Abstract
To evaluate the long-term effects of submucosal Medpor implants in patients with empty nose syndrome (ENS), using the Sinonasal Outcome Test (SNOT) score as a measure of clinical improvement. A comprehensive search of six databases was conducted up to October 2024. The analysis included studies that examined the impact of submucosal Medpor implants on refractory ENS symptoms, as assessed by various symptom-specific questionnaires. Post-intervention SNOT scores were evaluated during follow-up periods of over 12 months, showing a statistically significant improvement in ENS symptoms (standardized mean difference [95% confidence interval]= 1.4676 [1.2067; 1.7285]; I2=37.2%). This meta-analysis indicates that submucosal Medpor implantation in patients with ENS is associated with significant long-term improvements in nasal symptoms.
Keywords: Rhinitis, Atrophic; Surveys and questionnaires; Sino-Nasal Outcome Test
INTRODUCTION
Empty nose syndrome (ENS) is characterized by a paradoxical sensation of nasal obstruction despite the presence of normal nasal airflow. Symptoms vary among patients and may include difficulty breathing, a sense of excessive nasal openness, increased airflow sensation, shortness of breath, and feelings of suffocation. As inferior turbinate surgeries have become increasingly common as outpatient procedures for treating rhinitis, alongside the rise in endoscopic endonasal skull base surgeries, the prevalence of post-surgical ENS symptoms has also increased. Although conservative management remains the primary treatment approach for ENS, recent reports highlight the development of more precise intervention techniques aimed at providing long-term symptom relief. This review, therefore, examines the outcomes of submucosal Medpor (Porex Surgical, Inc., Newnan, GA, USA) implantation as a treatment option for ENS.
METHODS
Search strategy
The population, intervention, comparison, outcomes, and study (PICOS) framework for this study was as follows: 1) population: patients diagnosed with ENS or atrophic rhinitis; 2) intervention: submucosal Medpor implantation; 3) comparison: not restricted; 4) outcomes: Sinonasal Outcome Test (SNOT) scores; 5) study design: no limitations. Review articles and studies lacking analyzable data were excluded. Two independent reviewers, DHK and SHH, screened the titles and abstracts of all identified studies, excluding those unrelated to questionnaire score changes following procedures in ENS patients. The search encompassed several databases, including PubMed, Scopus, Embase, Web of Science, Google Scholar, and the Cochrane database, covering articles published up to October 2024. Studies involving patients with coexisting nasal diseases, such as sinusitis or tumors, were also excluded.
Data extraction
We extracted data from selected studies on patient demographics, including numbers, sex, age, and nationality, as well as scores for nasal symptoms and p-values for comparisons between pre-procedure and post-procedure scores [1-5]. The analyzed outcome was changes in SNOT scores from pre-procedure to post-procedure.
Statistical analysis
Meta-analyses were conducted using the R statistical software (version 4.4.2; R Foundation for Statistical Computing, Vienna, Austria). For continuous variables, the meta-analysis utilized either the standardized mean difference (SMD) or the mean difference (MD). The SMD was chosen to estimate effect sizes because of the differing scales employed across studies to measure SNOT scores, which include SNOT-20, SNOT-22, and SNOT-25.
RESULTS
Five articles with 156 participants were included in the analysis. The characteristics of the studies are listed in Table 1.
Table 1.
Study characteristics
| Study (year) | Study type | Sample size | Age, yr (mean, range, or standard deviation) | Sex (male/female) | Nation | Outcomes |
|---|---|---|---|---|---|---|
| Jiang 2013 [3] | Cohort | 19 | 32.2 (18–64) | 15/4 | China | SNOT-20 |
| Jiang 2014 [4] | Cohort | 24 | 32.4 (18–64) | 18/6 | China | SNOT-25 |
| Tam 2014 [5] | Case series | 16 | 31–68 | 10/6 | Taiwan | SNOT-22 |
| Fu 2021 [1] | Case series | 43 | 44.7 (13.2) | 37/6 | Taiwan | SNOT-25 |
| Huang 2021 [2] | Case series | 54 | 50.9±12.2 | 36/18 | China | SNOT-25 |
SNOT, Sinonasal Outcome Test
Change of SNOT scores between pretreatment and posttreatment
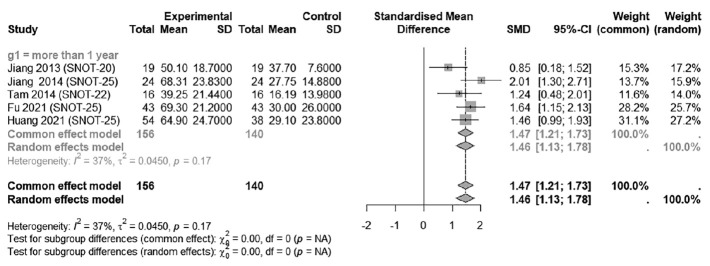
The improvement in symptoms, as measured by the pre-post SNOT score difference after 1 year, was statistically significant in patients with ENS following procedures, with an SMD of 1.4676 (95% CI: 1.2067; 1.7285; I2=37.2%) (Fig. 1).
Fig. 1.
Improvements in Sinonasal Outcome Test (SNOT) scores after inferior meatus augmentation or inferior turbinate augmentation. The standardized mean difference (SMD) was utilized to quantify the changes in SNOT scores. SD, standard deviation, CI, confidence interval.
DISCUSSION
In this study, SNOT scores demonstrated significant improvement for over 1 year following interventions, including the submucosal Medpor implant, in ENS patients.
In managing ENS, conservative treatments such as saline sprays, various emollients, and moisturization are generally recommended. Patients with this syndrome not only suffer from discomfort due to respiratory symptoms like paradoxical nasal obstruction but also frequently experience the onset or worsening of anxiety and depression. As a result, conservative treatments alone may not adequately address the condition. This study demonstrates that active intervention in treating ENS leads to significant long-term improvements in nasal-specific quality of life. The interventions were designed to mimic pre-surgery or pre-injury nasal conditions by reducing the size of the nasal passages. This reduction aids in the warming and humidification of inhaled air and helps decrease airflow turbulence in the enlarged nasal passages, which occurs when airflow passes through narrowed nostrils. The most common site for implantation is the anterior-inferior lateral nasal wall because this location facilitates easier elevation of the mucous membrane compared to the nasal floor. It is also more accessible for insertion than areas that are difficult to dissect due to fibrosis, such as sites of turbinate excision. Typically, a cotton test is performed at the intended implantation site to assess symptom improvement before proceeding with the implantation.
Synthetic materials are widely used in the treatment of ENS. Porous polyethylene, commonly known as Medpor, is the most frequently used implant. Other materials, including silicone sheets and β-tricalcium phosphate implants (SINUSUP Implants), are also utilized. These synthetic options are relatively inexpensive and easy to manipulate. However, they pose a greater risk of foreign body reactions, extrusion, and infection compared to autologous materials.
In conclusion, submucosal Medpor implantation for the treatment of ENS improved nasal symptoms for more than 1 year.
Acknowledgments
None
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Do Hyun Kim and Se Hwan Hwang who are on the editorial board of the Journal of Rhinology were not involved in the editorial evaluation or decision to publish this article.
Author Contributions
Conceptualization: Se Hwan Hwang. Data curation: Se Hwan Hwang. Formal analysis: Se Hwan Hwang. Methodology: Se Hwan Hwang. Project administration: Se Hwan Hwang. Resources: Se Hwan Hwang. Software: Se Hwan Hwang. Supervision: Se Hwan Hwang. Validation: Do Hyun Kim. Visualization: Do Hyun Kim. Writing—original draft: Do Hyun Kim. Writing—review & editing: Do Hyun Kim, Se Hwan Hwang.
Funding Statement
None
References
- 1.Fu CH, Chen HC, Huang CC, Chang PH, Lee TJ. Serum high-sensitivity C-reactive protein is associated with postoperative psychiatric status in patients with empty nose syndrome. Diagnostics (Basel) 2021;11(12):2388. doi: 10.3390/diagnostics11122388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang CC, Wu PW, Lee CC, Chang PH, Huang CC, Lee TJ. Suicidal thoughts in patients with empty nose syndrome. Laryngoscope Investig Otolaryngol. 2022;7(1):22–8. doi: 10.1002/lio2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang C, Shi R, Sun Y. Study of inferior turbinate reconstruction with Medpor for the treatment of empty nose syndrome. Laryngoscope. 2013;123(5):1106–11. doi: 10.1002/lary.23908. [DOI] [PubMed] [Google Scholar]
- 4.Jiang C, Wong F, Chen K, Shi R. Assessment of surgical results in patients with empty nose syndrome using the 25-item Sino-Nasal Outcome Test Evaluation. JAMA Otolaryngol Head Neck Surg. 2014;140(5):453–8. doi: 10.1001/jamaoto.2014.84. [DOI] [PubMed] [Google Scholar]
- 5.Tam YY, Lee TJ, Wu CC, Chang PH, Chen YW, Fu CH, et al. Clinical analysis of submucosal Medpor implantation for empty nose syndrome. Rhinology. 2014;52:35–40. doi: 10.4193/Rhino13.086. [DOI] [PubMed] [Google Scholar]