at this depth26. The brush was meticulously cleaned before each sampling event to prevent potential contamination, as recommended by previous studies5. Following collection, all soil samples were carefully sealed in zip lock bags, with their respective GPS locations clearly labeled for accurate identification. Subsequently, to remove extraneous materials such as twigs, leaves, stones, and cobbles, the samples underwent drying and sieving through a 2 mm sieve27,28. This preparation ensured the integrity of the soil samples for further analysis. Soil samples were also named as being similar to indoor and street dust. The summer samples collected were RSS (n = 10), JSS (n = 12), BSS (n = 13), and DSS (n = 10) for Ranchi, Jamshedpur, Bokaro, and Dhanbad. Similarly, for monsoons, they were named RMS (n = 10), JMS (n = 12), BMS (n = 15), DMS (n = 11), and for winters, RWS (n = 13), JWS (n = 20), BWS (n = 13), DWS (n = 14).
Pseudo-total metal analysis
The pseudo-total metal content in the samples was determined following the 3050B USEPA method29. Initially, oven-dried samples were passed through a 2 mm mesh-size sieve, from which 0.1 g was selected for digestion. Digestion was done using 10 mL of aqua regia in an Advanced Microwave Digestor (Milestone Connect ETHOS EASY) operating at 1800 MW, 220 °C, and 35 bar pressure. After digestion, the final volume was adjusted to 50 mL using 1% HNO3, and the solution was centrifuged. The supernatant was carefully transferred to acid-resistant vessels for subsequent analysis. The heavy metal content of each sample was determined using ICP-OES (Perkin Elmer, USA Optical 2100 V ICP-OES).
Quality assurance and control
Analytical grade reagents were utilized to prepare internal standards of varying dilutions. ICP multi-element standard solutions IV (Al) and XVI (Cr, Cd, Cu, Ni, Pb, Zn, As, Co, Mn) from MERCK, Germany were employed as standard solutions. Triplicate measurements were conducted, and the average values were reported, with a relative percentage difference below 20% observed to ensure precision. Additionally, to ensure quality control, a NIST-certified soil reference material (GBW07411) from China was analyzed with each batch, resulting in recovery rates of 122.963% for Zn, 121.972% for Cu, 103.634% for As, 103.00% for Pb, and 100.912% for Ni, confirming accurate metal concentrations. A continuous check of variation (CCV) was performed at intervals of 10 samples using a metal standard of 30 ppb in 2% HNO3 solution to assess instrument precision. Furthermore, each sample was diluted in a 2% HNO3 solution to minimize matrix effects. Metal detectable ranges were as follows: Cd (0.01–10 ppm), Cr (0.02–20 ppm), Cu (0.04–40 ppm), Fe (0.01–10 ppm), Mn (0.001–10 ppm), Ni (0.05–50 ppm), and Pb (0.01–100 ppm). Dilutions were carried out for samples recording metal concentrations above the detectable range.
Geochemical indices
Geo-accumulation index (Igeo)
Igeo is a valuable tool for assessing contamination levels by comparing them with background concentrations22 and calculating them using (1).
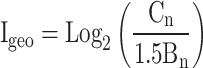 |
1 |
Cn represents the amount of each element in the street dust; Bn is the background geochemical value. The categorizations are given in S2.
Enrichment factor (EF)
To normalize metal concentrations for Iron (Fe), which is a primary sorbent phase for metal(loid)s, the Enrichment Factor (EF) is calculated using (2)30. This equation compares metal amounts in the soil to those in the earth’s crust31. The EF values provide insights into the enrichment status of metals in the sampled areas.
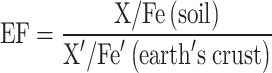 |
2 |
X and X′ are the metal amounts in soil and the earth’s crust, respectively, while Fe′ and Fe and Fe′ are the amount of iron in soil and the earth’s crust, respectively. The categorizations are given in S2.
Contamination factor (CF)
CF assesses the metal content in street dust relative to its background level32. It is determined using (3), where Cn represents the metal concentration in street dust, and Bn is the geochemical background value. The categorizations are outlined in S2.
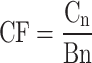 |
3 |
Ecological risk (ER)
ER assesses the adverse effects of specific metals on the environment, while ERI evaluates cumulative effects considering all metals. Ecological risks are quantified using the ER index, calculated using (4), which considers toxic response factors, metal concentrations in street dust (Ci), and background values (Co)33. The sum of all metal risk factors denoted as ERI, is calculated using (5), offering a comprehensive assessment of potential ecological risks.
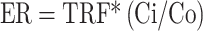 |
4 |
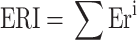 |
5 |
TRF represents the toxic response factor associated with a substance, incorporating considerations of its toxicity and sensitivity requirements, while i denotes the monomial potential ecological risk factor. The categorizations are summarized in S2.
Total organic carbon
The soil organic matter was estimated using Walkley and Black’s34 rapid titration method. 1.0 g oven-dried, ground, and sieved fly ash amended soil was taken in a 500 ml titration flask, adding 20 ml of 1N K2Cr2O7 and 20 ml concentrated H2SO435. After half an hour, the flask contents were diluted with 200 ml distilled water. Before titrating it against 0.5 N (NH₄)₂Fe(SO₄)₂·6H₂O (FAS), 10 ml of 85% H3PO4 and 1.0 ml of C12H11N indicator were added. At the endpoint, the dark blue color changed to grassy green. A blank was also run simultaneously to standardize the normality of the FAS solution used. The TOC values were calculated as given in (6).
 |
6 |
Metal bio-availabilities
The ecological and environmental health effects initially depend on the elements’ mobility and availability, which can be tested by sequential extraction. The modified Tessier technique was employed for the chemical fractionation analysis of the samples, providing insights into metal bioavailability36. Pseudo-total metal content was evaluated using the USEPA3051 microwave digestion method with the ETHOS EASY microwave digester. It is based on elemental concentrations in various fractions, including exchangeable (F1), carbonate-bound (F2), Fe–Mn oxide-bound (F3), organic matter-bound (F4), and residual fractions (F5).
Mobility factor (MF)
MF quantifies the accessibility of metal ions in dust, with higher values indicating increased mobility and potential toxicity, and is calculated using (7)37. Greater MF values signify heightened availability to biological systems due to increased mobility.
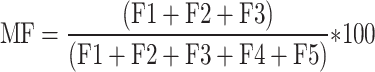 |
7 |
Results and discussion
Physicochemical parameters
Indoor dust samples generally exhibited neutral to basic pH levels, with a few exceptions (Table 1). In contrast, street dust samples displayed a wider pH range, from 1.37 to 8.66. Some street dust samples showed acidic pH levels, such as RSSD (3.19), JSSD (4.07), and DMSD (5.39). Few soil samples had acidic pH (DSS = 3.86, BSS = 3.96, JS = 6.1, JMS = 6.76, JWS = 6.7, BMS = 5.97, DMS = 5.89). EC (mS/cm) values ranged from 0.25 (BWS) to 2.64 (DSID), while TOC (%) values varied significantly, from 0.90 (BWID) to 12 (RMS, BMID, JSS, DMS). Most samples had TOC (%) values of ≤ 6, except for a few cases (JSSD = 7.10, BSSD = 7.35, RMSD = 7.70, RSSD = 9.30). The indoor dust samples exhibited TOC (%) ranging from 0.90 (BWID) to 5.20 (DWID), except for a remarkably high TOC (%) of 12 in BMID. The highest TOC (%) values were observed exclusively in the street dust of Ranchi during summers (9.30). In contrast, very high TOC values were found in soil samples only during the monsoon seasons of Ranchi and the summer season of Jamshedpur (12%). Such elevated TOC levels may be attributed to high organic inputs, slow decomposition rates, or soil clay content. TOC (%) ranged from 1 in BMS to 5.70% in RWS for other samples, and lower TOC values could indicate leaching, insufficient organic inputs, or rapid mineralization of organic matter.
Table 1.
pH, EC, and TOC of samples.
| pH | EC (mS/cm) | TOC (%) | |
|---|---|---|---|
| RSID (n = 11) | 7.6 ± 1.31 | 0.92 ± 0.01 | 5.00 ± 0.30 |
| RMID (n = 21) | 4.62 ± 0.82 | 0.96 ± 0.01 | 3.50 ± 0.80 |
| RWID (n = 21) | 7.29 ± 1.78 | 1.72 ± 0.20 | 1.10 ± 0.40 |
| JSID (n = 23) | 7.38 ± 1.23 | 0.47 ± 0.03 | 2.80 ± 0.20 |
| JMID (n = 13) | 6.27 ± 0.88 | 1.1 ± 0.19 | 6.00 ± 0.06 |
| JWID (n = 15) | 3.9 ± 0.98 | 0.58 ± 0.09 | 5.00 ± 0.10 |
| BSID (n = 29) | 8.25 ± 1.89 | 2.35 ± 0.19 | 4.45 ± 1.10 |
| BMID (n = 14) | 5.82 ± 0.36 | 1.07 ± 0.21 | 12.00 ± 3.20 |
| BWID (n = 24) | 7.76 ± 1.19 | 0.31 ± 0.19 | 0.90 ± 0.20 |
| DSID (n = 50) | 7.72 ± 0.98 | 2.64 ± 0.29 | 4.95 ± 1.20 |
| DMID (n = 14) | 4.77 ± 0.07 | 0.91 ± 0.19 | 2.20 ± 0.30 |
| DWID (n = 20) | 7.31 ± 1.89 | 1.21 ± 0.13 | 5.20 ± 0.12 |
| RSSD (n = 10) | 3.19 ± 0.19 | 0.65 ± 0.12 | 9.30 ± 0.60 |
| RMSD (n = 31) | 6.21 ± 0.21 | 0.66 ± 0.17 | 7.70 ± 1.20 |
| RWSD (n = 27) | 7.56 ± 0.69 | 0.453 ± 0.12 | 2.0 ± 0.04 |
| JSSD (n = 10) | 4.07 ± 1.29 | 1.028 ± 0.29 | 7.10 ± 0.10 |
| JMSD (n = 23) | 6.62 ± 0.10 | 0.30 ± 0.19 | 1.00 ± 0.50 |
| JWSD (n = 35) | 7.73 ± 0.21 | 0.29 ± 0.06 | 2.20 ± 0.11 |
| BSSD (n = 30) | 8.66 ± 1.02 | 1.03 ± 0.29 | 7.35 ± 0.10 |
| BMSD (n = 18) | 7.67 ± 0.02 | 0.34 ± 0.19 | 1.50 ± 0.50 |
| BWSD (n = 16) | 6.47 ± 1.29 | 0.58 ± 0.09 | 1.50 ± 0.01 |
| DSSD (n = 10) | 1.37 ± 0.39 | 1.13 ± 0.19 | 4.00 ± 1.00 |
| DMSD (n = 13) | 5.39 ± 0.05 | 0.53 ± 0.19 | 2.30 ± 1.10 |
| DWSD (n = 10) | 7.4 ± 1.0 | 0.49 ± 0.21 | 3.20 ± 0.50 |
| RSS (n = 10) | 7.55 ± 1.98 | 1.02 ± 0.02 | 5.00 ± 0.10 |
| RMS (n = 10) | 6.79 ± 1.99 | 0.35 ± 0.12 | 12.00 ± 1.50 |
| RWS (n = 13) | 7.32 ± 1.49 | 0.37 ± 0.11 | 5.70 ± 1.20 |
| JSS (n = 12) | 6.1 ± 1.50 | 0.49 ± 0.11 | 12.00 ± 2.20 |
| JMS (n = 12) | 6.76 ± 0.89 | 0.25 ± 0.05 | 2.30 ± 0.05 |
| JWS (n = 20) | 6.78 ± 1.09 | 0.27 ± 0.07 | 1.10 ± 0.10 |
| BSS (n = 13) | 3.96 ± 0.59 | 0.28 ± 0.12 | 2.50 ± 0.05 |
| BMS (n = 15) | 5.97 ± 1.03 | 0.26 ± 0.03 | 1.00 ± 0.10 |
| BWS (n = 13) | 7.63 ± 0.86 | 0.25 ± 0.05 | 1.50 ± 0.20 |
| DSS (n = 10) | 3.86 ± 0.29 | 0.49 ± 0.11 | 2.20 ± 0.80 |
| DMS (n = 11) | 5.89 ± 0.39 | 0.68 ± 0.01 | 12.00 ± 2.12 |
| DWS (n = 14) | 7.52 ± 1.99 | 0.26 ± 0.02 | 2.00 ± 1.20 |
Pseudo-total metal concentration in indoor dust, street dust, and soil
Pseudo-total metals in indoor dust
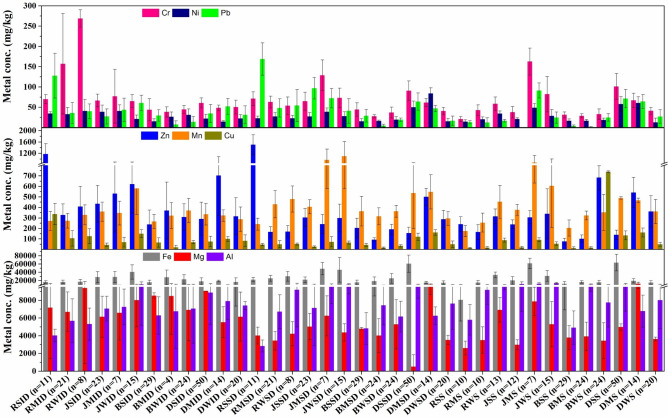
In all four cities, metal concentrations in indoor dust were studied seasonally. Interestingly, most metals exhibited their highest concentrations in all cities during winter except Dhanbad. Analyzing the trends in indoor dust composition revealed that in Ranchi and Jamshedpur, the predominant sequence of metal concentrations was Zn > Cr > Cu > Pb > Ni (Fig. 2a). For Bokaro and Dhanbad, the order was Zn > Cu > Cr > Pb > Ni. Notably, indoor dust samples from Ranchi displayed elevated concentrations of Cr, Fe, Mg, Mn, and Ni during winters, while Cu, Pb, and Zn levels peaked during the summer. Similarly, in Jamshedpur, most metals followed a similar seasonal pattern, except for Cr and Ni, which exhibited peak concentrations during the monsoons. Moreover, indoor dust samples from Bokaro primarily showcased heightened metal concentrations during winters, except Fe and Zn, which were highest during the monsoons, and Mg and Pb, which peaked during summers. The seasonal variability of metal concentrations was even more pronounced in Dhanbad. While metals such as Al, Mg, Cr, and Mn reached their highest concentrations during summers, Ni was the only metal with peak levels during winters. However, during monsoons, most metals recorded their highest concentrations in Dhanbad, suggesting a complex interplay of seasonal factors influencing metal deposition in indoor environments across these cities.
Fig. 2.
Pseudo-total metal concentrations in (a) indoor dust, (b) street dust, and (c) soil of the cities.
Yoshinaga identified metals like Sb, Sn, and plastic as sources of Pb in the house dust of 100 Japanese residences38. House age and dust metal concentrations had significant relationships for Cd, Zn, and Pb39. Significant risks to toddlers from Pb and BDE 209 in an e-waste recycling area in South China where the median concentrations of Pb, Cd, Cr, and Cu (except Zn) were higher in urban areas39. Traffic sources were identified as the primary sources of metal pollution in indoor dust, and the correlation coefficients were significant between Cu, Cr, Ni, Cd, and Fe. Mo, Sb, Cd, Sn, Cu, Pb, and Zn concentrations were more than 10 times higher compared to outdoors, but there were no significant health risks40. Even in Iran, there were no health risks from exposure to indoor dust41. Indoor dust has also been studied for preschool children42, school dust43, and different micro-environments44.
Pseudo-total metals in street dust
In the street dust samples of Ranchi and Jamshedpur, the highest concentrations of most metals were recorded during winter. However, Bokaro exhibited the highest concentrations during summer (Cr, Cu, Mn, Pb, Zn) and winter (Fe, Mg, Ni). Interestingly, no metal recorded its peak concentrations during winter in Dhanbad’s street dust samples. Instead, the highest metal concentrations were observed in summers (Cr, Fe, Pb) and monsoons (Cu, Mg, Mn, Ni, Zn). Fe is used in steel and motor industries, while Cu is used in electrical equipment such as wiring and motors. Zn concentrations (mg/kg) in street dust ranged from 93.43 to 500.67, with Jamshedpur showing comparably higher levels of Cr during the monsoon season (129 mg/kg) (Fig. 2b). Ranchi’s street dust had the highest Pb concentrations (168.96 mg/kg) during summer, while Ni was predominant in Dhanbad during the monsoons (84.10 mg/kg). In Dhanbad, Zn, followed by Cu, exhibited the highest metal concentrations, sourced from various industries, including steel processing, paint, cosmetics, batteries, textiles, ammunition, electrical equipment manufacturing, waste combustion, and mining of refined petroleum products45. The control site had the lowest metal concentrations due to its serene unpolluted environment, even during the winter season (Cd = 5.5 mg/kg, Cr = 31 mg/kg, Mn = 265.5 mg/kg, Ni = 8 mg/kg, Pb = below detection limit, Zn = 30 mg/kg). Their corresponding levels in monsoon season were Cd = 0.6 mg/kg, Cr = Ni = Pb = BDL, Mn = 22.9 mg/kg, Zn = 4.2 mg/kg.
Several studies on heavy metal concentrations have recently been reported worldwide in Spain, Poland, China, Iran, Saudi Arabia, and Bangladesh46–51. The metal concentration ranges in Jharia coalfield (Jharkhand) were:- Pb = 12.55–14.99 mg/kg, Cd = 2.29–3.49 mg/kg, Cr = 43.8–62.8 mg/kg, Cu = 9.15–19.85 mg/kg, Mn = 181.8–251.7 mg/kg, Zn = 18.5–29 mg/kg52. Pb values in street dust of Jamshedpur fell within the range as reported in Anand City (65.91–105.39 mg/kg), probably as an after–effect of the phasing out of leaded gasoline53. In contrast, very high values of Pb and Zn were observed in Kolkata54, majorly due to its sampling from major roadway intersections with high traffic volume, attrition of tires, and its use as additives in lubricating oils.
Pseudo-total metals in soil
In Ranchi and Bokaro, the highest metal concentrations in soil were recorded during the winters, encompassing all metals except Fe, Mg, and Al in Bokaro, which peaked during the monsoons. Conversely, in Jamshedpur, most metals reached their highest concentrations during the monsoon. Dhanbad’s soil exhibited varied seasonal variations, with metals like Cr, Fe, Mn, Pb, and Al peaking during summer. At the same time, Cu, Mg, Ni, and Zn recorded their highest concentrations in the monsoons (Fig. 2c). Other studies too have reported seasonal variations in metal concentrations of soil owing to temperature moisture, vegetation growth, pH, chemical and biological reactions, and fertilizer applications55–58.
Alarmingly, Zn levels exceeded WHO permissible limits in all street dust and soil samples across cities, along with Cu (RWS = 88.55, JMS = 91.71, JWS = 54.70, BWS = 738.83, DSS = 133.81, DMS = 160.78), Pb (JMS = 91.33), Cr (JMS = 162.72, DSS = 101.22), and Ni (JMS = 49.01, DSS = 57.94, DMS = 60.67)22,59.
In northern Telangana, agricultural soils were studied for different metal concentrations and pollution. Few metals like Cu and Zn had higher concentrations than those prescribed in Canadian soil quality guidelines. Anthropogenic activity (total variance > 53.02%) was identified as the primary source of pollution, as per PCA. The surface soils were evaluated for metal concentrations in the soils of Neyshabur60. Pb was responsible for very high contamination in the soils of this region. The farmland soils showed different degrees of pollution from Zn, Hg, and Pb in the different areas.
In Jiedong district, agricultural practices. industrial activities, traffic emissions, and natural sources were identified as the significant sources of pollution61. Industrial activities contributed 49.71%, 48.11%, and 47.15% in construction land, woodlands, and farmland, respectively. Agricultural practices were the primary source of pollution in farmlands and woodland, while industrial activities were the primary source of construction land.
In China, heavy metals and their consequent health risks were evaluated62. Distinct metals were identified as pollution sources for separate districts. There were no carcinogenic or non-carcinogenic risks posed.
In Iran63, the concentration trend of metals was as Zn < Ni < Pb < As < Cd in surface soils. Industrial activities were the primary source of pollution in the Khayyam industrial zone. Cu, Zn, Pb, Cr, Ni, and Co levels exceeded the background geochemical values in Ranga Reddy district of Telangana64. Traffic was the primary source contributing to Zn and Pb pollution.
Statistical analysis
The detailed statistical analysis is given in S3 of Supplementary. ANOVA (S4), for studying the variations in metals for each season, with separate analyses conducted for indoor dust (S5), street dust (S6), and soils (S7), is given in the supplementary.
Geo-accumulation index (Igeo)
The highest contamination was from Cu (1.21–7.68), and moderate contamination of Pb (1.06–4.95) and Zn (1.07–4.39) (Table 2). The street dust of Ranchi during the summer, monsoons, and soil during the summers recorded the maximum contamination from Cu, Pb, and Zn. Igeo values of 7.68 and 6.19 due to Cu in Ranchi’s street dust and soils. Similar Igeo values were recorded from Pb (4.95) and Zn (4.39) during summers in street dust and soils, respectively. The corresponding contaminations from Zn were 3.62 and 2.41. Comparatively, very low contaminations were recorded in street dust during monsoon (Cu = 0.46, Pb = 0.42, Zn = 0.48).
Table 2.
Igeo values of street dust and soils of Ranchi, Jamshedpur, Bokaro, and Dhanbad.
| Cu | Pb | Zn | |
|---|---|---|---|
| RSSD (n = 10) | 7.68 ± 0.35 | 4.95 ± 0.35 | 3.62 ± 0.35 |
| RSS (n = 10) | 6.19 ± 0.87 | 2.41 ± 1.14 | 4.39 ± 0.73 |
| RMSD (n = 31) | 0.46 ± 0.54 | 0.42 ± 0.29 | 0.48 ± 0.95 |
| RMS (n = 10) | BDL | BDL | BDL |
| RWSD (n = 27) | BDL | 0.635 ± 0.69 | 0.198 ± 0.15 |
| RWS (n = 13) | 0.20 ± 0.15 | BDL | 0.76 ± 0.67 |
| JSSD (n = 10) | BDL | 2.70 ± 0.39 | 0.73 ± 0.09 |
| JSS (n = 12) | BDL | 0.94 ± 0.35 | 0.38 ± 0.08 |
| JMSD (n = 23) | BDL | 0.78 ± 0.38 | 0.56 ± 0.46 |
| JMS (n = 12) | BDL | 0.88 ± 0.71 | 0.88 ± 1.45 |
| JWSD (n = 35) | BDL | 0.66 ± 0.45 | 0.39 ± 0.34 |
| JWS (n = 20) | BDL | 2.97 ± 1.20 | 0.95 ± 0.58 |
| BSSD (n = 30) | BDL | 0.67 ± 0.34 | 0.63 ± 0.49 |
| BSS (n = 13) | BDL | BDL | BDL |
| BMSD (n = 18) | BDL | BDL | BDL |
| BMS (n = 15) | BDL | BDL | BDL |
| BWSD (n = 16) | BDL | BDL | 0.44 ± 0.30 |
| BWS (n = 13) | BDL | BDL | 0.99 ± 0.31 |
| DSSD (n = 10) | 0.93 ± 0.2 | 1.21 ± 0.30 | 0.25 ± 0.38 |
| DSS (n = 10) | 0.93 ± 0.45 | 1.19 ± 0.94 | 0.37 ± 0.22 |
| DMSD (n = 13) | 1.24 ± 0.23 | 0.63 ± 0.24 | 1.80 ± 0.24 |
| DMS (n = 11) | 1.21 ± 0.34 | 1.06 ± 0.34 | 1.74 ± 0.34 |
| DWSD (n = 10) | 0.37 ± 0.34 | 0.41 ± 0.02 | 1.07 ± 0.31 |
| DWS (n = 14) | 0.08 ± 0.10 | 0.63 ± 0.22 | 1.36 ± 0.46 |
BDL = below detection limit. IgeoCr = BDL, IgeoNi = BDL.
Enrichment factor (EF)
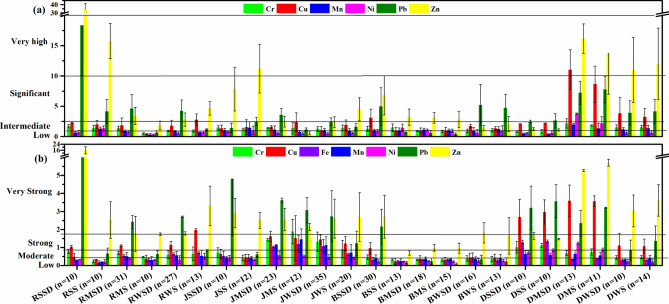
Street dust was highly enriched only in Ranchi during summers (34.4). Significant enrichments were also found in street dust from Cu in Dhanbad during monsoons (11.03), from Pb in Ranchi during summers (18.36), and from Zn in soils of Ranchi (15.75) and Jamshedpur (11.21) during summers (Fig. 3a). Such high enrichment values indicate the anthropogenic origins of these metals, such as mining, metal processing industries, and agricultural practices. Jamshedpur had significant Zn enrichment in street dust (7.8) and soils (11.21) during summer. Bokaro had significant enrichment in its street dust samples during summers from Zn (6.8), followed by Pb (5.21) during winters. Compared to other cities, Dhanbad recorded very high enrichment values from Zn during monsoon (DMSD = 16.16, DMS = 13.77) and winter (DWS = 12, DWSD = 11) seasons. Cu, Ni, Pb, and Zn were moderately enriched in most samples. Similar high enrichments of these metals of anthropogenic origin were also observed in the street dust of Asansol and Australia65,66.
Fig. 3.
(a) Enrichment factors and (b) contamination factors of various samples.
Contamination factor (CF)
CF identifies specific metals of immediate concern by comparing them with background values. Season-wise contamination factors for each city are presented in Fig. 3b. Pb and Zn majorly contributed to the contamination of street dust and soils in Ranchi, Jamshedpur, and Bokaro. Most samples recorded low to moderate CF values. Strong contaminations were seen only in soil samples of Ranchi during winters, JSSD, JMSD, JMS, and DSSD. Exceptionally substantial contamination from Zn (15.81) was observed in soil samples of Ranchi during winters.
Ecological risks (ER) and ecological risk indices (ERI)
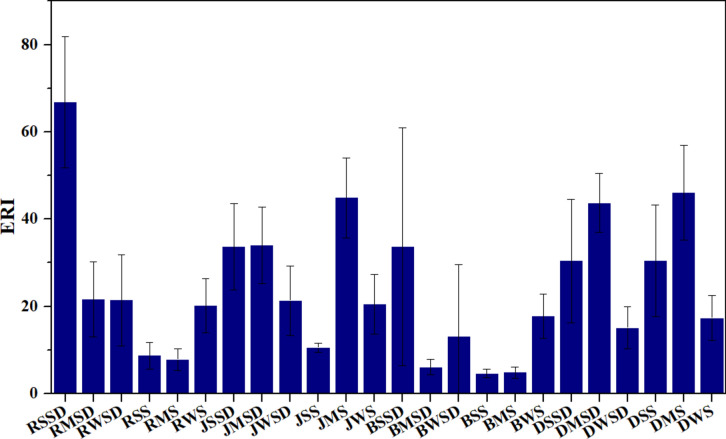
All ER values were below 40, indicating low ecological risks for three seasons: summer, monsoon, and winter (Table 3). The respective ER values of the metals ranged as follows: Zn (0.69–15.82), Pb (0.79–6.80), Ni (1.03–6.18), Mn (0.19–1.43), Cu (1.14–8.07) and Cr (0.43–3.61). Zn recorded the highest ER value in the street dust of Ranchi during summers. Among the metals, Pb contributed significantly higher ER values across the cities (RMSD = 11.13, RWSD = 13.41, JMS = 22.83, JSSD = 24.2, JMSD = 18.10, DSS = 17.8, DMS = 16.03, DSSD = 12.08, DMSD = 11.76). Cu showed higher ER values only in Bokaro and Dhanbad (BSSD = 19.28, DSS = 14.86, DMS = 17.86, DSSD = 10.82, DMSD = 17.97). These metals’ pollution sources need to be controlled to avoid future risks. Pb sources (vehicular, paints) in all cities and Cu (wires, electroplating industries) in Bokaro and Dhanbad are significant concerns. Comparing ERI values, the highest was recorded in the street dust of Ranchi and Bokaro during summers (66.76 and 33.66) and in the soils of Jamshedpur and Dhanbad during monsoons (44.89 and 46.06) (Fig. 4). As all ERI values were below 150, the ecological risks from these metals were very low. These results align with findings in Tianjin, Iran, where industries, automobiles, and urbanization were identified as the leading causes67.
Table 3.
Ecological risks of indoor dust, street dust, and soil.
| Cr | Cu | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|
| RSS | 0.43 ± 0.15 | 1.49 ± 0.54 | 0.19 ± 0.07 | 1.03 ± 0.37 | 3.12 ± 1.11 | 2.38 ± 0.85 |
| RMS | 0.95 ± 0.19 | 1.52 ± 0.72 | 0.29 ± 0.09 | 1.43 ± 0.39 | 1.85 ± 1.40 | 1.76 ± 0.73 |
| RWS | 1.23 ± 0.43 | 7.99 ± 3.89 | 0.53 ± 0.22 | 2.25 ± 0.72 | 4.24 ± 1.64 | 3.91 ± 3.32 |
| RSSD | 1.58 ± 0.37 | 5.16 ± 1.22 | 0.28 ± 0.07 | 1.67 ± 0.39 | 42.24 ± 10.01 | 15.82 ± 3.75 |
| RMSD | 1.39 ± 0.33 | 5.15 ± 3.88 | 0.47 ± 0.13 | 1.81 ± 0.39 | 11.13 ± 5.83 | 1.79 ± 0.56 |
| RWSD | 1.17 ± 0.48 | 5.57 ± 1.58 | 0.55 ± 0.16 | 1.63 ± 0.56 | 13.41 ± 9.56 | 1.75 ± 0.60 |
| JSS | 0.83 ± 0.32 | 2.11 ± 1.02 | 0.44 ± 0.06 | 1.53 ± 0.21 | 3.09 ± 0.64 | 2.51 ± 0.45 |
| JMS | 3.61 ± 0.74 | 10.19 ± 2.07 | 1.43 ± 0.29 | 3.60 ± 0.73 | 22.83 ± 4.66 | 3.21 ± 0.65 |
| JWS | 1.83 ± 0.97 | 6.07 ± 1.95 | 0.71 ± 0.44 | 2.13 ± 1.03 | 6.17 ± 3.41 | 3.57 ± 2.49 |
| JSSD | 1.44 ± 0.49 | 2.92 ± 0.81 | 0.47 ± 0.08 | 2.02 ± 0.71 | 24.25 ± 31.59 | 3.20 ± 0.91 |
| JMSD | 2.86 ± 0.84 | 8.07 ± 5.34 | 1.13 ± 0.45 | 2.85 ± 0.68 | 18.1 ± 5.92 | 2.54 ± 0.94 |
| JWSD | 1.62 ± 0.53 | 5.80 ± 1.69 | 1.28 ± 0.61 | 2.08 ± 0.79 | 10.28 ± 7.52 | 3.15 ± 1.39 |
| BSS | 0.64 ± 0.10 | 1.14 ± 0.88 | 0.24 ± 0.13 | 1.10 ± 0.11 | 0.79 ± 0.28 | 0.69 ± 0.12 |
| BMS | 0.60 ± 0.10 | 1.37 ± 1.00 | 0.42 ± 0.10 | 1.25 ± 0.50 | 0.57 ± 0.01 | 0.78 ± 0.11 |
| BWS | 0.73 ± 0.28 | 2.17 ± 1.13 | 0.41 ± 0.20 | 1.15 ± 0.59 | 1.25 ± 1.36 | 1.79 ± 1.11 |
| BSSD | 1.08 ± 0.38 | 19.28 ± 21.37 | 0.45 ± 0.18 | 1.32 ± 0.68 | 12.65 ± 10.28 | 1.06 ± 1.31 |
| BMSD | 0.66 ± 0.15 | 1.84 ± 0.83 | 0.37 ± 0.09 | 1.18 ± 0.14 | 1.03 ± 0.93 | 0.98 ± 0.18 |
| BWSD | 0.77 ± 0.35 | 3.88 ± 5.66 | 0.43 ± 0.17 | 1.42 ± 0.58 | 4.53 ± 9.95 | 2.00 ± 0.91 |
| DSS | 2.24 ± 0.71 | 14.86 ± 4.68 | 0.58 ± 0.01 | 4.26 ± 1.34 | 17.85 ± 5.62 | 1.47 ± 0.46 |
| DMS | 1.48 ± 0.39 | 17.86 ± 4.76 | 0.55 ± 0.02 | 4.47 ± 1.10 | 16.03 ± 4.27 | 5.69 ± 1.52 |
| DWS | 0.91 ± 0.19 | 5.35 ± 2.01 | 0.42 ± 0.14 | 1.10 ± 0.69 | 6.80 ± 4.20 | 3.61 ± 0.87 |
| DSSD | 1.65 ± 0.69 | 10.82 ± 4.91 | 0.92 ± 0.53 | 2.82 ± 1.54 | 12.08 ± 7.21 | 2.10 ± 0.83 |
| DMSD | 1.37 ± 0.22 | 17.97 ± 2.89 | 0.64 ± 0.19 | 6.18 ± 0.99 | 11.76 ± 1.89 | 5.27 ± 0.85 |
| DWSD | 0.90 ± 0.20 | 5.55 ± 3.41 | 0.35 ± 0.07 | 1.14 ± 0.52 | 4.2 ± 2.85 | 3.03 ± 0.88 |
Fig. 4.
Ecological risk indices of samples.
Assessment of bioavailability and mobility of metals
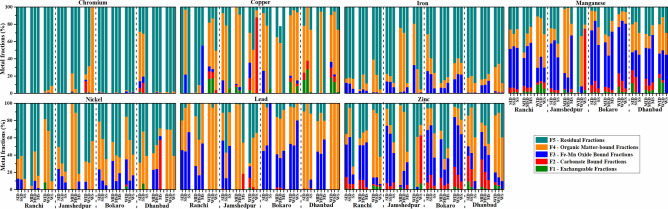
Chemical fractionation and mobility of metals
Metal availability was tested by conducting sequential extraction, following the Tessier procedure on the samples (Fig. 5). Metals in exchangeable fractions indicate their easy availability for plant uptake68.
Fig. 5.
Chemical speciation of metals in each fraction of Tessier method in indoor dust, street dust, and soil samples.
Cr
The maximum Cr fraction was in the residual fraction (91.46–99.93%) throughout the cities, seasons, and matrices. This may be caused by replacing Fe3+ and Al3+ in silicate minerals with Cr3+ in soils69. Exceptions were the soil of Jamshedpur (winter) and indoor and street dust in Dhanbad (summer). The Cr in exchangeable fractions was very low (0–0.00136) throughout, except for the indoor dust of Jamshedpur during winters (F2 = 13.20) and street dust of Dhanbad during summers (F2 = 12.01). The high concentration in mobile fractions is probably due to anthropogenic input from Ni–Cr electroplating, stainless steel, and other alloy industries70. In these cases, the maximum portion was in the organic-bound phase, which is not bioavailable. The high affinity for silicate crystal lattices may bind Cr strongly to the residual fractions.
Cu
In Ranchi, the residual fraction had the maximum Cu availability in indoor dust and soil during summers and indoor and street dust during the monsoons. High Cu concentration in the residual fraction denotes its strong affinity to the mineral matrix71. Cu can also form compounds with Fe–Mn oxides due to their high scavenging capacity and absorption area72. During winters, metals available in each fraction followed F4 > F5 > F1 > F2 > F3 trend. Cu is primarily an organic complex in F4 fractions33. Metals in F4 fractions are generally bound to the organic phase, making them the least available. Carbonate-bound fractions had the least (3.69–8.84%) Cu concentration, followed by exchangeable fractions.
In Jamshedpur, about 78.11% and 78.63% of fractions were present during summers, and 88.98% and 83.88% in indoor and street dust during monsoons, respectively. The soils had about 94% (summer) and 100% (monsoon) fractions in the residual fractions. During winters, indoor dust bioavailable fractions followed the trend F4(34.63%) > F5(29.92%) > F3(17.73%) > F2(14.42%) > F1(3.28%). In street dust, the maximum portion was carbonate-bound in the F2 fraction (88.03%), hence more bioavailable.
In Bokaro, the percentage of available metal in the F4 fraction constituted 67.11% and 78.63% (summer) and 65.21% and 58.23% (monsoon) in indoor and street dust, respectively. Residual fractions comprised the highest bioavailable fractions in the soil during both summers and monsoons. In winter, the metal bioavailability distribution was maximum in the F4 fraction throughout indoor dust (69.94%), street dust (96.27%), and soils (78.76%). The high adsorbing capacity of Fe–Mn oxides and Fe2⁺ replacing capacity of Cu are responsible for its higher concentrations in F4 fractions.
In Dhanbad, the maximum proportion of metal was consistently available in the F4 fraction in all seasons and matrices, except in indoor dust and soils during monsoons, where 100% bioavailable fraction was present in the residual fraction. F4 fractions are associated with organic content and sulfide groups due to the increased stability of organometallic complexes, affinity with functional groups of humic matter, and its chalcophile character73. In some cases, high Cu concentrations in Fe–Mn oxide fractions (F3 fractions) can be available even with slight changes in redox potentials of the water column or soil pH74.
Fe
Throughout all seasons, maximum concentrations of Fe were found in the F5 fractions across the cities: Ranchi (61.0–95.5%), Jamshedpur (77.6–87.8%), Bokaro (59.4–88.1%), and Dhanbad (65.9–88.2%). These results indicate that Fe originates from the lithosphere and is in crystalline iron oxides75. Exceptions were noted in the indoor dust of Jamshedpur during the monsoon (74.32%) and winter (47.82%), Bokaro during summer (42.95%), and street dust of Jamshedpur during the monsoon (67.85%), where Fe was predominant in the F4 fraction. In the F4 fraction, Fe is mostly present in aluminosilicates, oxides, and sulfides of the crystal lattice73 and in hematite, goethite, limonite, and magnetite73. The high sorption of Fe in F5 fractions is due to the dominance of its crystalline lattice, like goethite and magnetite76. Fe is the least available due to its maximum retention in the residual fraction, with hydroxides and oxides of Fe acting to help in metal adsorption77.
Mn
In Ranchi, Mn was mostly associated with the F3 fraction during summers in all matrices (RSID = 44.70%, RSSD = 47.99%, RSS = 47.90%) and the F4 fractions during winters (RWID = 9.44%, RWSD = 44.50%, RWS = 38.46%). Its association with F3 fractions is likely due to its strong adherence to metal oxides or hydroxides as a surface layer or stable complexes with organic matter78. In F4 fractions, Mn is retained weakly by electrostatic interactions and becomes available through ion exchange or dissociation of the Mn-carbonate phase79. Activities like metal casting or steel manufacturing can be sources of anthropogenic Mn in soil80.
In Jamshedpur, Mn was primarily associated with the F3 phase during summer (JSID = 51.08%, JSSD = 57.69%) and monsoon (JMS = 62.01%). During the monsoon season, the highest association was seen indoors (74.50%) and street dust (80.34%). Mn also co-precipitates with Fe-oxy-hydroxides as a coating on minerals and organic matter in the soil66. In Bokaro, the highest Mn concentrations were available in the F3 fractions, ranging from 37.17% (BMSD) to 74.82% (BWS) throughout all seasons and matrices. In Dhanbad, F4 fractions had the maximum metals during summers and winters, while the monsoons showed the highest concentration in the F3 fractions (DMID = 35.93%, DMSD = 42.73%, DMS = 54.91%).
Ni
In Ranchi the maximum percentage of Ni was present in the residual fraction: summer (indoor dust = 64.54%, street dust = 61.07%, soil = 87.71%) and monsoon (indoor dust = 95.54%, street dust = 55.92%, soil = 83.95%). The highest crystal field stabilization of Ni2⁺ leads to its enrichment in clay or soil particles65,81. High concentrations of Ni in soil/dust are detrital in nature, becoming occluded by silicates during the weathering process. Only during winter does the F4 fraction hold the most significant proportion, with 73.32% in indoor dust and 68.11% in street dust. The F5 fraction had the highest (90.48%) in soil samples, consistent with other studies33,82,83.
In Jamshedpur, the highest concentrations were bound to the residual fraction in all three matrices during summer: indoor dust (50.74%), street dust (69.64%), and soil (79.23%). During monsoons, the residual portion dominated only the soils (81.74%), while both indoor (98.25%) and street dust (89.10%) had maximum metals bound to the F4 fraction. In winter, maximum portions were found in the indoor and street dust residual fractions, whereas the F4 fraction dominated the soil.
In Bokaro, the soil matrix had the maximum bioavailable fractions across all seasons: summer (64.87%), monsoon (62.72%), and winter (59.58%). Ni can be associated with metal casting, alloy manufacturing, coal use, and smelting84. During summer and winter, the highest percentages were F4 fractions indoors (summer = 55.53%, winter = 49.48%) and street dust (summer = 47.11%, winter = 48.21%). During monsoons, the maximum percentage was in the residual fractions: indoor dust (44.78%), street dust (60.57%), and soil (62.72%).
F4 fractions indicated high Ni concentrations in indoor and street dust, likely due to its adsorption by Mn oxides. Mn can substitute Ni2⁺ and form Mn oxides85. Fe–Mn oxides and hydroxides act as natural scavengers of Ni86. Ni becomes mobilized and co-precipitates with Fe-oxides under anoxic conditions87. The mobility and bioavailability of Ni are strongly governed by environmental pH and EH conditions88.
Pb
In Ranchi, during both monsoons (indoor dust = 57.79%, soil = 46.73%) and winters (indoor dust = 100%, street dust = 94.82%, soil = 99.16%), the highest percentage of metal was available in the F4 fraction in all three matrices except street dust (F5 = 42.25%) and soil (F3 = 53.26%) during monsoons. The association of Pb with clay minerals is due to its affinity for Fe–Mn hydroxides, which scavenge Pb at alkaline pH (> 7)68,80.
In Jamshedpur, the percentage availability was highest in F3 fractions for indoor dust (55.75%) and soil (50.59%) during summer. F4 fractions had the highest metal available indoors (99.12%) and in street dust during monsoons, as well as in street dust (57.17%) and soil (65.64%) during winters. F5 fractions held the maximum bioavailable metal fractions in street dust (57.01%) and soil (45.45%) during monsoon and indoor dust (69.43%) during all seasons. These results align with studies conducted in urban and agricultural soils of Poland and South Africa89,90.
In Bokaro, during summer, the maximum percentage of metal was bioavailable in the F4 fraction for indoor dust and the F3 fractions for street dust (51.08%) and soil (100%). During monsoon, the maximum percentage of metal was available in the F4 fraction (indoor dust = 43.45%, street dust = 45.49%, soil = 47.40%). During winter, the bioavailable fractions were highest in the F3 fractions (indoor dust = 52.92%, street dust = 51.08%, soil = 80.13%). In Dhanbad, the maximum metal fraction was available in the F4 fractions during summer (indoor dust = 89.36%, street dust = 100%, soil = 76.39%) and winters. A slightly different trend was observed during the monsoon, where the maximum percentage of metal was in F3 fractions for indoor dust (43.91%), soil (57.90%), and street dust (47.66%).
In a few cases, F5 fractions had the maximum concentrations (Jamshedpur summer street dust and winter indoor dust), consistent with findings from previous studies91,92. The higher concentrations of metal in F3 fractions are due to the adsorption and eventual fixation of Pb cations on hydrous oxides of Fe–Mn. Pb predominance in F3 fractions occurs mainly by chemisorption in soil, released only with long-term source contamination78. Identical outcomes were reported in the soils of an abandoned metal smelting site in China, the Jialing River, and peat, compost, and biochar prepared in Brazil93–95. In a few cases, higher bioavailable metals were present in F4 fractions, where Pb forms stable organic complexes96. The adsorbed Pb of F3 and F4 fractions is only bioavailable when released under changing Fe–Mn oxidation states, acting as a source of long-term contaminants97.
Zn
In Ranchi, the F3 fractions contained the highest metals during summers (indoor dust = 37.25%, street dust = 54.53%) and monsoons (street dust = 40.32%, soil = 52.03%), similar to those reported in the sediments of the Yangtze River and aerosols over tropical peninsular India98,99. The F5 fraction had the maximum bioavailable metals in soil (73.21%) during summer and indoor dust (40.64%) during monsoon. In winter, the metal was more significantly present in the organic-bound phase (indoor dust = 78.50%, street dust = 74.48%) and soil residual fractions (62.01%). This was similar to the findings reported in pumpkin (Dhaka), rice, winter oilseed rape plants (Poland), and sediments100.
In Jamshedpur, the highest concentrations of metals were in F3 fractions during summer (indoor dust = 66.43%, street dust = 60.66%) and F4 fractions during monsoon (indoor dust = 66.43%, street dust = 60.66%) seasons. The high stability constant of Zn oxides and their retention in oxide and oxyhydroxide phases of Mn and Fe leads to high absorption by carbonates, clay minerals, and hydrous oxides101. Zn2⁺ is the most soluble divalent cation in acidic conditions, resulting in phytotoxicity in soil84. Zn complexes are primarily associated with carbonate phases with very high solubilities102.
In Bokaro, the F3 fraction contained the maximum metal (summer: indoor dust = 60.71%, street dust = 57.42%; monsoon: indoor dust = 44.60%; winter: indoor dust = 57.62%, street dust = 57.42%, soil = 55.01%). The highest bioavailable metals were in F5 fractions for soil samples in summer (34.74%), street dust (64.42%), and soil (52.17%) during monsoon. In Dhanbad, the maximum metal was in the F4 fraction in all three matrices (summer: indoor dust = 35.42%, street dust = 52.87%, soil = 50.89%; winter: indoor dust = 66.10%, street dust = 74.83%, soil = 50.89%). During monsoons, the highest bioavailable metal was in F3 fractions for indoor dust (31.91%) and street dust (29.90%), while soil had the most metal in the F5 fraction (46.85%). The high concentrations of Zn in Fe–Mn oxide fractions are due to the increased stability constants of Zn oxides, similar to those reported previously33. The second most dominant fraction was carbonate-bound, likely due to pH conditions favoring adsorption by forming stable complexes103.
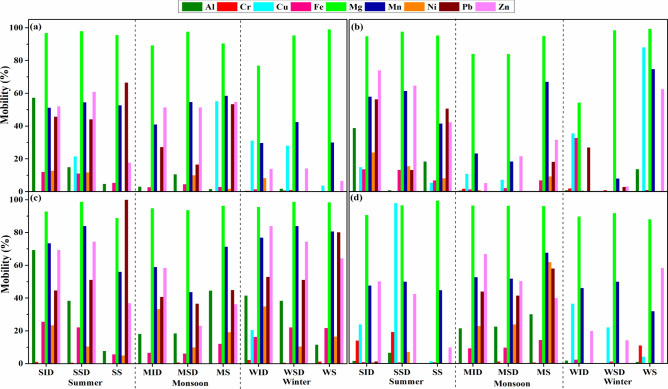
Mobility factor
The bioavailability of any element depends on its mobility and solubilization from extraction procedures. Metal mobility, influenced by solubility, follows the order: F1 > F2 > F3 > F4 > F5. Mobile metals can transform into more harmful forms, such as the methylation of mercury (Hg) into methylmercury (Me-Hg), which is more toxic than elemental Hg. Mobility depends on various geochemical conditions and physicochemical properties104. The distribution of metals in different fractions indicates their binding ability and remobilization extent105, distinguishing between natural and anthropogenic origins. Metals in exchangeable fractions are primarily anthropogenic, while those in residual fractions are mostly lithogenic104.
This study evaluated the metal mobilities during the three seasons (Fig. 6). Mg was the most mobile element across all cities and seasons (Ranchi = 76.93–98.97%, Jamshedpur = 83.92–99.33%, Bokaro = 88.92–98.77%, Dhanbad = 89.66–99.35%) due to its poor soil binding, consistent with other studies106,107 During summers and monsoons in Ranchi, Zn (51.28–60.94%) and Mn (40.95–58.52%) had similar mobilities. Overall, Mg, Mn, and Zn were the most mobile metals. The mobile fractions of Zn varied significantly: 6–60% in Ranchi, 3–73% in Jamshedpur, 36.91–83.91% in Bokaro, and 14.12–66.77% in Dhanbad. Fe showed similar mobility across all cities and seasons, primarily associated with residual fractions108. In Ranchi, metals were most mobile in the soil across all seasons. Al showed the highest mobility indoors during all seasons. In Dhanbad, all metals were highly mobile during the monsoons. During winter, the order of metal mobility in the soils of Dhanbad was Mg > Mn > Cd > Cu > Zn > Fe > Al > Cr > Pb = Ni. Low mobilities of Cr and Ni were observed, similar to steel-industrial areas of South China109.
Fig. 6.
The mobility factor of different metals in different matrices of the cities (a) Ranchi (b) Jamshedpur (c) Bokaro (d) Dhanbad.
With a geogenic origin, Pb showed higher bioavailability in anthropogenic fractions108. Pb exhibited considerable mobility in Ranchi during summer and monsoon (16.44–66.53%). In Jamshedpur, its mobility was highest during summer in indoor dust (56.31%) and street dust (50.59%). In Bokaro, Pb was most mobile in the soil during summer (100%), closely followed by winter (80.13%), with general mobility ranging from 40.74 to 52.92%. In Dhanbad, Pb was mobile-only during the monsoons (41.42% in street dust and 57.90% in soil). Bokaro showed higher mobilities of Zn and Pb across all matrices and seasons and Cd in indoor dust during winter (52.51%).
Among heavy metals, Zn (RSID = 52.04, RMID = 51.28, RSSD = 60.94, RMSD = 51.43, RMS = 54.70) and Pb (RSID = 45.71, RSSD = 44.15, RSS = 66.52, RMS = 53.27) had higher mobilities during summer and monsoon in indoor and street dust of Ranchi. Its strong association with Fe–Mn oxides is due to the high stability constants of Zn oxides110. Zn and Pb in F2 and F3 fractions were 57.58% and 28.19%, respectively. Metals are released upon changes in oxidation states in reducing environments. Cr exhibited deficient mobility, with the highest in Ranchi (0.72%), Jamshedpur (1.93%), and Bokaro (2.25%). Similar low mobilities were observed in Anshan City and Volos (Greece)111. Only in Dhanbad was the mobility of Cr comparatively higher indoors (13.93%), in street dust (19.19%) during summer, and in soil (10.95%) during winter.
Cu showed mobility up to 88.04% during winter in the soils of Jamshedpur and 97% in the street dust of Dhanbad. Otherwise, its mobility ranged from 0 to 55% in Ranchi, 0 to 14% in Jamshedpur, 0 to 20.35% in Bokaro, and 0 to 36.44% in Dhanbad. Cu was predominantly in F4 fractions in Anshan City, indicating slow leaching under strong oxidation or reduction conditions109. The highest contribution of Cu in residual fractions was also noted in Pakistan108.
Cd exhibited high mobilities in indoor and street dust during winter (RWID = 60, RWSD = 100) and in soils during summer (64.78) and monsoon (60.98). In Jamshedpur, Zn (JSID = 73.95, JSSD = 64.64, JWS = 62.59) and Cd (JSID = 57.44, JMID = 56.92, JSSD = 59.05, JSS = 54.33) had higher mobilities, followed by Pb (JSID = 56.30, JSS = 49.72). In Dhanbad, Cd mobility was high in indoor dust during summer and winter (DSID = 69.23, DWID = 52.57) and street dust (DWSD = 69.23). Zn (DMID = 66.77) and Pb (DMS = 57.90) followed. While Ni, Cr, and Cu generally had low mobilities, Cr showed higher mobility in Jamshedpur’s winter indoor dust (JWID = 92.93), Cu in soils of Ranchi in summers (RSS = 92.06), and street dust of Dhanbad (DSSD = 97.78), and Ni in soils of Dhanbad (DMS = 61.82). The major health risks from metals include vomiting, body pain, nausea from Zn, liver, kidney, and intestinal dysfunction from Cu, and severe neuro-muscular and DNA damage from Pb. Metal mobilities estimate their bioavailability, highlighting the need to manage metal contamination to mitigate health risks.
Conclusions
This study evaluated heavy metal contaminations, ecological risks, bioavailabilities, and mobility in Ranchi, Jamshedpur, Bokaro, and Dhanbad. Zn concentrations were highest in indoor dust, street dust, and soil, irrespective of season and city. It was followed by Cr, Cu, Pb, and Ni. Indoor dust had higher metal concentrations than street dust and soil, indicating its in-house origin. The various indoor origins of these metals can be electronic parts, ceramic glazes, cosmetics, pharmaceutical products, paint, and wood preservatives for Zn. Cr can originate from tobacco smoke, paints, and asbestos lining, Pb from deteriorated lead-based house paints, Cu from wiring, roofing, and decorative art, and Ni from stainless steel production and coal combustion, fuel, spent batteries, and diesel oil.
Geo-accumulation indices were highest for Cu, indicating strongest contamination (1.21–7.68), followed by moderate contamination of Pb (1.06–4.95) and Zn (1.07–4.39). Among all cities throughout the seasons, enrichment factor values were highest in street dusts of Ranchi during summers (Zn = 34.4, Pb = 18.36). The contamination factors from Pb and Zn were high in all the cities. Only in Dhanbad, Cu, Pb, and Zn posed severe contaminations. Yet, the ecological risk indices indicated only low to moderate levels of ecological risks.
Geological speciation studies suggested that Zn, Cu, Mn, Ni, and Pb were primarily of anthropogenic origin, appearing mainly in F1 fractions. Cr, Fe, and Ni were present principally in residual fractions, indicating their occurrence in silicate minerals. Pb and Zn showed significant mobilities across various matrices and seasons, posing risks in the long term. The mobilities of metals can be affected by climatic conditions, their geogenic dominance, and various anthropogenic activities. Hence, routine monitoring, time-scale studies, and implementation of strategies for reducing metal sources are essential. This study’s results can help develop regulations governing industrial emissions, waste management, and agricultural practices. Biochar use can be beneficial in controlling the mobility of hazardous metals, thereby reducing their bioavailability and ecological impact. Collaboration between environmental scientists, public health officials, and community organisations can create a holistic approach to metal exposure. Integrating research on bioavailability can help refine and adapt policies as new information emerges.
Identifying the metals and their bioavailabilities is not enough to identify the sources. Stable isotope ratios of metals can identify the natural and anthropogenic contribution. It can distinguish between sources and trace the route from source to sink or burial. This helps assess the effect of remedial actions to reduce emissions from a particular source. This was a significant limitation of this study.
In indoor dust, high metal concentrations are a matter of concern, and some policy intervention is needed to control the metal content in household products. Industrial and vehicular emissions, coal combustions, and mining activities are the predominant metal sources in these cities’ street dust. Clean production options can manage these sources. As this work has extensively identified metals of concern in dust and soil, further focus seeks solution-oriented research to curb metal pollution levels. Comprehensive dust quality assessments and regular multi-compartmental environmental surveillance and remediation programs are required.
Supplementary Information
Acknowledgements
The authors thank the Department of Civil and Environmental Engineering and Central Instrumentation Facility, BIT-Mesra for sample analyses and provisioning of reagents, glassware, and instruments.
Author contributions
A.R.—Conceptualization, Methodology, Investigation, Resources, Data curation, Writing—original draft preparation; T.B.—Supervision, Conceptualization. M.K.—Investigation, Validation, Resources, Resources, Methodology. A.K.—Investigation, Resources, Writing—review & editing.
Funding
This manuscript is part of a project funded by the Science and Engineering Research Board, Department of Science and Technology, Government of India (Sanction Order No. ECR/2017/000695, Ledger no. GO 9244).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83574-2.
References
- 1.Liu, W. et al. The mechanistic investigation of geochemical fractionation, bioavailability and release kinetic of heavy metals in contaminated soil of a typical copper-smelter. Environ. Pollut.306, 119391 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Abbasi, S. et al. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut.244, 153–164 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Tang, Z. et al. Contamination and health risks of heavy metals in street dust from a coal-mining city in eastern China. Ecotoxicol. Environ. Saf.138, 83–91 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Doabi, S. A., Karami, M., Afyuni, M. & Yeganeh, M. Pollution and health risk assessment of heavy metals in agricultural soil, atmospheric dust and major food crops in Kermanshah province, Iran. Ecotoxicol. Environ. Saf.163, 153–164 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Han, Q. et al. Health risk assessment and bioaccessibilities of heavy metals for children in soil and dust from urban parks and schools of Jiaozuo, China. Ecotoxicol. Environ. Saf.191, 110157 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Chardi, A. Biomonitoring potential of five sympatric Tillandsia species for evaluating urban metal pollution (Cd, Hg and Pb). Atmos. Environ.131, 352–359 (2016). [Google Scholar]
- 7.Oudejans, L. et al. Remediating indoor pesticide contamination from improper pest control treatments: Persistence and decontamination studies. J. Hazard. Mater.397, 122743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan, H. et al. Co-existence of organophosphate Di- and Tri-esters in house dust from south China and midwestern United States: Implications for human exposure. Environ. Sci. Technol.53, 4784–4793 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Singh, S. et al. Ambient black carbon particulate matter in the coal region of Dhanbad. India. Sci. Total Environ.615, 955–963 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Singh, A. K. et al. Precipitation chemistry and occurrence of acid rain over Dhanbad, coal city of India. Environ. Monit. Assess.125, 99–110 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Kumari, S., Kumar, M. & Elumalai, S. P. Assessment of pollution and health risks of heavy metals in particulate matter and road dust along the road network of Dhanbad. India. J. Heal. Pollut.11(29), 210305 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prathap, A. & Chakraborty, S. Assessment of surface water quality around opencast coal mines for sustainable utilization potentials: a case study in Jharkhand, India. Environ. Dev. Sustain.22, 3179–3205 (2020). [Google Scholar]
- 13.ENVIS Centre on Control of Pollution Water Air and Noise. National Ambient Air Quality Monitoring Programme (NAMP) Data. (2019).
- 14.Yue, H., He, C., Huang, Q., Yin, D. & Bryan, B. A. Stronger policy required to substantially reduce deaths from PM2.5 pollution in China. Nat. Commun.11, 1462 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambade, B., Kumar, A., Kumar, A. & Sahu, L. K. Temporal variability of atmospheric particulate-bound polycyclic aromatic hydrocarbons (PAHs) over central east India: sources and carcinogenic risk assessment. Air Qual. Atmos. Heal.15, 115–130 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh, S. K., Singh, R. K., Singh, K. K. K., Singh, R. K. & Singh, S. Particulate triggers spread of Covid-19, a case study of industrial clustered region of Jharkhand, India. Int. J. Innov. Eng. Sci. Res.5, 2581–4591 (2021). [Google Scholar]
- 17.Sur, K., Verma, V. K. & Pateriya, B. Variation of tropospheric NO2 over Indo-Gangetic plain during COVID-19 outbreak in India. Spat. Inf. Res.29, 841–855 (2021). [Google Scholar]
- 18.Jena, S. Assessment of impacts of vehicular emission on ambient air quality along the National Highway-32 at Dhanbad. (2018).
- 19.Kumar, A. & Kumar, A. Assessing human and carbon footprint of ranchi urban environment using remote sensing technology. J. Urban Environ. Eng.13, 257–264 (2019). [Google Scholar]
- 20.Dinda, S., Ghosh, S. & Das Chatterjee, N. An analysis of transport suitability, modal choice and trip pattern using accessibility and network approach: A study of Jamshedpur city, India. Spat. Inf. Res.27, 169–186 (2019). [Google Scholar]
- 21.Jharkhand Transport. Total Regsiter Vehicle in Jharkhand. (2022).
- 22.Roy, A., Bhattacharya, T. & Kumari, M. Air pollution tolerance, metal accumulation and dust capturing capacity of common tropical trees in commercial and industrial sites. Sci. Total Environ.722, 137622 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Iwegbue, C. M. A. et al. Distribution, sources and risk of exposure to polycyclic aromatic hydrocarbons in indoor dusts from electronic repair workshops in southern Nigeria. Emerg. Contam.5, 23–30 (2019). [Google Scholar]
- 24.Bhattacharya, T., Kriplani, L. & Chakraborty, S. Seasonal variation in air pollution tolerance index of various plant species of Baroda city. Univ. J. Environ. Res. Technol.3, 199–208 (2013). [Google Scholar]
- 25.USEPA. Method 3051a, Microwave Assisted Acid Dissolution of Sediments, Sludges, Soils, and Oils, Revision 1. United States Environmental Protection Agency, Washington, DC. (2007).
- 26.Yao, X., Zhang, N., Zeng, H. & Wang, W. Effects of soil depth and plant–soil interaction on microbial community in temperate grasslands of northern China. Sci. Total Environ.630, 96–102 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Zhang, H., Luo, Y., Makino, T., Wu, L. & Nanzyo, M. The heavy metal partition in size-fractions of the fine particles in agricultural soils contaminated by waste water and smelter dust. J. Hazard. Mater.248–249, 303–312 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Lanzerstorfer, C. Heavy metals in the finest size fractions of road-deposited sediments. Environ. Pollut.239, 522–531 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Roy, A. & Bhattacharya, T. Air pollution tolerance, dust capturing capacity of native tropical trees for green belt development in Dhanbad and Bokaro city, Jharkhand, India. J. Indian Chem. Soc.97, 635–643 (2020). [Google Scholar]
- 30.Lu, X. et al. Contamination assessment of mercury and arsenic in roadway dust from Baoji, China. Atmos. Environ.43, 2489–2496 (2009). [Google Scholar]
- 31.Khillare, P. S., Balachandran, S. & Meena, B. R. Spatial and temporal variation of heavy metals in atmospheric aerosol of Delhi. Environ. Monit. Assess.90, 1–21 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Ravankhah, N., Mirzaei, R. & Masoum, S. Evaluation of geoaccumulation index, contamination factor, and principal component analysis for estimating soil contamination. Iran. J. Heal. Env.8, 345–356 (2015). [Google Scholar]
- 33.Gope, M., Masto, R. E., George, J. & Balachandran, S. Tracing source, distribution and health risk of potentially harmful elements (PHEs) in street dust of Durgapur, India. Ecotoxicol. Environ. Saf.154, 280–293 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Walkley, A. & Black, I. A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci.37, 29–38 (1934). [Google Scholar]
- 35.Jha, P. et al. Predicting total organic carbon content of soils from Walkley and black analysis. Commun. Soil Sci. Plant Anal.45, 713–725 (2014). [Google Scholar]
- 36.Pandey, S. K., Bhattacharya, T. & Chakraborty, S. Metal phytoremediation potential of naturally growing plants on fly ash dumpsite of Patratu thermal power station, Jharkhand, India. Int. J. Phytoremediation18, 87–93 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Khadhar, S., Sdiri, A., Chekirben, A., Azouzi, R. & Charef, A. Integration of sequential extraction, chemical analysis and statistical tools for the availability risk assessment of heavy metals in sludge amended soils. Environ. Pollut.263, 114543 (2020). [Google Scholar]
- 38.Yoshinaga, J. et al. Lead and other elements in house dust of Japanese residences—source of lead and health risks due to metal exposure. Environ. Pollut.189, 223–228 (2014). [DOI] [PubMed] [Google Scholar]
- 39.He, C.-T.T. et al. Organic contaminants and heavy metals in indoor dust from e-waste recycling, rural, and urban areas in South China: Spatial characteristics and implications for human exposure. Ecotoxicol. Environ. Saf.140, 109–115 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Kurt-Karakus, P. B. Determination of heavy metals in indoor dust from Istanbul, Turkey: Estimation of the health risk. Environ. Int.50, 47–55 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Neisi, A. et al. Study of heavy metal levels in indoor dust and their health risk assessment in children of Ahvaz city, Iran. Toxin Rev.35, 16–23 (2016). [Google Scholar]
- 42.Latif, M. T. et al. Composition of heavy metals in indoor dust and their possible exposure: A case study of preschool children in Malaysia. Air Qual. Atmos. Heal.7, 181–193 (2014). [Google Scholar]
- 43.Rehman, A. et al. Characterizing pollution indices and children health risk assessment of potentially toxic metal(oid)s in school dust of Lahore. Pakistan. Ecotoxicol. Environ. Saf.190, 110059 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Hashemi, S. E. et al. Occurrence, potential sources, in vitro bioaccessibility and health risk assessment of heavy metal in indoor dust from different microenvironment of Bushehr. Iran. Environ. Geochem. Health42, 3641–3658 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Sevik, H., Cetin, M., Ozel, H. B. & Pinar, B. Determining toxic metal concentration changes in landscaping plants based on some factors. Air Qual. Atmos. Heal.12, 983–991 (2019). [Google Scholar]
- 46.Valido, I. et al. Physico-chemical characterization of playground sand dust, inhalable and bioaccessible fractions. Chemosphere190, 454–462 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Trojanowska, M. & Świetlik, R. Investigations of the chemical distribution of heavy metals in street dust and its impact on risk assessment for human health, case study of Radom (Poland). Hum. Ecol. Risk Assess.26, 1907–1926 (2020). [Google Scholar]
- 48.Xiao, Q., Zong, Y., Malik, Z. & Lu, S. Source identification and risk assessment of heavy metals in road dust of steel industrial city (Anshan), Liaoning, Northeast China. Hum. Ecol. Risk Assess.26, 1359–1378 (2020). [Google Scholar]
- 49.Moradi, Q. et al. The concentration, characteristics, and probabilistic health risk assessment of potentially toxic elements (PTEs) in street dust: A case study of Kashan, Iran. Toxin Rev.40, 1421–1430 (2021). [Google Scholar]
- 50.Ghanavati, N., Nazarpour, A. & De Vivo, B. Ecological and human health risk assessment of toxic metals in street dusts and surface soils in Ahvaz, Iran. Environ. Geochem. Health41, 875–891 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Dhaka, Bangladesh.Safiur Rahman, M., et al. Assessing risk to human health for heavy metal contamination through street dust in the Southeast Asian Megacity. Sci. Total Environ.660, 1610–1622 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Raj, D., Chowdhury, A. & Maiti, S. K. Ecological risk assessment of mercury and other heavy metals in soils of coal mining area: A case study from the eastern part of a Jharia coal field, India. Hum. Ecol. Risk Assess. An Int. J.23, 767–787 (2017). [Google Scholar]
- 53.Bhattacharya, T., Chakraborty, S., Bhumika, F. & Bhattacharya, P. Heavy metal concentrations in street and leaf deposited dust in Anand city, India. Res. J. Chem. Sci.1, 61–66 (2011). [Google Scholar]
- 54.Das, A. et al. Lead isotopic ratios in source apportionment of heavy metals in the street dust of Kolkata. India. Int. J. Environ. Sci. Technol.15, 159–172 (2018). [Google Scholar]
- 55.Nguyen, B. T. et al. Seasonal, spatial variation, and pollution sources of heavy metals in the sediment of the Saigon River. Vietnam. Environ. Pollut.256, 113412 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Men, C. et al. Temporal variations of levels and sources of health risk associated with heavy metals in road dust in Beijing from May 2016 to April 2018. Chemosphere270, 129434 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Men, C. et al. Spatial-temporal characteristics, source-specific variation and uncertainty analysis of health risks associated with heavy metals in road dust in Beijing, China. Environ. Pollut.278, 116866 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Dhaliwal, S. S. et al. Assessment of seasonal variations and human health risks due to heavy metals in water, soils and food crops using multi-indices approach. Environ. Earth Sci.80, 411 (2021). [Google Scholar]
- 59.WHO. World Health Organization - Permissible limits of heavy metals in soil and plants. Geneva, Switz. (1996).
- 60.Esmaeilzadeh, M. et al. Investigation of the extent of contamination of heavy metals in agricultural soil using statistical analyses and contamination indices. Hum. Ecol. Risk Assess.25, 1125–1136 (2019). [Google Scholar]
- 61.Jiang, H. H. et al. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ.701, 134466 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Zhaoyong, Z. et al. Pollution assessment and health risks evaluation of (metalloid) heavy metals in urban street dust of 58 cities in China. Environ. Sci. Pollut. Res.26, 126–140 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Mohammadi, A. A. et al. Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur. Iran. Biol. Trace Elem. Res.195, 343–352 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Adimalla, N., Chen, J. & Qian, H. Spatial characteristics of heavy metal contamination and potential human health risk assessment of urban soils: A case study from an urban region of South India. Ecotoxicol. Environ. Saf.194, 110406 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Gope, M., Masto, R. E., George, J., Hoque, R. R. & Balachandran, S. Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol. Environ. Saf.138, 231–241 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Jayarathne, A., Egodawatta, P., Ayoko, G. A. & Goonetilleke, A. Assessment of ecological and human health risks of metals in urban road dust based on geochemical fractionation and potential bioavailability. Sci. Total Environ.635, 1609–1619 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Keshavarzi, B. et al. Heavy metals and polycyclic aromatic hydrocarbons in surface sediments of Karoon River, Khuzestan province. Iran. Environ. Sci. Pollut. Res.22, 19077–19092 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Verma, A., Yadav, S. & Kumar, R. Geochemical fractionation, bioavailability, ecological and human health risk assessment of metals in topsoils of an emerging industrial cluster near New Delhi. Environ. Geochem. Health10.1007/s10653-023-01536-5 (2023). [DOI] [PubMed] [Google Scholar]
- 69.Forghani, G., Mokhtari, A. R., Kazemi, G. A. & Davoodi Fard, M. Total concentration, speciation and mobility of potentially toxic elements in soils around a mining area in central Iran. Geochemistry75, 323–334 (2015). [Google Scholar]
- 70.Liu, S. et al. Mechanistic study of Ni–Cr–P alloy electrodeposition and characterization of deposits. J. Electroanal. Chem.897, 115582 (2021). [Google Scholar]
- 71.Yiika, L. P., Tita, M. A., Suh, C. E., Mimba, M. E. & Jean-Lavenir, N. M. Heavy metal speciation by Tessier sequential extraction applied to artisanal gold mine tailings in Eastern Cameroon. Chem. Africa6, 2705–2723 (2023). [Google Scholar]
- 72.Sarmah, K. & Pratihar, S. Synthesis, characterization, and photocatalytic application of iron oxalate capped Fe, Fe–Cu, Fe–Co, and Fe–Mn oxide nanomaterial. ACS Sustain. Chem. Eng.5, 310–324 (2017). [Google Scholar]
- 73.Soltani, N. et al. In vitro bioaccessibility, phase partitioning, and health risk of potentially toxic elements in dust of an iron mining and industrial complex. Ecotoxicol. Environ. Saf.212, 111972 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Hansda, A. & Kumar, V. Influence of Cu fractions on soil microbial activities and risk assessment along Cu contamination gradient. CATENA151, 26–33 (2017). [Google Scholar]
- 75.Lagroix, F., Banerjee, S. K. & Jackson, M. J. Geological occurrences and relevance of iron oxides. Iron Oxides Nat. Appl.10.1002/9783527691395.ch2 (2016). [Google Scholar]
- 76.Sipos, P., Choi, C., Németh, T., Szalai, Z. & Póka, T. Relationship between iron and trace metal fractionation in soils. Chem. Speciat. Bioavailab.26, 21–30 (2014). [Google Scholar]
- 77.Bradl, H. B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci.277, 1–18 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Gope, M., Masto, R. E., George, J. & Balachandran, S. Exposure and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the street dust of Asansol city, India. Sustain. Cities Soc.38, 616–626 (2018). [Google Scholar]
- 79.Tessier, A., Campbell, P. G. C. & Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem.51, 844–851 (1979). [Google Scholar]
- 80.Pavlović, D. et al. Fractionation, mobility, and contamination assessment of potentially toxic metals in urban soils in four industrial Serbian Cities. Arch. Environ. Contam. Toxicol.75, 335–350 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Rinklebe, J. & Shaheen, S. M. Redox chemistry of nickel in soils and sediments—a review. Chemosphere179, 265–278 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Ma, L. Q. & Rao, G. N. Chemical fractionation of cadmium, copper, nickel, and zinc in contaminated soils. J. Environ. Qual.26, 259–264 (1997). [Google Scholar]
- 83.Banerjee, A. D. K. K. Heavy metal levels and solid phase speciation in street dusts of Delhi. India. Environ. Pollut.123, 95–105 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Demir, A. Speciation, risk assessment and bioavailability of metals in the agricultural soils of the Göksu Delta, Turkey. Soil Sediment Contam. An Int. J.30, 292–313 (2021). [Google Scholar]
- 85.Verma, A. & Yadav, S. Chemical speciation, bioavailability and human health risk assessment of metals in surface dust from an industrial cluster in India. Arch. Environ. Contam. Toxicol.84, 267–283 (2023). [DOI] [PubMed] [Google Scholar]
- 86.Soliman, N. F., Younis, A. M. & Elkady, E. Chemical speciation and comprehensive risk assessment of metals in sediments from Nabq protectorate, the Red Sea using individual and synergistic indices. Mar. Pollut. Bull.201, 116219 (2024). [DOI] [PubMed] [Google Scholar]
- 87.Ao, M. et al. Natural source of Cr(VI) in soil: The anoxic oxidation of Cr(III) by Mn oxides. J. Hazard. Mater.433, 128805 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Shaheen, S. M., Rinklebe, J., Rupp, H. & Meissner, R. Temporal dynamics of pore water concentrations of Cd Co, Cu, Ni, and Zn and their controlling factors in a contaminated floodplain soil assessed by undisturbed groundwater lysimeters. Environ. Pollut.191, 223–231 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Matong, J. M., Nyaba, L. & Nomngongo, P. N. Fractionation of trace elements in agricultural soils using ultrasound assisted sequential extraction prior to inductively coupled plasma mass spectrometric determination. Chemosphere154, 249–257 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Adamiec, E., Jarosz-Krzemińska, E. & Bilkiewicz-Kubarek, A. Adverse health and environmental outcomes of cycling in heavily polluted urban environments. Sci. Rep.12, 148 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeong, H. & Ra, K. Source apportionment and health risk assessment for potentially toxic elements in size-fractionated road dust in Busan Metropolitan city, Korea. Environ. Monit. Assess.194, 350 (2022). [DOI] [PubMed] [Google Scholar]
- 92.Miazgowicz, A., Krennhuber, K. & Lanzerstorfer, C. Metals concentrations in road dust from high traffic and low traffic area: A size dependent comparison. Int. J. Environ. Sci. Technol.17, 3365–3372 (2020). [Google Scholar]
- 93.Zanin Lima, J., Monici Raimondi Nauerth, I., Ferreira da Silva, E., José Pejon, O. & Guimarães Silvestre Rodrigues, V. Competitive sorption and desorption of cadmium, lead, and zinc onto peat, compost, and biochar. J. Environ. Manag.344, 118515 (2023). [DOI] [PubMed] [Google Scholar]
- 94.Zhang, T. et al. Assessing the remobilization and fraction of cadmium and lead in sediment of the Jialing River by sequential extraction and diffusive gradients in films (DGT) technique. Chemosphere257, 127181 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Yang, J. et al. Cadmium, lead and arsenic contamination in an abandoned nonferrous metal smelting site in southern China: Chemical speciation and mobility. Ecotoxicol. Environ. Saf.239, 113617 (2022). [DOI] [PubMed] [Google Scholar]
- 96.Zimdahl, R. L. & Skogerboe, R. K. Behaviour of lead in soil. Environ. Sci. Technol.11, 1202–1207 (1977). [Google Scholar]
- 97.Xu, D. M., Li, H. K., Xu, Z. L. & Fu, R. B. How do the occurrence patterns of potentially toxic elements (PTEs) control their release behaviours from Pb/Zn smelter contaminated soils?. J. Clean. Prod.434, 140334 (2024). [Google Scholar]
- 98.Liang, Y. et al. Geochemical controls on the distribution and bioavailability of heavy metals in sediments from Yangtze River to the East China Sea: Assessed by sequential extraction versus diffusive gradients in thin-films (DGT) technique. J. Hazard. Mater.452, 131253 (2023). [DOI] [PubMed] [Google Scholar]
- 99.Boreddy, S. K. R., Hegde, P. & Aswini, A. R. Geochemical characteristics of trace elements in size-resolved coastal urban aerosols associated with distinct air masses over tropical peninsular India: Size distributions and source apportionment. Sci. Total Environ.763, 142967 (2021). [DOI] [PubMed] [Google Scholar]
- 100.Wojtkowska, M. The influence of mineral parameters on the geochemistry of heavy metals in bottom sediments. Adv. Sci. Technol. Innov.10.1007/978-3-031-18165-8_4 (2023). [Google Scholar]
- 101.Górka-Kostrubiec, B., Świetlik, R., Szumiata, T., Dytłow, S. & Trojanowska, M. Integration of chemical fractionation, Mössbauer spectrometry, and magnetic methods for identification of Fe phases bonding heavy metals in street dust. J. Environ. Sci.124, 875–891 (2023). [DOI] [PubMed] [Google Scholar]
- 102.Golui, D. et al. Assessing geoavailability of zinc, copper, nickel, lead and cadmium in polluted soils using short sequential extraction scheme. Soil Sediment Contam.30, 74–91 (2021). [Google Scholar]
- 103.Tournassat, C., Tinnacher, R. M., Grangeon, S. & Davis, J. A. Modeling uranium(VI) adsorption onto montmorillonite under varying carbonate concentrations: A surface complexation model accounting for the spillover effect on surface potential. Geochim. Cosmochim. Acta220, 291–308 (2018). [Google Scholar]
- 104.Baran, A. & Tarnawski, M. Assessment of heavy metals mobility and toxicity in contaminated sediments by sequential extraction and a battery of bioassays. Ecotoxicology24, 1279–1293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turki, A. Metal speciation (Cd, Cu, Pb and Zn) in sediments from Al Shabab Lagoon, Jeddah, Saudi Arabia. J. King Abdulaziz Univ. Sci.18, 191–210 (2007). [Google Scholar]
- 106.Rehman, H., Alharby, H. F., Alzahrani, Y. & Rady, M. M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem.126, 97–105 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Senbayram, M., Gransee, A., Wahle, V. & Thiel, H. Role of magnesium fertilisers in agriculture: Plant-soil continuum. Crop Pasture Sci.66, 1219–1229 (2015). [Google Scholar]
- 108.Parveen, R., Khan, Y. K., Toqeer, M. & Shah, M. H. Study of fractionation, mobility and risk assessment of selected metals in suburban, urban and roadside soil from Pakistan. Environ. Earth Sci.80, 584 (2021). [Google Scholar]
- 109.Zong, Y., Xiao, Q., Malik, Z. & Lu, S. Environmental risk assessment of heavy metals by exploring chemical fractions, leachability, bioavailability in road dusts from steel-industrial city (Anshan), Northeastern China. 1–16 (2021).
- 110.Parveen, R., Saini, R. & Taneja, A. Chemical characterization and health risk assessment of soil and airborne particulates metals and metalloids in populated semiarid region, Agra, India. Environ. Geochem. Health40, 2021–2035 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Botsou, F. et al. Estimating remobilization of potentially toxic elements in soil and road dust of an industrialized urban environment. Environ. Monit. Assess.194, 526 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].