Abstract
Iron is limiting in the human host, and bacterial pathogens respond to this environment by regulating gene expression through the ferric uptake regulator protein (Fur). In vitro studies have demonstrated that Neisseria gonorrhoeae controls the expression of several critical genes through an iron- and Fur-mediated mechanism. While most in vitro experiments are designed to determine the response of N. gonorrhoeae to an exogenous iron concentration of zero, these organisms are unlikely to be exposed to such severe limitations of iron in vivo. To determine if N. gonorrhoeae expresses iron- and Fur-regulated genes in vivo during uncomplicated gonococcal infection, we examined gene expression profiles of specimens obtained from male subjects with urethral infections. RNA was isolated from urethral swab specimens and used as a template to amplify, by reverse transcriptase PCR (RT-PCR), gonococcal genes known to be regulated by iron and Fur (tbpA, tbpB, and fur). The constitutively expressed gonococcal rmp gene was used as a positive control. RT-PCR analysis indicated that gonorrhea-positive specimens where rmp expression was seen were also 93% (51/55) fbpA positive, 87% (48/55) tbpA positive, and 86% (14 of 16 tested) tbpB positive. In addition, we detected a fur transcript in 79% (37 of 47 tested) of positive specimens. We also measured increases in levels of immunoglobulin G antibody against TbpA (91%) and TbpB (73%) antigens in sera from infected male subjects compared to those in uninfected controls. A positive trend between tbpA gene expression and TbpA antibody levels in sera indicated a relationship between levels of gene expression and immune response in male subjects infected with gonorrhea for the first time. These results indicate that gonococcal iron- and Fur-regulated tbpA and tbpB genes are expressed in gonococcal infection and that male subjects with mucosal gonococcal infections exhibit antibodies to these proteins.
Neisseria gonorrhoeae, the causative agent of gonorrhea, is one of the most common causes of sexually transmitted infections in the world, with over 62 million new cases estimated by the World Health Organization in 1999 alone (see http://whqlibdoc.who.int/hq/2001/WHO_HIV_AIDS_2001.02.pdf). Control of gonorrhea has been complicated by the development of resistance to antimicrobial agents. Manifestations of gonococcal disease include urethritis and epididymitis in men and urethritis, cervicitis, salpingitis, and endometritis in women. If left untreated, gonococcal infection in women may lead to the development of pelvic inflammatory disease, which can result in infertility or ectopic pregnancy. Recent data also suggest that gonorrhea upregulates production of human immunodeficiency virus (HIV) in seminal plasma of men coinfected with both agents and is accompanied by increased transmission of HIV to female sex partners (9).
Bacteria are limited in their capacity to multiply in vivo by their hosts' “iron-withholding” defense mechanism (32, 41). Bacteria require iron (0.3 to 1.8 μM) for optimal growth (6), but as bacteria colonize and then proliferate in the host, they utilize elaborate mechanisms to acquire iron from the host. The best-characterized mechanism is to scavenge iron; this involves the synthesis of siderophores, which bind iron with high affinity. Pathogenic Neisseria, however, does not produce siderophores, but instead has evolved outer membrane receptors that bind directly to host iron sources, such as transferrin, lactoferrin, and hemoglobin. All gonococcal isolates can utilize iron from transferrin and hemoglobin (29), but only 50 to 70% of strains can internalize iron bound to lactoferrin (28). The transferrin receptor consists of a highly conserved integral outer membrane receptor, TbpA (10), and a variable surface-exposed lipoprotein, TbpB (2, 11). Together, these proteins bind human transferrin, specifically facilitating the removal of iron by Neisseria in an energy-dependent manner (12). Once iron is removed from transferrin, it is bound by periplasmic ferric binding protein (FbpA), which ferries it to a cytoplasmic membrane acceptor (FbpB), where it is internalized by an energy-dependent process (8). In the human male urethral challenge model of gonococcal infection, expression of a functional transferrin uptake system (but not necessarily the lactoferrin system) is essential for gonococcal colonization after urethral installation of the challenge inoculum, thereby emphasizing the importance of this system in human infection (13).
The expression of genes that encode gonococcal transferrin-binding proteins is controlled at the transcriptional level by the iron-dependent regulatory protein Fur (ferric uptake regulatory protein) (31). Fur functions as a general global regulator and controls the expression of genes required for iron transport and also controls genes that are required for virulence (20, 39). Fur forms a dimer with ferrous iron and binds to a consensus sequence (Fur-box) that overlaps the promoters of iron-regulated genes and results in inhibition of transcription. Although Fur may also act as a positive regulator in controlling gene expression (15-17, 25), the interactions between the operator regions of the iron-activated genes have not been studied in detail. We have determined previously that the gonococcal Fur protein binds to the promoter regions of several well-defined iron transport genes in Neisseria and to additional genes involved in catabolic, secretory, and recombination pathways. These include tonB, fur, recN, secY, sodB, hemO, hmbR, fumC, and the opa family of genes (39). Furthermore, we recently demonstrated with DNA microarray technology, using Neisseria meningitidis strain MC58, that ∼10% of the entire bacterial genome is regulated in response to growth with iron (20). While these recent observations demonstrate that pathogenic Neisseria may regulate the expression of specific genes globally in response to in vitro iron, little is known about gene expression in response to iron in vivo.
In this study, we have directly assessed the expression of the iron- and Fur-regulated genes fbpA, tbpA, tbpB, and fur in urethral samples obtained from male subjects with uncomplicated gonococcal infections. Levels of antibody directed to a subset of the proteins encoded by these genes were also measured to assess the immunogenic capacities of these iron- and Fur-regulated gene products when they are expressed in vivo.
MATERIALS AND METHODS
Study population.
Male subjects 18 years of age and older with uncomplicated gonorrhea were enrolled from the Public Health Clinics at Boston Medical Center (BMC), Boston, Mass., and the Medical University of South Carolina (MUSC), Charleston, S.C. Men were excluded if they had been treated with antibiotics in the past month or were HIV infected. Informed consent was obtained and a current and past sexual history recorded. Routine laboratory examination of urethral swab specimens, including enumeration of polymorphonuclear leukocytes and nucleic acid amplification testing for Chlamydia trachomatis, was performed. Separate urethral swabs were obtained for this study from men who were diagnosed with gonococcal infections as evidenced by Gram's stains of urethral exudate that showed gram-negative intracellular diplococci or who exhibited positive tests for neisserial H8 antigen by use of immunochromatographic detection assays (27). The diagnoses were confirmed by the growth of N. gonorrhoeae on Thayer-Martin media or by positive hybridization tests (Gen-Probe, San Diego, CA) or transcription-mediated amplification assays (Gen-Probe, San Diego, CA) performed on the urethral specimens. The separate urethral swabs to be used for this study were placed in 1 ml TRIZOL reagent (Invitrogen, Carlsbad, CA) for subsequent RNA isolation and stored at −80°C. Specimens from MUSC were shipped on dry ice by overnight delivery to Boston Medical Center, and specimens from both sites were processed within 2 days. All 55 samples were analyzed for fbpA and tbpA mRNA transcripts. Forty-seven samples were tested for fur transcripts and 16 for tbpB transcripts. At MUSC, sera were also collected to measure levels of immunoglobulin G (IgG) antibody against gonococcal TbpA and TbpB antigens and gonococcal porin isoforms IA (PIA) and IB (PIB), with the latter two used as control antigens. Control sera were also obtained from five uninfected volunteers with no history of gonococcal infection or contact with gonococcal antigens.
In vitro growth of N. gonorrhoeae strain F62 and RNA isolation.
To determine the minimal concentration of RNA required to detect specific gonococcal mRNA transcripts by reverse transcriptase (RT)-PCR, in vitro, we grew N. gonorrhoeae strain F62 and isolated RNA from organisms grown under iron-depleted and iron-sufficient conditions. Strain F62 was grown in chemically defined medium (CDM) supplemented with 4.2% NaHCO3 and in CDM plus an iron chelator, 25 μM Desferal (CDM/25 μM Desferal) (Ciba-Geigy), for 3 h aerobically at 37°C. Organisms grown under iron-restricted conditions were then washed, resuspended, divided, and inoculated into fresh CDM/12.5 μM Desferal (iron-depleted liquid cultures) or CDM/100 μM ferric nitrate (iron-sufficient liquid cultures), each beginning with an absorbance at 660 nm (A660) of 0.06. Growth was monitored and samples collected hourly for a total of 5 h (30).
RT-PCR of N. gonorrhoeae strain F62 RNA.
Total RNA was isolated from N. gonorrhoeae strain F62 using the RNeasy kit (QIAGEN, Valencia, CA). Samples were treated with DNase I (Invitrogen) before performing an RT-PCR using the SuperScript one-step RT-PCR with the Platinum Taq kit (Invitrogen, Carlsbad, CA). To the DNA-free RNA samples (200 ng), we added 25 μl of 2× reaction mix, 100 ng of each primer (Table 1), 1 μl RT/Taq mix, and diethyl pyrocarbonate (DEPC)-treated water to final volumes of 50 μl. Samples were heated to 50°C for 30 min and subsequently predenaturated at 94°C for 2 min. PCR amplifications were then carried out using the following parameters: denaturation at 94°C for 30 s, annealing at 56°C for 45 s, and elongation at 72°C for 45 s, for 25 cycles. For each sample, a control was also included to ensure the absence of DNA contamination by performing a PCR lacking reverse transcriptase enzyme with the isolated RNA sample using the rmp gene-specific primers for amplification.
TABLE 1.
Primers used for RT-PCR in this study
| Gene | Primera
|
Internal fragment size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| rmp | GAAACCATTTCCCTGTCTGC | GTTACGATGCTGCGGATTTT | 347 |
| fbpA | TATCCGATACGCACTGCTTG | CAGTGCCACCCAGTCTTTTT | 349 |
| tbpA | GGATAAATTGCCCGAAGGTT | CACGGAGGGTGAAGTGTTTT | 347 |
| tbpB | ATCTGAAATACGGGCTGCTG | GGCGTCAATGGTAAAGGTTG | 335 |
| fur | CGCGTTTGAAGATTTTGGAT | TGCACACGCCGTACATATAAA | 348 |
Primers are written in the 5′ to 3′ direction.
RNA isolation and RT-PCR of clinical samples.
Total RNA was isolated from TRIZOL-preserved urethral swab specimens according to the manufacturer's instructions. Briefly, the sample in TRIZOL was repeatedly pipetted to disrupt cells. The samples were incubated for 5 min at room temperature to permit complete dissociation of nucleoprotein complexes, 0.25-ml portions of chloroform were added, and the samples were centrifuged at 12,000 × g for 15 min at 4°C. Five milligrams of RNase-free glycogen and 0.5 ml of isopropyl alcohol were introduced to precipitate nucleic acids for 15 min at room temperature, and the pellets were washed with 75% ethanol (in DEPC-treated water). Pellets were resuspended in DEPC-treated water, and DNase I (Invitrogen) treatment was performed according to the manufacturer's instructions. The total volume of isolated RNA was divided equally for each amplification reaction, and all RT-PCRs for a single specimen were performed simultaneously using parameters the same as those described above for RNA from gonococcal strain F-62, except that the number of amplification cycles was increased to 35. Amplification was performed using gene-specific primers of gonococcal genes known to be regulated by iron and Fur (fbpA, tbpA, tbpB, and fur) and by the constitutively expressed rmp gene (Table 1). For each sample, a control sample was also included to ensure the absence of DNA contamination of RNA prepared from specimen samples by performing a PCR, lacking reverse transcriptase enzyme, with the isolated RNA sample using rmp gene-specific primers for amplification.
Semiquantitative densitometry analysis of amplified cDNA bands.
Amplified cDNA fragments isolated by the RT-PCR methods indicated above were run on a 1% agarose gel in 1× TAE (Tris-acetate-EDTA) buffer with 0.5 μg/ml ethidium bromide and then visualized under UV light (38). The density of each DNA band on the 1% agarose gel was measured using Bio-Rad QUANTITY ONE 4.1.1 quantitation software. Background measurements were subtracted, and a relative number was assigned to each band intensity (20).
Antigen production.
TbpA protein was purified from F62 that was grown in iron-deficient media using an affinity isolation procedure described earlier (5). Gonococcal porins were purified by previously published purification procedures using detergent extraction and column chromatography (4). Recombinant gonococcal outer membrane protein TbpB (36) was purified from Escherichia coli (DH5α) expressing the maltose-binding fusion protein that contained TbpB by affinity purification using an amylose resin column (36).
ELISA and antibody quantification.
Levels of anti-TbpA- and anti-TbpB-specific IgG antibody were measured in the sera of subjects infected with N. gonorrhoeae and in the sera of uninfected volunteers by use of quantitative enzyme-linked immunosorbent assay (ELISA) (40, 42). Antibodies directed against PIA and PIB were measured as positive controls, because previous studies have shown the presence of PIA- and PIB-specific antibodies in all sera from subjects with local (7, 23, 26, 33, 34) or disseminated (21, 37) gonococcal disease. The Mann-Whitney U test was used to compare IgG antibody levels in sera from subjects with control levels. We also compared levels of IgG antibody against TbpA and TbpB in subject sera with those in control sera by determining the number of subject sera that displayed antibody levels greater than 2 standard errors of the mean (SEMs) (geometric mean) above the geometric mean(s) of control sera. Fisher's exact test was used to assess the difference between levels of IgG antibody levels against TbpA and TbpB in subjects and those of control sera. A possible correlation between tbpA gene expression and TbpA isoantibody levels was assessed with Pearson's linear correlation (InStat; GraphPad, San Diego, CA).
RESULTS
Sensitivity of RNA isolation and detection of gonococcal transcripts.
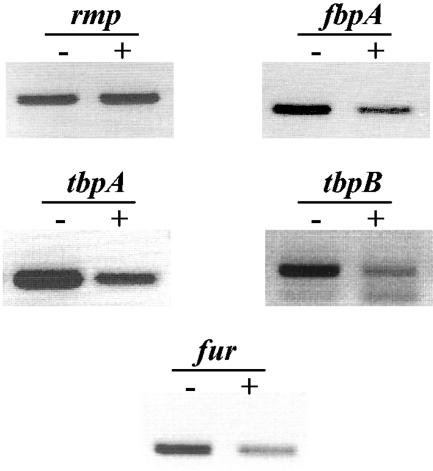
Total RNA isolated from cultures of N. gonorrhoeae strain F62 in iron-depleted and -sufficient conditions was analyzed for differential gene expression of the iron- and Fur-regulated fbpA, tbpA, tbpB, and fur genes. The rmp gene was also amplified at different time points in iron-depleted and -sufficient conditions, and no variability of expression was observed between the growth conditions, indicating that the rmp gene was constitutively expressed in each of the growth conditions. We confirmed that in iron-depleted conditions, the expression of the iron-regulated genes was increased compared to the expression of the constitutively expressed rmp gene (Fig. 1). Total RNA isolated from N. gonorrhoeae strain F62 grown in iron-sufficient conditions was also utilized to determine the sensitivity of RT-PCR under the experimental conditions used in this study. We amplified an rmp PCR product with as little as 1 ng of total RNA obtained from cultures grown in iron-sufficient conditions (data not shown).
FIG. 1.
RT-PCR analysis of N. gonorrhoeae strain F62. Differential iron-regulated gene expression was monitored in growing cultures of N. gonorrhoeae strain F62 under iron-depleted (−) and -sufficient (+) conditions. Total bacterial RNA was isolated from a culture sample collected at 2 h, and RT-PCR was performed as described in Materials and Methods.
Detection of gonococcal transcripts in urethral specimens from male subjects.
Using the methodology described above, we next analyzed gonococcal gene expression in specimens from male subjects with uncomplicated gonococcal infections. The total amount of RNA isolated from urethral specimens (host plus organism) typically ranged from 50 ng to 600 ng. Differential net gene expression of specific iron- and Fur-regulated genes from urethral specimens was assessed following RT-PCR and semiquantitative densitometry analysis of each amplified product. Each gene examined in this manner was assigned a relative densitometry value with Bio-Rad QUANTITY ONE 4.1.1 quantitation software, and a ratio of the relative densitometry values of the fbpA, tbpA, tbpB, and fur gene transcripts was calculated against the rmp value for each specimen. A ratio (deemed the expression ratio) of <1.0 was arbitrarily taken to represent a decrease in gene expression versus that of rmp mRNA, and an expression ratio of >1.0 was taken to represent increased gene expression compared to rmp gene expression. Over 90% of gonorrhea-positive specimens expressed the rmp gene by RT-PCR. Of the 55 rmp-positive specimens, 51 (93%) expressed the fbpA gene (Table 2). The genes encoding the transferrin-binding proteins TbpA and TbpB were expressed, respectively, in 48 (87%) specimens positive for rmp transcripts and in 14 out of 16 (86%) rmp-positive specimens that were also tested for the tbpB transcript. We also detected a fur transcript in 37 of 47 (79%) specimens positive for rmp transcripts that were also tested for the fur transcript (Table 2). None of the RNA specimens prepared from urethral swab specimens were contaminated with DNA, as determined by PCRs lacking reverse transcriptase enzyme with rmp-specific primers (data not shown).
TABLE 2.
Expression found by RT-PCR of iron- and Fur-regulated genes: fbpA, tbpA, fur, and tbpB transcripts and rmp transcripts amplified from total RNA isolated from urethral specimens from gonorrhea-infected males
| Gene | % of specimens expressing gene (no. expressing/no. expressing rmp)a | Median (mean ± SEM)b |
|---|---|---|
| fbpA | 93 (51/55) | 0.64 (0.23 ± 0.012) |
| tbpA | 87 (48/55) | 0.66 (0.15 ± 0.013) |
| fur | 79 (37/47) | 0.50 (0.07 ± 0.013) |
| tbpB | 86 (14/16) | 0.22 (0.06 ± 0.014) |
When both genes were examined in the same specimen.
Medians and geometric means (mean ± SEM) of the expression ratios defined as the relative densitometry measurements of the amplified transcripts of fbpA, tbpA, fur, and tbpB genes compared to the level of the constitutively expressed rmp gene measured in the same specimens. The expression ratio of the band intensity of the transcript of interest divided by the band intensity of the rmp transcript in 1% agarose gels was measured using Bio-Rad quantitation software (QUANTITY ONE 4.1.1).
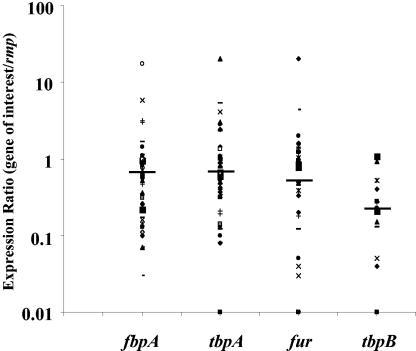
Levels of the amplified transcripts for the iron-regulated genes, relative to that of the rmp gene (the expression ratio), varied greatly from subject to subject (Fig. 2 and 3). Expression ratios ranged from 0.03 to 17.4 for fbpA transcripts; from 0.01 to 20.4 and from 0.01 to 20.1 for tbpA and fur transcripts, respectively; and from 0.01 to 1.09 for the tbpB transcript (Fig. 2). Overall, 73% of the specimens exhibited expression ratios for fbpA, tbpA, and fur transcripts of <1.0, and 27% had expression ratios of >1.0. Two specimens demonstrated no differences in fbpA or tbpA gene expression compared to rmp gene expression (expression ratio, 1.0). Although expression ratios for fbpA, tbpA, and fur genes fell above (28%, 28%, and 24%) and below (72%, 72%, and 76%) 1.0, among the 14 specimens positive for tbpB transcripts, expression ratios were <1.0 in 13.
FIG. 2.
Ratio of densitometry measurements of iron-regulated fbpA, tbpA, fur, and tbpB genes to the constitutively expressed rmp gene, termed the expression ratio. Each individual specimen is represented by a distinct symbol. The median value of the expression ratio for each gene is marked as a straight line.
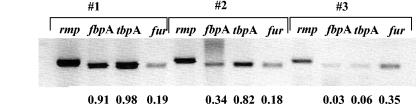
FIG. 3.
Differential expression of iron-regulated fbpA, tbpA, and fur genes found by RT-PCR in three urethral specimens from males with gonococcal infections (#1, #2, #3). The expression ratio is indicated below each lane.
Antibody responses to iron-regulated proteins in male subjects.
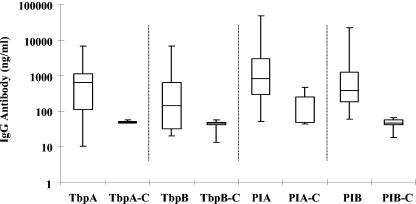
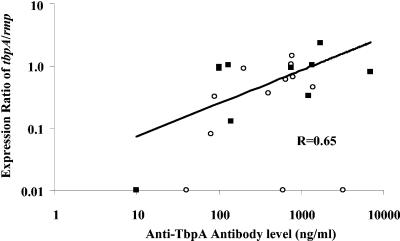
We measured IgG antibody responses to gonococcal transferrin-binding proteins TbpA and TbpB in sera from male subjects with gonorrhea by use of quantitative ELISA to assess whether IgG antibody responses correlated with upregulation of the genes encoding these proteins. IgG antibodies against TbpA and TbpB were measured because they exhibit complement-dependent bactericidal activity in Neisseria-infected mice, which may be protective in neisserial infection (24). Levels of IgG antibody against TbpA ranged widely, from 10 to 6,970 ng/ml (median, 630 ng/ml; geometric mean ± SEM, 379 ± 1.4 ng/ml). Levels of IgG against TbpB also ranged widely (20 to 6,700 ng/ml) (median, 134 ng/ml; geometric mean ± SEM, 164 ± 1.5 ng/ml) (Fig. 4). Measured levels of IgG antibody against TbpA and TbpB from gonorrhea-infected subjects were significantly higher than the corresponding levels (49 ± 1 and 36 ± 1.3 ng/ml) measured in control sera (P = 0.003 and P = 0.02, respectively). Ninety-one percent of subjects had TbpA IgG antibody levels that exceeded the geometric mean (± 2 SEM) level of the control sera (P = 0.004), compared to 73% for TbpB (P = 0.04). As expected, levels of IgG against PIA and PIB antigens were also higher than levels in control sera (Table 3). These results indicate that subjects exhibit above-normal levels of IgG antibody to these iron-regulated protein antigens during natural gonococcal infection. A correlation of tbpA gene expression to antibody levels against TbpA in sera from infected male specimens was determined by calculating the correlation coefficient using the InStat program (version 3.06; GraphPad Software, San Diego, CA). The expression ratios of tbpA to rmp versus IgG antibody levels in 10/22 subjects infected with N. gonorrhoeae were plotted (Fig. 5). These 10 subjects reported first-time gonococcal infections; the remaining 12 had had gonorrhea before, and some exhibited elevated antibody levels in the absence of tbpA gene expression. The correlation coefficient (r) in the 10 subjects was 0.65 (P = 0.04) (Fig. 5).
FIG. 4.
Levels of IgG antibody in sera from male subjects with uncomplicated gonococcal infection directed against TbpA purified from gonococcal strain F62 and recombinant TbpB antigens (see Materials and Methods). Levels are represented as (log10) ng/ml; values in control sera for antibodies against specific antigens are depicted as TbpA-C and TbpB-C (anti-PIA and -PIB antibody levels in infected subjects are also compared to control levels). The data are represented as box-whisker plots, in which the lower and upper levels of the boxes represent the 25th and 75th percentiles, respectively, and the whiskers represent the ranges of data points; the median values are depicted as horizontal lines in the boxes.
TABLE 3.
Percentages of samples displaying levels of IgG antibody against the indicated antigens from male subjects with uncomplicated gonococcal infection greater than 2 SEM above the geometric means of levels measured in control sera
| Antigen | % of samples (no. of positive sera/no. tested) | P valuea |
|---|---|---|
| TbpA | 91 (20/22) | 0.004 |
| TbpB | 73 (16/22) | 0.04 |
| PIA | 91 (20/22) | 0.03 |
| PIB | 100 (22/22) | 0.003 |
Comparison of antibody levels in subjects and controls by Fisher's exact test.
FIG. 5.
Expression ratios of the tbpA gene to the rmp gene versus levels of anti-TbpA IgG displayed for 22 gonorrhea-infected male subjects. Each symbol represents the expression ratio for an individual subject. Infected subjects with no known history of previous gonococcal infection (n = 10) are represented as closed boxes. Infected subjects with prior histories of gonococcal infection (n = 12) are represented as open circles. The regression line and the r value were determined for infected subjects with no known prior history of gonococcal infection (r = 0.65; P = 0.04). There was no correlation in infected subjects with known prior histories of gonococcal infection (note that three subjects had antibody levels whose expression ratios were minimal). The r value was calculated using Pearson's linear correlation (InStat; GraphPad).
DISCUSSION
We have confirmed that a subset of gonococcal iron- and Fur-regulated genes are expressed in men with uncomplicated gonococcal infections. Furthermore, we have demonstrated that these subjects exhibit antibodies to TbpA and TbpB proteins. In the majority of subjects with gonococcal infections, we detected fbpA, tbpA, tbpB, and fur transcripts. The Neisseria Fur appears to act as a global regulator with the ability to act both as a repressor and as an activator of gene transcription. While several studies have recently demonstrated fur expression during in vitro growth (14, 39), our study is the first to describe the expression of the fur transcript during natural gonococcal infection (in 79% of infected samples).
Our studies also demonstrated that a high proportion of male subjects with uncomplicated gonococcal infections exhibited levels of IgG antibody against TbpA and TbpB antigens that were significantly higher than levels measured in uninfected controls. The majority of sera from infected subjects in our study also contained anti-PIA and anti-PIB IgG antibody levels that were elevated relative to the levels in control sera (Table 3). Previous studies have shown measurable levels of IgG antibody to gonococcal porins in infected subjects (7, 23, 26, 40). Despite elevated levels of TbpA antibodies measured in gonorrhea-infected men, bactericidal function against TbpA is highly dependent on activity directed against native or conformational epitopes (1). Several studies have also suggested that TbpB should be considered as a candidate for a possible vaccine against N. meningitidis infection (1-3, 19). TbpB antibodies can be measured in convalescent-phase sera from patients with meningococcal disease (18, 19, 22); they are protective in a mouse model of infection, and they are also bactericidal in laboratory animals (24). However, TbpB is highly variable in different strains of N. gonorrhoeae and, taken together with lower tbpB transcript amounts produced in subject samples, may explain why we observed lower titers of IgG antibody against TbpB antigen than against TbpA. The gene-specific primers that we used for RT-PCR may have lacked the homology necessary to recognize all the separate tbpB genes. Interestingly, we have found that tbpB, when examined by microarray analysis (unpublished data), is expressed at levels higher than those found with RT-PCR, such as we have reported here. In the microarray analysis, we used a 50-bp oligonucleotide conserved across all the known tbpB genes to represent the tbpB gene, compared to a 350-bp internal tbpB fragment that was used here in RT-PCR analysis, containing both conserved and unique (variable) tbpB sequences.
Recently, Price et al. (35) reported IgG anti-TbpA and -TbpB antibody levels similar to those we report here for gonorrhea-infected male subjects but indicated that these were not different from the levels in uninfected controls (35). This may be explained by differences in the sources of control sera used to measure antibody specificity. In our study, control sera were obtained from normal volunteers with no previous history of neisserial disease and no contact with gonococcal antigens. In comparison, control sera used by Price et al. (35) were heavily weighted to include subjects from a sexually transmitted disease clinic who were culture negative for N. gonorrhoeae at the time blood was drawn for antibody determinations and who had no known prior history of gonococcal infection, reflecting antibody levels ∼10 times higher than those seen in our controls and those found by others (19).
In our study, a trend between tbpA gene expression and antibody levels in sera was observed only in subjects with initial gonococcal infections, suggesting that the increases in antibody levels over a low baseline (e.g., control sera) may come about from single gonococcal infections. Those with previous gonococcal infection(s) exhibited antibody levels, but this bore no relationship to tbpA gene expression at the time of the current infection and suggests the possibility of carryover of IgG antibodies from previous infection.
Cross-reactivity between gonococcal and meningococcal Tbp's cannot be ruled out. However, the following three observations reported here indicate that much of the IgG antibody against TbpA/TbpB was the result of past and present gonococcal infection. (i) Antibody levels in serum taken from male subjects with gonorrhea are displayed at significantly higher levels (7.7-fold higher for anti-TbpA and 4.6-fold higher for anti-TbpB) than those from normal sera obtained from individuals with no history of gonorrhea and no contact with gonococcal antigens. (ii) In N. gonorrhoeae-infected male subjects who also had prior histories of gonococcal infection, anti-TbpA and anti-TbpB levels were 8.3-fold higher than in the normal controls. These subjects did not show correlations of their antibody levels with normalized expressions of tbpA (expression ratios), indicating possible carryover of antibody from previous gonococcal infection. (iii) In first-time gonococcal infection, a correlation was found between anti-TbpA levels and normalized expression of tbpA (expression ratio) (Fig. 5).
In conclusion, we have shown that iron-regulated and Fur-regulated fbpA, tbpA, tbpB, and fur genes are expressed in vivo and that men with gonorrhea express measurable antibodies in their sera directed against certain of these gene products (TbpA and TbpB). We have also demonstrated that the iron- and Fur-regulated genes are differentially expressed in mucosal samples. Levels of antibody to TbpAB are present in male subjects with uncomplicated gonorrhea; in the case of TbpA, antibody levels correlate with the expression of the tbpA gene.
Acknowledgments
This study was supported by grants AI48611 (C.A.G.), AI40944 (L.M.W.), and U19AI38515 (P.A.R.).
We thank Andrea Dandridge, Cresene Sanglap, Stephanie Crane, Rosalyn Liu, Faye Huang, and Linda Richard from Boston Medical Center Public Health Clinic and Faye LeBoeuf, Beth Collins-Sharp, Lisa Steed, Emily Betsille, and Sandy Hirschmann from the Medical University of South Carolina, Charleston, for their invaluable help in collecting subject samples.
Editor: V. J. DiRita
REFERENCES
- 1.Ala'Aldeen, D. A., P. Stevenson, E. Griffiths, A. R. Gorringe, L. I. Irons, A. Robinson, S. Hyde, and S. P. Borriello. 1994. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect. Immun. 62:2984-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald, F. S., and I. W. DeVoe. 1980. Iron acquisition by Neisseria meningitidis in vitro. Infect. Immun. 27:322-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake, M. S., and E. C. Gotschlich. 1982. Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect. Immun. 36:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnah, R. A., R. Yu, and A. B. Schryvers. 1995. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb. Pathog. 19:285-297. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., and H. Killman. 1999. Bacterial solutions to the iron-supply problem. Trends Biochem. Sci. 24:104-109. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, G. F., and C. J. Lammel. 1989. Humoral immune response to gonococcal infections. Clin. Microbiol. Rev. 2(Suppl):S5-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C. Y., S. A. Berish, S. A. Morse, and T. A. Mietzner. 1993. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol. Microbiol. 10:311-318. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, J. J. Eron, Jr., et al. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868-1873. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen, C. N., J. E. Anderson, I. C. Boulton, and P. F. Sparling. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect. Immun. 68:4725-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect. Immun. 65:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Energy-dependent changes in the gonococcal transferrin receptor. Mol. Microbiol. 26:25-35. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 14.Delany, I., R. Rappuoli, and V. Scarlato. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 52:1081-1090. [DOI] [PubMed] [Google Scholar]
- 15.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2001. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol. Microbiol. 42:1297-1309. [DOI] [PubMed] [Google Scholar]
- 16.Dubrac, S., and D. Touati. 2000. Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J. Bacteriol. 182:3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubrac, S., and D. Touati. 2002. Fur-mediated transcriptional and post-transcriptional regulation of FeSOD expression in Escherichia coli. Microbiology 148:147-156. [DOI] [PubMed] [Google Scholar]
- 18.Ferreiros, C. M., L. Ferron, and M. T. Criado. 1994. In vivo human immune response to transferrin-binding protein 2 and other iron-regulated proteins of Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 8:63-68. [DOI] [PubMed] [Google Scholar]
- 19.Gorringe, A. R., R. Borrow, A. J. Fox, and A. Robinson. 1995. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine 13:1207-1212. [DOI] [PubMed] [Google Scholar]
- 20.Grifantini, R., S. Sebastian, E. Frigimelica, M. Draghi, E. Bartolini, A. Muzzi, R. Rappuoli, G. Grandi, and C. A. Genco. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 100:9542-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadfield, S. G., and A. A. Glynn. 1982. Analysis of antibodies in local and disseminated Neisseria gonorrhoeae infections by means of gel electrophoresis-derived ELISA. Immunology 47:283-288. [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, A. S., A. R. Gorringe, A. J. Fox, R. Borrow, and A. Robinson. 1997. Analysis of the human Ig isotype response to individual transferrin binding proteins A and B from Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 19:159-167. [DOI] [PubMed] [Google Scholar]
- 23.Lammel, C. J., R. L. Sweet, P. A. Rice, J. S. Knapp, G. K. Schoolnik, D. C. Heilbron, and G. F. Brooks. 1985. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J. Infect. Dis. 152:990-1001. [DOI] [PubMed] [Google Scholar]
- 24.Lissolo, L., G. Maitre-Wilmotte, P. Dumas, M. Mignon, B. Danve, and M. J. Quentin-Millet. 1995. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect. Immun. 63:884-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMillan, A., G. McNeillage, and H. Young. 1979. Antibodies to Neisseria gonorrhoeae: a study of the urethral exudates of 232 men. J. Infect. Dis. 140:89-95. [DOI] [PubMed] [Google Scholar]
- 27.McQuillen, D. P., S. Gulati, S. Ram, A. K. Turner, D. B. Jani, T. C. Heeren, and P. A. Rice. 1999. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J. Infect. Dis. 179:124-135. [DOI] [PubMed] [Google Scholar]
- 28.Mickelsen, P. A., E. Blackman, and P. F. Sparling. 1982. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect. Immun. 35:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morse, S. A., and L. Bartenstein. 1980. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can. J. Microbiol. 26:13-20. [DOI] [PubMed] [Google Scholar]
- 31.Morton, D. J., J. M. Musser, and T. L. Stull. 1993. Expression of the Haemophilus influenzae transferrin receptor is repressible by hemin but not elemental iron alone. Infect. Immun. 61:4033-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne, S. M. 1993. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1:66-69. [DOI] [PubMed] [Google Scholar]
- 33.Plummer, F. A., H. Chubb, J. N. Simonsen, M. Bosire, L. Slaney, I. Maclean, J. O. Ndinya-Achola, P. Waiyaki, and R. C. Brunham. 1993. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J. Clin. Investig. 91:339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plummer, F. A., H. Chubb, J. N. Simonsen, M. Bosire, L. Slaney, N. J. Nagelkerke, I. Maclean, J. O. Ndinya-Achola, P. Waiyaki, and R. C. Brunham. 1994. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J. Clin. Investig. 93:1748-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price, G. A., M. M. Hobbs, and C. N. Cornelissen. 2004. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect. Immun. 72:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Retzer, M. D., R. H. Yu, and A. B. Schryvers. 1999. Identification of sequences in human transferrin that bind to the bacterial receptor protein, transferrin-binding protein B. Mol. Microbiol. 32:111-121. [DOI] [PubMed] [Google Scholar]
- 37.Rice, P. A., H. E. Vayo, M. R. Tam, and M. S. Blake. 1986. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 164:1735-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., and D. Russel (ed.). 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 1, p. 5.14-5.17. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sebastian, S., S. Agarwal, J. R. Murphy, and C. A. Genco. 2002. The gonococcal Fur regulon: identification of additional genes involved in major catabolic, recombination, and secretory pathways. J. Bacteriol. 184:3965-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson S. D., Y. Ho, P. A. Rice, and L. M. Wetzler. 1999. T lymphocyte response to Neisseria gonorrhoeae porin in individuals with mucosal gonococcal infections. J. Infect. Dis. 180:762-773. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg, E. D. 1993. The development of awareness of iron-withholding defense. Perspect. Biol. Med. 36:215-221. [DOI] [PubMed] [Google Scholar]
- 42.Wetzler, L. M., M. S. Blake, K. Barry, and E. C. Gotschlich. 1992. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J. Infect. Dis. 166:551-555. [DOI] [PubMed] [Google Scholar]