Abstract
The objective of this study was to evaluate potential mechanisms of Trichomonas vaginalis involvement in human immunodeficiency virus type 1 (HIV-1) transmission. Polarized monolayer integrity of primary cervical and prostate epithelial cells or cell lines cultured with T. vaginalis was measured by monitoring transepithelium resistance. The effect of T. vaginalis isolates on HIV-1 passage through polarized epithelial cell monolayers was evaluated for HIV-1 p24gag in the basolateral supernatants. Coincubation with T. vaginalis isolates induced disruption of monolayer integrity and resulted in passage of virus to the basolateral side of the monolayer. Furthermore, there was isolate variability in which two isolates induced greater monolayer damage and increased HIV-1 passage than did the other two isolates. Coincubation of T. vaginalis isolates with acutely HIV-1-infected peripheral blood mononuclear cells enhanced HIV-1 replication. This enhancement was associated with cellular proliferation and activation, as well as with tumor necrosis factor alpha production. In contrast to the monolayer disruption, the effect of T. vaginalis on HIV-1 replication was not isolate dependent. Thus, two mechanisms have been identified that could contribute to the epidemiologic association of trichomoniasis with the sexual transmission of HIV-1. (i) T. vaginalis disruption of urogenital epithelial monolayers could facilitate passage of HIV-1 to underlying layers. (ii) Activation of local immune cells by T. vaginalis in the presence of infectious HIV-1 might lead to increased viral replication. Collectively, these data suggest the need for more vigilant efforts in the diagnosis and treatment of T. vaginalis in women and men, especially in countries with a high prevalence of HIV-1.
Throughout the world, 75% of human immunodeficiency virus type 1 (HIV-1) infections are acquired through heterosexual contact (41). Lesions in the mucosal surfaces of the urogenital tract provide portals of entry for pathogens, such as HIV-1. Physical trauma, damage from other sexually transmitted pathogens (STPs), and a vigorous host immune response compromise the integrity of mucosal surfaces, thereby potentially enhancing susceptibility to infection. Several epidemiologic studies have shown that ulcerative (Treponema pallidum, Haemophilus ducreyi, and herpes simplex virus) (28, 30, 39) and nonulcerative STPs (Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis) (7, 11, 12, 27) are associated with increased risk of HIV-1 infection. Moreover, HIV-1 shedding into genital secretions is increased by coinfecting STPs but subsequently returns to baseline after successful treatment (9, 16, 29). These data suggest that STPs are important cofactors in HIV-1 transmission. However, little is known about the underlying mechanisms involved in the interactions between these pathogens, the host, and HIV-1.
Trichomonas vaginalis is the most common nonviral STP worldwide, reported to have an annual incidence of 170 million cases (44). T. vaginalis is a flagellated parasitic protozoan that elicits a broad range of clinical symptoms (32, 42). It is estimated that up to 50% of infected women are asymptomatic (with normal vaginal pH and flora) (15, 43), with about one third of these women developing symptoms within 6 months (35). In acute symptomatic infections, clinical manifestations can include punctate hemorrhagic spots on the vaginal and cervical mucosa and yellow-green discharge (5, 8, 43). In chronic infections, symptoms are milder and may include itching and pain during sexual intercourse (35). Trichomoniasis has also been associated with cervical cancer (48), atypical pelvic inflammatory disease (20), and infertility (19). Pregnant women with T. vaginalis infections are predisposed to premature rupture of the placental membranes, premature labor, and low-birth-weight babies (10). Men generally remain asymptomatic and are classified as carriers, although some develop urethritis (22, 23) and prostatitis (24, 31).
Given the worldwide distribution of T. vaginalis, data suggesting an epidemiologic link with HIV-1, and their similar routes of transmission, the effects of trichomoniasis on the urogenital tract in relation to HIV-1 bear further study. The objectives for this study were to determine whether T. vaginalis affects the integrity of urogenital epithelial cells, thereby removing a barrier to HIV-1 transmission, and to determine whether coinfection with T. vaginalis influences HIV-1 replication in the underlying immune cells of the urogenital tract.
MATERIALS AND METHODS
Cell lines, viral isolates, and Trichomonas vaginalis strains.
Primary prostate epithelial cells (PrEC) and ectocervical epithelial cells (CerEC) were purified from normal tissues negative for HIV-1 and hepatitis B and C viruses (BioWhittaker, San Diego, CA) and grown as previously described (13). The CerEC medium was supplemented with 0.4 mM CaCl2 to permit the formation of intercellular cadherin and desmosomal junctions (18, 38) when the cells were plated in transwells (see below). Due to inconsistent growth of the primary epithelial cells (which is typical of all primary cells), the endometrial epithelial cell line, HEC1A, was also utilized. The HEC1A epithelial cell line, known to stratify and form a monolayer on transwell supports, was obtained from the American Type Culture Collection (Manassas, VA) (26). HEC1A cells were grown in McCoy's 5A medium (Invitrogen Corp., Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 mM l-glutamine. Normal human peripheral blood mononuclear cells (PBMCs) were obtained by leukophoresis from HIV-1-negative blood donors, purified by differential centrifugation, and stored in the gas phase of liquid nitrogen until needed. PBMCs were maintained in Cellgro serum-free medium (Mediatech, Inc., Herndon, VA) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. For HIV-1 infection, CD8+ cells were depleted from PBMCs using anti-CD8-conjugated magnetic beads (Dynal, Lake Success, NY) according to the manufacturer's instructions. Epithelial cell lines, primary cells, and PBMCs were maintained at 37°C in an incubator with 7% CO2.
The syncytium-inducing, tissue culture-adapted isolate HIV-1LAI was obtained from stocks at the Centers for Disease Control and Prevention. The non-syncytium-inducing, tissue culture-adapted isolate HIV-1Ba-L was obtained from Advanced Biotechnologies, Inc. (Columbia, MD).
A variety of T. vaginalis isolates were assessed for their effects on urogenital epithelial cells and ability to induce HIV-1 replication. Two laboratory isolates of T. vaginalis, Balt42 and JH31 (21, 25) were obtained from the American Type Culture Collection (Manassas, VA). In addition, primary isolates (Tv1 to Tv4) obtained from patients attending a sexually transmitted disease clinic in the area of Atlanta, Georgia, were evaluated. Two of these isolates were obtained from wet-mount-positive patients with an asymptomatic presentation (Tv1 and Tv2), and two isolates (Tv3 and Tv4) were obtained from patients with cervicitis and vaginal discharge (40). T. vaginalis isolates were maintained in Diamond's modified Trypticase-yeast-maltose medium (TYM) supplemented with 10% heat-inactivated fetal bovine serum at 37°C. The trichomonads were in culture no more than 10 weeks prior to evaluation in the assays described below.
Measurement of polarized epithelial monolayer and passage of HIV-1.
PrEC and CerEC were plated at 5 × 105 cells/well in a 10-mm transwell plate (membrane porosity, 3.0 μm) (Corning Inc., Corning, NY). HEC1A epithelial cells were plated at 2.5 × 105 cells/well in a 6.5-mm transwell plate (membrane porosity, 0.4 μm; collagen-coated) (Corning Inc.). Resistance across the membrane was measured daily with a Millicell-ERS resistance system (Millipore Corp., Bedford, MA). After transepithelium resistance (TER) plateaued, washed trichomonads were suspended in epithelial cell culture medium and added to the apical side of the transwell cultures. Resistance was measured over time and expressed as ohms × cm2 minus the medium alone background resistance and presented as the percentage of resistance at time zero. In some experiments, HIV-1Ba-L at a multiplicity of infection of 0.5 with or without trichomonads was added to the apical side of the HEC1A transwells. The basolateral supernatant was tested for the presence of HIV-1 using a p24gag enzyme-linked immunosorbent assay (ELISA) kit (Coulter Corp., Miami, FL).
Effects of T. vaginalis on HIV-1 infection and cytokine production.
Normal PBMCs were infected with HIV-1LAI at a multiplicity of infection of 0.01 using a resting, CD8-depleted acute infection model described previously (14), with the exception of using serum-free medium. T. vaginalis strains were centrifuged, suspended in serum-free mammalian culture medium, and added at the indicated concentrations at the time of infection. Supernatants from the infected cultures were harvested and replenished with fresh medium every other day. Culture supernatants were assayed for HIV-1 replication using a p24gag ELISA kit (Coulter Corp.) and for tumor necrosis factor alpha (TNF-α) levels with a human TNF-α ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's specifications. To evaluate the role of TNF-α in enhancing HIV-1 infection in this system, a parallel experiment was performed with the addition of neutralizing anti-TNF-α monoclonal antibody (10 μg/ml; R&D Systems) or with an isotype control monoclonal antibody (10 μg/ml; R&D Systems). The percent decrease in p24 production was determined.
T. vaginalis-induced PBMC proliferation and activation.
Proliferation was assessed by [3H]thymidine uptake in 2 × 105 CD8-depleted PBMCs cultured in serum-free medium along with T. vaginalis. The cells were cultured for 5 days at 37°C in 7% CO2 and labeled with 0.5 μCi/well [3H]thymidine for 18 h prior to harvest. Incorporated counts per minute were determined using a Matrix 96 direct beta counter (Packard Instruments Co., Downers Grove, IL). Assays were performed in triplicate, and average counts per minute was determined for treated and control cultures. Proliferation was reported as a stimulation index (SI) using the formula: SI = counts per minute in treatment wells/wells with PBMCs alone.
The expression of cellular activation markers and chemokine receptors was assessed by flow cytometry on CD8-depleted PBMCs cultured with the T. vaginalis isolates. On day 2 of culture, cells were collected and stained with peridinin-chlorophyll protein-anti-CD3, fluorescein isothiocyanate-conjugated anti-CD4, and either phycoerythrin (PE)-conjugated anti-HLA-DR, PE-conjugated anti-CD69, PE-conjugated anti-CCR5, PE-conjugated anti-CXCR4, or isotype control antibody. All monoclonal antibodies were obtained from BD Pharmingen, San Jose, CA. Forward and side scatter were used to ascertain the lymphocyte population. A FACScan (BD Pharmingen) was used to collect 10,000 events, and the samples were analyzed for activation marker or chemokine receptor expression by gating on CD3+/CD4+ cells using Cell Quest software (BD Pharmingen).
Statistical analysis.
Statistical analyses were performed with InStat version 3.0 (GraphPad Software, Inc., San Diego, CA). To determine whether there were significant differences between group means, nonparametric Kruskal-Wallis analysis of variance followed by Dunn's multiple comparisons after the statistical tests were performed. Evaluation of correlations was performed using nonparametric Spearman's rank analysis.
RESULTS
Effect of T. vaginalis on epithelial monolayer integrity.
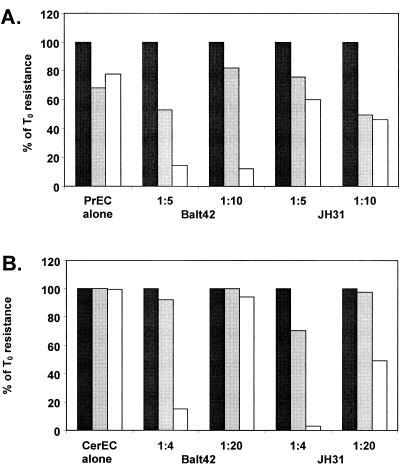
T. vaginalis isolates are cytotoxic to urogenital epithelial cells (1, 2, 17, 34, 37). To determine whether T. vaginalis isolates also affect the integrity of polarized monolayers of urogenital epithelial cells, PrEC, CerEC, and HEC1A cells were cultured on transwell supports. After TER plateaued, T. vaginalis strains were added. By 4 h after addition of either T. vaginalis isolate Balt42 or JH31, the integrity of a PrEC monolayer was affected, as demonstrated by a reduction in the monolayer TER (Fig. 1A). After 18 h, the PrEC monolayer was completely breached by Balt42. JH31 caused approximately 50% reduction in TER across the monolayer by 18 h. The stratified CerEC integrity was minimally affected by either T. vaginalis strain after 4 h (Fig. 1B). After 18 h, the integrity of the stratified CerEC was eliminated with both Balt42 and JH31 at a ratio of one protozoan to four epithelial cells. However, the more dilute Balt42 affected the CerEC monolayer only minimally, while the more dilute JH31 reduced TER by approximately 50% after 18 h. Using the endometrial epithelial cell line HEC1A, a reduction in TER across the polarized HEC1A monolayer was seen by 24 h and resistance was <20% by 72 h after the addition of Balt42 and JH31 cells at a ratio of one protozoan to five epithelial cells (Fig. 2A). Motile parasites could be observed at all time points. These findings were extended using T. vaginalis isolates obtained from symptomatic and asymptomatic patients. T. vaginalis isolates Tv3 and Tv4 reduced the HEC1A TER to levels similar to those of Balt42 and JH31 by 48 h. However, T. vaginalis isolates Tv1 and Tv2 reduced the HEC1A TER ≤50% by 72 h. Because cell disruption was observed among the three epithelial cell types using the low parasite to epithelial cell ratio, further evaluation was done using HEC1A cells.
FIG. 1.
Deterioration of polarized primary monolayers of A) prostate (PrEC) epithelial cells and B) cervical (CerEC) epithelial cells after culturing with T. vaginalis strains Balt42 and JH31. Deterioration was determined by a decrease in the resistance of a polarized monolayer of cells. Ratios given are protozoan to cell. Black bar, 0 h; grey bar, 4 h; white bar, 18 h after T. vaginalis addition. Data presented are representative of two independent experiments. T0, time zero.
FIG. 2.
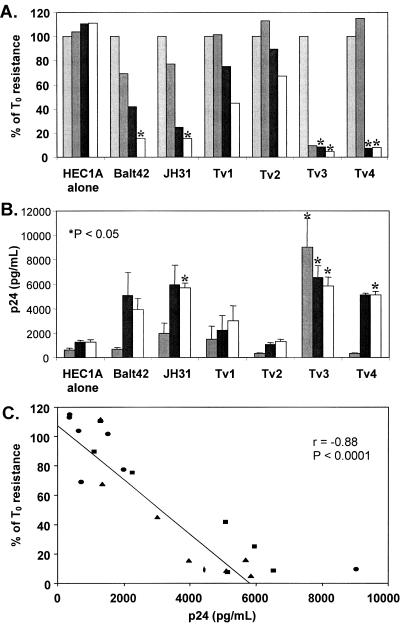
Deterioration of a polarized monolayer of an endometrial epithelial cell line (HEC1A) after exposure to T. vaginalis. A) Resistance across the HEC1A monolayer at 0, 24, 48, and 72 h after treatment with T. vaginalis (1 protozoan to 5 cells). Resistance presented as a percentage of the resistance at time zero (T0) and is the mean of three independent experiments. Light grey bar, 0 h; dark grey bar, 24 h; black bar, 48 h; white bar, 72 h. B) HIV-1 p24 levels in the basolateral supernatants at 24, 48, and 72 h of culture. Data presented are the means ± standard errors of the means (error bars) from three independent experiments. Dark grey bar, 24 h; black bar, 48 h; white bar, 72 h. Values that were significantly different (P < 0.05) from the value for the culture of HEC1A cells alone are indicated by asterisks. C) Correlation between resistance across the HEC1A monolayer and HIV-1 p24 levels at all time points. There is an inverse relationship between resistance and p24 levels (Spearman rank correlation of r = −0.88 [P < 0.0001]). Circles represent 24 h, squares represent 48 h, and triangles represent 72 h time points.
Effects of T. vaginalis on HIV-1 passage through epithelial monolayers.
While T. vaginalis was shown to disrupt the integrity of urogenital epithelial cells in a time- and dose-dependent manner, these experiments were unable to determine whether perforations caused by T. vaginalis allowed HIV-1 to cross the monolayer. After HEC1A cells reached a plateau TER, HIV-1 with or without T. vaginalis strains was added to the apical side of the cultures. Changes in the resistance across the monolayer were monitored over time, and the basolateral supernatants were assayed for HIV-1 p24gag. As the monolayer TER was diminished by the Balt42 and JH31 strains (Fig. 2A), HIV-1 p24 levels increased in the basolateral compartment such that by 48 h after addition of HIV-1, p24 levels were 4- to 4.6-fold higher than background (Fig. 2B). Similarly, Tv3 and Tv4 isolates caused a complete disruption of the HEC1A monolayer with a concurrent four- to fivefold rise in basolateral HIV-1 p24 levels over background (P < 0.05) (Fig. 2B). In contrast, Tv1 and Tv2 isolates induced much lower monolayer disruption, coinciding with basolateral HIV-1 p24 levels that were not significantly different from background. Collectively, these data show that maintenance of the TER was inversely proportional to the amount of virus present in the basolateral supernatant (Fig. 2C) (Spearman rank correlation of r = −0.88 [P < 0.0001]).
T. vaginalis activates immune cells and results in HIV-1 replication via a TNF-α pathway.
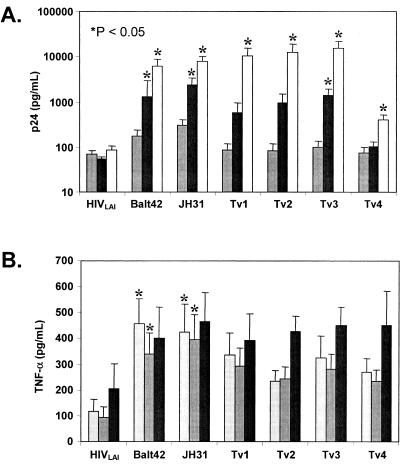
Because infectious virus could pass through breached epithelial layers, the effect of T. vaginalis on HIV-1 replication was studied. T. vaginalis isolates were cocultured with HIV-1-infected PBMCs using a previously described acute, resting CD8-depleted infection model (14). HIV-1 replication increased over time to a greater extent in cultures containing trichomonads than in the cultures that contained virus alone (Fig. 3A). In contrast to the monolayer disruption studies where differences were observed between T. vaginalis isolates Tv1 and Tv2 compared to isolates Tv3 and Tv4, all isolates induced similar levels of viral replication. For all isolates, a significant difference could be observed by day 8 after initiation of coculture compared to the day 8 HIVLAI-only culture (P < 0.05).
FIG. 3.
T. vaginalis-induced HIV-1 replication and TNF-α in resting PBMCs. A) HIV-1 p24 levels on days 4 (grey bar), 6 (black bar), and 8 (white bar) in HIV-1-infected, resting PBMCs incubated with T. vaginalis (1 protozoan to 100 cells). Cells stimulated with 0.5 μg/ml of phytohemagglutinin (positive control) produced 852,600 pg/ml of TNF-α on day 6. B) TNF-α levels on days 2 (light grey bar), 4 (dark grey bar), and 6 (black bar) in the experiment described above. Cells stimulated with 0.5 μg/ml of phytohemagglutinin (positive control) produced 1,483 pg/ml of TNF-α and peaked on day 2. Data presented are the means ± standard errors of the means (error bars) from three independent experiments. Values that were significantly different (P < 0.05) from the values for cultures with HIVLAI alone on the same day are indicated by asterisks.
To investigate the mechanism(s) involved in the induction of HIV-1 replication, T. vaginalis isolates were evaluated for induction of TNF-α production by PBMCs. TNF-α production induced by other protozoan parasites has been shown to promote HIV-1 replication in PBMCs (45, 46). Culture supernatants were obtained from CD8-depleted, resting, HIV-1-infected PBMCs with or without the addition of T. vaginalis laboratory or primary isolates. Elevated levels (two- to fourfold) of TNF-α were detected in all culture supernatants compared with virus alone at each time point (Fig. 3B). To evaluate whether TNF-α was directly involved in HIV-1 induction, a neutralizing monoclonal antibody was used to block its effect. Addition of anti-TNF-α antibody significantly blocked >50% of the HIV-1 replication in cultures containing T. vaginalis (P < 0.05), while the isotype control antibody did not reduce HIV-1 replication (Table 1). This result indicates that induction of virus replication was in part due to TNF-α resulting from the presence of parasite. To assess other responses that could promote non-TNF-α-induced HIV-1 replication, the abilities of trichomonads to stimulate nonspecific cellular activation and proliferation were tested. Coculture of T. vaginalis with PBMCs from healthy donors induced CD69 (approximately 30-fold) and HLA-DR (2- to 3-fold) cell surface expression but did not affect CCR5 or CXCR4 cell surface expression (data not shown). Similarly, parasites induced PBMC proliferation at a ratio of one trichomonad to four PBMCs. Peak proliferation (stimulation index of >12) was noted at a 1:16 parasite-to-PBMC ratio and returned to baseline at a ratio of 1:256. Collectively, these data show that T. vaginalis induces HIV-1 replication through TNF-α production, with concomitant cellular activation and proliferation that may contribute to the increased viral production.
TABLE 1.
Replication of HIV-1 in PBMCs in the presence of T. vaginalis is partially TNF-α dependenta
| T. vaginalis isolate | HIV-1 p24 level
|
% Decrease in HIV-1 p24 | HIV-1 p24 level in isotype control antibody-treated cultures | % Decrease in HIV p24 | |
|---|---|---|---|---|---|
| No treatment | Anti-TNF-α treated cultures | ||||
| Balt42 | 23,157 | 11,535 | 50 | 27,142 | −17 |
| JH31 | 29,702 | 10,460 | 65 | 34,314 | −16 |
HIV-1 p24 levels (pg/ml) were measured on day 6 of culture. Some cultures were treated with 10 μg/ml of anti-TNF-α antibody or an isotype control monoclonal antibody. Anti-TNF-α monoclonal antibody-treated cultures had significantly reduced HIV p24 levels (P < 0.05) compared to the untreated cultures, while the isotype control monoclonal antibody-treated cultures did not exhibit reductions in the level of HIV-1 p24. Data presented are representative of two independent experiments.
DISCUSSION
Worldwide, trichomoniasis is the most common nonviral STP and has been implicated in the transmission of HIV-1 (7, 27). Previous studies have shown that pathology associated with T. vaginalis infection is due to contact-dependent cytotoxicity of cervical cell lines and primary vaginal epithelial cells (1, 17). Because trichomoniasis infection can lead to ectocervicitis in women (20) and can lead to urethritis and prostatitis in men (22-24, 31), the interactions between T. vaginalis isolates and primary epithelial cells and cell lines were examined. First, T. vaginalis isolates were shown to disrupt the monolayer integrity of primary CerEC and PrEC as well as the endometrial cell line HEC1A. Perforations of the polarized epithelial monolayer allowed passage of HIV-1 through the monolayer. Second, T. vaginalis isolates were shown to activate PBMCs, as evidenced by an increase in HIV-1 replication in infected PBMCs, and this activation occurred in part through a TNF-α pathway. Collectively, the results presented here suggest two discrete explanations for the association of T. vaginalis infection with the dissemination and transmission of HIV-1 in infected persons.
The intact epithelial lining provides a protective, mechanical barrier against environmental pathogens. Multiple layers of stratified, squamous epithelial cells cover the vagina and ectocervix, while the prostate and uterus are lined with a single layer of simple columnar epithelial cells that form tight junctions. While differences were noted in the responses of primary epithelial cells and the HEC1A cell line monolayer, coincubation of these monolayers with the T. vaginalis laboratory isolate Balt42 or JH31, as well as primary isolates obtained from women with symptomatic infections, resulted in disruption of monolayer integrity. Interestingly, primary isolates of T. vaginalis obtained from women with asymptomatic infections induced less epithelial monolayer disruption. This finding could explain the clinical observations of punctate lesions associated with some trichomonas infections but absent in others (15, 43). However, it is possible that host factors also contributed to the clinical presentation noted in the infections from which the recent isolates were obtained. Thus, further research with more isolates would be useful to solidify the observation that isolates from women with symptomatic infections cause more damage to epithelial monolayers than the isolates obtained from women with asymptomatic infections.
T. vaginalis strains Balt42, JH31, Tv3, and Tv4 compromised the intact monolayer and enhanced the concentration of virus in the basolateral supernatants of the transwell. Recently, HIV-1 was shown to attach to trichomonads in vitro (36). If this were to occur in vivo, HIV-1 could be carried along with trichomonads as they permeate through the epithelium to the underlying tissue and possibly have a better opportunity to infect the local immune cells. Perturbations in the epithelial lining would ultimately allow infectious virus to reach the underlying lamina propria that is rich in HIV-1 targets. Collectively, these data suggest that infection with T. vaginalis can lead to epithelial cell death and the breakdown of the epithelial lining, allowing trichomonads and HIV-1 the opportunity to interact with underlying immune cells in the lamina propria.
The lamina propria is rich in immune cells, serving as a fertile target area for HIV-1 infection. T. vaginalis induced resting lymphocyte activation and replication and the production of proinflammatory cytokines. Recent work showed that induction of proinflammatory cytokines, specifically TNF-α, by vaginal washes from T. vaginalis-infected women was through cells expressing Toll-like receptor 4 (i.e., leukocytes) (47). If these cells become infected with HIV-1, T. vaginalis also may exacerbate viral replication. Proinflammatory cytokines play a central role in up-regulating HIV-1 replication. Previous studies showed that TNF-α and interleukin 1β induced HIV-1 from latency (6, 33). Moreover, several infectious diseases up-regulate HIV-1 replication through the TNF-α/NF-κB pathway. Specifically, Plasmodium falciparum and Cryptosporidium parvum induce HIV-1 replication through the induction of TNF-α in primary PBMCs (45, 46). Salmonella enterica serovar Typhimurium and Leishmania donovani also activate HIV-1 replication in chronically infected cell lines through the induction of TNF-α (3, 4). All T. vaginalis isolates induced HIV-1 replication and TNF-α production in culture independent of their ability to disrupt the epithelial monolayer. Blocking TNF-α inhibited replication by >50%. These data indicate that TNF-α induction is, in part, responsible for increased levels of HIV-1 in a trichomonad coinfection; however, since the decrease in viral replication was not complete, it would suggest that other mechanisms of T. vaginalis-associated HIV-1 viral replication exist. Indeed, we have previously shown that clinical Mycobacterium avium isolates induce HIV-1 replication through a process not associated with inflammatory cytokines (14). Further, data showed that incubation of T. vaginalis with PBMCs resulted in activation and proliferation in PBMCs that were not specific for trichomonas antigens, emphasizing a generalized activation possibly through the innate immune response via cells expressing Toll-like receptor 4.
We have described two possible mechanisms for T. vaginalis in promoting sexual transmission of HIV-1. Contact with T. vaginalis damages the epithelium, the primary line of defense against infection, causing cytotoxicity and epithelial cell disruption, and allowing HIV-1 access to underlying immune cells. Trichomonads also induce HIV-1 replication through cytokine pathways, such as TNF-α. We found it intriguing that there was variability in the effects of isolates on epithelial cell disruption and HIV-1 translocation, while the effects on TNF-α production and HIV-1 replication were similar among all isolates. Our data provide two mechanistic explanations for the role that T. vaginalis may have in the epidemiologic observation that trichomoniasis is associated with the enhanced sexual transmission of HIV-1. One or both of these mechanisms may lead to T. vaginalis-infected patients being more susceptible to HIV-1 infection. Augmented viral replication in coinfected patients would make them more likely to infect their sexual partners, thereby increasing HIV-1 transmission. Together, these findings suggest a need for improved testing and treatment of T. vaginalis infections and further evaluation of their possible effect on the sexual transmission of HIV-1.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Alderete, J. F., and G. E. Garza. 1985. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect. Immun. 50:701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete, J. F., and E. Pearlman. 1984. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br. J. Vener. Dis. 60:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreana, A., S. Gollapudi, C. H. Kim, and S. Gupta. 1994. Salmonella typhimurium activates human immunodeficiency virus type 1 in chronically infected promonocytic cells by inducing tumor necrosis factor-alpha production. Biochem. Biophys. Res. Commun. 201:16-23. [DOI] [PubMed] [Google Scholar]
- 4.Bernier, R., B. Barbeau, M. J. Tremblay, and M. Olivier. 1998. The lipophosphoglycan of Leishmania donovani up-regulates HIV-1 transcription in T cells through the nuclear factor-kappaB elements. J. Immunol. 160:2881-2888. [PubMed] [Google Scholar]
- 5.Brown, M. T. 1972. Trichomoniasis. Practitioner 209:639-644. [PubMed] [Google Scholar]
- 6.Butera, S. T., B. D. Roberts, and T. M. Folks. 1993. Regulation of HIV-1 expression by cytokine networks in a CD4+ model of chronic infection. J. Immunol. 150:625-634. [PubMed] [Google Scholar]
- 7.Buve, A., H. A. Weiss, M. Laga, E. Van Dyck, R. Musonda, L. Zekeng, M. Kahindo, S. Anagonou, L. Morison, N. J. Robinson, and R. J. Hayes. 2001. The epidemiology of trichomoniasis in women in four African cities. AIDS 15(Suppl. 4):S89-S96. [DOI] [PubMed] [Google Scholar]
- 8.Catterall, R. D. 1972. Trichomonal infections of the genital tract. Med. Clin. N. Am. 56:1203-1209. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, J. J. Eron, Jr., and the AIDSCAP Malawi Research Group. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868-1873. [DOI] [PubMed] [Google Scholar]
- 10.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, G. G. Rhoads, and The Vaginal Infections and Prematurity Study Group. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 11.Deschamps, M. M., J. W. Pape, A. Hafner, and W. D. Johnson. 1996. Heterosexual transmission of HIV in Haiti. Ann. Intern. Med. 125:324-330. [DOI] [PubMed] [Google Scholar]
- 12.de Vincenzi, I., for the European Study Group on Heterosexual Transmission of HIV. 1994. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. N. Engl. J. Med. 331:341-346. [DOI] [PubMed] [Google Scholar]
- 13.Dezzutti, C. S., P. C. Guenthner, J. E. Cummins, Jr., T. Cabrera, J. H. Marshall, A. Dillberger, and R. B. Lal. 2001. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J. Infect. Dis. 183:1204-1213. [DOI] [PubMed] [Google Scholar]
- 14.Dezzutti, C. S., W. E. Swords, P. C. Guenthner, D. R. Sasso, L. M. Wahl, A. H. Drummond, G. W. Newman, C. H. King, F. D. Quinn, and R. B. Lal. 1999. Involvement of matrix metalloproteinases in human immunodeficiency virus type 1-induced replication by clinical Mycobacterium avium isolates. J. Infect. Dis. 180:1142-1152. [DOI] [PubMed] [Google Scholar]
- 15.Fouts, A. C., and S. J. Kraus. 1980. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J. Infect. Dis. 141:137-143. [DOI] [PubMed] [Google Scholar]
- 16.Ghys, P. D., K. Fransen, M. O. Diallo, V. Ettiegne-Traore, I. M. Coulibaly, K. M. Yeboue, M. L. Kalish, C. Maurice, J. P. Whitaker, A. E. Greenberg, and M. Laga. 1997. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cóte d'Ivoire. AIDS 11:F85-F93. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert, R. O., G. Elia, D. H. Beach, S. Klaessig, and B. N. Singh. 2000. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect. Immun. 68:4200-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorodeski, G. I., W. Jin, and U. Hopfer. 1997. Extracellular Ca2+ directly regulates tight junctional permeability in the human cervical cell line CaSki. Am. J. Physiol. 272:C511-C524. [DOI] [PubMed] [Google Scholar]
- 19.Grodstein, F., M. B. Goldman, and D. W. Cramer. 1993. Relation of tubal infertility to history of sexually transmitted diseases. Am. J. Epidemiol. 137:577-584. [DOI] [PubMed] [Google Scholar]
- 20.Heine, P., and J. A. McGregor. 1993. Trichomonas vaginalis: a reemerging pathogen. Clin. Obstet. Gynecol. 36:137-144. [DOI] [PubMed] [Google Scholar]
- 21.Honigberg, B. M., P. K. Gupta, M. R. Spence, J. K. Frost, K. Kuczynska, L. Choromanski, and A. Warton. 1984. Pathogenicity of Trichomonas vaginalis: cytopathologic and histopathologic changes of the cervical epithelium. Obstet. Gynecol. 64:179-184. [PubMed] [Google Scholar]
- 22.Krieger, J. N. 1995. Trichomoniasis in men: old issues and new data. Sex. Transm. Dis. 22:83-96. [PubMed] [Google Scholar]
- 23.Krieger, J. N., C. Jenny, M. Verdon, N. Siegel, R. Springwater, C. W. Critchlow, and K. K. Holmes. 1993. Clinical manifestations of trichomoniasis in men. Ann. Intern. Med. 118:844-849. [DOI] [PubMed] [Google Scholar]
- 24.Kuberski, T. 1980. Trichomonas vaginalis associated with nongonococcal urethritis and prostatitis. Sex. Transm. Dis. 7:135-136. [DOI] [PubMed] [Google Scholar]
- 25.Kuczynska, K., L. Choromanski, and B. M. Honigberg. 1984. Comparison of virulence of clones of two Trichomonas vaginalis strains by the subcutaneous mouse assay. Z. Parasitenkd. 70:141-146. [DOI] [PubMed] [Google Scholar]
- 26.Kuramoto, H., S. Tamura, and Y. Notake. 1972. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am. J. Obstet. Gynecol. 114:1012-1019. [DOI] [PubMed] [Google Scholar]
- 27.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, and M. Alary. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 28.Limpakarnjanarat, K., T. D. Mastro, S. Saisorn, W. Uthaivoravit, J. Kaewkungwal, S. Korattana, N. L. Young, S. A. Morse, D. S. Schmid, B. G. Weniger, and P. Nieburg. 1999. HIV-1 and other sexually transmitted infections in a cohort of female sex workers in Chiang Rai, Thailand. Sex. Transm. Infect. 75:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClelland, R. S., C. C. Wang, K. Mandaliya, J. Overbaugh, M. T. Reiner, D. D. Panteleeff, L. Lavreys, J. Ndinya-Achola, J. J. Bwayo, and J. K. Kreiss. 2001. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS 15:105-110. [DOI] [PubMed] [Google Scholar]
- 30.Nelson, K. E., S. Eiumtrakul, D. Celentano, I. Maclean, A. Ronald, S. Suprasert, D. R. Hoover, S. Kuntolbutra, and J. M. Zenilman. 1997. The association of herpes simplex virus type 2 (HSV-2), Haemophilus ducreyi, and syphilis with HIV infection in young men in northern Thailand. J. Acquir. Immune Defic. Syndr. 16:293-300. [DOI] [PubMed] [Google Scholar]
- 31.Ohkawa, M., K. Yamaguchi, S. Tokunaga, T. Nakashima, and S. Fujita. 1992. The incidence of Trichomonas vaginalis in chronic prostatitis patients determined by culture using a newly modified liquid medium. J. Infect. Dis. 166:1205-1206. [DOI] [PubMed] [Google Scholar]
- 32.Petrin, D., K. Delgaty, R. Bhatt, and G. Garber. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poli, G., A. L. Kinter, and A. S. Fauci. 1994. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. USA 91:108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen, S. E., M. H. Nielsen, I. Lind, and J. M. Rhodes. 1986. Morphological studies of the cytotoxicity of Trichomonas vaginalis to normal human vaginal epithelial cells in vitro. Genitourin. Med. 62:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rein, M. 1990. Clinical manifestations of urogenital trichomoniasis in women, p. 225-234. In B. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, N.Y.
- 36.Rendon-Maldonado, J., M. Espinosa-Cantellano, C. Soler, J. V. Torres, and A. Martinez-Palomo. 2003. Trichomonas vaginalis: in vitro attachment and internalization of HIV-1 and HIV-1-infected lymphocytes. J. Eukaryot. Microbiol. 50:43-48. [DOI] [PubMed] [Google Scholar]
- 37.Rendon-Maldonado, J. G., M. Espinosa-Cantellano, A. Gonzalez-Robles, and A. Martinez-Palomo. 1998. Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp. Parasitol. 89:241-250. [DOI] [PubMed] [Google Scholar]
- 38.Schon, M., and J. G. Rheinwald. 1996. A limited role for retinoic acid and retinoic acid receptors RAR alpha and RAR beta in regulating keratin 19 expression and keratinization in oral and epidermal keratinocytes. J. Investig. Dermatol. 107:428-438. [DOI] [PubMed] [Google Scholar]
- 39.Serwadda, D., R. H. Gray, N. K. Sewankambo, F. Wabwire-Mangen, M. Z. Chen, T. C. Quinn, T. Lutalo, N. Kiwanuka, G. Kigozi, F. Nalugoda, M. P. Meehan, R. Ashley Morrow, and M. J. Wawer. 2003. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J. Infect. Dis. 188:1492-1497. [DOI] [PubMed] [Google Scholar]
- 40.Snipes, L. J., P. M. Gamard, E. M. Narcisi, C. B. Beard, T. Lehmann, and W. E. Secor. 2000. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J. Clin. Microbiol. 38:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.UNAIDS Joint United Nations Program on HIV/AIDS. 2003. AIDS epidemic update: December 2002. UNAIDS Joint United Nations Program on HIV/AIDS, United Nations, New York, N.Y.
- 42.Reference deleted.
- 43.Wisdom, A. R., and E. M. Dunlop. 1965. Trichomoniasis: study of the disease and its treatment in women and men. Br. J. Vener. Dis. 41:90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. 1995. An overview of selected curable sexually transmitted diseases, p. 2-27. World Health Organization Global Program on AIDS, Geneva, Switzerland.
- 45.Xiao, L., R. B. Lal, and A. A. Lal. 1999. Effect of immune activation induced by Cryptosporidium parvum whole antigen on in vitro human immunodeficiency virus type 1 infection. J. Infect. Dis. 180:559-563. [DOI] [PubMed] [Google Scholar]
- 46.Xiao, L., S. M. Owen, D. L. Rudolph, R. B. Lal, and A. A. Lal. 1998. Plasmodium falciparum antigen-induced human immunodeficiency virus type 1 replication is mediated through induction of tumor necrosis factor-alpha. J Infect. Dis. 177:437-445. [DOI] [PubMed] [Google Scholar]
- 47.Zariffard, M. R., S. Harwani, R. M. Novak, P. J. Graham, X. Ji, and G. T. Spear. 2004. Trichomonas vaginalis infection activates cells through toll-like receptor 4. Clin. Immunol. 111:103-107. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Z. F., and C. B. Begg. 1994. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int. J. Epidemiol. 23:682-690. [DOI] [PubMed] [Google Scholar]