Abstract
Placenta-sequestering Plasmodium falciparum involved in the pathogenesis of pregnancy-associated malaria (PAM) in otherwise clinically immune women expresses particular variant surface antigens (VSAPAM) on the surface of infected erythrocytes that differ from VSA found in parasitized nonpregnant individuals (non-PAM type VSA). We studied levels of immunoglobulin G (IgG) and IgG subclasses with specificity for VSAPAM and for non-PAM type VSA in pregnant and nonpregnant women from two sites with different endemicities in Cameroon. We found that VSAPAM-specific responses depended on the pregnancy status, parity, gestational age, and parasite transmission intensity, whereas only the parasite transmission intensity influenced the levels of IgG specific for non-PAM type VSA. For both types of VSA, the responses were dominated by the cytophilic subclass IgG1, followed by IgG3. In pregnant women, the levels of VSAPAM-specific antibodies either were very low or negative or were very high, whereas the levels of the antibodies specific for non-PAM type VSA were uniformly high. Interestingly, the levels of VSAPAM-specific IgG1 increased with increasing gestational age, while the levels of the corresponding IgG3 tended to decrease with increasing gestational age. The IgG subclass responses with specificity for non-PAM type VSA did not vary significantly with gestational age. Taken together, our data indicate that IgG1 and to a lesser extent IgG3 are the main subclasses involved in acquired VSAPAM-specific immunity to pregnancy-associated malaria.
A number of studies have indicated that parasite-encoded variant surface antigens (VSA) on the surface of infected erythrocytes are important targets of acquired protective immunity following exposure to Plasmodium falciparum parasites (5, 11, 17, 19, 30). The case is particularly strong for VSAPAM-specific immunoglobulin G (IgG) in protection against adverse pregnancy outcomes as a consequence of pregnancy-associated malaria (PAM) (8, 31). However, only three previous studies have provided data on VSA-specific IgG subclass responses (6, 14, 23). Two of these studies included longitudinal data (6, 14), but the researchers did not study pregnant women or VSAPAM-specific responses. Studies of the relationship between levels of endemicity and VSA-specific antibody responses are also rare (1, 20), and to our knowledge no longitudinal studies comparing VSAPAM-specific antibody responses in areas where the parasite transmission intensities are different have been conducted. Here we present the results of a study in which we investigated plasma levels of IgG and IgG subclasses with specificity for VSA expressed by parasites infecting nonpregnant individuals (non-PAM type VSA) and by parasites capable of accumulating in the placentas of pregnant women (VSAPAM). We compared the levels of VSA-specific antibodies in sympatric pregnant and nonpregnant women and in pregnant women living in areas where transmission intensities are very different, and we studied the relationship among VSA type, level of endemicity, pregnancy status, parity, and gestational age.
MATERIALS AND METHODS
Study sites and plasma donors.
In the present study, we used plasma samples from previous longitudinal cohort studies performed between 1996 and 1998 at two health centers in Cameroon, Biyem Assi Hospital in Yaounde and Etoa Health Center in Etoa. Malaria transmission is perennial at both sites, but it is considerably lower in urban Yaounde (entomological inoculation rate, 0.1 to 1.1/month) (15) than in rural Etoa (EIR, 0.4 to 2.4/day) (24). The study sites and populations have been described in detail elsewhere (33). We used plasma samples collected from 283 Cameroonian women. Of these samples, 215 were from pregnant women, each of whom donated blood samples at antenatal visits between estimated gestational weeks 8 and 41 in Yaounde (186 women) and Etoa (29 women). Sixty-eight samples were from nonpregnant women (parity, 0 to 9) from Yaounde. None of these plasma donors had malaria at the time of blood sampling, but some had low-grade, asymptomatic P. falciparum parasitemia. Informed consent was obtained from all the women in the study, which was approved by the National Ethical Committee, Ministry of Health, Cameroon, and the Institutional Review Board at Georgetown University, Washington D.C.
Plasma samples from 20 Danish adults never exposed to P. falciparum infection were included as negative controls.
Parasite lines and selection protocols.
For all the experiments reported here we used two sublines of the long-term in vitro-adapted P. falciparum FCR-3 line (13). The sublines were established by repeated panning essentially as described previously (26). To select for FCR-3 expressing non-PAM type VSA, we used Chinese hamster ovary 745 (CHO-745) cells that do not express chondroitin sulfate phosphoglycan (9). To select for FCR-3 expressing VSAPAM type antigens (25, 30), we selected infected erythrocytes which had been preselected for nonadhesion to the CHO-745 cells by using wild-type CHO-K1 cells that express the main placental adhesion ligand chondroitin sulfate phosphoglycan. The genotypic stabilities and identities of the parasite sublines used were confirmed by regular profiling at the polymorphic msp1 and msp2 loci (25).
Measurement of VSA-specific IgG and IgG subclasses by flow cytometry.
We used flow cytometry to measure plasma levels of IgG and IgG subclasses with specificity for VSA expressed on the surface of intact erythrocytes infected with trophozoite and schizont stages of the parasite sublines mentioned above. Preparation of infected erythrocytes and subsequent analysis with a FACScan flow cytometer (Becton Dickinson, San Jose, CA) were performed essentially as described in detail elsewhere (29). For analysis of IgG and IgG3 levels, we used affinity-purified fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (FI-3080; Vector, Burlingame, CA) and FITC-conjugated sheep anti-human IgG3 (AF008; The Binding Site, Birmingham, United Kingdom), respectively. For the remaining IgG subclasses we used purified mouse monoclonal antibodies against human IgG1 (clone JDC-1; BD PharMingen, San Diego, CA), IgG2 (clone 6014; Southern Biotechnology, Birmingham, AL), or IgG4 (clone 6025; Southern Biotechnology). For analysis of IgG1, IgG2, and IgG4, the primary monoclonal antibodies were followed by biotinylated rabbit anti-mouse IgG (E0354; Dako, Glostrup, Denmark) and FITC-conjugated streptavidin (BD PharMingen). All reagents were used at predetermined optimal dilutions. For each sample, the mean fluorescence index (MFI) was recorded and used as a measure of the VSA-specific antibody level.
Statistical analysis.
Pairwise intergroup differences were evaluated by the Mann-Whitney rank-sum test. Parameter association was evaluated by using Spearman's rank-order correlation coefficient (rs). Differences in the proportions of positive VSA-specific antibody responses were evaluated by the χ2 test, using the mean plus two standard deviations obtained for unexposed control donors as the negative cutoff. For non-PAM type VSA, we used the 20 Danish donors who were not exposed to any P. falciparum parasites to calculate the negative cutoff. For VSAPAM, we used the 35 nulligravidae from Yaounde (who had been exposed to non-PAM P. falciparum infections but had never been exposed to PAM) to calculate the negative cutoff. Although these women were significantly younger (median age, 22 years) than the remaining nonpregnant women from Yaounde (median age, 34 years), there were no statistically significant differences in the levels of non-PAM type VSA antibodies (P > 0.21 in all cases) between these two groups of women. Multiple linear regression was used in multivariate analysis of VSA-specific antibody responses. We used the SigmaStat (SPSS, Chicago, IL), JMP (SAS Institute, Cary, NC), and CIA (2) software packages for the statistical analyses.
RESULTS
Baseline characteristics of study participants.
The age ranges of the three study groups were similar (Table 1). The proportion of nulligravidae for the nonpregnant women and the proportion of primigravidae for the pregnant women from Yaounde were very similar (P = 0.6 as determined by the χ2 test), as were the proportions of primigravidae in Yaounde and Etoa (Table 1). The distributions of gestational ages were similar for pregnant women in Yaounde and Etoa and for women with different parities (P > 0.6 as determined by the Mann-Whitney rank-sum test in all cases). Together, these data suggest that the study groups were comparable with respect to maternal age, gestational age, and parity.
TABLE 1.
Baseline characteristics of study participants
| Parameter | Yaounde
|
Etoa (pregnant) | P value (Yaounde)a | P value (Yaounde/ Etoa)b | |
|---|---|---|---|---|---|
| Non- pregnant | Preg- nant | ||||
| n | 68 | 186 | 29 | ||
| Age range (yr) | 16-46 | 15-40 | 14-38 | ||
| % <20 yr old | 18 | 22 | 39 | 0.56 | 0.08 |
| % Primigravidae | 47 | 48 | 0.98 | ||
| % Nulligravidae | 52 | ||||
| Gestational age range (wk) | 8-41 | 14-37 | 0.7 | ||
| % Parasitemicc | 4 | 17 | 38 | 0.02 | 0.02 |
| % Anemicd | 23 | 45 | 38 | 0.003 | 0.60 |
| % Receiving prophylaxise | 6 | 36 | 17 | <0.001 | 0.08 |
Significance for a comparison of nonpregnant and pregnant women from Yaounde as determined by the χ2 test.
Significance for a comparison of pregnant women from Yaounde and Etoa as determined by the χ2 test (except for the comparison of gestational age distributions, for which we used the Mann-Whitney rank-sum test).
Peripheral asexual P. falciparum parasitemia.
Packed cell volume, <33%.
Women reporting regular use of chemoprophylaxis against malaria.
In Yaounde, more pregnant women than nonpregnant women were parasitemic, and more pregnant women from Etoa than from Yaounde were parasitemic (Table 1). Furthermore, pregnant women were anemic more often than nonpregnant women (Table 1). These findings correspond well with the high malaria endemicity in Etoa compared to Yaounde (see Materials and Methods) and with the impact of pregnancy and P. falciparum infection on the prevalence of anemia.
The reported use of chemoprophylaxis was more frequent in pregnant women than in nonpregnant women, suggesting that advice given at antenatal clinics had some impact (Table 1).
Plasma levels of IgG specific for non-PAM type VSA depend on endemicity but not on pregnancy status.
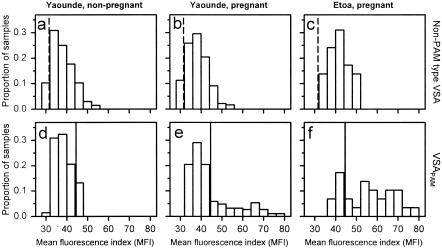
Most women from Yaounde had significant levels of IgG with specificity for non-PAM type VSA. The median IgG levels were similar in nonpregnant and pregnant women (Fig. 1 and Table 2). Similarly, the proportions of samples from nonpregnant and pregnant women with levels above the negative cutoff (Danish control samples) were similar (Table 3). As far as we are aware, this is the first time that it has been shown directly that pregnancy does not affect the levels of IgG with specificity for non-PAM type VSA.
FIG. 1.
Distribution of plasma levels of VSA-specific IgG in Cameroonian women. The levels of IgG with specificity for VSA expressed by parasites infecting nonpregnant individuals (non-PAM type VSA) (a to c) and by parasites infecting the placentas of pregnant women (VSAPAM) (d to f) in nonpregnant (a and d) and pregnant (b and e) women from Yaounde and in pregnant women from Etoa (c and f) are shown. Levels were measured by flow cytometry and are expressed in MFI units. The negative cutoff levels (means plus two standard deviations of MFI values) obtained with plasma from nonexposed control donors (vertical dashed lines) and with plasma from sympatric nonpregnant nulligravidae (vertical solid lines) are shown for reference.
TABLE 2.
Plasma levels of non-PAM type VSA-specific and VSAPAM-specific antibodies
| Immunoglobulin | Plasma levels (MFI units)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (A) | Yaounde
|
Etoa (pregnant) (D) | Statistical significance (Mann-Whitney rank-sum test)
|
||||||
| Nonpregnant (B) | Pregnant (C) | P (A vs B) | P (A vs C) | P (A vs D) | P (B vs C) | P (C vs D) | |||
| Non-PAM type VSA | |||||||||
| IgG | 29.3 (28.1-31.6)a | 37.5 (29.6-54.5) | 37.4 (28.5-54.4) | 41.5 (33.5-51.2) | <0.0001 | <0.0001 | <0.0001 | 0.97 | <0.0001 |
| IgG1 | 32.1 (30.9-36.0) | 44.6 (30.7-64.6) | 45.9 (31.7-74.6) | 49.4 (37.6-66.3) | <0.0001 | <0.0001 | <0.0001 | 0.58 | 0.005 |
| IgG2 | 35.3 (31.6-38.9) | 37.2 (31.6-43.9) | 36.9 (31.1-45.6) | 37.3 (33.7-41.3) | 0.01 | 0.006 | 0.009 | 0.80 | 0.79 |
| IgG3 | 31.7 (28.8-36.4) | 35.6 (32.3-50.0) | 36.0 (31.5-49.7) | 37.0 (32.4-49.3) | <0.0001 | <0.0001 | <0.0001 | 0.43 | 0.06 |
| IgG4 | 33.9 (30.8-36.6) | 33.2 (28.8-40.1) | 33.3 (28.6-44.1) | 33.7 (30.5-40.2) | 0.44 | 0.76 | 0.56 | 0.47 | 0.18 |
| VSAPAM | |||||||||
| IgG | 33.9 (32.0-40.7) | 38.1 (28.5-47.4) | 40.1 (32.0-78.1) | 57.6 (38.3-80.3) | <0.0001 | <0.0001 | <0.0001 | 0.002 | <0.0001 |
| IgG1 | 39.6 (37.1-42.0) | 43.5 (38.1-70.8) | 44.7 (37.1-115.7) | 87.5 (41.0-115.3) | <0.0001 | <0.0001 | <0.0001 | 0.004 | <0.0001 |
| IgG2 | 36.7 (35.8-40.8) | 38.3 (36.0-44.5) | 38.8 (34.6-47.2) | 39.5 (35.2-48.8) | <0.0001 | <0.0001 | <0.0001 | 0.08 | 0.10 |
| IgG3 | 35.0 (32.3-38.8) | 38.4 (32.2-44.2) | 38.1 (31.1-54.6) | 42.3 (35.1-54.1) | <0.0001 | <0.0001 | <0.0001 | 0.54 | <0.0001 |
| IgG4 | 36.7 (34.0-51.1) | 40.4 (36.9-51.2) | 40.1 (36.9-48.2) | 41.7 (36.5-46.7) | <0.0001 | <0.0001 | <0.0001 | 0.24 | 0.01 |
Median (range).
TABLE 3.
Proportions of plasma sample with positive levels of non-PAM type VSA-specific and VSAPAM-specific antibodies
| Immunoglobulin | Negative cutoff value (MFI)a | Proportion
|
||||
|---|---|---|---|---|---|---|
| Yaounde
|
Etoa (pregnant) (C) | Statistical significance (χ2 test)
|
||||
| Nonpregnant (A) | Pregnant (B) | P (A vs B) | P (B vs C) | |||
| Non-PAM type VSA | ||||||
| IgG | 31.62 | 0.93 (0.84-0.97)b | 0.90 (0.85-0.93) | 1.00 (0.89-1.00) | 0.65 | 0.13 |
| IgG1 | 35.35 | 0.88 (0.79-0.94) | 0.95 (0.91-0.97) | 1.00 (0.89-1.00) | 0.09 | 0.45 |
| IgG2 | 39.71 | 0.13 (0.07-0.23) | 0.18 (0.13-0.24) | 0.23 (0.11-0.40) | 0.51 | 0.69 |
| IgG3 | 34.90 | 0.66 (0.54-0.76) | 0.71 (0.64-0.77) | 0.77 (0.60-0.89) | 0.56 | 0.60 |
| IgG4 | 37.17 | 0.06 (0.02-0.14) | 0.11 (0.07-0.16) | 0.07 (0.02-0.21) | 0.35 | 0.68 |
| VSAPAM | ||||||
| IgG | 44.50 | 0.12 (0.06-0.22) | 0.29 (0.23-0.36) | 0.77 (0.60-0.89) | 0.008 | <0.001 |
| IgG1 | 49.66 | 0.12 (0.06-0.22) | 0.35 (0.29-0.42) | 0.94 (0.79-0.98) | <0.001 | <0.001 |
| IgG2 | 42.28 | 0.03 (0.01-0.10) | 0.08 (0.05-0.12) | 0.19 (0.09-0.36) | 0.30 | 0.08 |
| IgG3 | 41.44 | 0.04 (0.02-0.12) | 0.19 (0.14-0.25) | 0.52 (0.35-0.68) | 0.008 | <0.001 |
| IgG4 | 47.06 | 0.03 (0.01-0.10) | 0.01 (0.00-0.03) | 0.00 (0.00-0.02) | 0.36 | 0.31 |
Levels were considered positive if they exceeded subclass-specific negative cutoff values calculated from results obtained with unexposed control donors (donors without exposure to any P. falciparum parasites for non-PAM type VSA and nulligravidae from Yaounde for VSAPAM). See Materials and Methods for details.
The values in parentheses are 95% confidence interval.
Furthermore, the median levels of IgG with specificity for non-PAM type VSA were significantly higher for pregnant women from Etoa, where transmission of malaria parasites is intense, than in samples from women living in Yaounde, where the intensity of transmission is low (Fig. 1 and Table 2). The proportion of samples with levels above the negative cutoff was also marginally higher in samples from pregnant women in Etoa than in samples from pregnant women in Yaounde (Table 3). These findings are in accordance with previous data showing that adults living in areas with endemic transmission of P. falciparum parasites generally have significant levels of non-PAM type VSA-specific antibodies and that the levels of these antibodies depend on endemicity (16, 20).
The distribution of plasma VSAPAM-specific IgG is different from that of non-PAM type VSA-specific IgG.
In contrast to non-PAM type VSA-specific IgG, VSAPAM-specific IgG is exclusively found in women who are or have recently been pregnant (gender or sex specificity), and the levels of VSAPAM-specific IgG in pregnant women increase with parity (parity dependence) (3, 11, 25, 30). We found that nonpregnant women in Yaounde had lower plasma levels of VSAPAM-specific IgG than corresponding pregnant women (Fig. 1 and Table 2). Most nonpregnant women (all parities) had levels below the negative VSAPAM-specific cutoff (see Materials and Methods for details), and the remaining samples (all from multigravidae) had levels just above the cutoff (Fig. 1 and Table 3). In marked contrast, a significant proportion of the samples from pregnant women in Yaounde had levels greater than the negative VSAPAM-specific cutoff, and many had very high levels (Fig. 1 and Table 3). The samples from pregnant women in Etoa showed a similar pattern, although a much higher proportion had high to very high VSAPAM-specific IgG levels (Fig. 1 and Table 3). Thus, our data agree with previous observations from Ghana (25) and Kenya (31) and are consistent with our hypothesis that the distribution of VSAPAM-specific IgG has essentially two peaks (women having either no or very little VSAPAM-specific IgG and other women having very high levels) and thus very different from the bell-shaped distribution seen with non-PAM type VSA-specific IgG (31).
VSA-specific plasma IgG is dominated by IgG1.
Only a few previous studies have addressed the IgG subclass distribution of VSA-specific antibody responses in general (6, 14), and none have examined responses in relation to pregnancy or VSAPAM-specific responses. We found higher levels (Table 2) and proportions (Table 3) of non-PAM type VSA-specific IgG1 and IgG3 in plasma from each of the three groups of Cameroonian women than in plasma from unexposed control donors. The levels and proportions of IgG2 were only marginally higher, while the IgG4 levels were not significantly greater than the negative control levels (Tables 2 and 3). The distributions of non-PAM type VSA-specific antibody levels were bell shaped for all subclasses (data not shown). The levels and proportions of all non-PAM type VSA-specific IgG subclasses were similar in nonpregnant and pregnant women from Yaounde (Tables 2 and 3), while the levels of non-PAM type VSA-specific IgG1 and IgG3 were higher in Etoa than in Yaounde (Table 2).
The plasma levels (Table 2) of all VSAPAM-specific IgG subclasses were significantly higher in women in Cameroon than in control donors, and the most prominent differences in proportions were seen for IgG1 and IgG3. The distributions of VSAPAM-specific subclass levels resembled those of total VSAPAM-specific IgG (data not shown). The levels and proportions of VSAPAM-specific IgG1 and the proportions of VSAPAM-specific IgG3 were higher in pregnant women than in nonpregnant women from Yaounde (Tables 2 and 3). Both the levels and the proportions of all VSAPAM-specific IgG subclasses were generally higher in Etoa than in Yaounde (Tables 2 and 3). Overall, our data indicate that the antibody responses to both non-PAM type VSA and VSAPAM are dominated by IgG1 and to a lesser extent by IgG3, although a direct quantitative comparison of subclasses was not possible.
Plasma levels of all VSAPAM-specific IgG subclasses increase with parity.
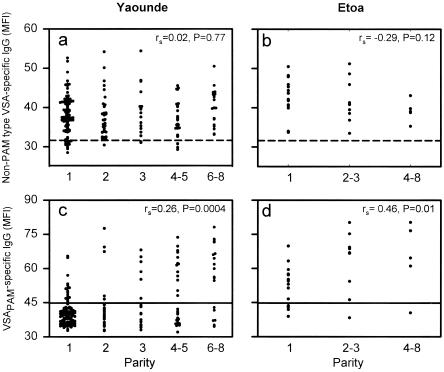
The levels of VSAPAM-specific IgG have previously been shown to correlate with parity, whereas the levels of IgG with specificity for non-PAM type VSA do not (11, 25). This finding was confirmed here (Fig. 2 and Table 4). In addition, our data show that there were significant relationships between parity and VSAPAM-specific IgG1 (both sites) and between parity and VSAPAM-specific IgG3 (only significant for Yaounde) (Table 4). Finally, we show here for the first time that the correlation between VSAPAM-specific antibodies and parity depends on the intensity of transmission, as it is much stronger for the high-endemicity study area (Etoa) than for the low-endemicity study area (Yaounde) (Table 4). This is mainly due to the presence of substantial proportions of samples with very low levels of VSAPAM-specific antibodies in all parity groups in the low-transmission area, which was not seen in the high-transmission area (Fig. 2). These findings imply that while the likelihood of acquiring a placental P. falciparum infection during the course of pregnancy obviously depends on endemicity, once such an infection has been acquired, it generally induces a very marked VSAPAM-specific antibody response.
FIG. 2.
Relationship between plasma levels of VSA-specific IgG and parity in Cameroonian pregnant women. Individual levels of IgG with specificity for VSA expressed by parasites infecting nonpregnant individuals (non-PAM type VSA) (a and b) and by parasites capable of sequestering in the placenta (VSAPAM) (c and d) in pregnant women from Yaounde (a and c) and Etoa (b and d) are shown. The negative cutoff levels (means plus two standard deviations of MFI values) obtained with plasma from nonexposed control donors (horizontal dashed lines) and with plasma from sympatric nonpregnant nulligravidae (horizontal solid lines) are shown for reference.
TABLE 4.
Relationships (Spearman's rank-order correlation coefficients, rs) between donor parity and levels of VSA-specific IgG subclasses in Cameroonian pregnant women
| Immuno- globulin | VSAPAM type responses
|
Non-PAM VSA type responses
|
||||||
|---|---|---|---|---|---|---|---|---|
| Yaounde (n = 186)
|
Etoa (n = 29)
|
Yaounde (n = 186)
|
Etoa (n = 29)
|
|||||
| rs | P | rs | P | rs | P | rs | P | |
| IgG | 0.26 | 0.0004 | 0.46 | 0.01 | 0.02 | 0.77 | −0.29 | 0.12 |
| IgG1 | 0.30 | <0.0001 | 0.42 | 0.02 | 0.02 | 0.80 | −0.06 | 0.75 |
| IgG2 | 0.10 | 0.16 | 0.44 | 0.01 | −0.08 | 0.26 | 0.07 | 0.71 |
| IgG3 | 0.18 | 0.01 | 0.22 | 0.24 | −0.07 | 0.38 | −0.16 | 0.39 |
| IgG4 | 0.12 | 0.09 | 0.25 | 0.17 | −0.10 | 0.16 | −0.27 | 0.14 |
The relationship between VSAPAM-specific IgG subclasses and gestational age depends on transmission intensity.
In two previous studies, both performed in Yaounde, we investigated the relationship between VSA-specific antibodies and gestational age. The data showed that the levels of VSAPAM-specific IgG increased steadily as pregnancy progressed (22, 30), while the non-PAM type VSA IgG levels were only marginally affected (30). The present data for IgG specific for non-PAM type VSA and VSAPAM in plasma samples from primigravidae support our earlier findings and extend them to an area where endemicity is high (Table 5). The correlations between VSAPAM-specific IgG were much stronger for samples from primigravidae in Etoa than for samples from primigravidae in Yaounde, probably reflecting the much higher risk of infection in the former area and the relatively slow development of primary VSAPAM-specific responses in primigravidae (22). With respect to subclass responses, IgG1 followed the IgG pattern both for VSAPAM and non-PAM type VSA and in primigravidae and multigravidae. This is perhaps not surprising since IgG1 is generally the dominant IgG subclass, accounting for about 70% of the total IgG. Only minimal changes in the very low levels of IgG2 with specificity for VSAPAM and non-PAM type VSA were observed. In contrast to the increasing levels of VSAPAM-specific IgG1 with advancing pregnancy in primigravidae, the levels of VSAPAM-specific IgG3 decreased with increasing gestational age in women from Yaounde (Table 5). This pattern was only partially reproduced in Etoa, probably due to the higher level of endemicity at this site, less drug use (Table 1), and/or insufficient sample size. With the exception of IgG4 levels in primigravidae from Yaounde, the levels of IgG subclasses with specificity for non-PAM type VSA did not depend on gestational age (Table 5). The changes in the levels of non-PAM type VSA-specific IgG4 are unlikely to be biologically relevant as the levels of this type of antibody were very low (Tables 1 and 2). Taken together, these results support the evidence described above that IgG1 dominates VSA-specific IgG responses, while IgG2 and IgG4 are much less prominent.
TABLE 5.
Relationships (Spearman's rank-order correlation coefficients, rs) between gestational age and levels of VSA-specific IgG subclasses in Cameroonian pregnant women
| Immuno- globulin | VSAPAM type responses
|
Non-PAM VSA type responses
|
||||||
|---|---|---|---|---|---|---|---|---|
| Yaounde
|
Etoa
|
Yaounde
|
Etoa
|
|||||
| rs | P | rs | P | rs | P | rs | P | |
| Primigravidaea | ||||||||
| IgG | 0.29 | 0.007 | 0.78 | 0.0006 | −0.10 | 0.37 | −0.02 | 0.95 |
| IgG1 | 0.21 | 0.05 | 0.80 | 0.0003 | −0.07 | 0.55 | 0.28 | 0.31 |
| IgG2 | −0.24 | 0.02 | 0.19 | 0.50 | 0.20 | 0.06 | 0.64 | 0.01 |
| IgG3 | −0.43 | <0.001 | 0.31 | 0.26 | 0.05 | 0.63 | −0.33 | 0.22 |
| IgG4 | 0.03 | 0.76 | −0.36 | 0.18 | 0.33 | 0.002 | 0.10 | 0.72 |
| Multigravidaeb | ||||||||
| IgG | 0.03 | 0.75 | 0.37 | 0.16 | −0.19 | 0.06 | 0.05 | 0.87 |
| IgG1 | 0.03 | 0.75 | 0.28 | 0.29 | −0.19 | 0.06 | 0.04 | 0.87 |
| IgG2 | −0.11 | 0.27 | 0.33 | 0.21 | 0.24 | 0.02 | 0.15 | 0.59 |
| IgG3 | −0.33 | 0.0009 | −0.20 | 0.46 | 0.01 | 0.95 | −0.43 | 0.09 |
| IgG4 | −0.01 | 0.93 | 0.19 | 0.48 | 0.13 | 0.20 | −0.16 | 0.56 |
N = 88 for Yaounde and n = 15 for Etoa.
N = 98 for Yaounde and n = 14 for Etoa.
Multivariate analysis of VSA-specific antibody responses.
Because of the covariation of maternal age, parity, and gestational age influencing VSA-specific antibody levels in the univariate analyses described above and the potential effect of asymptomatic parasitemia and drug usage, we tested the impact of these variables together by multiple linear regression analysis. All variables were generally poor predictors of IgG- and subclass-specific responses to non-PAM type VSA. However, maternal age and parity were predictors of the levels of non-PAM type VSA-specific IgG and IgG3 in pregnant women from Yaounde (P < 0.003 in all cases), while gestational age could predict levels of this type of IgG and IgG2 in pregnant women from Yaounde and/or Etoa (P < 0.002 in all cases). This suggests that pregnancy-associated malaria can boost antibody responses to non-PAM type VSA. For VSAPAM-specific responses, parity was a significant predictor of levels of all subclasses in pregnant women from Yaounde and/or Etoa (P < 0.005 in all cases). Gestational age could significantly predict levels of IgG, IgG1, IgG2, and IgG3 in samples from these women (P < 0.008 in all cases). Taken together, these data corroborate the results of the univariate analysis.
DISCUSSION
Two studies of VSA-specific IgG subclass responses that preceded the present study indicated that IgG3 is the dominant subclass in children (14) or that IgG2 and IgG3 are the dominant subclasses in adults and IgG3 and IgG4 are the dominant subclasses in children (6). The data presented here suggest that IgG1 is the dominant subclass involved in the VSA-specific IgG response in adults and that IgG3 is the only other subclass that is significantly involved. In this respect, our study supports the conclusion of the third previous study of VSA-specific IgG subclass responses (23) and extends it to include the type of VSA (VSAPAM) involved in the pathogenesis of pregnancy-associated malaria. The discrepancy between the present findings and the Kenyan study (14) is probably related to the fact that responses to autologous parasites in children with limited preexisting immunity were studied in the latter study. In contrast, the disagreement with the Gabonese study (6) is more likely to reflect differences in methods and interpretation.
Previous studies have shown that pregnancy either affects (10, 18) or does not affect (7) levels of antibodies. We report here for the first time that the levels and prevalence of IgG and subclasses of IgG with specificity for the non-PAM type VSA that are expressed by parasites not involved in the pathogenesis of pregnancy-associated malaria are only marginally affected by pregnancy. We also show that the levels and prevalence of non-PAM type VSA-specific IgG and IgG subclasses depend only on the level of endemicity in pregnant women. This finding supports and extends previous findings regarding VSA-specific IgG in nonpregnant individuals (1, 20). With respect to VSAPAM-specific IgG, which mediates protection against an adverse pregnancy outcome due to placental P. falciparum infection (8, 31), the present data are consistent with our earlier finding of a dichotomous distribution of the IgG specificities that is fundamentally different from the single-peak, bell-shaped distribution of IgG with specificity for non-PAM type VSA (31). This observation implies that the levels of VSAPAM-specific IgG decline fairly rapidly in the absence of antigenic stimulation, which can occur only during pregnancy. This inference is reinforced by the observed endemicity-dependent difference in the relative magnitude of the two peaks and is in line with our previous data (30). It is likely that antibody responses to non-PAM type VSA are in fact similarly transient in nature (14) and that the significant amounts of antibodies with these specificities found in most long-term residents of endemic areas reflect regular reinfection and/or persistent low-grade (probably asymptomatic) infections in such individuals.
The developing placenta appears to be able to sustain an infection from the beginning of the second trimester (12), and peripheral parasitemia, probably originating from a placental focus (21), peaks around 13 to 16 weeks of gestation (4). This corresponds well with the finding that VSAPAM-specific IgG responses appear around weeks 18 to 20 in primigravidae and somewhat earlier in multigravidae (22, 30). The weak, but highly significant, negative association between VSAPAM-specific IgG3 and gestational age that we found in samples from Yaounde may thus reflect catabolic antibody decay toward the end of pregnancy following VSAPAM-specific antibody-mediated control of placental parasite multiplication (11, 30-32). The fact that this was not seen for IgG1 was probably related to the longer half-life of this subclass, and the fact that it was not seen in the samples from Etoa may be related to the higher endemicity, less drug usage (Table 1), and/or inadequate sample size.
In conclusion, the data from this first study of VSA-specific IgG subclass responses in pregnant women indicate that VSA-specific IgG1 and, less prominently, IgG3 are the main subclasses of the VSA-specific IgG response. This is important for antibodies with specificity for the VSAPAM-type parasite antigens, as there is strong evidence that VSAPAM-specific IgG is directly involved in acquired protection against adverse consequences of pregnancy-associated malaria in both expectant mothers and their offspring (8, 31). The fact that IgG1 and IgG3 are cytophilic subclasses suggests that opsonization of infected erythrocytes may be an important element in the control of placental parasitemia in addition to interference with adhesion to placental proteoglycans (30). While we acknowledge the limitation that we compared only VSAPAM-specific and non-PAM type VSA-specific antibody responses for a single parasite line, we believe that our findings are important in relation to the current intensive efforts to develop VSA-based vaccines against P. falciparum malaria, including pregnancy-associated malaria (27, 28).
Acknowledgments
We are indebted to all the individuals who donated parasite and plasma samples for the study. We also gratefully acknowledge the contributions to the study made by the entire malaria research team at the Biotechnology Center, University of Yaounde I, Yaounde, Cameroon. Kirsten Pihl and Maiken Christensen are thanked for excellent technical assistance in the laboratory.
This work received financial support from the Commission of European Union (grant QLK2-CT-2001-01302, PAMVAC), from the Danish Medical Research Council (grants 22-02-0571 and 22-03-0333), and from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant UO1 AI43888).
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aguiar, J. C., G. R. Albrecht, P. Cegielski, B. M. Greenwood, J. B. Jensen, G. Lallinger, A. Martinez, I. A. McGregor, J. N. Minjas, J. Neequaye, M. E. Patarroyo, J. A. Sherwood, and R. J. Howard. 1992. Agglutination of Plasmodium falciparum-infected erythrocytes from East and West African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 47:621-632. [DOI] [PubMed] [Google Scholar]
- 2.Altman, D. G., D. Machin, T. N. Bryant, and M. J. Gardner (ed.). 2000. Statistics with confidence. British Medical Journal, London, United Kingdom.
- 3.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 5.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera, G., C. Yone, A. E. Tebo, J. Van Aaken, B. Lell, P. G. Kremsner, and A. J. Luty. 2004. Immunoglobulin G isotype responses to variant surface antigens of Plasmodium falciparum in healthy Gabonese adults and children during and after successive malaria attacks. Infect. Immun. 72:284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deloron, P., G. H. Campbell, D. Brandling-Bennett, J. M. Roberts, I. K. Schwartz, J. S. Odera, A. A. Lal, C. O. Osanga, V. de la Cruz, and T. M. McCutchan. 1989. Antibodies to Plasmodium falciparum ring-infected erythrocyte surface antigen and P. falciparum and P. malariae circumsporozoite proteins: seasonal prevalence in Kenyan villages. Am. J. Trop. Med. Hyg. 41:395-399. [DOI] [PubMed] [Google Scholar]
- 8.Duffy, P. E., and M. Fried. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71:6620-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fievet, N., M. Cot, P. Ringwald, J. Bickii, B. Dubois, J. Y. Le Hesran, F. Migot, and P. Deloron. 1997. Immune response to Plasmodium falciparum antigens in Cameroonian primigravidae: evolution after delivery and during second pregnancy. Clin. Exp. Immunol. 107:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried, M., F. Nosten, A. Brockman, B. T. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 12.Garnham, P. C. C. 1938. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans. R. Soc. Trop. Med. Hyg. 32:13-34. [Google Scholar]
- 13.Jensen, J. B., and W. Trager. 1978. Plasmodium falciparum in culture: establishment of additional strains. Am. J. Trop. Med. Hyg. 27:743-746. [DOI] [PubMed] [Google Scholar]
- 14.Kinyanjui, S. M., P. Bull, C. I. Newbold, and K. Marsh. 2003. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J. Infect. Dis. 187:667-674. [DOI] [PubMed] [Google Scholar]
- 15.Manga, L., V. Robert, J. Messi, M. Desfontaine, and P. Carnevale. 1992. Le paludisme urbain a Yaoundé, Cameroun: l'etude entomologique dans deux quartiers centraux. Mem. Soc. R. Belge Entomol. 35:155-162. [Google Scholar]
- 16.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 17.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 18.McGregor, I. A., D. S. Rowe, M. E. Wilson, and W. Z. Billewicz. 1970. Plasma immunoglobulin concentrations in an African (Gambian) community in relation to season, malaria and other infections and pregnancy. Clin. Exp. Immunol. 17:51-74. [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen, M. A., T. Staalsoe, J. A. L. Kurtzhals, B. Q. Goka, D. Dodoo, M. Alifrangis, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and non-severe malaria and is modified by acquired immunity. J. Immunol. 168:3444-3450. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen, M. A., L. S. Vestergaard, J. Lusingu, J. A. L. Kurtzhals, H. Giha, B. Grevstad, B. Q. Goka, M. M. Lemnge, J. B. Jensen, B. D. Akanmori, T. G. Theander, T. Staalsoe, and L. Hviid. 2004. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect. Immun. 72:3531-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ofori, M. F., T. Staalsoe, V. Bam, M. Lundquist, K. P. David, E. N. L. Browne, B. D. Akanmori, and L. Hviid. 2003. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect. Immun. 71:1584-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill-Dunne, I., R. N. Achur, S. T. Agbor-Enoh, M. Valiyaveettil, R. S. Naik, C. F. Ockenhouse, A. Zhou, R. Megnekou, R. Leke, D. W. Taylor, and D. C. Gowda. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69:7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piper, K. P., D. J. Roberts, and K. P. Day. 1999. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp. Parasitol. 91:161-169. [DOI] [PubMed] [Google Scholar]
- 24.Quakyi, I. A., R. G. Leke, R. Befidi-Mengue, M. Tsafack, D. Bomba-Nkolo, L. Manga, V. Tchinda, E. Njeungue, S. Kouontchou, J. Fogako, P. Nyonglema, L. T. Harun, R. Djokam, G. Sama, A. Eno, R. Megnekou, S. Metenou, L. Ndountse, A. Same-Ekobo, G. Alake, J. Meli, J. Ngu, F. Tietche, J. Lohoue, J. L. Mvondo, E. Wansi, R. Leke, A. Folefack, J. Bigoga, C. Bomba-Nkolo, V. Titanji, A. Walker-Abbey, M. A. Hickey, A. H. Johnson, D. W. Taylor, and L. Ndountse. 2000. The epidemiology of Plasmodium falciparum malaria in two Cameroonian villages: Simbok and Etoa. Am. J. Trop. Med. Hyg. 63:222-230. [PubMed] [Google Scholar]
- 25.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 26.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe, J. A., and S. A. Kyes. 2004. The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol. Microbiol. 53:1011-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, J. D., and K. W. Deitsch. 2004. Pregnancy-associated malaria and the prospects for syndrome-specific antimalaria vaccines. J. Exp. Med. 200:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 30.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 31.Staalsoe, T., C. E. Shulman, J. N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against the clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363:283-289. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, D. W., A. Zhou, L. E. Marsillio, L. W. Thuita, E. B. Leke, O. Branch, D. C. Gowda, C. Long, and R. F. G. Leke. 2004. Antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A and to the C terminus of merozoite surface protein 1 correlate with reduced placental malaria in Cameroonian women. Infect. Immun. 72:1603-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, A., R. Megnekou, R. Leke, J. Fogako, S. Metenou, B. Trock, D. W. Taylor, and R. F. Leke. 2002. Prevalence of Plasmodium falciparum infection in pregnant Cameroonian women. Am. J. Trop. Med. Hyg. 67:566-570. [DOI] [PubMed] [Google Scholar]