Abstract
Chemokines regulate the host immune response to a variety of infectious pathogens. Since the role of chemokines in regulating host immunity in children with Plasmodium falciparum malaria has not previously been reported, circulating levels of β-chemokines (MIP-1α, MIP-1β, and RANTES) and their respective transcriptional profiles in ex vivo peripheral blood mononuclear cells (PBMCs) were investigated. Peripheral blood MIP-1α and MIP-1β levels were significantly elevated in mild and severe malaria, while RANTES levels decreased with increasing disease severity. β-Chemokine gene expression profiles in blood mononuclear cells closely matched those of circulating β-chemokines, illustrating that PBMCs are a primary source for the observed pattern of β-chemokine production during acute malaria. Statistical modeling revealed that none of the chemokines was significantly associated with either parasitemia or anemia. Additional investigations in healthy children with a known history of malaria showed that children with prior severe malaria had significantly lower baseline RANTES production than children with a history of mild malaria, suggesting inherent differences in the ability to produce RANTES in these two groups. Baseline MIP-1α and MIP-1β did not significantly differ between children with prior severe malaria and those with mild malaria. Additional in vitro experiments in PBMCs from healthy, malaria-naïve donors revealed that P. falciparum-derived hemozoin (Hz; malarial pigment) and synthetic Hz (β-hematin) promote a similar pattern of β-chemokine gene expression. Taken together, the results presented here demonstrate that children with severe malaria have a distinct profile of β-chemokines characterized by increased circulating levels of MIP-1α and MIP-1β and decreased RANTES. Altered patterns of circulating β-chemokines result, at least in part, from Hz-induced changes in β-chemokine gene expression in blood mononuclear cells.
Malaria is one of the greatest influences of infectious origin on morbidity and mortality in sub-Saharan Africa (49). In young children who are largely non- or semi-immune to Plasmodium falciparum malaria, severe disease primarily manifests as cerebral malaria and/or severe malarial anemia (31). In areas of hyperendemicity, such as Lambaréné, Gabon, severe malaria generally affects children less than 5 years of age due to the lack of naturally acquired immunity (3). The primary clinical manifestations of severe malaria in this area are severe malarial anemia and/or high-density parasitemia, with only rare occurrences of cerebral malaria (23, 36).
Although the molecular determinants of mild versus severe malaria are largely undefined, current evidence suggests a potential role for the relative balance between pro- and anti-inflammatory cytokines (9, 13, 34, 36, 43). Elevated levels of proinflammatory cytokines during the acute phase of infection appear to limit disease progression, while a bias towards an anti-inflammatory response appears to promote enhanced pathogenesis (13, 32, 33, 48). Additional soluble inflammatory mediators which may be important in malaria pathogenesis are chemokines, a superfamily of small, structurally related proteins (8 to 17 kDa) that regulate innate and adaptive immunity (26). Chemokines are categorized into four classes based on the number and spacing of N-terminal cysteine residues (2). These classes can be more broadly defined as CXC (or α-) chemokines (e.g., stromal cell derived factor 1 [SDF-1]) and the CC (or β-) chemokines (e.g., macrophage inflammatory protein 1α [MIP-1α], MIP-1β, and regulated on activation, normal T-cell expressed and secreted [RANTES]) (2). The actions of chemokines are mediated by a family of 7-transmembrane G protein-coupled receptors (26). Binding of chemokines to their cognate receptors results in an intracellular signaling cascade that culminates in diverse biological functions, such as chemotactic recruitment of inflammatory cells, leukocyte activation, angiogenesis, hematopoiesis, and antimicrobial effects (26).
In addition to their role in regulating the immune response during viral and bacterial infections, chemokines also appear to mediate the host immune response during protozoan infections (4). For example, recent studies have shown that P. falciparum infection during pregnancy is characterized by elevations in MIP-1α, monocyte chemoattractant protein 1 (MCP-1), I-309, and interleukin-8 (IL-8) transcripts, which are associated with increased monocyte density in the placental intervillous space (1, 42). Additional studies in women with malaria during pregnancy show that MIP-1α and MIP-1β are elevated in cultured intervillous leukocytes (8). Serum concentrations of MIP-1α and IL-8 are also increased in adults with severe and complicated falciparum malaria (6).
Recent studies in our laboratory have shown that malarial pigment (hemozoin [Hz]) is one of the primary parasitic products responsible for altered production of soluble immune mediators, such as nitric oxide and prostaglandin-E2 (PGE2) from peripheral blood mononuclear cells (PBMCs) during acute falciparum malaria in children (21, 22). Hz is a coordinated aggregation polymer of heme that is produced by parasites during the digestion of host hemoglobin within red blood cells (RBCs) (for review, see reference 15). Following lysis of parasitized RBCs, Hz is rapidly phagocytosed by neutrophils, monocytes, and tissue macrophages. Naturally acquired Hz is a conglomeration of host- and parasite-derived lipids, proteins, and nucleic acids which are adherent to the polymerized heme core structure, ferriprotoporphyrin IX (FP-IX) (16). Removal of proteins and lipids from the FP-IX core yields a compound which appears structurally identical to synthetic malarial pigment, β-hematin (sHz) (14). Recent findings in a murine model of malaria demonstrate that injection of Hz in BALB/c mice induces the expression of chemokines (MIP-1α, MIP-1β, MIP-2, and MCP-1) (20).
Although chemokines are important for bridging innate and adaptive immune responses and regulating hematopoietic maturation (5, 24), the profile of chemokine expression and their potential role in regulating disease severity in children with P. falciparum malaria have not been defined. As such, we determined circulating protein levels and transcript profiles of β-chemokines (MIP-1α, MIP-1β, and RANTES) and an α-chemokine (SDF-1) in plasma and PBMCs, respectively, in children with various degrees of P. falciparum malaria. Additional studies were conducted with cultured PBMCs to determine the effects of Hz and sHz on chemokine transcripts and protein.
(Portions of this work were presented at the 52nd American Society of Tropical Medicine and Hygiene [ASTMH] Annual Meeting, Philadelphia, PA, 3 to 7 December 2003.)
MATERIALS AND METHODS
Study participants.
Children (n = 43, age 2 to 7 years) were recruited from a longitudinal prospective study at the Albert Schweitzer Hospital in Lambaréné, Gabon, in the province of Moyen Ogooue. Classification of malaria was defined according to our previously published methods, with inclusion criteria for severe cases (n = 10) characterized by parasitemias greater than 200,000 parasites/μl and/or the presence of severe anemia (i.e., hemoglobin, ≤5 g/dl) (35). Matched mild malaria cases (n = 10) were defined by parasitemias less than 150,000 parasites/μl and the absence of any signs or symptoms of severe malaria. Healthy, malaria-exposed subjects (n = 23) were defined as those participants with a previous episode(s) of either mild or severe malaria and the absence of a positive thick blood film for malaria or any other illnesses within the last 4 weeks. All blood samples were obtained prior to treatment with antimalarials and/or antipyretics. Routine clinical evaluations and laboratory measures were used to evaluate the patients. Children with malaria were given antimalarials and the appropriate supportive therapy as required.
To determine the effects of Hz or sHz on the in vitro production of β-chemokines, healthy, malaria-naïve adult volunteers (n = 5) were recruited at the University of Pittsburgh, Pittsburgh, Pa. The study was approved by the ethics committee of the International Foundation of the Albert Schweitzer Hospital, Duke University Medical Center Investigational Review Board, and the University of Pittsburgh Investigational Review Board.
Isolation and culture of peripheral blood mononuclear cells.
Venous blood (3 ml from children and 50 ml from adult volunteers) was drawn into EDTA-containing vials. Plasma was separated and PBMCs were prepared using Ficoll-Hypaque according to our previously described methods (45). PBMCs were washed twice with Dulbecco's modified Eagle's medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10 mM HEPES and 10 mM penicillin-streptomycin. Plasma and washed PBMC pellets were immediately frozen and kept at −70°C until use.
To assess the effects of Hz or sHz on the production of chemokines, PBMCs were isolated from healthy adult U.S. donors and plated at a density of 1 × 106 cells/ml in Dulbecco's modified Eagle's medium supplemented with 10 mM HEPES, 10 mM penicillin-streptomycin, and 10% pooled human serum (heat inactivated at 56°C for 30 min). Cells were incubated with medium alone (control) or physiologic amounts of Hz or sHz representing a concentration comparable to that during severe malaria (i.e., 10 μg/ml) (22). Hz and sHz preparations were extensively sonicated prior to addition to the cultures. Cells were kept at 37°C in a humid atmosphere of 5% CO2. Culture supernatants and PBMCs were collected at 4, 24, and 48 h.
Preparation of crude hemozoin and synthetic hemozoin.
Crude Hz and sHz were prepared according to our previously described methods (21). In brief, Hz was prepared from in vitro cultures of P. falciparum-infected red blood cells (strain Pf-D6) harvested when the parasitemia level was 3 to 5%, and late trophozoites and early schizonts were the predominant forms. Infected RBCs were spun at 2,000 rpm for 10 min and resuspended in 40 ml of 0.01 M phosphate-buffered saline (pH 7.2) with 2 ml of 1% saponin solution for 10 min. Cell lysates were then subjected to ultracentrifugation (14,000 rpm for 15 min) and cell pellets were washed 3 to 5 times in 1× phosphate-buffered saline to remove adherent lipid membranes. Following the final wash step, the pellet was dried overnight, weighed, and resuspended in filter-sterilized H2O at a concentration of 1.0 mg/ml.
sHz was prepared in a 4.5 M acidic acetate solution as previously described (14). In brief, hemin chloride (Sigma, St. Louis, MO) was added to a 0.1 M solution of NaOH followed by addition of HCl at 60°C. sHz was crystallized by addition of an acidic solution of acetate, and the mixture was incubated at 60°C for 150 min. The solution was then centrifuged and washed three times with distilled water and dried at 60°C under vacuum. The final pellet was weighed and resuspended in filter-sterilized H2O at a concentration of 1.0 mg/ml. The presence of endotoxin in the Hz and sHz preparations was tested with the Limulus amebocyte lysate test (BioWitttaker Inc, Walersville, MD) and found to be less than 0.1 endotoxin unit/ml.
Chemokine ELISAs.
Quantitative enzyme-linked immunosorbent assay (ELISA) was performed with commercially available assays to determine MIP-1α, MIP-1β, and RANTES (Biosource International, Camarillo, CA) and SDF-1 (DuoSet: R&D Minneapolis, MN) levels in plasma or culture supernatants. The standard deviation of replicate samples was less than 10% of the mean in all experiments. Sample concentrations of each chemokine were quantified from standard curves generated with recombinant chemokines and analyzed with Softmax software (Molecular Devices Corporation, Sunnyvale, CA). The lower limit of detection for the chemokines was 15 pg/ml.
Quantitative real-time RT-PCR.
Total RNA was extracted from PBMCs by the guanidinium isothiocyanate method (10). Following isopropanol precipitation, RNA was pelleted at 13,000 rpm for 15 min and resuspended in RNase-free water to a final concentration of 1.0 μg/μl. Extracted RNA (1.0 μg) was reverse transcribed using random hexamers to generate cDNA. One-tenth (2.0 μl) of the resulting cDNA was used as a template for Taqman real-time reverse transcription-PCR (RT-PCR) amplification on an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Each PCR was set up in duplicate with 2.0 μl of cDNA template in a total volume of 25 μl of Taqman Universal Master mix and specific primer/probe sets for chemokine transcripts: MIP-1α (Hs00234142_mL), MIP-1β (Hs00605740_mL), RANTES (Hs00174575_mL), and SDF-1 (Hs00171022_mL) (Applied Biosystems, Foster City, CA). Amplification of β-actin (accession number NM_001101; Applied Biosystems, Foster City, CA) served as an endogenous control to normalize loading of cDNA samples. For experiments with ex vivo PBMCs from Gabonese children, data were compared by subtracting the β-actin cycle threshold (CT) value from the experimental gene CT for each sample, and expressed as fold change relative to β-actin levels (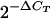 ). For experiments with cultured PBMCs, data were compared using the −ΔΔCT method as previously described (22).
). For experiments with cultured PBMCs, data were compared using the −ΔΔCT method as previously described (22).
Statistical analyses.
Mann-Whitney U tests (statistical significance set at P < 0.05) were used to analyze the differences in the concentrations of chemokines in plasma and for comparisons of chemokine gene expression profiles between groups. Pearson correlations were used to examine the linear associations among the variables of interest. For the in vitro experiments, chemokine concentrations in culture supernatants and gene expression levels were compared between Hz- or sHz-stimulated cells and unstimulated control cells using Mann-Whitney U tests (P < 0.05).
RESULTS
Patient characteristics.
Children (3 to 7 years of age) with acute P. falciparum malaria were recruited from the Albert Schweitzer Hospital in Lambarènè, Gabon. Healthy, age-matched controls (HC; n = 23) were recruited from a longitudinal cohort study. At the time of sampling, all children in the HC group had been parasite free for at least 4 weeks. Children with mild malaria (MM; n = 10) and severe malaria (SM; n = 10) were recruited upon presentation at hospital. The clinical and laboratory characteristics of the groups are presented in Table 1. The difference in age between the three groups was not significant (P = 0.99). Peripheral parasitemia was significantly different between mild and severe cases (P < 0.01). Hemoglobin concentrations and axillary temperature were significantly different between the groups (P < 0.001 and P < 0.01, respectively).
TABLE 1.
Clinical and laboratory measures in childrena
| Characteristic | Result for group
|
P | ||
|---|---|---|---|---|
| Healthy controls | Mild malaria | Severe malaria | ||
| No. of children | 23 | 10 | 10 | |
| Age (yr) | 4.9 (0.5) | 5.1 (0.5) | 4.9 (0.6) | 0.99 |
| No. male/female | 11/12 | 6/4 | 4/6 | 0.66b |
| Parasitemia | ||||
| No. of parasites/μl | 0 | 52,899 (10,437) | 355,571 (58,050) | <0.01c |
| Geometric mean | 0 | 39,890 | 330,092 | |
| Hemoglobin (g/dl) | 10.6 (0.3) | 10.1 (0.5) | 6.7 (0.5) | <0.001 |
| Axillary temp (°C) | 37.8 (0.18) | 38.2 (0.2) | 39.6 (0.4) | <0.01 |
Healthy controls (aparasitemic for 4 weeks or greater) were recruited from a longitudinal cohort study. Acute malaria cases were recruited upon presentation at hospital. Mild malaria was defined by hemoglobin between 5.1 and 11.0 g/dl, with parasitemia less than 150,000 parasites/μl, while severe malaria was defined by hemoglobin less than or equal to 5.0 g/dl and/or parasitemia >200,000 parasites/μl. Heel- or finger-prick blood (∼100 μl) was obtained to determine parasitemia and anemia status. Peripheral blood smears were prepared and stained with Giemsa reagent and examined under oil immersion for malaria parasites. Asexual malaria parasites were counted against 300 leukocytes, and parasite densities were estimated assuming a count of 8,000 white blood cells per microliter of blood. Hemoglobin levels were measured using a HemoCue system (HemoCue AB, Angelholm, Sweden). Data are presented as the mean (standard error in parentheses) and comparisons between groups were performed by Kruskal-Wallis test unless otherwise noted.
Differences between the proportions were determined by chi-square analysis.
Differences between mild and severe cases determined by Mann-Whitney U test.
Circulating levels of chemokines in children with malaria.
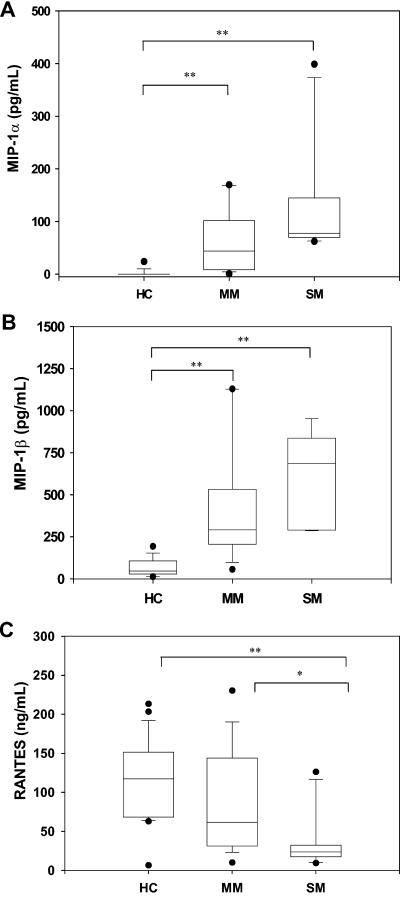
To determine if circulating levels of β-chemokines differed between the groups, plasma samples were obtained from children with various degrees of disease severity and analyzed by ELISA. MIP-1α was largely undetectable in the plasma of the HC group, but was elevated in children with MM (P < 0.001) and SM (P < 0.001, Fig. 1A). Similarly, levels of MIP-1β were higher in children with MM (P < 0.001) and SM (P < 0.001) compared to HC (Fig. 1B). Although the SM group had higher levels of MIP-1α and MIP-1β than the MM group, levels were not significantly different between the groups (P = 0.80 and P = 0.31, respectively; Fig. 1A and 1B). In contrast to MIP-1α and MIP-1β, circulating levels of RANTES were lower in children with MM (P = 0.14) and SM relative to HC (P < 0.01, Fig. 1C). Furthermore, RANTES levels in children with MM were significantly higher than in children with SM (P < 0.05, Fig. 1C). Additional experiments revealed that SDF-1 was not detectable in plasma from any of the children in the present study (data not shown).
FIG. 1.
Peripheral blood levels of chemokines in children with malaria. (A) MIP-1α, (B) MIP-1β, and (C) RANTES levels were determined in plasma of children from the HC (n = 23), MM (n = 10), and SM (n = 10) groups by ELISA. Each box plot represents the range (25th to 75th percentiles) of chemokine concentration for each group, with the median value at the intersect. Statistical significance was determined by Mann-Whitney U test. * denotes P < 0.05 relative to HC; ** denotes P < 0.01 relative to HC.
β-Chemokine transcript profiles.
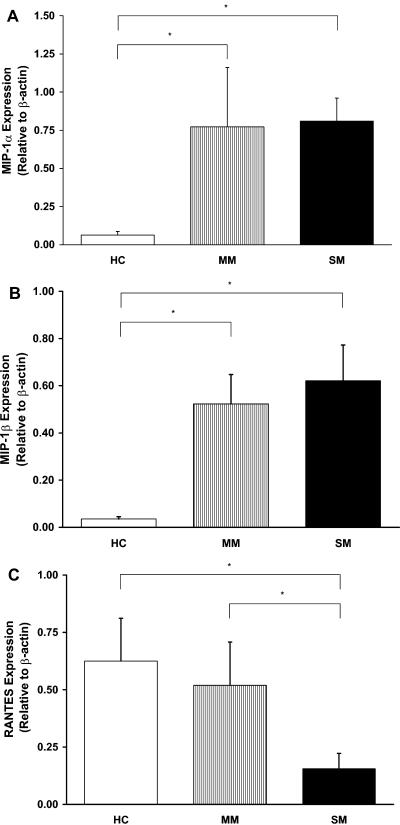
To determine the potential cellular source(s) of circulating chemokines, gene expression profiles for MIP-1α, MIP-1β, and RANTES were determined by Taqman real-time RT-PCR in ex vivo PBMCs isolated from children with various degrees of malaria. Relative to HC, MIP-1α expression was significantly increased in children with either MM (P < 0.05) or SM (P < 0.05; Fig. 2A). Similarly, MIP-1β gene expression was significantly elevated in children with MM and SM compared to HC (P < 0.05 and P < 0.01, respectively; Fig. 2B). In contrast, RANTES was slightly lower in children with MM (P = 0.71) and significantly decreased in children with SM (P < 0.01; Fig. 2C). RANTES gene expression was significantly lower in children with SM relative to children with MM (P < 0.05; Fig. 2C). SDF-1 transcripts were not detectable in any of the specimens analyzed (data not presented).
FIG. 2.
Transcriptional profiles of chemokines in children with malaria. Total RNA was isolated from PBMCs obtained from children from the HC (n = 23), MM (n = 10), and SM (n = 10) groups. (A) MIP-1α, (B) MIP-1β, and (C) RANTES gene expression was determined by real time RT-PCR. Data are presented for each chemokine relative to expression of endogenous β-actin. Statistical significance was determined by Mann-Whitney U test. * denotes P < 0.05 relative to HC.
Association of β-chemokine expression with prior disease severity.
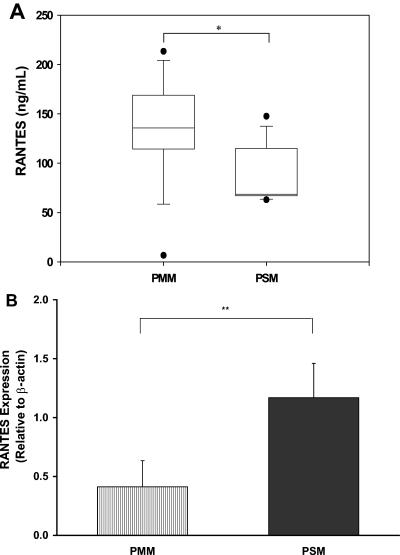
Since the history of prior malaria exposure was available for children in the HC group, data were further stratified into those children with previous mild malaria (PMM, n = 13) and those with previous severe malaria (PSM, n = 10). These groups were analyzed for circulating levels of RANTES and transcript levels in ex vivo PBMCs. As shown in Fig. 3A, the PMM group had significantly higher plasma RANTES levels than the PSM group (P < 0.05). In contrast, RANTES transcripts were significantly lower in children with PMM than in children with PSM (P < 0.01; Fig. 3B). There were no significant differences in the levels of MIP-1α and MIP-1β protein (P = 0.92 and P = 0.11, respectively) and transcripts (P = 0.10 and P = 0.89, respectively) in the PMM group versus the PSM group.
FIG. 3.
Circulating RANTES and ex vivo PBMC mRNA levels in healthy children with prior malaria exposure. (A) Plasma RANTES levels were determined by ELISA in children from the HC group with a history of either mild malaria (PMM; n = 14) or severe malaria (PSM; n = 9). (B) RANTES gene expression was determined by real-time RT-PCR on RNA isolated from PBMCs. Data are presented relative to expression of endogenous β-actin. Statistical significance was determined by Mann-Whitney U test. * denotes P < 0.05 relative to PMM; ** denotes P < 0.001 relative to PMM.
Association of β-chemokines with hemoglobin concentrations and parasite density.
To examine the linear associations between the chemokines and clinical parameters (i.e., anemia and parasitemia), bivariate Pearson correlations were conducted. Before conducting the analyses, all relevant variables were examined for departures from normality. Since MIP-1α and parasitemia were moderately positively skewed, these variables were normalized by square root transformations prior to performing the analyses. Although there was a significant negative correlation between parasitemia and hemoglobin (r = −0.402, P < 0.05), none of the other correlations was statistically significant (see Table 2). As a follow-up to the correlation analyses, we conducted two linear multiple regression analyses on the mild and severe malaria groups, one each for the dependent variables of hemoglobin and parasitemia. The purpose of these analyses was to examine the unique predictive ability of each of the three chemokines (i.e., MIP-1α, MIP-1β, and RANTES) after controlling for the remaining two chemokines and age. Both of the regression equations were statistically nonsignificant (P = 0.43 and P = 0.12, respectively), and, likewise, all of the standardized partial regression coefficients (beta weights) were nonsignificant, indicating that none of the chemokines uniquely predicted anemia and parasitemia.
TABLE 2.
Correlations between β-chemokines and hemoglobin and parasitemiaa
| Variable | rb | Pd | rc | Pd |
|---|---|---|---|---|
| Age | 0.105 | 0.62 | −0.297 | 0.12 |
| MIP-1α | 0.148 | 0.60 | 0.033 | 0.90 |
| MIP-1β | −0.316 | 0.27 | 0.230 | 0.39 |
| RANTES | 0.062 | 0.81 | −0.309 | 0.20 |
Pearson correlation coefficients were used (mild and severe malaria groups only).
Hemoglobin.
Parasitemia.
All P values are two-tailed.
Effect of Hz and sHz on PBMC chemokine production.
Previous studies show that parasite-derived products, such as Hz, augment production of MIP-1α and MIP-1β from cultured human PBMCs (40). In addition, we have previously shown that Gabonese children with severe malaria have significantly higher circulating levels of Hz-containing monocytes and neutrophils than children with mild malaria (27). The effect of malarial pigment on β-chemokine production was, therefore, determined in PBMCs from healthy, malaria-naïve adult donors (n = 5). MIP-1α and MIP-1β levels were undetectable under baseline (control) conditions at all time points examined. Treatment of cultures with Hz or sHz increased MIP-1α and MIP-1β production by 24 h, which remained elevated throughout the 48-h time course (P < 0.01 and P < 0.01, respectively, at 24 and 48 h; Fig. 4A and 4B). Relative to the 4-h time point, baseline RANTES production decreased significantly at 48 h in control cells (P < 0.05), while the addition of Hz or sHz resulted in significantly higher RANTES production than that observed in control cells at 48 h (P < 0.05 and P < 0.05, respectively, Fig. 4C). SDF-1 was undetectable in culture supernatants from stimulated and unstimulated cells at all time points (data not shown).
FIG. 4.
Effects of P. falciparum-derived hemozoin and synthetic hemozoin on PBMC chemokine production. PBMCs (2 × 106/well) from healthy, malaria-naïve adult volunteers (n = 5) were stimulated with medium alone (controls; solid gray line), Hz (10 μg/ml; solid black line), or sHz (10 μg/ml; dashed black line) for 4, 24, and 48 h. (A) MIP-1α, (B) MIP-1β, and (C) RANTES levels were determined in culture supernatants by ELISA. Statistical significance was determined by Mann-Whitney U test. * denotes P < 0.05 relative to control; ** denotes P < 0.01 relative to control.
Effect of Hz and sHz on PBMC chemokine transcripts.
To determine if alterations in β-chemokine production by Hz and sHz were regulated at the transcriptional level, gene expression profiles for MIP-1α, MIP-1β, RANTES, and SDF-1 were determined by real-time RT-PCR. Relative to nonstimulated control cells, expression of MIP-1α and MIP-1β was significantly increased by Hz and sHz at 4, 24, and 48 h (P < 0.05 for all comparisons; Fig. 5A and 5B), except for MIP-1β transcripts, which were not significantly elevated in cultures stimulated with sHz at 48 h (P = 0.83). In contrast, RANTES expression was not altered by either Hz or sHz at 4 h (P = 0.13 and P = 0.17, respectively); however, after 24 h in culture, PBMCs stimulated with sHz, but not Hz, had comparatively higher expression of RANTES than under baseline conditions (P < 0.05; Fig. 5C). RANTES gene expression in cells stimulated with Hz for 48 h was not significantly altered (P = 0.32), while cells stimulated with sHz had suppressed RANTES mRNA expression (P < 0.05; Fig. 5C). SDF-1 transcripts were not detectable under any of the culture conditions examined (data not shown).
FIG. 5.
Effects of P. falciparum-derived hemozoin and synthetic hemozoin on PBMC chemokine gene expression. PBMCs (2 × 106/well) from healthy, malaria-naïve adult volunteers (n = 5) were stimulated with medium alone (controls; solid gray line), Hz (10 μg/ml; solid black line), or sHz (10 μg/ml; dashed black line), and cells were harvested at 4, 24, and 48 h. (A) MIP-1α, (B) MIP-1β, and (C) RANTES mRNA expression was determined by real-time RT-PCR. Statistical significance was determined by Mann-Whitney U test. * denotes P < 0.05 relative to control; ** denotes P < 0.01 relative to control.
DISCUSSION
The role of chemokines in the regulation of host-parasite interactions appears to be highly variable in protozoan infections (4). Chemokines have been shown to have direct antiprotozoal activity against Toxoplasma gondii, Leishmania donovani, and Trypanosoma cruzi (4). Although the ability of chemokines to regulate intraerythrocytic growth of the malaria parasite has not been reported, a functional role for altered expression patterns of chemokines during malaria has been associated with mononuclear cell infiltration during placental malaria (1, 42). In addition, increased expression of chemokines has been shown in both murine and human cerebral malaria (19, 38). Previous results also showed that serum concentrations of MIP-1α are elevated in adults with P. falciparum malaria in Southeast Asia; levels of MIP-1α were highest on day 14 after admission and following treatment (6). Our studies show that MIP-1α is also elevated in children with acute falciparum malaria prior to treatment with antipyretics and/or antimalarials, demonstrating that elevated circulating concentrations of MIP-1α are the direct result of a malaria infection and not due to treatment interventions. Analysis of MIP-1α transcripts in ex vivo PBMCs illustrated that MIP-1α gene expression was more than 50% higher in children with malaria than in their healthy, age-matched controls. These results suggest that changes in MIP-1α during acute disease occur, at least in part, through enhanced MIP-1α mRNA transcription in PBMCs. Investigation of MIP-1β revealed similar results in which acute malaria was characterized by elevated circulating levels of MIP-1β that appear to result from malaria-induced upregulation of MIP-1β gene expression in PBMCs.
Currently, it is not clear whether increased circulating levels of MIP-1α and MIP-1β in acute malaria are an appropriate physiologic response to the infection or if they contribute to enhanced pathophysiology. The previous observation that MIP-1α and IL-8 are induced in the acute phase of malaria and remain elevated even after parasite clearance (6) suggests that the immunologic effects of chemokines may occur over prolonged periods. Since a number of studies have shown that MIP-1α suppresses hematopoiesis (5, 7, 17, 18, 25, 41), sustained elevated levels of MIP-1α may contribute to malarial anemia. Previous studies illustrating that enhanced MIP-1β production from bone marrow aspirates is associated with decreased hemoglobin concentrations in human immunodeficiency virus-positive adults further support a potential role of chemokines in the promotion of anemia (12). However, results presented here failed to find a significant association between MIP-1α and MIP-1β levels and hemoglobin concentrations. It is important to note that disease severity in the present study was defined according to both parasitemia and anemia. As such, with disease severity based on these two parameters, it may not be possible to fully elucidate the contribution of MIP-1α and MIP-1β to the etiology of malarial anemia. Since many of the severe cases had high-density parasitemia in the absence of severe anemia, we are currently investigating the role of MIP-1α and MIP-1β in the promotion of malarial anemia in a pediatric population in western Kenya in which the classification of disease severity is based solely on anemia status.
Of particular interest in the current study was the finding that RANTES is suppressed in children with severe malaria at both the mRNA level and protein level. There is growing evidence that RANTES provides protection against protozoan diseases (29, 37, 44). Although the molecular basis of this protection remains unclear, it may be related to bridging innate and adaptive immune responses, since RANTES is a specific chemoattractant for memory T cells and augments polarization of a T helper 1 (Th1) response (30). Thus, in the context of malaria, reduced RANTES production may result in an ineffective immunologic response. This hypothesis is supported by the fact that immunosuppression is a prominent feature of malaria, as evidenced by increased susceptibility to a number of bacterial and viral infections, including salmonellosis (28) and reactivation of chronic and latent herpesvirus zoster (11), herpes simplex virus (39), and Epstein-Barr virus (46, 47) infections. Data presented here showing that circulating levels of RANTES are significantly reduced in healthy children with a history of severe malaria relative to those that previously experienced mild malaria suggest that elevated levels of RANTES may provide protection against severe disease. Interestingly, transcriptional analysis of RANTES revealed that children with PSM had significantly higher RANTES mRNA expression than those with PMM. Additional experiments are required to determine if there is an inherent deficiency in RANTES transcript stability and/or posttranslational modifications that can explain the high levels of RANTES transcripts in the presence of low circulating levels of RANTES protein observed in children with PSM.
Although a number of parasite-derived factors, such as glycosylphospatidylinositols, soluble antigens, and Hz, could account for altered β-chemokine expression, experiments presented here using PBMCs from healthy, malaria-naïve adults illustrate that malarial pigment appears to be, at least in part, responsible for dysregulation in β-chemokine production. Comparable results obtained here using a crude Hz preparation and an sHz moiety (prepared in a cell-free system) demonstrate that the core heme polymer, FP-IX, and not additional parasite-derived products, is likely responsible for altered β-chemokine mRNA and protein expression. One important feature of the current work is that, unlike many of the previous studies, a physiologically relevant concentration of P. falciparum-derived Hz and sHz was used for the in vitro experiments. The profile of MIP-1α and MIP-1β mRNA and protein in the in vitro experiments corresponds more closely to the results observed in children with acute malaria than the results for RANTES. For example, protein levels of RANTES decreased under baseline conditions by 48 h, while RANTES increased in cells treated with Hz and sHz at the same time points. Analyses of RANTES transcripts, however, revealed that the mRNA declined by 48 h in culture, suggesting that there would be a subsequent decrease in RANTES protein over prolonged periods.
Taken together, the results presented here provide the first reported evidence that children with acute falciparum malaria have dysregulation of β-chemokines characterized by elevated MIP-1α and MIP-1β and decreased RANTES at the mRNA and protein level. This distinct profile of β-chemokine expression appears to be due to phagocytosis of Hz by circulating blood mononuclear cells. Additional studies aimed at defining the role of β-chemokines in the promotion of malarial anemia and the impact of decreased RANTES on malaria-induced immunosuppression may provide important information about the pathophysiology of malaria.
Acknowledgments
We thank the following staff members of Albert Schweitzer Hospital in Lambaréné, Gabon, for technical assistance with the study: Anita van den Biggerlaar, Judith Jans, Hanna Knoop, Doris Luckner, Barbara Moritz, Anselme Ndzengue, Mercel Nkeyi, Daniela Schmid, and Milena Sovric.
This study was conducted at the University of Pittsburgh, and supported in part by the National Institutes of Health grants AI-51305-01 (to D.J.P.) and AI-41764 (to J.B.W.), the VA Research Service (J.B.W.), and Fogarty training grant 5D43-TW00588-4 (to D.J.P.).
There is no conflict of interest for any of the authors of the manuscript due to either commercial or other affiliations.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abrams, E. T., H. Brown, S. W. Chensue, G. D. Turner, E. Tadesse, V. M. Lema, M. E. Molyneux, R. Rochford, S. R. Meshnick, and S. J. Rogerson. 2003. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J. Immunol. 170:2759-2764. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, K., M. Baggiolini, H. Broxmeyer, R. Horuk, I. Lindley, A. Mantovani, K. Maysushima, P. Murphy, H. Nomiyama, J. Oppenheim, A. Rot, T. Schall, M. Tsang, R. Thorpe, J. Van Damme, M. Wadhwa, O. Yoshie, A. Zlotnik, and K. Zoon. 2002. Chemokine/chemokine receptor nomenclature. J. Interferon Cytokine Res. 22:1067-1068. [DOI] [PubMed] [Google Scholar]
- 3.Breman, J. G., A. Egan, and G. T. Keusch. 2001. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 64:iv-vii. [DOI] [PubMed] [Google Scholar]
- 4.Brenier-Pinchart, M. P., H. Pelloux, D. Derouich-Guergour, and P. Ambroise-Thomas. 2001. Chemokines in host-protozoan-parasite interactions. Trends Parasitol. 17:292-296. [DOI] [PubMed] [Google Scholar]
- 5.Broxmeyer, H. E. 2001. Regulation of hematopoiesis by chemokine family members. Int. J. Hematol. 74:9-17. [DOI] [PubMed] [Google Scholar]
- 6.Burgmann, H., U. Hollenstein, C. Wenisch, F. Thalhammer, S. Looareesuwan, and W. Graninger. 1995. Serum concentrations of MIP-1 alpha and interleukin-8 in patients suffering from acute Plasmodium falciparum malaria. Clin. Immunol. Immunopathol. 76:32-36. [DOI] [PubMed] [Google Scholar]
- 7.Cantor, A. B., and S. H. Orkin. 2002. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 21:3368-3376. [DOI] [PubMed] [Google Scholar]
- 8.Chaisavaneeyakorn, S., J. M. Moore, L. Mirel, C. Othoro, J. Otieno, S. C. Chaiyaroj, Y. P. Shi, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2003. Levels of macrophage inflammatory protein 1α (MIP-1α) and MIP-1β in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin. Diagn. Lab. Immunol. 10:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiyaroj, S. C., A. S. Rutta, K. Muenthaisong, P. Watkins, M. Na Ubol, and S. Looareesuwan. 2004. Reduced levels of transforming growth factor-beta1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta Trop. 89:319-327. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 11.Cook, I. F. 1985. Herpes zoster in children following malaria. J. Trop. Med. Hyg. 88:261-264. [PubMed] [Google Scholar]
- 12.Dallalio, G., M. North, S. W. McKenzie, and R. T. Means, Jr. 1999. Cytokine and cytokine receptor concentrations in bone marrow supernatant from patients with HIV: correlation with hematologic parameters. J. Investig. Med. 47:477-483. [PubMed] [Google Scholar]
- 13.Dodoo, D., F. M. Omer, J. Todd, B. D. Akanmori, K. A. Koram, and E. M. Riley. 2002. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 185:971-979. [DOI] [PubMed] [Google Scholar]
- 14.Egan, T. J., W. W. Mavuso, and K. K. Ncokazi. 2001. The mechanism of beta-hematin formation in acetate solution. Parallels between hemozoin formation and biomineralization processes. Biochemistry 40:204-213. [DOI] [PubMed] [Google Scholar]
- 15.Francis, S. E., D. J. Sullivan, Jr., and D. E. Goldberg. 1997. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51:97-123. [DOI] [PubMed] [Google Scholar]
- 16.Goldie, P., E. Roth Jr., J. Oppenheim, and J. Vanderberg. 1990. Biochemical characterization of Plasmodium falciparum hemozoin. Am. J. Trop. Med. Hyg. 43:584-596. [DOI] [PubMed] [Google Scholar]
- 17.Graham, G. J., and I. B. Pragnell. 1992. SCI/MIP-1 alpha: a potent stem cell inhibitor with potential roles in development. Dev. Biol. 151:377-381. [DOI] [PubMed] [Google Scholar]
- 18.Graham, G. J., E. G. Wright, R. Hewick, S. D. Wolpe, N. M. Wilkie, D. Donaldson, S. Lorimore, and I. B. Pragnell. 1990. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature 344:442-444. [DOI] [PubMed] [Google Scholar]
- 19.Hanum, P. S., M. Hayano, and S. Kojima. 2003. Cytokine and chemokine responses in a cerebral malaria-susceptible or -resistant strain of mice to Plasmodium berghei ANKA infection: early chemokine expression in the brain. Int. Immunol. 15:633-640. [DOI] [PubMed] [Google Scholar]
- 20.Jaramillo, M., I. Plante, N. Ouellet, K. Vandal, P. A. Tessier, and M. Olivier. 2004. Hemozoin-inducible proinflammatory events in vivo: potential role in malaria infection. J. Immunol. 172:3101-3110. [DOI] [PubMed] [Google Scholar]
- 21.Keller, C. C., J. B. Hittner, B. K. Nti, J. B. Weinberg, P. G. Kremsner, and D. J. Perkins. 2004. Reduced peripheral PGE2 biosynthesis in Plasmodium falciparum malaria occurs through hemozoin-induced suppression of blood mononuclear cell cyclooxygenase-2 gene expression via an interleukin-10-independent mechanism. Mol. Med. 10:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller, C. C., P. G. Kremsner, J. B. Hittner, M. A. Misukonis, J. B. Weinberg, and D. J. Perkins. 2004. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect. Immun. 72:4868-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kun, J., R. Schmidt-Ott, L. Lehman, B. Lell, D. Luckner, B. Greve, P. Matousek, and P. Kremsner. 1998. Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Trans. R. Soc. Trop. Med. Hyg. 92:110-114. [DOI] [PubMed] [Google Scholar]
- 24.Lillard, J. W., Jr., U. P. Singh, P. N. Boyaka, S. Singh, D. D. Taub, and J. R. McGhee. 2003. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood 101:807-814. [DOI] [PubMed] [Google Scholar]
- 25.Lord, B. I., C. M. Heyworth, and L. B. Woolford. 1993. Macrophage inflammatory protein: its characteristics, biological properties and role in the regulation of haemopoiesis. Int. J. Hematol. 57:197-206. [PubMed] [Google Scholar]
- 26.Luster, A. D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129-135. [DOI] [PubMed] [Google Scholar]
- 27.Luty, A. J. F., D. J. Perkins, B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, J. B. Weinberg, and P. G. Kremsner. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabey, D. C., A. Brown, and B. M. Greenwood. 1987. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J. Infect. Dis. 155:1319-1321. [DOI] [PubMed] [Google Scholar]
- 29.Machado, F. S., G. A. Martins, J. C. Aliberti, F. L. Mestriner, F. Q. Cunha, and J. S. Silva. 2000. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation 102:3003-3008. [DOI] [PubMed] [Google Scholar]
- 30.Mackay, C. R. 2001. Chemokines: immunology's high impact factors. Nat. Immunol. 2:95-101. [DOI] [PubMed] [Google Scholar]
- 31.Maitland, K., P. Bejon, and C. R. Newton. 2003. Malaria. Curr. Opin. Infect. Dis. 16:389-395. [DOI] [PubMed] [Google Scholar]
- 32.Malaguarnera, L., R. M. Imbesi, S. Pignatelli, J. Simpore, M. Malaguarnera, and S. Musumeci. 2002. Increased levels of interleukin-12 in Plasmodium falciparum malaria: correlation with the severity of disease. Parasite Immunol. 24:387-389. [DOI] [PubMed] [Google Scholar]
- 33.Musumeci, M., L. Malaguarnera, J. Simpore, A. Messina, and S. Musumeci. 2003. Modulation of immune response in Plasmodium falciparum malaria: role of IL-12, IL-18 and TGF-beta. Cytokine 21:172-178. [DOI] [PubMed] [Google Scholar]
- 34.Othoro, C., A. Lal, B. Nahlen, D. Koech, A. Orago, and V. Udhayakumar. 1999. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic region in western Kenya. J. Infect. Dis. 179:279-282. [DOI] [PubMed] [Google Scholar]
- 35.Perkins, D. J., P. G. Kremsner, and J. B. Weinberg. 2001. Inverse relationship of plasma prostaglandin E2 and blood mononuclear cell cyclooxygenase-2 with disease severity in children with Plasmodium falciparum malaria. J. Infect. Dis. 183:113-118. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988-992. [DOI] [PubMed] [Google Scholar]
- 37.Santiago H. D. C., C. F. Oliveira, L. Santiago, F. O. Ferraz, D. D. G. de Souza, L. A. De-Freitas, L. C. C. Afonso, M. M. Teixeira, R. T. Gazzinelli, and L. Q. Vieira. 2004. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect. Immun. 72:4918-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarfo, B. Y., S. Singh, J. W. Lillard, A. Quarshie, R. K. Gyasi, H. Armah, A. A. Adjei, P. Jolly, and J. K. Stiles. 2004. The cerebral-malaria-associated expression of RANTES, CCR3 and CCR5 in post-mortem tissue samples. Ann. Trop. Med. Parasitol. 98:297-303. [DOI] [PubMed] [Google Scholar]
- 39.Scott, J. G. 1944. Herpes simplex cornea in malaria. Br. Med. J. 2:213-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherry, B. A., G. Alava, K. J. Tracey, J. Martiney, A. Cerami, and A. F. Slater. 1995. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J. Inflamm. 45:85-96. [PubMed] [Google Scholar]
- 41.Su, S. B., N. Mukaida, J. B. Wang, Y. Zhang, A. Takami, S. Nakao, and K. Matsushima. 1997. Inhibition of immature erythroid progenitor cell proliferation by macrophage inflammatory protein-1-alpha by interacting mainly with a C-C chemokine receptor, CCR1. Blood 90:605-611. [PubMed] [Google Scholar]
- 42.Suguitan, A. L., Jr., R. G. Leke, G. Fouda, A. Zhou, L. Thuita, S. Metenou, J. Fogako, R. Megnekou, and D. W. Taylor. 2003. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 188:1074-1082. [DOI] [PubMed] [Google Scholar]
- 43.Torre, D., F. Speranza, M. Giola, A. Matteelli, R. Tambini, and G. Biondi. 2002. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 9:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villalta, F., Y. Zhang, K. E. Bibb, J. C. Kappes, and M. F. Lima. 1998. The cysteine-cysteine family of chemokines RANTES, MIP-1α, and MIP-1β induce trypanocidal activity in human macrophages via nitric oxide. Infect. Immun. 66:4690-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg, J. B., J. J. Muscato, and J. E. Niedel. 1981. Monocyte chemotactic peptide receptor. Functional characteristics and ligand-induced regulation. J. Clin. Investig. 68:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittle, H. C., J. Brown, K. Marsh, M. Blackman, O. Jobe, and F. Shenton. 1990. The effects of Plasmodium falciparum malaria on immune control of B lymphocytes in Gambian children. Clin. Exp. Immunol. 80:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittle, H. C., J. Brown, K. Marsh, B. M. Greenwood, P. Seidelin, H. Tighe, and L. Wedderburn. 1984. T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum malaria. Nature 312:449-450. [DOI] [PubMed] [Google Scholar]
- 48.Winkler, S., M. Willheim, K. Baier, D. Schmid, A. Aichelburg, W. Graninger, and P. G. Kremsner. 1998. Reciprocal regulation of Th1- and Th2-cytokine-producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect. Immun. 66:6040-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization, Communicable Diseases Cluster. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]