Abstract
We investigated the dissemination of pathogenicity island (PAI) IIJ96-like elements (hra, hly, cnf1, and pap) among 455 Escherichia coli isolates from children and adults with urinary tract infection (UTI), neonates with meningitis or colonized healthy neonates, and 74 reference strains by means of PCR phylogenetic grouping, ribotyping, and PCR analysis of virulence genes. Colocalization of these genes was documented by pulsed-field gel electrophoresis followed by Southern hybridization and long-range PCR (LRPCR) between the hra and the papG alleles. Site-specific insertion of the PAI was determined by LRPCR between hra and tRNA flanking sequences. hra, hly, and cnf1 were found in 113 isolates and consistently colocalized, constituting the backbone of PAI IIJ96-like domains. The prevalence of PAI IIJ96-like domains was significantly higher among UTI isolates than among neonatal meningitis and commensal isolates. These domains were restricted to a few ribotypes of group B2. In contrast to the consistent colocalization of hra, hly, and cnf1, the pap operon was varied: 12% of strains exhibited an allelic exchange of the papG class III allele (papGIII) for the papG class II allele (papGII) (only UTI isolates), and the pap operon was deleted in 23% of strains. No strains harbored papGIII outside the PAI, which appears to be the only source of this allele. PAI IIJ96-like domains were inserted in the vicinities of three different tRNAs—pheU (54%), leuX (29%), and pheV (15%)—depending on the genetic backgrounds and origins of the isolates. Multiple insertional events restricted by the genetic background have thus led to PAI IIJ96 acquisition. Specific genetic backgrounds and insertion sites may have played a role in additional recombination processes for E. coli adaptation to different ecological niches.
Escherichia coli is a normal inhabitant of the human intestinal tract but is also a leading cause of community-acquired infections. In addition to causing intestinal infections, E. coli is the most frequent cause of gram-negative bacterial infections such as cystitis, pyelonephritis, bacteremia, and neonatal menin-gitis. These extraintestinal pathogenic E. coli (ExPEC) strains (38) differ from commensal E. coli strains in two major respects. First, among the four main phylogenetic groups of E. coli (A, B1, B2, and D), ExPEC strains belong mostly to group B2 and, to a lesser extent, to group D, whereas commensal strains belong mainly to group A (2, 8, 25, 36). Second, ExPEC strains harbor many genetic virulence determinants and other fitness factors. Most of these genes are acquired by horizontal transfer and constitute the so-called “ectochromosomal” DNA or “flexible gene pool.” The pathogenicity island (PAI) is one of the most important elements of ectochromosomal DNA (17). These large chromosomal regions (>10 kb), differing in their G+C contents from that of the core genome, are located near tRNA genes and contain both genetic virulence determinants and mobility genes (19).
Although the relationship between phylogenetic groups and extraintestinal virulence genes is well documented, few studies have focused on the relationship between the PAI and the genetic background of recipient E. coli isolates from different clinical sources (14, 36).
The aim of this study was to examine the interaction between the genetic background of E. coli strains and the integration and evolution of PAIs according to the ecological niche. Among the archetypal PAIs described for ExPEC, PAI IIJ96 appeared to be a good candidate for such an investigation. PAI IIJ96, initially described to occur in the uropathogenic E. coli strain J96, is one of the largest PAIs described to date (∼110 kb) (7, 23). It contains at least four genes or operons, including those coding for hemolysin (hly), cytotoxic necrotizing factor (cnf1), P fimbriae (pap) with the variant allelic adhesin (papG class III allele [papGIII]), and heat-resistant agglutinin (hra) (7, 40). PAI IIJ96 contributes to the virulence of cystitis, pyelonephritis, and neonatal meningitis strains (8, 15, 19, 22, 30). A previous study of urosepsis isolates suggests that three colocalized genes—hly, cnf1, and hra—constitute the backbone of a PAI IIJ96-like domain (6). Here we examined the distribution and insertion sites of PAI IIJ96-like domains in a large collection of ExPEC isolates from various clinical settings with regard to their phylogenetic groups and subgroups.
MATERIALS AND METHODS
Bacterial strains.
We analyzed 455 clinical E. coli isolates recovered from 1997 to 2000. They consisted of a previously described series of 100 French adult urosepsis isolates (6), 134 international strains of E. coli causing neonatal meningitis (ECNM) (8), and 84 urinary tract infection (UTI) isolates from French infants (<90 days) (9), as well as unpublished French collections of 75 UTI isolates from children aged from 3 months to 10 years and 62 isolates colonizing healthy neonates. All isolates were stored at −80°C until characterization.
Reference strains carrying a PAI IIJ96-like domain, uropathogenic E. coli (UPEC) strains AD110 and J96, and the 72 strains of the ECOR collection were studied for comparison (23, 34, 41).
Phylogenetic grouping and subgrouping.
The main phylogenetic groups (A, B1, B2, and D) were determined for all strains by using previously described PCR methods (11), and B2 strains were subgrouped by ribotyping with the restriction enzyme HindIII and with 16S and 23S rRNAs as the probes (1, 3-5).
Detection of virulence genes and characterization of PAI IIJ96-like domains.
Each strain was screened for hlyC, hlyA, cnf1, papC, papG class II and class III alleles, and hra by means of PCR, as previously described (Table 1) (6, 24, 33, 35), and their colocalizations were detected by pulsed-field gel electrophoresis using the restriction enzyme NotI followed by Southern hybridization (6). When strains harbored the two papG alleles, long-range PCR between the forward papG allele primers and the reverse hra primer hra.1 was used to determine which papG allele belonged to the PAI IIJ96-like domain; the Expand Long Template PCR system (Roche) was used as previously described (6). The insertion sites of PAI IIJ96-like domains were determined by using the same long-range PCR method between hra (primer hra.1) and either pheU (formerly pheR) or leuX tRNA flanking sequences, as previously described (Table 1) (6). When these PCRs were negative, isolates were screened for an insertion in pheV tRNA by long-range PCR with primers homologous to hra (primer hra.1) and the pheV tRNA flanking sequence (primer PheV.1) (Table 1). This tRNA was chosen because it has been described as a second insertion site for a PAI also inserted in pheU (31). To control the integrity of the archetypal pheU tRNA insertion site, we performed PCR of the flanking sequences of the archetypal insertion site pheU with primers pheR.1 and pheR.2 (Table 1).
TABLE 1.
Oligonucleotide primers used to amplify virulence-associated genes and for long-range PCR
| Primer designation | Primer sequence | Targeta | Size of PCR product (bp) | Reference of source |
|---|---|---|---|---|
| chuA.1 | 5′-GACGAACCAACGGTCAGGAT-3′ | chuA | 279 | 11 |
| chuA.2 | 5′-TGCCGCCAGTACCAAAGACA-3′ | |||
| TspE4C2.1 | 5′-GAGTAATGTCGGGGCATTCA-3′ | tspE4.C2 | 152 | 11 |
| TspE4C2.2 | 5′-CGCGCCAACAAAGTATTACG-3′ | |||
| yjaA.1 | 5′-TGAAGTGTCAGGAGACGCTG-3′ | yjaA | 211 | 11 |
| yjaA.2 | 5′-ATGGAGAATGCGTTCCTCAAC-3′ | |||
| hly.1 | 5′-AGGTTCTTGGGCATGTATCCT-3′ | hlyC/A | 556 | 2 |
| hly.2 | 5′-TTGCTTTGCAGACTGCAGTGT-3′ | |||
| cnfl.1 | 5′-CAGTGACCGGATCTCCGTTAT-3′ | cnfl | 230 | 35 |
| cnfl.2 | 5′-CGTGTAATTCTTCTGTACTTCC-3′ | |||
| hra.1 | 5′-CAGAAAACAACCGGTATCAG-3′ | hra | 260 | 6 |
| hra.2 | 5′-ACCAAGCATGATGTCATGAC-3′ | |||
| papC.1 | 5′-GACGGCTGTACTGCAGGGTGTGGCG-3′ | papC | 328 | 33 |
| papC.2 | 5′-ATATCCTTTCTGCAGGGATGCAATA-3′ | |||
| papG.II.1 | 5′-GGGATGAGCGGGCCTTTGAT-3′ | papGII | 190 | 24 |
| papG.II.2 | 5′-CGGGCCCCCAAGTAACTCG-3′ | |||
| papG.III.1 | 5′-GGCCTGCAATGGATTTACCTGG-3′ | papGIII | 258 | 24 |
| papG.III.2 | 5′-CCACCAAATGACCATGCCAGAC-3′ | |||
| pheR.1 | 5′-GCCGCAATCTTAAGCAGTTG-3′ | pheU | 350 | 6 |
| pheR.2 | 5′-GCACGACATTTCACGTCAGT-3′ | |||
| pheR.1 | 5′-GCCGCAATCTTAAGCAGTTG-3′ | pheU (LR PCR) | 6 | |
| yjgB.1 | 5′-ACCTTGCTCGCAGTTGATCT-3′ | leuX (yjgB) (LR PCR) | 6 | |
| PheV.1 | 5′-AACCGGATTACGCATCTGTG-3′ | pheV (LR PCR) | This study |
LR PCR, long-range PCR with hra.1. C2, anonymous DNA fragment issued from subtractive hybridization (11).
Statistical analysis.
Fisher's exact test was used. P values of <0.05 were considered statistically significant.
RESULTS
Among the 455 clinical isolates and the reference strains, hly, hra, and cnf1 were present simultaneously, and always colocalized, in 113 strains (104 of the 455 clinical isolates [23%], 7 of the 72 ECOR strains [10%], and the UPEC reference strains J96 and AD110). None of our 455 clinical isolates carried cnf1 without hly and hra. The distribution of these strains is shown in Table 2 according to their clinical sources. PAI IIJ96-like domains were significantly more frequent among UTI isolates (adult urosepsis and children and infant UTI; 30% of 259 isolates) than among ECNM and neonatal colonization isolates (10% of 134 isolates and 19% of 62 isolates, respectively) (P < 0.01). In our ECNM collection, PAI IIJ96-like domains were mostly found among the 38 O18:K1 strains (in 9 strains, or 24%).
TABLE 2.
Presence of a PAI IIJ96-like domain according to the phylogenetic group and subgroup among collections of E. coli strains of various origins
| Phylogenetic group/subgroupa | Adults with urosepsis (n = 100)
|
Children with UTI (n = 75)
|
Infants with UTI (n = 84)
|
Neonates with meningitis (n = 134)
|
Neonates with colonization (n = 62)
|
ECOR collection (n = 72)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | PAI IIJ96b | No. (%)c | PAI IIJ96 | No. (%) | PAI IIJ96 | No. (%) | PAI IIJ96 | No. (%) | PAI IIJ96 | No. (%) | PAI IIJ96 | |
| A (n = 71) | 11 | 0 | 3 (4) | 0 | 7 (8) | 0 | 12 (9) | 0 | 13 (21) | 0 | 25 (35) | 0 |
| B1 (n = 25) | 1 | 0 | 1 | 0 | 2 | 0 | 3 (2) | 0 | 2 | 0 | 16 (22) | 0 |
| B2 (n = 320) | 61 | 21 (34) | 51 (68) | 32 (63) | 60 (71) | 25 (42) | 96 (72) | 14 (15) | 37 (60) | 11 (30) | 15 (21) | 6 (40) |
| Ribotype I | 20 | 13 (65) | ND | 19 | 16 (19) | 12 (75) | 4 (3) | 2 | 1 | 1 | 6 (8) | 3 (50) |
| Ribotype II | 19 | 0 | ND | 0 | 23 (27) | 1 | 55 (41) | 9 (16) | 18 (29) | 1 | 2 | 0 |
| Ribotype III | 12 | 3 (25) | ND | 8 | 15 (18) | 9 (60) | 15 (11) | 3 (20) | 8 (13) | 3 (38) | 2 | 1 |
| Ribotype IX | 4 | 3 (75) | ND | 3 | 2 | 2 | 6 (5) | 0 | 6 (10) | 6 (100) | 0 | 0 |
| Ribotype X | 4 | 0 | ND | 0 | 2 | 0 | 2 | 0 | 4 (7) | 0 | 1 | 0 |
| Ribotype XI | 2 | 2 | ND | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 3 (4) | 2 |
| Other ribotypes | 0 | 0 | ND | 0 | 1 | 0 | 14 (10) | 0 | 0 | 0 | 1 | 0 |
| D (n = 107) | 27 | 0 | 20 (27) | 0 | 15 (18) | 0 | 23 (17) | 0 | 10 (16) | 1 | 12 (17) | 1 |
| Other (n = 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (5) | 0 |
| Total | 100 | 21 (21) | 75 | 32 (43) | 84 | 25 (30) | 134 | 14 (10) | 62 | 12 (19) | 72 | 7 (10) |
Each subgroup corresponds to a different ribotype, as described previously (6).
Number (and percentage) of strains harboring a PAI IIJ96-like domain among each phylogenetic group or subgroup of the collection.
ND, not determined for the entire collection.
The distribution of these PAI IIJ96-like domains was restricted to a limited number of genetic backgrounds. Indeed, our clinical collection comprised 46, 9, 305, and 95 isolates from phylogenetic groups A, B1, B2, and D, respectively (Table 2), while all 113 strains harboring a PAI IIJ96-like domain belonged only to group B2, apart from two group D strains.
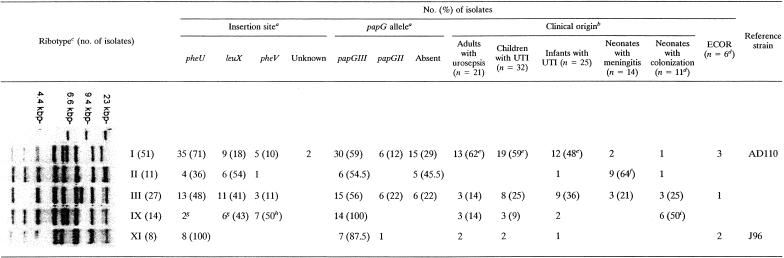
Twelve ribotypes were identified among the 305 group B2 clinical isolates, whereas strains harboring a PAI IIJ96-like domain belonged to only five ribotypes (Table 3).
TABLE 3.
Molecular characterization of the PAI IIJ96-like domains and clinical origins of group B2 strains according to ribotype
Numbers in parentheses are percentages of strains among each ribotype.
Numbers in parentheses are percentages of strains among each clinical collection.
The number of the ribotype corresponds to the ribotyping patterns described previously (6).
One strain of this collection belonged to group D.
By the Fisher test, P was <0.05 in a comparison with values for meningitis and colonization isolates.
By the Fisher test, P was <0.05 in a comparison with values for UTI and colonization isolates.
One isolate of ribotype IX harbored two PAI IIJ96-like domains inserted in pheU and leuX.
By the Fisher test, P was <0.05 in a comparison with values for the other ribotypes.
By the Fisher test, P was <0.05 in a comparison with values for meningitis and UTI isolates.
In contrast to the consistent association of hra, hly, and cnf1, the pap operon was more varied: 23% of hra-, hly-, and cnf1-positive strains did not harbor a colocalized pap operon, and 12% bore the papGII allele instead of the papGIII allele within the PAI, as shown by positive papGII-hra long-range PCR (Table 4). The 23% of pap-negative strains harboring a PAI IIJ96-like domain included six ECNM isolates (43%), three urosepsis isolates (14%), five childhood UTI isolates (20%), nine infantile UTI isolates (28%), and two colonization isolates (16%). Three different PAI IIJ96-like domains were defined on the basis of pap operon variations, namely, the papGIII-positive, papGII-positive, and pap-negative domains. The papGIII allele was found in none of the 351 isolates negative for the PAI IIJ96-like domain, and no isolates harbored a papGIII allele outside the PAI IIJ96-like domain. In contrast, papGII was found outside the PAI in 56% of UTI isolates versus in 7% and 8% of ECNM and colonization isolates, respectively (P < 0.01). When located outside the PAI IIJ96-like domain, the papGII allele was physically linked to hly in seven strains.
TABLE 4.
Presence of papG alleles and origin of the strains according to the tRNA insertion sites
| Location of tRNA insertion site (no. of isolates) | No. (%) of isolates
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
papG allelea
|
Clinical originb
|
ECOR (n = 7) | |||||||
| Class III (65%) | Class II (12%) | Absent (23%) | Adults with urosepsis (n = 21c) | Children with UTI (n = 32) | Infants with UTI (n = 25) | Neonates with meningitis (n = 14) | Neonates with colonization (n = 12) | ||
| pheU (62d) | 36d (58) | 9 (15) | 17 (27) | 18 (86) | 21 (66) | 9 (36) | 7 (50) | 2 | 3 |
| leuX (33) | 31 (94) | 2 | 4 (19) | 5 (15) | 12 (48) | 7 (50) | 3 (25) | 2 | |
| pheV (17) | 8 (47) | 9 (53) | 6 (19) | 2 | 7 (58e) | 2 | |||
| Unknown (2) | 2 | 2 | |||||||
Numbers in parentheses are percentages of strains among each insertion site.
Numbers in parentheses are percentages of strains among each clinical collection.
One isolate of ribotype IX harbored two PAI IIJ96-like domains inserted in pheU and leuX.
The two UPEC reference strains J96 and AD110 have their PAI IIJ96 inserted in pheU with a papGIII allele.
By the Fisher test, P was <0.05 in a comparison with values for meningitis and UTI isolates.
The PAI IIJ96-like domains were inserted in three different tRNAs, namely, pheU (formerly pheR) (54%), leuX (29%), and pheV (15%). The insertion site was unknown for two isolates (Table 4). When a PAI IIJ96-like domain was inserted in pheU, pheU PCR was negative owing to tRNA disruption. When PAI IIJ96-like domains were inserted in leuX or pheV, pheU PCR was positive in 64% and 94% of cases, respectively, showing that the pheU site was free. The insertion site differed according to the genetic background determined by ribotyping. In ribotype I and ribotype XI isolates, the insertion occurred mostly in the pheU tRNA (71% and 100%, respectively, versus 35% for other ribotypes; P < 0.01), whereas it occurred mainly in pheV in ribotype IX isolates (Table 3). When the insertion occurred in leuX, long-range PCR between hra and leuX yielded a 4.5-kbp product for all ribotype IX isolates and an 8-kbp product for isolates of other ribotypes. When the insertion occurred in pheU, long-range PCR between hra and pheU yielded a 5.2-kbp product for all isolates belonging to ribotypes II, IX, and XI and a product of either 5.2 kbp or 7.5 kbp for isolates belonging to ribotypes I and III.
The frequencies of papGIII differed according to the insertion site. papGIII was significantly more frequent in PAI IIJ96-like domains inserted in leuX than in PAI IIJ96-like domains inserted in pheU or pheV (94% versus 58% or 47%, respectively; P < 0.01) (Table 4).
The replacement of papGIII by papGII observed in PAI IIJ96-like domains inserted in pheU (15%) and, to a lesser extent, in leuX (two isolates) was not observed in domains inserted in pheV (Table 4).
DISCUSSION
The consistent colocalization of hly, hra, and cnf1 in 113 of 529 E. coli strains of various origins, contrasting with the marked plasticity of the pap operon, considerably extends our previous results for adult urosepsis isolates, in which the hly, cnf1, and hra gene triplet constitutes the backbone of the PAI IIJ96-like domain (6). The presence of these specific genes does not necessarily imply the presence of the complete PAI IIJ96, as we did not analyze the flanking sequences. Therefore, for a given strain, the simultaneous detection of these three genes may be considered the signature of this ectochromosomal domain. Although the consistent association of hly and cnf1 could be explained by a combined cytotoxic effect, the role of hra in this group of genes remains to be determined (15, 32). Interestingly, in the overall strain collection studied here, the papGIII allele was found in only 64% of strains harboring this backbone and was consistently colocalized. Other authors studying both papGIII and cnf1 have never found isolates harboring papGIII without cnf1 (28, 29). This suggests that archetypal PAI IIJ96 may be the sole source of the papGIII allele and that it has evolved by the allele substitution of papG or by the deletion of the pap operon (6). In contrast, the papGII allele was found instead of papGIII within and/or outside the PAI (data not shown). The colocalization of papGII with hly outside the PAI IIJ96-like domain in seven strains suggests that papGII is located in another PAI. Deletion may optimize the structure of PAI elements and reduce the genetic burden by eliminating genes whose products are no longer used (17). However, E. coli strains that lack virulence factors are able to cause extraintestinal infections, including UTI, in compromised hosts (6, 26, 28).
As previously reported, PAI IIJ96-like domains were almost exclusively restricted to group B2 (6); the only exceptions were two group D strains. Furthermore, among group B2 strains, PAI IIJ96-like domains were restricted to only 5 of the 12 ribotypes identified among all strains studied. This points to a strong association between PAI IIJ96-like domains and a few B2 genetic backgrounds. Two scenarios of PAI IIJ96 acquisition may explain these data. In the first scenario, PAI IIJ96 was acquired once, by chance, by a common B2 ancestor of these five subgroups and was subsequently transmitted vertically and eventually rearranged and deleted with additional recombination processes leading to additions or deletions within the PAIs (19, 27, 40). In the second scenario, the integration of this PAI in a group B2 E. coli strain occurred by multiple insertional events which were restricted to these five genetic backgrounds because they are compatible with PAI integration and expression (19). Other studies have also suggested that specific genetic backgrounds are required for the integration, retention, and expression of PAIs acquired by several horizontal transfers among ExPEC strains (14, 28). Acquired sequences are effective only if their expression is coordinated with that of the rest of the chromosome and with the life cycle of the microbial host (16). The second scenario appears more likely, because we found that PAI IIJ96-like domains were inserted within at least three different tRNAs. To our knowledge, this is the first description of three different insertion sites for a given PAI-like domain in E. coli. Only the high-pathogenicity island in Yersinia spp. displays such a distribution of insertion sites (10, 39). However, a multiplicity of high-pathogenicity island insertion sites can occur in a single strain of Yersinia spp., due to the sequence identity of the different asn tRNAs; this is not the case for pheU and leuX in PAI IIJ96-like domains.
Virulence factors carried by the genetic background may influence PAI acquisition. Indeed, in our clinical isolates, the papGIII-positive PAI IIJ96-like domain was always associated with papGII outside the PAI in isolates of ribotype I, while the papGIII-positive PAI IIJ96-like domain, with or without papGII, was present in isolates belonging to the other four ribotypes (data not shown). The fact that papGII alone (without the PAI IIJ96-like domain) was present in isolates of ribotype I, contrary to what occurs with papGIII, suggests that papGII was acquired first by isolates of ribotype I. So, it is tempting to speculate that the PAI IIJ96-like domain was acquired secondarily, only on a ribotype I background still carrying papGII or genetic determinants belonging to PAIs containing papGII. Of note, we found no isolates harboring papC without a papGII or papGIII allele within the PAI IIJ96-like domain. Thus, the papGI allele was not present in the PAI IIJ96-like domains of our collection.
The genetic background also seems to influence the insertion site of the mobile genetic element carrying the PAI. Indeed, both the frequencies of site-specific PAI insertion in the different tRNAs and the lengths of the PCR product between hra and the tRNA differed with the ribotype. To examine whether insertion in a given tRNA occurs randomly or is restricted by free insertion sites, we performed PCR of the flanking sequences of the archetypal insertion site pheU. When PAI IIJ96-like domains were inserted in leuX or pheV, pheU PCR was positive in 64% or 94% of cases, respectively, showing that the pheU site was free. These results support the possible influence of the genetic background on the insertion site.
The key features of this PAI that render it incompatible with many other genetic backgrounds remain to be determined. cnf1, which has never been described to occur in another ectochromosomal DNA, may offer one line of investigation (32).
Of particular interest was the influence of the insertion site on the plasticity of the PAI. Indeed, the PAI IIJ96-like domains inserted in leuX displayed lower degrees of variation than those inserted in other sites, as the pap operon with the papGIII allele was present in 94% of cases, compared to 58% in pheU tRNA and 47% in pheV tRNA (P < 0.01). Dobrindt et al. found that, in E. coli 536, leuX was required for the efficient expression of several virulence genes, such as hly and type 1 fimbriae (13, 37). Our results show that PAI plasticity is dependent on the insertion site, whatever the B2 subgroup or the clinical origin. Interestingly, the allelic exchange of papGII for papGIII, observed mostly in PAI IIJ96-like domains inserted in pheU, was not observed in pheV. This difference in papG alleles may influence pathogenicity, particularly as it was encountered only among UTI isolates (18% versus 0% of ECNM and colonization isolates; P < 0.01). Moreover, 56% of UTI isolates harbored a chromosomal papGII allele outside the PAI IIJ96-like domain. The allelic switch to papGII within the PAI IIJ96-like domain, and/or the acquisition of papGII outside this PAI, may allow the bacterium to colonize the urinary tract.
When we examined the distribution of genetic backgrounds among clinical isolates of different sources carrying PAI IIJ96-like domains, we found that ribotype II predominated among ECNM isolates and ribotype I predominated among UTI isolates (Table 3). However, this distribution also reflects the predominance of these ribotypes in the overall strain collections (Table 2). Houdouin et al. found that, in ribotype II strains, this PAI contributed to bacterial survival in blood by inducing high-level bacteremia, a step preceding blood-brain barrier penetration (22). Thus, ECNM isolates of ribotype II lacking PAI IIJ96 may carry other virulence factors. The predominance of ribotype IX in neonatal colonization isolates carrying a PAI IIJ96-like domain contrasted with the significantly lower prevalence of this ribotype in the entire collection of neonatal colonization isolates (Table 2). Moreover, all but one of the colonization isolates of ribotype IX had their PAI IIJ96-like domains inserted in pheV, with a papGIII-positive pap operon. This raises the possibility that PAI insertion in pheV on a particular genetic background may alter virulence gene expression or favor the expression of fitness factors contributing to increasing survival in the gut, thus forming a “saprophytic island” (12, 20, 21).
In conclusion, the simultaneous detection of hly, cnf1, and hra may be considered the signature of a PAI IIJ96-like domain in a given strain of E. coli and could be used for further epidemiological studies. Multiple insertional events at at least three different sites, restricted by the genetic background, have thus led to PAI IIJ96-like domain acquisition. Specific genetic backgrounds and insertion sites may have played a role in additional recombination processes for E. coli adaptation to different ecological niches (18, 20).
Editor: J. B. Bliska
REFERENCES
- 1.Bingen, E., E. Denamur, N. Lambert-Zechovsky, Y. Aujard, N. Brahimi, P. Geslin, and J. Elion. 1992. Analysis of DNA restriction fragment length polymorphism extends the evidence for breast milk transmission in Streptococcus agalactiae late-onset neonatal infection. J. Infect. Dis. 165:569-573. [DOI] [PubMed] [Google Scholar]
- 2.Bingen, E., B. Picard, N. Brahimi, S. Mathy, P. Desjardins, J. Elion, and E. Denamur. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642-650. [DOI] [PubMed] [Google Scholar]
- 3.Bingen, E. H., E. Denamur, and J. Elion. 1994. Use of ribotyping in epidemiological surveillance of nosocomial outbreaks. Clin. Microbiol. Rev. 7:311-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingen, E. H., E. Denamur, N. Y. Lambert-Zechovsky, A. Bourdois, P. Mariani-Kurkdjian, J.-P. Cezard, J. Navarro, and J. Elion. 1991. DNA restriction fragment length polymorphism differentiates crossed from independent infections in nosocomial Xanthomonas maltophilia bacteremia. J. Clin. Microbiol. 29:1348-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingen, E. H., E. Denamur, B. Picard, P. Goullet, N. Y. Lambert-Zechovsky, N. Brahimi, J.-C. Mercier, F. Beaufils, and J. Elion. 1992. Molecular epidemiology unravels the complexity of neonatal Escherichia coli acquisition in twins. J. Clin. Microbiol. 30:1896-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingen-Bidois, M., O. Clermont, S. Bonacorsi, M. Terki, N. Brahimi, C. Loukil, D. Barraud, and E. Bingen. 2002. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect. Immun. 70:3216-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum, G., V. Falbo, A. Caprioli, and J. Hacker. 1995. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and alpha-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett. 126:189-195. [DOI] [PubMed] [Google Scholar]
- 8.Bonacorsi, S., O. Clermont, V. Houdouin, C. Cordevant, N. Brahimi, A. Marecat, C. Tinsley, X. Nassif, M. Lange, and E. Bingen. 2003. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates: identification of a new virulent clone. J. Infect. Dis. 187:1895-1906. [DOI] [PubMed] [Google Scholar]
- 9.Bonacorsi, S., S. Lefevre, O. Clermont, V. Houdouin, A. Bourrillon, C. Loirat, Y. Aujard, and E. Bingen. 2005. Escherichia coli strains causing urinary tract infection in uncircumcised infants resemble urosepsis-like adult strains. J. Urol. 173:195-197. [DOI] [PubMed] [Google Scholar]
- 10.Buchrieser, C., R. Brosch, S. Bach, A. Guiyoule, and E. Carniel. 1998. The high-pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asn tRNA genes. Mol. Microbiol. 30:965-978. [DOI] [PubMed] [Google Scholar]
- 11.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connell, H., M. Hedlund, W. Agace, and C. Svanborg. 1997. Bacterial attachment to uro-epithelial cells: mechanisms and consequences. Adv. Dent. Res. 11:50-58. [DOI] [PubMed] [Google Scholar]
- 13.Dobrindt, U., L. Emödy, I. Gentschev, W. Goebel, and J. Hacker. 2002. Efficient expression of the alpha-haemolysin determinant in the uropathogenic Escherichia coli strain 536 requires the leuX-encoded tRNA5Leu. Mol. Genet. Genomics 267:370-379. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Paramo, P., O. Clermont, A. B. Blanc-Potard, H. Bui, C. Le Bouguenec, and E. Denamur. 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 21:1085-1094. [DOI] [PubMed] [Google Scholar]
- 15.Falbo, V., M. Famiglietti, and A. Caprioli. 1992. Gene block encoding production of cytotoxic necrotizing factor 1 and hemolysin in Escherichia coli isolates from extraintestinal infections. Infect. Immun. 60:2182-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groisman, E. A., and H. Ochman. 1997. How Salmonella became a pathogen. Trends Microbiol. 5:343-349. [DOI] [PubMed] [Google Scholar]
- 17.Hacker, J., L. Bender, M. Ott, J. Wingender, B. Lund, R. Marre, and W. Goebel. 1990. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb. Pathog. 8:213-225. [DOI] [PubMed] [Google Scholar]
- 18.Hacker, J., G. Blum-Oehler, I. Muhldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. [DOI] [PubMed] [Google Scholar]
- 19.Hacker, J., G. Blum-Oehler, B. Janke, G. Nagy, and W. Goebel. 1999. Pathogenicity islands of extraintestinal Escherichia coli, p. 59-76. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 20.Hacker, J., and E. Carniel. 2001. Ecological fitness, genomic islands and bacterial pathogenicity. A Darwinian view of the evolution of microbes. EMBO Rep. 2:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hacker, J., U. Hentschel, and U. Dobrindt. 2003. Prokaryotic chromosomes and disease. Science 301:790-793. [DOI] [PubMed] [Google Scholar]
- 22.Houdouin, V., S. Bonacorsi, N. Brahimi, O. Clermont, X. Nassif, and E. Bingen. 2002. A uropathogenicity island contributes to the pathogenicity of Escherichia coli strains that cause neonatal meningitis. Infect. Immun. 70:5865-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hull, R. A., R. E. Gill, P. Hsu, B. H. Minshew, and S. Falkow. 1981. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun. 33:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, J. R., and J. J. Brown. 1996. A novel multiply primed polymerase chain reaction assay for identification of variant papG genes encoding the Gal(alpha 1-4)Gal-binding PapG adhesins of Escherichia coli. J. Infect. Dis. 173:920-926. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, J. R., M. A. Kuskowski, T. T. O'Bryan, and J. N. Maslow. 2002. Epidemiological correlates of virulence genotype and phylogenetic background among Escherichia coli blood isolates from adults with diverse-source bacteremia. J. Infect. Dis. 185:1439-1447. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, J. R., T. T. O'Bryan, M. Kuskowski, and J. N. Maslow. 2001. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect. Immun. 69:5363-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 30.Kim, K. S. 2003. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat. Rev. Neurosci. 4:376-385. [DOI] [PubMed] [Google Scholar]
- 31.Lalioui, L., and C. Le Bouguénec. 2001. afa-8 gene cluster is carried by a pathogenicity island inserted into the tRNAPhe of human and bovine pathogenic Escherichia coli isolates. Infect. Immun. 69:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landraud, L., M. Gibert, M. R. Popoff, P. Boquet, and M. Gauthier. 2003. Expression of cnf1 by Escherichia coli J96 involves a large upstream DNA region including the hlyCABD operon, and is regulated by the RfaH protein. Mol. Microbiol. 47:1653-1667. [DOI] [PubMed] [Google Scholar]
- 33.Le Bouguenec, C., M. Archambaud, and A. Labigne. 1992. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 30:1189-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picard, B., P. Duriez, S. Gouriou, I. Matic, E. Denamur, and F. Taddei. 2001. Mutator natural Escherichia coli isolates have an unusual virulence phenotype. Infect. Immun. 69:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picard, B., C. Journet-Mancy, N. Picard-Pasquier, and P. Goullet. 1993. Genetic structures of the B2 and B1 Escherichia coli strains responsible for extra-intestinal infections. J. Gen. Microbiol. 139:3079-3088. [DOI] [PubMed] [Google Scholar]
- 37.Ritter, A., D. L. Gally, P. B. Olsen, U. Dobrindt, A. Friedrich, P. Klemm, and J. Hacker. 1997. The PAI-associated leuX specific tRNA5Leu affects type 1 fimbriation in pathogenic Escherichia coli by control of FimB recombinase expression. Mol. Microbiol. 25:871-882. [DOI] [PubMed] [Google Scholar]
- 38.Russo, T. A., and J. R. Johnson. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. Epub 15 May 2000. [DOI] [PubMed] [Google Scholar]
- 39.Schubert, S., A. Rakin, and J. Heesemann. 2004. The Yersinia high-pathogenicity island (HPI): evolutionary and functional aspects. Int. J. Med. Microbiol. 294:83-94. [DOI] [PubMed] [Google Scholar]
- 40.Swenson, D. L., N. O. Bukanov, D. E. Berg, and R. A. Welch. 1996. Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect. Immun. 64:3736-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Die, I., I. van Megen, W. Hoekstra, and H. Bergmans. 1984. Molecular organisation of the genes involved in the production of F7(2) fimbriae, causing mannose-resistant haemagglutination, of a uropathogenic Escherichia coli 06:K2:H1:F7 strain. Mol. Gen. Genet. 194:528-533. [DOI] [PubMed] [Google Scholar]