Abstract
Listeriolysin O (LLO), a major virulence factor of Listeria monocytogenes, is a member of the cholesterol-dependent cytolysin family and plays important roles not only in survival of this bacterium in phagocytes but also in induction of various cellular responses, including cytokine production. In this work, we examined the involvement of LLO in induction of the cytokine response in intestinal epithelial cells, the front line of host defense against food-borne listeriosis. Infection of Caco-2 cells with wild-type L. monocytogenes induced persistent expression of interleukin-6 (IL-6) mRNA. In contrast, IL-6 expression was observed only transiently during infection with non-LLO-producing strains. A sublytic dose of recombinant LLO (rLLO) induced the expression of IL-6 via formation of membrane pores. Under conditions of LLO-induced pore formation without extensive cell lysis, Ca2+ influx was observed, and the IL-6 expression induced by rLLO was inhibited by pretreatment with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), an intracellular Ca2+ chelator. LLO secreted by cytoplasmic L. monocytogenes appeared to induce pore formation in the membrane and to enable the trafficking of intracellular and extracellular molecules. Pretreatment with BAPTA-AM inhibited persistent IL-6 expression in Caco-2 cells infected with wild-type L. monocytogenes. These results suggest that LLO is involved in IL-6 production in the late phase of infection through the formation of Ca2+-permeable pores and subsequent Ca2+-dependent modulation of signaling and gene expression.
Listeria monocytogenes is a gram-positive, facultative, intracellular parasitic bacterium which causes food-borne listeriosis in humans (11, 13). L. monocytogenes invades and multiplies within the cytoplasm of various types of cells, including macrophages, epithelial cells, hepatocytes, and fibroblasts (12, 61). An array of virulence factors have been reported to support the intracellular parasitism of L. monocytogenes at various steps, including invasion of cells, escape from the endosomal compartment, utilization of a cytosolic nutrient source, and cell-to-cell spread (4, 61). Listeriolysin O (LLO), a 56-kDa protein encoded by the hly gene, is a member of the cholesterol-dependent cytolysin family and plays an essential role in escape from phagosomes, because a mutant strain lacking the hly gene is avirulent, incapable of escaping from phagosomes, and killed by host phagocytes (5).
In listeriosis, L. monocytogenes is thought to invade the host via intestinal epithelial cells. Based on this assumption, the Caco-2 cell line, a human intestinal epithelial cell line, has been used widely as a model for L. monocytogenes infection in vitro (8, 26, 33). Caco-2 cells express E-cadherin, which mediates the internalization of L. monocytogenes into cells as the receptor for bacterial internalin A (33).
Cytokines play a pivotal role in the resistance against L. monocytogenes infection (40). Gamma interferon is one of the most effective cytokines that enhance the bactericidal activity of macrophages (29). However, in the case of Caco-2 cells, it has been reported that interleukin-6 (IL-6) induces antilisteria activity that is stronger than that induced by gamma interferon through induction of inducible nitric oxide synthase and an unknown mechanism(s) (48). Furthermore, IL-6 induced the activation of NF-κB in Caco2-BBE cells and resulted in the expression of intracellular adhesion molecule 1, which plays an important role in inflammation through some form of leukocyte-epithelial cell adhesion (62).
These observations imply that IL-6 plays an essential role in the intestinal barrier in an autocrine or paracrine manner, because one previous report showed that L. monocytogenes induced the expression of IL-6 in human colon intestinal epithelial cells (25) and another study in which the microarray technique was used showed that there was up-regulation of IL-6 expression in L. monocytogenes-infected Caco-2 cells (2).
LLO plays crucial roles not only in the survival of L. monocytogenes inside phagocytes but also in the activation of macrophages, endothelial cells, and epithelial cells to secret cytokines and chemokines and to express adhesion molecules (27, 28, 45, 59, 65). In this study, we examined the involvement of LLO in IL-6 expression in Caco-2 cells during L. monocytogenes infection in vitro. We found that wild-type L. monocytogenes induced persistent IL-6 production in Caco-2 cells, whereas non-LLO-producing strains induced only transient IL-6 expression. The involvement of LLO in persistent IL-6 production in L. monocytogenes-infected Caco-2 cells was demonstrated by using recombinant LLO (rLLO).
MATERIALS AND METHODS
Bacterial strains.
A hemolytic and virulent strain of L. monocytogenes EGD (serovar 1/2a) was used as the L. monocytogenes wild-type strain (L. monocytogenes wt). L. monocytogenes EGD hly (hly mutant), kindly provided by Eva Ng (Wuerzburg University), was an attenuated isogenic strain with plasmid integration into the hly gene and was not capable of producing functional LLO (64). Another non-LLO-producing strain used in this study was L. monocytogenes ATCC 15313, which has naturally occurring defects in the gene cluster for hly, plcA, and a portion of the prfA promoter (44; unpublished data).
Cell culture.
Caco-2 cells and HEK293 cells were purchased from the American Type Culture Collection (Rockville, Md.). Caco-2 cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen Corporation, Carlsbad, Calif.) containing 5 μg/ml of gentamicin (Wako Pure Chemical Industries, Osaka, Japan) and 10% fetal calf serum (Invitrogen Corporation). HEK293 cells were maintained in RPMI 1640 (Invitrogen Corporation) containing 5 μg/ml of gentamicin and 10% FSC.
Infection and determination of intracellular bacterial growth.
Caco-2 cells were seeded into 24-well microplates (2 × 105 cells/well) and grown for 24 to 48 h. The cells were washed three times with phosphate-buffered saline (PBS) and infected with 5 × 106 CFU of L. monocytogenes wt, the hly mutant, or ATCC 15313 in gentamicin-free medium for 1 h. Then the cells were washed three times with PBS to eliminate extracellular bacteria and incubated in medium containing 5 μg/ml of gentamicin for 1, 3, or 5 h. For determination of the intracellular growth of L. monocytogenes, cells were washed three times with PBS and lysed in distilled water, and the number of viable bacteria was determined by a CFU assay. In some experiments, cytochalashin D (Sigma Aldrich, St. Louis, Mo.) was added to the culture 1 h before infection at a final concentration of 10 μg/ml.
Reverse transcription-PCR (RT-PCR) and real-time PCR.
Total cellular RNA was extracted from Caco-2 cells using Nucleospin RNA II (Macherey-Nagel, Düren, Germany), and cDNA was reverse transcribed from 0.2 μg of total RNA using a random primer and Superscript III RNase− reverse transcriptase (Invitrogen Corporation). A PCR was performed by using KOD-Plus DNA polymerase (TOYOBO, Osaka, Japan) and primer sets specific for human IL-6 and human β-actin. Each PCR cycle consisted of 94°C for 15 s, 54°C for 30 s, and 68°C for 60 s. The numbers of amplification cycles for IL-6 and β-actin were 35 and 25, respectively. The sequences of the oligonucleotide primers used were as follows: 5′-GTACATCCTCGACGGCATCTC-3′ and 5′-TGTGGTTGGGTCAGGGGTGGT-3′ for IL-6 and 5′-GCAAAGACCTGTACGCCAAC-3′ and 5′-CTAGAAGCATTTGCGGTGGAA-3′ for β-actin. A quantitative analysis of IL-6 expression was performed by real-time PCR using qPCR Mastermix for Syber Green I (Eurogentec, Seraing, Belgium) and the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, Calif.).
Immunofluorescent microscopy.
Caco-2 cells grown on coverslips were infected with 5 × 106 CFU of L. monocytogenes wt, the hly mutant, or ATCC 15313 as descried above. Then the cells were washed, incubated in medium containing 5 μg/ml of gentamicin for 5 h, and fixed with 4% paraformaldehyde. The cells were stained with phalloidin-Alexa 488 (Molecular Probes, Eugene, Oreg.) and goat anti-Listeria polyclonal antibody (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) and then with anti-goat immunoglobulin G antibody-Alexa 546 (Molecular Probes) in the presence of 1% saponin.
Preparation of rLLO proteins.
Full-length rLLO was prepared as described previously (31). Briefly, the hly gene was cloned into the pQE-31 vector (QIAGEN, Hilden, Germany), and Escherichia coli SG13009 was transformed with the recombinant vector. Recombinant LLO was produced in E. coli cells as a six-His-tagged protein by incubation of the transformants with 1 mM isopropyl-β-d-thiogalactopyranoside (Nacali Tesque, Kyoto, Japan). Then the E. coli cells were disrupted by vortexing with zirconia-silica beads, and rLLO was purified from the soluble fraction using a nickel-nitrilotriacetic acid column (QIAGEN). For construction of rLLOW492A, a mutant LLO with substitution of Ala (GCG) for 492Trp (TGG), the hly gene was amplified by PCR using a mutagenic primer (5′-TTGGGAATGGGCGAGACGGTAA-3′) as the 5′ primer and hly529 (31) as the 3′ primer. Secondary PCR amplification of the hly gene was performed using the resulting PCR product as the 3′ primer and hly26 (31) as the 5′ primer. Recombinant LLOW492A was prepared by a procedure similar to the procedure used for rLLO. Contaminating lipopolysaccharide was extensively removed by using a Detoxi-Gel endotoxin-removing gel (Pierce Chemical Company, Rockford, Ill.). The level of lipopolysaccharide was determined by the Limulus Color KY test (Wako Pure Chemical Industries) and was found to be less than 5 pg/ml when the preparation was suspended in PBS at a protein concentration of 10 μg/ml. To inhibit the cytolytic activity, rLLO was treated with 10 μg/ml of cholesterol for 30 min on ice (45).
Detection of LDH release.
Culture supernatants were collected, centrifuged at 200 × g for 5 min, and transferred to new tubes. Lactate dehydrogenase (LDH) activity was measured using an LDH cytotoxicity detection kit (TaKaRa BIO Inc., Shiga, Japan). The percentage of LDH release was calculated by using the following formula: percentage of release = 100 × (experimental LDH release − spontaneous LDH release)/(maximal LDH release − spontaneous LDH release). To determine the maximal LDH release, Caco-2 cells were treated with 1% Triton X-100.
WST-1 assay.
To measure the percentage of surviving cells, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) (DOJINDO, Kumamoto, Japan) was used. Caco-2 cells were seeded into 96-well microplates (5 × 104 cells/100 μl culture medium/well), grown for 24 to 48 h, and stimulated with rLLO or rLLOW492A for 3 h. Then 20 μl of WST-1 reagent (HEPES, pH 7.4, 0.2 mM 1-methoxy-5-methylphenazinium methyl sulfate, 5 mM WST-1) was added to cell culture and incubated for 30 min. Supernatants were transferred to new 96-well microplates, and the absorbance at 450 nm was determined. The percentage of surviving cells was calculated by using the following formula: percentage of surviving cells = 100 × (experimental optical density − cell-free optical density)/(untreated optical density − cell-free optical density).
Luciferase assay.
HEK293 cells were seeded into 24-well plates (105 cells/well), incubated overnight, and transfected with 0.9 μg of pNF-κB-luc (Stratagene, La Jolla, Calif.) and 0.1 μg of pRL-SV40 (Promega, Madison, Wis.) using the PolyFect transfection reagent (QIAGEN) according to the manufacturer's instructions. Cells were cultured for 18 h and stimulated with rLLO or rLLOW492A for 6 h, and then the cells were lysed with passive lysis buffer (Promega) and the luciferase activity was measured using the dual-luciferase reporter assay system (Promega) and a Wallac 1420 ARVOsx multilabel counter (Amersham Biosciences, Piscataway, N.J.).
Intracellular Ca2+ measurement.
The intracellular Ca2+ concentration was determined by microfluorometry using 1-(2-[5′-carboxyoxazol-2′-yl]-6-aminobenzofuran-5-oxy)-2(2ino-5′-methyl-phenoxy)ethane-N,N,N′,N′-tetraacetic acid, pentaacetoxy methyl ester (Fura 2-AM) (Molecular Probes) and the ARGUS-50/CA system (Hamamatsu Photonics, Japan). Caco-2 cells on coverslips were treated with 2 μM Fura 2-AM for 3 h and washed twice with HEPES, pH 7.2. Measurements were performed in HEPES (pH 7.2) with or without 1 mM CaCl2.
Ca2+ chelator and Ca2+ channel inhibitors.
1,2-bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) (Sigma Aldrich, St. Louis, Mo.) was dissolved in dimethyl sulfoxide and used at a final concentration of 5 or 10 μM. NiCl2 (Sigma Aldrich) was dissolved in PBS and used at a final concentration of 50 μM. Verapamil (Sigma Aldrich) was dissolved in ethanol and used at a final concentration of 10 μM.
Statistical analysis.
The statistical significance of the data was determined by Student's t test or Fisher's protected least-significant-difference test, and a P value of >0.05 was considered significant.
RESULTS
Requirement for LLO for persistent IL-6 expression in L. monocytogenes-infected Caco-2 cells.
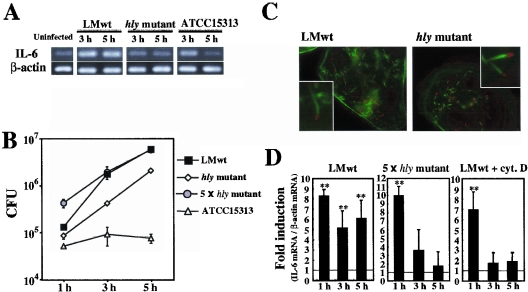
To examine the role of LLO in the induction of a cytokine response in intestinal epithelial cells, Caco-2 cells were infected with various strains of L. monocytogenes, including virulent wild-type strain EGD (L. monocytogenes wt), an isogenic strain producing inactive LLO protein (hly mutant), and strain ATCC 15313, which lacks the genomic region that includes plcA, hly, and part of the prfA promoter, and the expression of IL-6 mRNA was analyzed by RT-PCR. All the strains induced expression of IL-6 in Caco-2 cells 1 h after infection (Fig. 1A). The expression of IL-6 mRNA induced by the non-LLO-producing strains (the hly mutant and ATCC 15313) disappeared by 5 h after infection, whereas the expression induced by L. monocytogenes wt lasted for over 5 h (Fig. 1A). It was found that IL-6 mRNA was still expressed even 7 h after infection with L. monocytogenes wt (data not shown). This result suggested that LLO was involved in the persistent and/or late-phase expression of IL-6 in L. monocytogenes-infected Caco-2 cells.
FIG. 1.
L. monocytogenes induces persistent IL-6 expression in Caco-2 cells in an LLO-dependent manner. (A) Kinetics of IL-6 expression in L monocytogenes-infected Caco-2 cells. Caco-2 cells were infected with L. monocytogenes wt (LMwt), the hly mutant, or ATCC 15313, and the expression of IL-6 was analyzed by RT-PCR. The results are representative of the results of three similar experiments. (B) Intracellular growth of L. monocytogenes. Caco-2 cells were infected with L. monocytogenes wt, the hly mutant, a fivefold-higher dose of the hly mutant (5 × hly mutant), or ATCC 15313, and the number of intracellular bacteria was determined by the CFU assay. The results are representative of the results of two similar experiments. The data are the means ± standard deviations for three determinations. (C) Cytoplasmic invasion by L. monocytogenes. Caco-2 cells were infected with L. monocytogenes wt and the hly mutant for 5 h. The cells were then fixed and stained with phalloidin-Alexa 488 to detect actin nucleation (green) and with anti-Listeria goat polyclonal antibody and sequential anti-goat immunoglobulin G-Alexa 546 to detect L. monocytogenes (red). (D) Quantitative analysis of IL-6 expression. Caco-2 cells were infected with L. monocytogenes wt in the absence or presence of cytochalashin D (cyt. D) or with a fivefold-higher dose of the hly mutant. The expression of IL-6 at 5 h after infection was analyzed by real-time RT-PCR. The results were expressed relative to the level of β-actin mRNA. The data are the means ± standard deviations from three independent experiments. Two asterisks indicate that there was a significant difference compared to uninfected cells.
LLO is not an essential requirement for invasion of Caco-2 cytoplasm by L. monocytogenes.
LLO is known to play an essential role in the escape of L. monocytogenes from phagosomes into the cytosolic space inside professional phagocytes (52). We compared the intracellular growth of L. monocytogenes wt, the hly mutant, and ATCC 15313 in Caco-2 cells. L. monocytogenes wt was very capable of intracellular growth, as expected, but ATCC 15313 was not able to grow inside Caco-2 cells at all (Fig. 1B). Although there has been a report that an hly mutant hardly lysed the phagocytic vacuole in Caco-2 cells (42), the hly mutant used in this study showed significant intracellular growth. This finding was consistent with recent reports showing that non-LLO-producing strains are able to invade the cytoplasm in some types of human cell lines (22, 47).
A virulent strain of L. monocytogenes is known to induce host actin filament nucleation, followed by the formation of a tail-like structure called the actin tail or comet tail after invasion of the host cytoplasm (61). Caco-2 cells infected with each strain were stained with fluorescent phalloidin to visualize the actin tail. Both L. monocytogenes wt and the hly mutant were capable of forming an actin tail in Caco-2 cells (Fig. 1C), whereas ATCC 15313 did not form an actin tail (data not shown). The proportions of cells with a visible actin tail 5 h after infection were 42.5% ± 4.04% and 29.3% ± 3.21% for the Caco-2 cells infected with L. monocytogenes wt and with the hly mutant, respectively. Thus, the escape of the hly mutant in Caco-2 cells was confirmed morphologically. Taking these observations into consideration, it appeared that the difference in IL-6 expression shown in Fig. 1A was not due to the difference in evasion of the phagocytic vacuole.
Next, we wanted to confirm the difference in IL-6-inducing ability between L. monocytogenes wt and the hly mutant by using quantitative real-time RT-PCR. Although both strains exhibited intracellular growth in Caco-2 cells, there was a significant difference in the intracellular bacterial burden when the same number (5 × 106 CFU) of bacteria was employed. To overcome this difference, we changed the dose of the hly mutant and found that a fivefold increase in the amount of the hly mutant (2.5 × 107 CFU) resulted in a similar level of intracellular growth (Fig. 1B). Under these experimental conditions, it was observed that the expression of IL-6 in Caco-2 cells infected with the hly mutant was only transient (Fig. 1D), a finding consistent with the results shown in Fig. 1A. When cytochalasin D was added to the culture, the number of CFU of L. monocytogenes wt in Caco-2 cells was significantly reduced, to less than 5% of the number of intracellular CFU in the absence of cytochalasin D (data not shown). Cytochalasin D treatment almost completely abolished the late-phase expression of IL-6 (Fig. 1D). These results clearly indicated that both bacterial invasion of the cells and the presence of LLO are necessary for the induction of persistent and/or late-phase expression of IL-6 in L. monocytogenes-infected Caco-2 cells and that just bacterial invasion is not sufficient.
LLO-induced IL-6 production in Caco-2 cells is dependent on membrane permeation at sublytic doses of LLO.
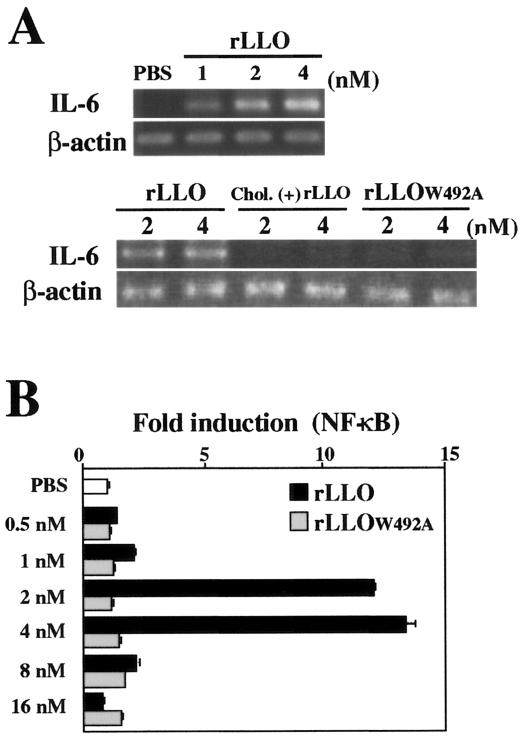
To determine the direct effect of LLO on IL-6 expression in Caco-2 cells, we constructed rLLO and stimulated the cells at different concentrations. LLO is known to form ring- or arc-shaped pores in the membrane, and this activity is completely blocked by cholesterol (24). We confirmed by electron microscopy that the full-length rLLO used in this experiment caused formation of numerous membrane pores in sheep erythrocyte ghosts under appropriate conditions (data not shown). A high level of IL-6 expression was observed in Caco-2 cells stimulated with a low dose (1 to 4 nM) of rLLO (Fig. 2A), but there was a decline in the IL-6 mRNA level when the stimulation dose was more than 8 nM (data not shown). Treatment of rLLO with cholesterol that abolished the pore-forming activity resulted in a complete loss of IL-6-inducing activity (Fig. 2A). We constructed a mutant LLO protein, rLLOW492A, by using a single-amino-acid substitution believed to impair the cytolytic activity (39). This mutant LLO protein was not able to induce IL-6 expression in Caco-2 cells (Fig. 2A). These results suggested that the formation of pores in the cell membrane was essential for the induction of IL-6 expression after stimulation with LLO.
FIG. 2.
LLO-induced IL-6 induction in Caco-2 cells is dependent on the pore-forming activity of recombinant protein. (A) Expression of IL-6 in Caco-2 cells. Caco-2 cells were stimulated with different doses of rLLO, cholesterol-treated rLLO [chol. (+) LLO], or rLLOW492A for 3 h, and the expression of IL-6 was analyzed by RT-PCR. The results are representative of the results of two similar experiments. (B) NF-κB activation induced by rLLO. HEK293 cells transfected with reporter vectors were stimulated with PBS or different doses of rLLO or rLLOW492A for 6 h, and NF-κB activation was assessed by the luciferase assay. The results are representative of the results of five similar experiments. The data are the means ± standard deviations for three determinations.
Transcription factor NF-κB is a central regulator of IL-6 gene expression (35, 49). We next examined the level of NF-κB activation by using HEK293 cells transfected with a reporter vector, as it was difficult to determine the level of NF-κB in Caco-2 cells. NF-κB activation was induced only by a limited range of doses of rLLO (2 and 4 nM), and rLLOW492A never induced NF-κB activation at the range of doses examined in this study (Fig. 2B).
LLO induces IL-6 production in Caco-2 cells at sublytic doses.
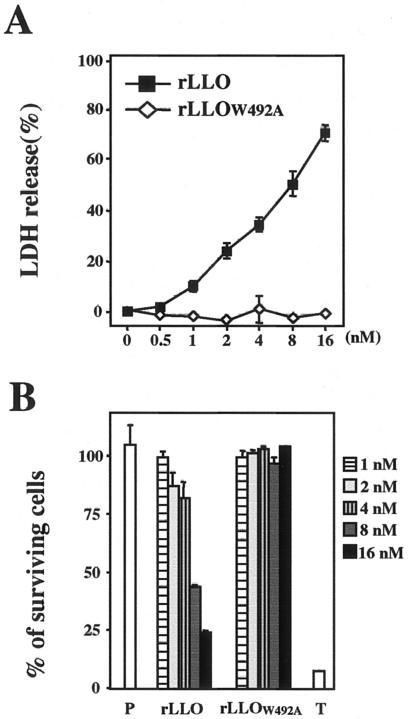
Because the IL-6 production induced by LLO appeared to be dependent on the pore-forming activity of LLO, we next examined the dose dependence of the cytolytic effect of LLO on Caco-2 cells. In this experiment, the amount of LDH released from cells indicated the level of cytolysis. LDH release from Caco-2 cells was induced by treatment with rLLO in a dose-dependent manner, whereas treatment with rLLOW492A did not result in LDH release (Fig. 3A). At the dose of rLLO that induced cytokine production (1 to 4 nM), release of 10 to 40% of the LDH was observed (Fig. 3A). Next, we measured the percentage of surviving cells after LLO treatment by using the WST-1 assay. Treatment with rLLO decreased the viability of cells in a dose-dependent manner. Notably, over 80% of the cells were still alive after treatment with a cytokine-inducible dose of rLLO, although 8 nM LLO decreased the level of surviving cells to about 40% (Fig. 3B). From these results, it became clear that LLO induced the production of IL-6 only at the sublytic doses.
FIG. 3.
Recombinant LLO induces IL-6 production only at a sublytic dose. (A) Cytolytic effect of rLLO on Caco-2 cells. Caco-2 cells were stimulated with different doses of rLLO and rLLOW492A for 3 h, and the LDH activity in culture supernatants was assessed. The results are representative of the results of three similar experiments. The data are the means ± standard deviations for three determinations. (B) Viability of Caco-2 cells after stimulation with rLLO. Caco-2 cells were treated with PBS (P) or 1% Triton X-100 (T) or were stimulated with different doses of rLLO or rLLOW492A for 3 h. Then the WST-1 reagent was added to the cultures, the cells were incubated for 30 min, and the absorbance at 450 nm of culture supernatants was measured. The results are representative of the results of two similar experiments. The data are the means ± standard deviations for three determinations.
LLO-formed pore mediates Ca2+ influx and leads to Ca2+-dependent IL-6 production.
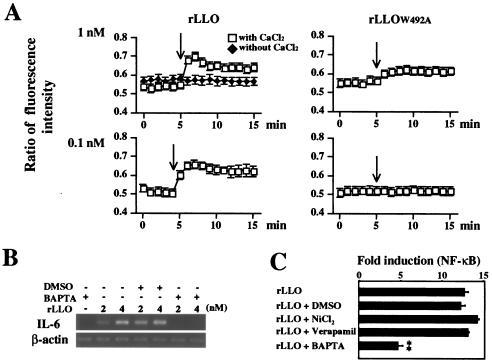
It has been reported that an LLO-formed pore is Ca2+ permeable and leads to intracellular Ca2+ oscillation in HEK293 cells (53). Since the intracellular Ca2+ ([Ca2+]in) acts as the second messenger and intracellular Ca2+ oscillation modulates cellular signaling and gene expression (7, 17, 60), the involvement of Ca2+ in LLO-induced IL-6 expression was examined. The concentration of [Ca2+]in inside Caco-2 cells increased immediately after treatment with 1 nM rLLO, and this effect lasted over 15 min (Fig. 4A). However, no significant increase in the [Ca2+]in concentration was observed in rLLOW492A-treated Caco-2 cells, and the effect of 1 nM rLLOW492A on [Ca2+]in was less than that of 0.1 nM rLLO (Fig. 4A). Furthermore, the absence of Ca2+ influx after treatment with rLLO under extracellular Ca2+-free conditions suggested that LLO-induced Ca2+ influx was not due to the release from intracellular Ca2+ stores (Fig. 4A). Pretreatment of cells with BAPTA-AM, an intracellular Ca2+ chelator, inhibited the expression of IL-6 induced by rLLO stimulation (Fig. 4B). Consistent with this observation, pretreatment with BAPTA-AM significantly reduced the activation of NF-κB after stimulation with rLLO, whereas the Ca2+ channel inhibitors NiCl2 and verapamil did not affect the response (Fig. 4C). Therefore, it could be concluded that Ca2+ influx mediated by LLO-formed pores resulted in the activation of an intracellular signaling cascade and subsequent IL-6 production.
FIG. 4.
Involvement of Ca2+ in LLO-induced IL-6 expression. (A) Effect of rLLO on intracellular Ca2+. Caco-2 cells were treated with Fura 2-AM. Recombinant LLO or rLLOW492A was added to cells at the time indicated by the arrow. Intracellular Ca2+ was assessed by microfluorometry, and the results are expressed as the ratio of fluorescence intensity (510 nm). The y axis is (emission with 340-nm excitation)/(emission with 380-nm excitation). The results are representative of the results of three similar experiments. (B) Inhibition of rLLO-induced IL-6 expression by chelating intracellular Ca2+. Caco-2 cells were treated with 10 μM BAPTA-AM for 1 h and then stimulated with different doses of rLLO for 3 h. The expression of IL-6 was analyzed by RT-PCR. The results are representative of the results of three similar experiments. DMSO, dimethyl sulfoxide. (C) Inhibition of rLLO-induced NF-κB activation by chelating intracellular Ca2+. HEK293 cells were transfected with reporter vectors and treated with 10 μM BAPTA-AM, 50 μM NiCl2, and 10 μM verapamil for 1 h. The cells were then stimulated with 4 nM rLLO for 6 h, and NF-κB activation was determined by the luciferase assay. The results are expressed in units relative to the activity of the internal control pRL-SV40. The results are representative of the results of two similar experiments. The data are the means ± standard deviations for three determinations. Two asterisks indicate that there was a significant difference compared to cells stimulated with rLLO without any pretreatment.
LLO secreted by cytoplasmic L. monocytogenes acts on the host cell membrane.
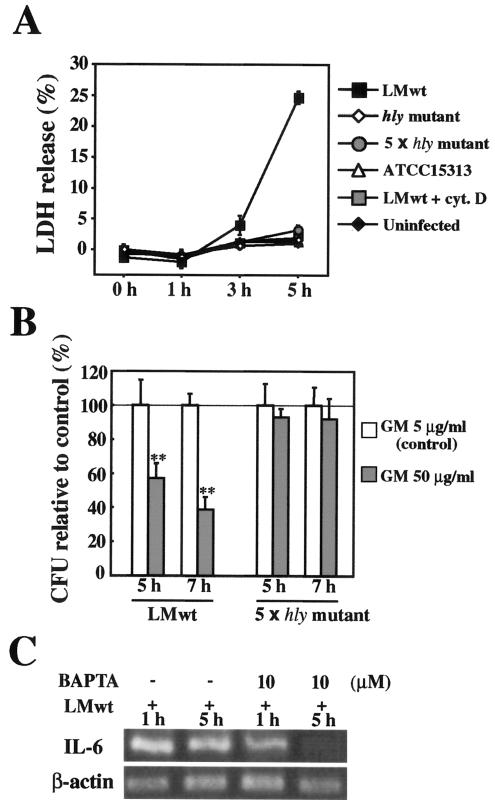
The expression of LLO has been reported to be up-regulated after invasion of the cytoplasm by L. monocytogenes (41). We examined the effect of LLO secreted by cytoplasmic L. monocytogenes on Caco-2 cells. To determine the cytolytic activity exhibited by the LLO produced in the cytoplasm, LDH release from L. monocytogenes-infected Caco-2 cells was assayed. Significant LDH release was observed at 5 h after infection with L. monocytogenes wt, but not after infection with strains that were not capable of producing LLO, including the hly mutant, a fivefold-higher dose of the hly mutant, and ATCC 15313. Cytochalashin D pretreatment also inhibited LDH release from L. monocytogenes wt-infected Caco-2 cells (Fig. 5A). This result indicated that LLO but not other virulence factors secreted by cytoplasmic L. monocytogenes was critical for the integrity of the host cell membrane. There was no significant difference in cell morphology as determined by phase-contrast microscopy between Caco-2 cells infected with L. monocytogenes wt for 5 h and uninfected Caco-2 cells (data not shown). Thus, the results suggested that LDH release induced by L. monocytogenes wt infection was due to incomplete membrane damage in most of the infected cells rather than to the death (complete cytolysis) of a limited number of cells. This might have been the result of actin tail-mediated spread of L. monocytogenes to neighboring cells (61). To confirm the involvement of cytoplasmic LLO in the influx of extracellular molecules, Caco-2 cells were infected with L. monocytogenes wt or the hly mutant and cultured in medium containing 5 or 50 μg/ml of gentamicin, and then the number of intracellular bacteria was determined. In a preliminary experiment, we were convinced that the susceptibility to gentamicin in vitro was the same for both the L. monocytogenes wt and hly mutant strains. The intracellular growth of the hly mutant was not affected even in the presence of 50 μg/ml of gentamicin (Fig. 5B), while the number of intracellular L. monocytogenes wt cells was decreased by the treatment with 50 μg/ml of gentamicin (Fig. 5B). This result indicated that the pores formed in the host cell membrane by cytoplasmic LLO mediated the influx of extracellular molecules, including Ca2+. Because LLO secreted by cytoplasmic L. monocytogenes, like extracellularly added rLLO, appeared to act on the host cell membrane, we next examined the possible involvement of Ca2+ in the persistent and/or late-phase expression of IL-6 by L. monocytogenes infection. It has been reported that EGTA, but not BAPTA-AM, inhibits the invasion of Hep-2 epithelial cells by L. monocytogenes (9), so BAPTA-AM was employed in this experiment. Treatment of cells with BAPTA-AM never affected the invasion and intracellular growth of L. monocytogenes wt (data not shown). When Caco-2 cells were pretreated with BAPTA-AM, the L. monocytogenes wt-induced IL-6 expression at 5 h was almost completely abolished, whereas expression at 1 h was still observed, although the level was reduced (Fig. 5C), indicating that the expression of IL-6 in the late phase of the infection was critically dependent on Ca2+, whereas the expression in the early phase of the infection was induced through both Ca2+-dependent and -independent mechanisms. These results suggested that the persistent IL-6 expression during L. monocytogenes infection was dependent on Ca2+ influx mediated by the membrane pores formed by cytoplasmic LLO.
FIG. 5.
Involvement of Ca2+ in L monocytogenes-induced persistent IL-6 expression. (A) LDH release from L. monocytogenes-infected Caco-2 cells. Caco-2 cells were infected with the hly mutant, a fivefold-higher dose of the hly mutant (5 × hly mutant), ATCC 15313, or L. monocytogenes wt (LMwt) in the presence or absence of cytochalashin D (cyt. D). Culture supernatants were collected at different times, and the LDH activity in culture supernatants was assayed. The results are representative of the results of three similar experiments. The data are the means ± standard deviations for three determinations. (B) Gentamicin (GM) influx induced by L. monocytogenes infection. Caco-2 cells were infected with L. monocytogenes wt or a fivefold-higher dose of the hly mutant and incubated in medium containing 5 μg/ml (control) or 50 μg/ml of gentamicin for 5 and 7 h. The number of intracellular bacteria was determined by the CFU assay, and the results were expressed as the number of CFU relative to the control. The solid line indicates the value for the control. The results are representative of the results of two similar experiments. The data are the means ± standard deviations for three determinations. Two asterisks indicate that there was a significant difference compared to cells treated with 5 μg/ml gentamicin. (C) Inhibition of L. monocytogenes-induced persistent IL-6 expression by chelating intracellular Ca2+. Caco-2 cells were pretreated with BAPTA-AM for 1 h and then infected with L. monocytogenes wt for 1 or 5 h. Then the expression of IL-6 was analyzed by RT-PCR. The results are representative of the results of two similar experiments.
DISCUSSION
In this study, the involvement of LLO in the cytokine response of L. monocytogenes-infected Caco-2 cells was examined. We found that there was a significant difference in the magnitude and persistence of IL-6 induction between wild-type L. monocytogenes and non-LLO-producing strains. Our results suggested that the IL-6 expression in the early phase of infection is rather LLO independent but that the IL-6 expression in the late phase of infection is highly LLO dependent. Furthermore, LLO exerted its effect on the Caco-2 cell membrane from both the intracellular and extracellular milieus to form Ca2+-permeable pores and to induce Ca2+-dependent IL-6 expression. These findings suggested that persistent expression of IL-6 in L. monocytogenes-infected Caco-2 cells is due to the formation of Ca2+-permeable pores by LLO.
As the induction of IL-6 in the early phase of in vitro infection appeared to be independent of both the presence of LLO and bacterial invasion of cells and as L. monocytogenes contains many candidate molecules that stimulate the innate immune system, it is possible that the recognition of bacterial components through cell surface receptors was responsible for the IL-6 expression in the early phase. Lipoteichoic acid fraction II (LTA II) of L. monocytogenes has been reported to stimulate the NF-κB pathway not only in P388D1 macrophages but also in Caco-2 cells (19, 20). Although Toll-like receptor 2 (TLR2), CD14, and scavenger receptor are known to be the potential cell surface receptors for LTA II (32, 58), how Caco-2 cells recognize LTA II is not known. Caco-2 cells are not responsive to TLR2 and TLR4 agonists due to the absence of TLR4 expression, the low level of expression of TLR2, TLR1, and TLR6, and the high level expression of the Toll inhibitory protein, Tollip (1, 38, 43). On the other hand, Caco-2 cells express TLR5 and are responsive to a TLR5 ligand, flagellin, which is a component of bacterial flagella (10, 21, 36). Although the expression of L. monocytogenes flagellin is temperature dependent and is shut off at 37°C (50), it has been reported that some laboratory-adapted strains and ∼20% of clinical isolates remain able to stimulate the TLR5 pathway even at 37°C (63). Thus, it is likely that the IL-6 expression in the early phase of infection was due to the recognition of LTA II and/or flagellin by Caco-2 cells.
The persistent expression of IL-6 was induced only by L. monocytogenes wt, which evades endosomes and produces LLO, and not by the hly mutant, which evades endosomes but does not produce LLO. It is known that two listerial phospholipases, phosphatidylinositol-specific phospholipase (PI-PLC) and phosphatidylcholine-specific phospholipase (PC-PLC), are involved in the escape of L. monocytogenes from the endosomal compartment (18, 37, 57), so the hly mutant may invade the cytoplasm of Caco-2 cells by using phospholipases even in the absence of functional LLO. A similar finding for the escape of an hly mutant into cytoplasm has been reported for some types of human cell lines (22, 47, 52). The results obtained by using five times more hly mutant ruled out the possibility that the absence of persistent IL-6 expression in Caco-2 cells was simply due to the small number of bacteria in the cells. As L. monocytogenes wt and the hly mutant are isogenic except for the hly gene coding for LLO, it was conceivable that LLO and not other components were responsible for the difference in the persistence of IL-6 expression.
A bacterium-sensing system mediated by Nod1 or Nod2 is known to be present in mammalian cell cytoplasm (54). Nod1 and Nod2 recognize peptidoglycan (PGN) components (γ-d-glutamyl-meso-diaminopimelic acid and muramyldipeptide, respectively), which results in the activation of NF-κB (3, 14, 15). It has been reported that Nod1, but not Nod2, is expressed in Caco-2 cells (23, 30). Based on our finding in the present study that persistent IL-6 expression was induced only by L. monocytogenes wt and not by the hly mutant even after adjustment of the number of intracellular bacteria by increasing the dose of the hly mutant strain, it was unlikely that the Nod1 pathway was responsible for the late-phase expression of IL-6. A previous report showed that although diaminopimelic acid is present in PGN of L. monocytogenes, extracts from gram-positive bacteria, including L. monocytogenes, did not activate NF-κB in digitonin-permeabilized HEK293 cells, whereas extracts from gram-negative bacteria did activate NF-κB (15). For this reason, it is conceivable that L. monocytogenes PGN is not recognized efficiently by Nod1.
Induction of IL-6 in Caco-2 cells was reproduced by stimulation with rLLO. Experiments using cholesterol-treated rLLO and rLLOW492A showed that the pore-forming activity of LLO was essential for induction of IL-6. However, IL-6 expression was induced only when Caco-2 cells were treated with sublytic doses of rLLO and not when the cells were treated with higher doses, resulting in complete cell destruction. Among the host cell responses linked to the formation of membrane pores, Ca2+ influx was the greatest possibility (53). Our data demonstrated clearly that a membrane pore formed by LLO was Ca2+permeable and induced Ca2+-dependent IL-6 expression. Although involvement of [Ca2+]in as the second messenger in modulation of the signal transduction pathway and gene expression has been reported (17), the detailed mechanism of Ca2+-dependent IL-6 induction has not been elucidated yet. LLO is known to induce phosphoinositide metabolism, resulting in the generation of inositol phosphates and diacylglycerol (DAG), probably through the activation of a host phospholipase(s) (55, 56). Therefore, a signaling pathway following activation of Ca2+-dependent protein kinase C (PKC) by [Ca2+]in and DAG may contribute to the IL-6 expression induced by LLO and L. monocytogenes infection. Indeed, we obtained preliminary results which showed that pretreatment with GF-109203X, a specific PKC inhibitor, significantly reduced the level of NF-κB activation induced by rLLO (data not shown). In addition, Ca2+ influx mediated by LLO-dependent pores may lead to host PC-PLC activation to generate DAG, because the activity of the phospholipase is critically dependent on Ca2+ (46, 51). Moreover, as PI-PLC and PC-PLC secreted by intracellular L. monocytogenes are enzymatically active and participate in the generation of DAG (57), it is possible that DAG generated by these listerial phospholipases also works on activation of Ca2+-dependent PKC and persistent IL-6 expression in L. monocytogenes-infected Caco-2 cells under LLO-induced Ca2+ influx conditions. A report indicating that PI-PLC and PC-PLC were necessary for persistent activation of NF-κB in L. monocytogenes-infected macrophages may support this idea (20).
If the expression of IL-6 in the late phase of infection reflected the IL-6-inducing activity of LLO, LLO should have exerted its effect inside the cells. Indeed, in the present study, LLO secreted by cytoplasmic L. monocytogenes appeared to act on the host cell membrane from the intracellular milieu, since LDH release and gentamicin influx were observed only after infection with an L. monocytogenes strain producing active LLO. LLO contains a PEST-like sequence located close to the N terminus, and this motif was shown to be possibly involved in the rapid degradation of LLO in the cytoplasm (6, 34). Moreover, an acidic pH in the phagosomal compartment is better than a neutral pH in the cytoplasm for cytolytic activity of LLO (16). These characteristics of LLO are thought to be the mechanisms that prevent severe damage to host cells, the “nest” for L. monocytogenes. However, LDH release was reportedly observed from L. monocytogenes-infected macrophages, although the level was significantly lower than the level of release from macrophages infected with L. monocytogenes producing ΔPEST-LLO or LLOL461T, a mutant LLO which is active at neutral pH (6, 16, 34). These findings suggest that the pore-forming activity of LLO is not completely controlled in the cytoplasm. Therefore, it is likely that cytoplasmically secreted LLO exerted its activity on the host cell membrane at a certain level, causing Ca2+ influx without inducing complete cell lysis.
Our findings suggest a novel mechanism for the sensing of L. monocytogenes, an enteroinvasive pathogen, by intestinal epithelial cells. Although LLO is a major virulence factor of L. monocytogenes, LLO also triggers the recognition system in host cells. Thus, LLO may be a useful but risky tool for L. monocytogenes; it may be like a double-edged sword, and the host surveillance system may target this pathogen's indispensable weak points. Further analysis of the relationship between virulence factors and pathogen recognition by hosts should increase our understanding of the innate defense mechanisms against pathogens.
Acknowledgments
We thank Chiyo Yamamoto and Shizuka Awata (University of Tokushima) for assistance with measurement of intracellular Ca2+.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (C) from The Ministry of Education, Science, Culture and Sports of Japan and by a Grant-in-Aid for Scientific Research (B) and (C) from The Japan Society for the Promotion of Science.
Editor: F. C. Fang
REFERENCES
- 1.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, D. N., V. Vanchinathan, P. O. Brown, and J. A. Theriot. 2003. A gene-expression program reflecting the innate immune response of cultured intestinal epithelial cells to infection by Listeria monocytogenes. Genome Biol. 4:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 4.Chico-Calero, I., M. Suarez, B. Gonzalez-Zorn, M. Scortti, J. Slaghuis, W. Goebel, and J. A. Vazquez-Boland. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA 99:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossart, P., M. F. Vicente, J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decatur, A. L., and D. A. Portnoy. 2000. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science 290:992-995. [DOI] [PubMed] [Google Scholar]
- 7.Dolmetsch, R. E., K. Xu, and R. S. Lewis. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392:933-936. [DOI] [PubMed] [Google Scholar]
- 8.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol. Microbiol. 9:931-1001. [DOI] [PubMed] [Google Scholar]
- 9.Dramsi, S., and P. Cossart. 2003. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect. Immun. 71:3614-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virag, G. Ross, F. G. Soriano, C. Szabo, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 11.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 14.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 15.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 16.Glomski, I. J., M. M. Gedde, A. W. Tsang, J. A. Swanson, and D. A. Portnoy. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross, V., B. Zhang, Y. Geng, P. M. Villiger, and M. Lotz. 1993. Regulation of interleukin-6 (IL-6) expression: evidence for a tissue-specific role of protein kinase C. J. Clin. Immunol. 13:310-320. [DOI] [PubMed] [Google Scholar]
- 18.Grundling, A., M. D. Gonzalez, and D. E. Higgins. 2003. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J. Bacteriol. 185:6295-62307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauf, N., W. Goebel, F. Fiedler, and M. Kuhn. 1999. Listeria monocytogenes infection of Caco-2 human epithelial cells induces activation of transcription factor NF-kappa B/Rel-like DNA binding activities. FEMS Microbiol. Lett. 178:117-122. [DOI] [PubMed] [Google Scholar]
- 20.Hauf, N., W. Goebel, F. Fiedler, Z. Sokolovic, and M. Kuhn. 1997. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-kappaB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IkappaBalpha and IkappaBbeta degradation. Proc. Natl. Acad. Sci. USA 94:9394-9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 22.Hense, M., E. Doman, S. Krusch, P. Wachholz, K. E. Dittmar, M. Rohde, J. Wehland, T. Chakraborty, and S. Weiss. 2001. Eukaryotic expression plasmid transfer from the intracellular bacterium Listeria monocytogenes to host cells. Cell. Microbiol. 3:599-609. [DOI] [PubMed] [Google Scholar]
- 23.Hisamatsu, T., M. Suzuki, H. C. Reinecker, W. J. Nadeau, B. A. McCormick, and D. K. Podolsky. 2003. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124:993-1000. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs, T., A. Darji, N. Frahm, M. Rohde, J. Wehland, T. Chakraborty, and S. Weiss. 1998. Listeriolysin O: cholesterol inhibits cytolysis but not binding to cellular membranes. Mol. Microbiol. 28:1081-1089. [DOI] [PubMed] [Google Scholar]
- 25.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karunasagar, I., B. Senghaas, G. Krohone, and W. Goebel. 1994. Ultrastructural study of Listeria monocytogenes entry into cultured human colonic epithelial cells. Infect. Immun. 62:3554-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 28.Kayal, S., A. Lilienbaum, O. Join-Lambert, X. Li, A. Israel, and P. Berche. 2002. Listeriolysin O secreted by Listeria monocytogenes induces NF-kappaB signalling by activating the IkappaB kinase complex. Mol. Microbiol. 44:1407-1419. [DOI] [PubMed] [Google Scholar]
- 29.Kiderlen, A. F., S. H. Kaufmann, and M. L. Lohmann-Matthes. 1984. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur. J. Immunol. 14:964-967. [DOI] [PubMed] [Google Scholar]
- 30.Kim, J. G., S. J. Lee, and M. F. Kagnoff. 2004. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by Toll-like receptors. Infect. Immun. 72:1487-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohda, C., I. Kawamura, H. Baba, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect. Immun. 70:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn, M., T. Pfeuffer, L. Greiffenberg, and W. Goebel. 1999. Host cell signal transduction during Listeria monocytogenes infection. Arch. Biochem. Biophys. 372:166-172. [DOI] [PubMed] [Google Scholar]
- 33.Lecuit, M., H. Ohayon, L. Braun, J. Mengaud, and P. Cossart. 1997. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun. 65:5309-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lety, M. A., C. Frehel, P. Berche, and A. Charbit. 2002. Critical role of the N-terminal residues of listeriolysin O in phagosomal escape and virulence of Listeria monocytogenes. Mol. Microbiol. 46:367-379. [DOI] [PubMed] [Google Scholar]
- 35.Libermann, T. A., and D. Baltimore. 1990. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 10:2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maaser, C., J. Heidemann, C. von Eiff, A. Lugering, T. W. Spahn, D. G. Binion, W. Domschke, N. Lugering, and T. Kucharzik. 2004. Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J. Immunol. 172:5056-5062. [DOI] [PubMed] [Google Scholar]
- 37.Marquis, H., V. Doshi, and D. A. Portnoy. 1995. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63:4531-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 39.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 40.Mocci, S., S. A. Dalrymple, R. Nishinakamura, and R. Murray. 1997. The cytokine stew and innate resistance to L. monocytogenes. Immunol. Rev. 158:107-114. [DOI] [PubMed] [Google Scholar]
- 41.Moors, M. A., B. Levitt, P. Youngman, and D. A. Portnoy. 1999. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect. Immun. 67:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mounier, J., A. Ryter, M. Coquis-Rondon, and P. J. Sansonetti. 1990. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect. Immun. 58:1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naik, S., E. J. Kelly, L. Meijer, S. Pettersson, and I. R. Sanderson. 2001. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J. Pediatr. Gastroenterol. Nutr. 32:449-453. [DOI] [PubMed] [Google Scholar]
- 44.Nishibori, T., K. Cooray, H. Xiong, I. Kawamura, M. Fujita, and M. Mitsuyama. 1995. Correlation between the presence of virulence-associated genes as determined by PCR and actual virulence to mice in various strains of Listeria spp. Microbiol. Immunol. 39:343-349. [DOI] [PubMed] [Google Scholar]
- 45.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nord, E. P. 1996. Signalling pathways activated by endothelin stimulation of renal cells. Clin. Exp. Pharmacol. Physiol. 23:331-336. [DOI] [PubMed] [Google Scholar]
- 47.O'Riordan, M., C. H. Yi, R. Gonzales, K. D. Lee, and D. A. Portnoy. 2002. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. USA 99:13861-13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouadrhiri, Y., Y. Sibille, and P. M. Tulkens. 1999. Modulation of intracellular growth of Listeria monocytogenes in human enterocyte Caco-2 cells by interferon-gamma and interleukin-6: role of nitric oxide and cooperation with antibiotics. J. Infect. Dis. 180:1195-1204. [DOI] [PubMed] [Google Scholar]
- 49.Parikh, A. A., A. L. Salzman, C. D. Kane, J. E. Fischer, and P. O. Hasselgren. 1997. IL-6 production in human intestinal epithelial cells following stimulation with IL-1 beta is associated with activation of the transcription factor NF-kappa B. J. Surg. Res. 69:139-144. [DOI] [PubMed] [Google Scholar]
- 50.Peel, M., W. Donachie, and A. Shaw. 1988. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and Western blotting. J. Gen. Microbiol. 134:2171-2178. [DOI] [PubMed] [Google Scholar]
- 51.Peterson, M. W., and M. E. Walter. 1992. Calcium-activated phosphatidylcholine-specific phospholipase C and D in MDCK epithelial cells. Am. J. Physiol. 263:C1216-C1224. [DOI] [PubMed] [Google Scholar]
- 52.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Repp, H., Z. Pamukci, A. Koschinski, E. Domann, A. Darji, J. Birringer, D. Brockmeier, T. Chakraborty, and F. Dreyer. 2002. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell. Microbiol. 4:483-491. [DOI] [PubMed] [Google Scholar]
- 54.Royet, J., and J. M. Reichhart. 2003. Detection of peptidoglycans by NOD proteins. Trends Cell Biol. 13:610-614. [DOI] [PubMed] [Google Scholar]
- 55.Sibelius, U., F. Rose, F., T. Chakraborty, A. Darji, J. Wehland, S. Weiss, W. Seeger, and F. Grimminger. 1996. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect. Immun. 64:674-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibelius, U., T. Chakraborty, B. Krogel, J. Wolf, F. Rose, R. Schmidt, J. Wehland, W. Seeger, and F. Grimminger. 1996. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J. Immunol. 157:4055-4060. [PubMed] [Google Scholar]
- 57.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsukada, H., I. Kawamura, T. Fujimura, K. Igarashi, M. Arakawa, and M. Mitsuyama. 1992. Induction of macrophage interleukin-1 production by Listeria monocytogenes hemolysin. Cell. Immunol. 140:21-30. [DOI] [PubMed] [Google Scholar]
- 60.Uhlen, P., A. Laestadius, T. Jahnukainen, T. Soderblom, F. Backhed, G. Celsi, H. Brismar, S. Normark, A. Aperia, and A. Richter-Dahlfors. 2000. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405:694-697. [DOI] [PubMed] [Google Scholar]
- 61.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, L., B. Walia, J. Evans, A. T. Gewirtz, D. Merlin, and S. V. Sitaraman. 2003. IL-6 induces NF-kappa B activation in the intestinal epithelia. J. Immunol. 171:3194-3201. [DOI] [PubMed] [Google Scholar]
- 63.Way, S. S., L. J. Thompson, J. E. Lopes, A. M. Hajjar, T. R. Kollmann, N. E. Freitag, and C. B. Wilson. 2004. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell. Microbiol. 6:235-242. [DOI] [PubMed] [Google Scholar]
- 64.Wuenscher, M. D., S. Kohler, W. Goebel, and T. Chakraborty. 1991. Gene disruption by plasmid integration in Listeria monocytogenes: insertional inactivation of the listeriolysin determinant lisA. Mol. Gen. Genet. 228:177-182. [DOI] [PubMed] [Google Scholar]
- 65.Xiong, H., I. Kawamura, T. Nishibori, and M. Mitsuyama. 1994. Cytokine gene expression in mice at an early stage of infection with various strains of Listeria spp. differing in virulence. Infect. Immun. 62:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]