Abstract
The humeral head is the second most common anatomical site of osteonecrosis after the femoral head. Studies have reported satisfactory clinical outcomes after shoulder arthroplasty to treat osteonecrosis of the humeral head (ONHH). However, there are concerns regarding implant longevity in relatively young patients. This study investigated the effectiveness of non-operative treatment for atraumatic ONHH in relatively young patients. Thirty-two patients (41 shoulders, 9 bilateral) were included and received non-operative ONHH treatment consisting of pain management and self-stretching exercises. Treatment failure was defined as conversion to surgical treatment owing to difficulty performing activities of daily living with resting pain (Visual Analog Scale [VAS] ≥ 6) after 6 months of staged treatment. The mean follow-up was 73.6 months. The mean ONHH stage was 3.6 initially and 3.8 at final follow-up. Stage progression was observed in 14.6% (6/41) of the shoulders. No treatment failures occurred during follow-up. Between the initial visit and final follow-up, no significant differences in clinical outcomes, including resting pain, Single Assessment Numeric Evaluation score, forward flexion, and external rotation were observed; only internal rotation range of motion was observed to significantly decrease (p < 0.001). Non-operative treatment showed satisfactory results in relatively young patients with advanced atraumatic ONHH.
Keywords: Osteonecrosis, Humeral head, Treatment, Shoulder arthroplasty
Subject terms: Prognosis, Rehabilitation
Introduction
Osteonecrosis of the bone, also known as avascular necrosis or aseptic necrosis, is a pathological process that defines the in situ death of a bone segment1. The humeral head is the second most frequent anatomical site of osteonecrosis after the femoral head2. Osteonecrosis of the humeral head (ONHH) is associated with numerous conditions and is classified as traumatic or atraumatic2–4. Among them, atraumatic ONHH usually occurs in relatively young patients and is associated with corticosteroid use, alcohol abuse, sickle cell hemoglobinopathies, and systemic disease like systemic lupus erythematosus (SLE), among other conditions5–8.
Studies have reported satisfactory clinical outcomes after shoulder arthroplasty for the treatment of ONHH9–11. However, arthroplasty in relatively young patients raises concerns about implant longevity7,12,13. A previous study reported that younger patients had higher expectations of outcomes and implant longevity after total shoulder arthroplasty14. In addition, Burrus et al. reported that shoulder arthroplasty performed for ONHH showed higher postoperative complication rates than shoulder arthroplasty performed for non-osteonecrosis6.
Previous studies have reported that non-surgical treatment showed good results in the early stage of ONHH; however, its success rate was not high in the advanced stage7,15,16. In a recent systematic review, core decompression showed superior clinical outcomes compared to non-operative treatment17. However, this systematic review included both atraumatic and traumatic patients in the initial stages of ONHH.
The purpose of this study was to investigate the effectiveness of non-operative treatment even in relatively young patients with advanced-stage atraumatic ONHH. Our hypothesis was that non-operative treatment, including pain management and self-stretching exercises, would show tolerable outcomes, even in relatively young patients with advanced atraumatic ONHH.
Results
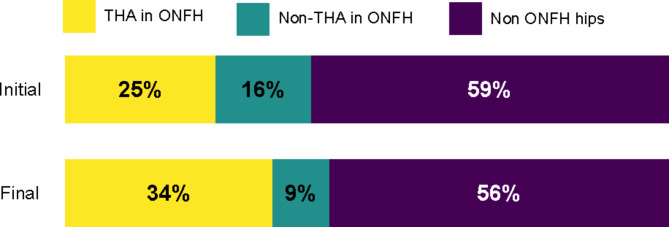
Thirteen men (four with bilateral involvement) and 19 women (five with bilateral involvement) with a mean age of 37.7 years old (range, 26–46) participated in this study. The mean symptomatic period was 32.3 months (range, 5–120) at initial presentation. The mean follow-up period was 73.6 months (range, 24–108). At the time of diagnosis of advanced ONHH, 40.6% of the 13 patients (13/32) had also been diagnosed with osteonecrosis of the femoral head. Of the 13 patients, 61.5% (8/13) (one unilateral, seven bilateral) had undergone a total hip arthroplasty for femoral head osteonecrosis. Among the 32 patients diagnosed with ONHH, one with SLE was newly diagnosed with bilateral osteonecrosis of the femoral head during the follow-up period. (Fig. 1) The underlying diseases were iatrogenic corticosteroid or organ transplantation in 16 patients (50%), SLE in 9 patients (28%), sickle cell anemia in 3 patients (9%), alcohol consumption in 2 patients (6%), and idiopathic in 2 patients (6%). Intra-articular corticosteroid injection was performed in 31%(13/42) of patients. Among 13 patients, the mean number of injection was 2.2 (range, 1–4).
Fig. 1.
Total hip arthroplasty rate among 32 ONHH patients’ ONFH. THA: Total hip arthroplasty, ONFH: osteonecrosis of the femoral head, ONHH: osteonecrosis of the humeral head.
Functional and radiological outcomes
The mean Visual Analog Scale (VAS) pain score, Single Assessment Numeric Evaluation (SANE) score, forward flexion angle, external rotation on the arm side did not show any significant difference initially to final follow-up except internal rotation. (Table 1)
Table 1.
Functional outcomes.
| Initial (N = 41) | Final follow-up (n = 41) | p-value | |
|---|---|---|---|
| Resting pain VAS* score | 2.8 ± 0.8 | 2.6 ± 1.0 | 0.370 |
| SANE** score | 73.8 ± 7.5 | 76.3 ± 6.1 | 0.107 |
| Forward flexion (scapular plane) | 123.8 ± 21.6 | 120.3 ± 14.0 | 0.278 |
| External rotation at arm side | 56.1 ± 7.0 | 56.7 ± 8.1 | 0.654 |
| Internal rotation*** | 11.8 ± 1.5 | 12.7 ± 1.0 | 0.001 |
*VAS: visual analogue scale, **SANE: Single Assessment Numeric Evaluation. ***Internal rotation was estimated by recording the highest spinal segment reached by the patient’s thumb. To facilitate statistical analysis, the spinal segments were converted into numbers: T1–T12 were designated as 1–12, L1–L5 as 13–17, and the sacrum as 18. Values are presented as the mean ± standard deviation.
No treatment failure occurred during the follow-up period. The mean Cruess stage was 3.6 initially and 3.8 at final follow-up. Stage progression was observed in 14.6% (6/41) of shoulders, while the initial stage was maintained in 85.4% (35/41) of shoulders. Cruess stage V shoulders were identified in 14.6% (6/41) of patients initially and 19.5% (8/41) at final follow-up. (Table 2)
Table 2.
Initial and final follow-up stage1 of osteonecrosis of the humeral head.
| Initial (N = 41) | Final follow-up (n = 41) | |
|---|---|---|
| Stage III | 21 | 17 |
| Stage IV | 14 | 16 |
| Stage V | 6 | 8 |
1 A modified Cruess stage was used for osteonecrosis of the humeral head classification evaluated by plain radiograph.
Discussion
The purpose of this study was to investigate the clinical outcomes of relatively young patients with atraumatic ONHH who underwent non-operative staged physiotherapy and to determine if the results were acceptable. After an average follow-up of more than 6 years, the average pain VAS score of the 32 patients was 2.8, indicating maintenance of a tolerable state. Although the radiological stage progressed in some patients, no treatment failures were observed. However, the restriction of internal rotation progressed significantly by approximately one segment.
The representative etiologies of atraumatic ONHH include SLE and sickle cell anemia; however, the etiology of this condition has not been fully elucidated18. The incidence of osteonecrosis has decreased significantly in patients with SLE or transplanted kidneys when comparing those treated in the 1980s and 1990s and those treated in the present day. This is because the use of other immunosuppressive agents has reduced the dose of glucocorticoids used19,20. However, additional research is needed to determine whether the trend of decreasing cumulative glucocorticoid use can reduce the incidence of osteonecrosis and delay disease progression, as well as how the effects of osteonecrosis of the femoral head and ONHH differ. The rate of ONHH progression varies depending on the underlying disease. ONHH eventually progresses to secondary osteoarthritis, and arthroplasty-type surgical treatment is considered. Hattrup and Cofield found that about 42% of early stage (stage I or II) patients, 55% of stage IV patients, and 79% of stage V patients required shoulder arthroplasty within 3 years16. However, in our study population, all the patients showed tolerable pain scores and relatively satisfactory outcomes. The patients also willingly agreed to undergo non-surgical treatment until they could not tolerate it after considering the limited longevity of arthroplasty.
When addressing the treatment of patients with ONHH, there is a lack of research on the etiology of the disease depending on the stage. Recently, Dubin, in a systematic review that included 15 articles, reported that core decompression in patients with stage I, II, and III ONHH had better clinical outcomes than non-operative treatment. However, the etiology was not separately distinguished in this study, and both traumatic and atraumatic cases were included. Therefore, additional studies are required to determine the etiologies of ONHH, depending on the varied causes of this condition21.
In terms of implant longevity, shoulder arthroplasty surgery should be carefully performed in relatively young patients. Sperling et al. reported that 48% (14/29) of patients aged 50 years or younger who underwent total shoulder arthroplasty showed unsatisfactory results after long-term follow-up of more than 15 years22. In this study, 85.4% (35/41) of patients at the final follow-up were stage III or IV patients with no glenoid involvement in the shoulder; these patients may be considered for hemiarthroplasty. However, glenoid erosion was reportedly observed in 60.9% (14/23) of shoulders after 12 years of follow-up after hemiarthroplasty in ONHH patients23. In addition, Levine et al. reported that only 25% of the patients with glenohumeral osteoarthritis were satisfied with the outcome of long-term observation after hemiarthroplasty24. Therefore, shoulder arthroplasty longevity problems may occur even when hemiarthroplasty is performed in patients without glenoid involvement. Thus, it is necessary to consider alternative methods before performing surgical treatments, such as hemiarthroplasty or total shoulder arthroplasty, in young patients. The results of this study are expected to provide evidence supporting non-operative treatment while delaying surgical treatment in relatively young patients with ONHH.
The hip joint is the most common site of osteoarthritis; therefore, osteonecrosis of the femoral head has been extensively studied25. When osteonecrosis of the femoral head and ONHH occur systemically, they are thought to have similar etiology, and both are known to respond well to conservative treatment in the early stage. However, in advanced stages, there may be some areas where clinical features and treatment methods may vary depending on the presence or absence of a weight-bearing joint. Of the 32 ONHH patients in our study, 40.6% (13/32) had femoral head osteonecrosis. Among these patients, 61.5% (8/13) underwent a total hip arthroplasty from the time of ONHH, and three additional patients underwent a total hip arthroplasty during the follow-up period, resulting in 78.6% (11/14) of patients having undergone a total hip arthroplasty. The difference in the rate of arthroplasty is likely due to the difference between the lower extremities, where weight-bearing is inevitable, and the upper extremities, where weight-bearing is avoidable, although there may also be a difference according to the lifespan of the artificial joint.
This study had some limitations. (1) It was a retrospective case series without a control group; (2) it had a follow-up loss as high as 40%, and therefore, the results were unknown and selection bias could not be fully avoided; (3) a sample calculation was not performed due to the limitations of observational studies; and (4) the sample size was small due to the low incidence of ONHH, with relatively narrow inclusion criteria.
In conclusion, non-operative treatment showed satisfactory results in relatively young patients with advanced atraumatic ONHH. It has been suggested that delaying surgical treatment and non-operative treatment, even in young patients with advanced atraumatic ONHH, could be beneficial.
Methods
Study design and participants
This observational study retrospectively reviewed 49 consecutive patients diagnosed with atraumatic ONHH by a single shoulder specialist orthopedic doctor (Y. M. C.) in a tertiary hospital (Severance Hospital, Seoul, Korea) between March 2009 and June 2018. The inclusion criteria were as follows: (1) atraumatic osteonecrosis of the humeral head, (2) age < 50 years at the time of diagnosis; and (3) Cruess stage III or higher at the time of diagnosis. Exclusion criteria were early stage ONHH (Cruess stage I or II) at the time of diagnosis or unavailability for a minimum 2-year follow-up evaluation. In cases with bilateral advanced-stage involvement, both sides were evaluated. After being diagnosed with ONHH, if the patient had never undergone investigations of their hip joint as an adult, a hip AP axial view was taken to confirm osteonecrosis of femoral head status. Finally, 32 patients (41 shoulders, nine bilateral) who met the inclusion criteria were included in this study. Data from radiographic images and medical records were retrospectively reviewed. Institutional Review Board of Severance Hospital waived Informed Consent due to the retrospective nature of the study, and the analysis used anonymous clinical data. A post hoc power analysis was conducted on the SANE score with an effect size of 0.4 and a significance level of 0.05 for this sample yielded an achieved power of 0.809.
Functional outcomes and radiological assessments
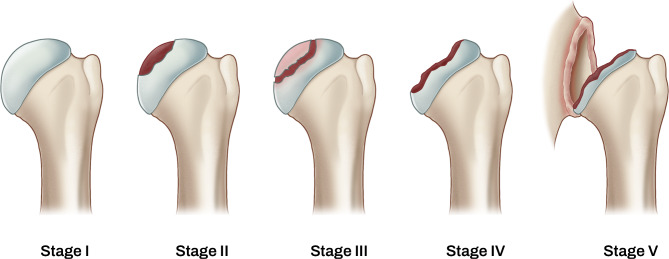
The functional outcomes and radiological findings were assessed at least annually. An independent therapist evaluated the functional outcomes, including the SANE26, resting VAS for pain, and passive range of motion (ROM) of the shoulder with three movements: forward flexion in the scapular plane, external rotation with the elbow at the side, and internal rotation. Internal rotation was examined by measuring the extent to which patients could reach the spine with their thumb. The spinal segments were assigned numerical equivalents: T1-T12 as 1–12; L1-L5 as 13–17, and sacrum as 18. An independent orthopedic surgeon (W. S. D.), who was blinded to the patients’ functional scores, assessed the radiologic value. True anteroposterior views of the shoulder and axillary views were taken at least annually. A modified Cruess stage was used for ONHH classification(Fig. 2), evaluated using plain radiography. [17] Stage III was defined as the presence of a ‘crescent sign’, indicating a subchondral bone fracture. Stage IV was defined as collapse progression, fragmentation, loose bodies, or secondary arthritis of the humeral head. Stage V was defined as degenerative changes extending into the glenoid.
Fig. 2.
A modified Cruess stage classification for ONHH. Stage I: pre-radiological stage with normal radiographic findings. Stage II: osteoporosis, sclerosis, or localized subchondral osteolysis without subchondral bone fracture. Stage III: The “crescent sign” appears subchondral fracture with mild loss of congruity. Stage IV: Characterized by extensive collapse of subchondral bone with severe articular incongruent surface. Stage V: Extending pathologic changes of the glenoid.
Patients were informed and agreed to undergo operative treatment in case of treatment failure. Treatment failure was defined as pain or difficulty in their daily-living activities with resting pain (VAS ≥ 6) even after 6 months of staged treatment.
Non-operative treatment protocol
We performed staged physiotherapy for pain control and passive stretching exercises to recover and maintain the ROM of the shoulder. For pain control, oral medication or intra-articular steroid injections were administered according to the VAS score, if the treatment was applicable. For a VAS ≤ 4, an oral selective COX-2 inhibitor once daily with a H-2 blocker or proton pump inhibitor were prescribed. Ultrasonography-guided intra-articular corticosteroid injections (triamcinolone 20 mg) were administered in cases of pain greater than or equal to a VAS score of 5. The interval between corticosteroid injections was at least three months. All patients diagnosed with ONHH were instructed to avoid active overhead activities that induce pain, and to avoid abduction or forward flexion over the shoulder level under resistance. [15] In addition, to improve and maintain shoulder ROM, passive stretching exercises were taught step-by-step. For these passive self-stretching exercises to be more effective, a hot tub bath was strongly recommended.
If the forward flexion angle in the scapular plane was less than 90°, passive circumduction exercises were performed. Table sliding forward flexion stretching exercises were added when a forward flexion of 90° or more was possible after 2–4 weeks of passive circumduction exercises. Thereafter, table sliding forward flexion stretching exercises were performed for 2–6 weeks. When a passive forward flexion of 120° or more and a VAS ≤ 3 became possible, passive abducted external rotation stretching exercises and passive internal rotation stretching exercises were added. Passive external rotation stretching exercises with the arm at a 90° abducted position were taught to be performed on a wall. Subsequently, passive internal rotation stretching exercises using the other arm were performed. In patients with bilateral involvement, passive internal rotation stretching exercises were restricted, and a towel was used for pulling.
Statistical analysis
SPSS software (version 25; IBM, Armonk, NY, USA) was used to analyze the data. A paired t-test was used to compare the initial and final continuous VAS, SANE shoulder score, and ROMs. Statistical significance was set at p < 0.05.
Author contributions
Conceptualization, Y. M. C.; methodology, H. H. C.; software, W. S. D.; validation, T. H. Y. and Y. M. C.; formal analysis, H. H. C.; investigation, W. S. D.; resources, J. R. L.; data curation, J. R. L.; writing—original draft preparation, J. R. L.; writing—review and editing, Y. M. C.; visualization, W. S. D.; supervision, Y.M.C. All authors reviewed and approved the final manuscript.
Funding
This study was supported by a faculty research grant from the Yonsei University College of Medicine (6-2024-0082).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Informed consent
Patient consent was waived owing to the retrospective nature of the study, and the analysis used anonymous clinical data.
Competing interests
The authors declare no competing interests.
Institutional review board statement
Institutional Review Board of Severance Hospital waived Informed Consent due to the retrospective nature of the study, and the analysis used anonymous clinical data.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mankin, H. J. Nontraumatic necrosis of bone (osteonecrosis). N. Engl. J. Med.326, 1473–1479 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Hasan, S. S. & Romeo, A. A. Nontraumatic osteonecrosis of the humeral head. J. Shoulder Elbow Surg.11, 281–298 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Feeley, B. T., Fealy, S., Dines, D. M., Warren, R. F. & Craig, E. V. Hemiarthroplasty and total shoulder arthroplasty for avascular necrosis of the humeral head. J. Shoulder Elbow Surg.17, 689–694 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Harreld, K. L., Marker, D. R., Wiesler, E. R., Shafiq, B. & Mont, M. A. Osteonecrosis of the humeral head. J. Am. Acad. Orthop. Surg.17, 345–355 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Levy, O. et al. Surface replacement arthroplasty for glenohumeral arthropathy in patients aged younger than fifty years: results after a minimum ten-year follow-up. J. Shoulder Elbow Surg.24, 1049–1060 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Burrus, M. T. et al. Shoulder arthroplasty for Humeral Head Avascular necrosis is Associated with increased postoperative complications. HSS Journal: Musculoskelet. J. Hosp. Special Surg.14, 2–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicak, N., Pećina, M. & Daković, M. Idiopathic osteonecrosis of the humeral head. Acta Med. Croatica: Casopis Hravatske Akademije Medicinskih Znanosti. 49, 93–98 (1995). [PubMed] [Google Scholar]
- 8.Gruson, K. I. & Kwon, Y. W. Atraumatic osteonecrosis of the humeral head. Bull. NYU Hosp. Joint Dis.67, 6–14 (2009). [PubMed] [Google Scholar]
- 9.Raiss, P. et al. Treatment of osteonecrosis of the humeral head with cementless surface replacement arthroplasty. J. Bone Joint Surg. Am. Vol.91, 340–349 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Uribe, J. W. Botto-Van Bemden, A. partial humeral head resurfacing for osteonecrosis. J. Shoulder Elbow Surg.18, 711–716 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Ristow, J. J. et al. Outcomes of shoulder replacement in humeral head avascular necrosis. J. Shoulder Elbow Surg.28, 9–14 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Bacle, G., Nové-Josserand, L., Garaud, P. & Walch, G. Long-term outcomes of reverse total shoulder arthroplasty: a follow-up of a previous study. J. Bone Joint Surg. Am. Vol.99, 454–461 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Hasan, S. S., Schwindel, L. E. & Fleckenstein, C. M. Prosthetic shoulder arthroplasty in patients 40 years or younger: outcomes stratified by diagnosis and surgery. Clin. Shoulder Elb.25, 311–320 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henn, R. F. et al. (ed ) Preoperative patient expectations of total shoulder arthroplasty. J. Bone Joint Surg. Am. Vol.93 2110–2115 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Cruess, R. L. Steroid-induced osteonecrosis: a review. Can. J. Surg.24, 567–571 (1981). [PubMed] [Google Scholar]
- 16.Hattrup, S. J. & Cofield, R. H. Osteonecrosis of the humeral head: relationship of disease stage, extent, and cause to natural history. J. Shoulder Elbow Surg.8, 559–564 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Dubin, J. A. et al. Core decompression is superior to nonoperative management for humeral head osteonecrosis: a systematic review. J. Shoulder Elbow Surg.32, 2192–2200 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Hernigou, P., Hernigou, J. & Scarlat, M. Shoulder osteonecrosis: Pathogenesis, causes, clinical evaluation, imaging, and classification. Orthop. Surg.12, 1340–1349 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takao, M. et al. Transitional changes in the incidence of hip osteonecrosis among renal transplant recipients. J. Orthop. Science: Official J. Japanese Orthop. Association. 25, 466–471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawata, K. et al. Transitional changes in the incidence of osteonecrosis in systemic lupus erythematosus patients: focus on immunosuppressant agents and glucocorticoids. Rheumatol. (Oxford England). 57, 844–849 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Scheidt, M. D., Aiyash, S., Salazar, D. & Garbis, N. Core decompression for early-stage avascular necrosis of the humeral head: current concepts and techniques. Clin. Shoulder Elb.26, 191–204 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling, J. W., Cofield, R. H. & Rowland, C. M. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J. Shoulder Elbow Surg.13, 604–613 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Smith, R. G., Sperling, J. W., Cofield, R. H., Hattrup, S. J. & Schleck, C. D. Shoulder hemiarthroplasty for steroid-associated osteonecrosis. J. Shoulder Elbow Surg.17, 685–688 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Levine, W. N. et al. Long-term follow-up of shoulder hemiarthroplasty for glenohumeral osteoarthritis. J. Bone Joint Surg. Am. Vol.94, e164 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Park, J. W. et al. Trends in Surgical treatment of femoral Head Osteonecrosis in South Korea: an analysis using Nationwide Claims Database. Clin. Orthop. Surg.14, 500–506 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor, C. M. & Ring, D. Correlation of single Assessment Numeric evaluation (SANE) with other patient reported outcome measures (PROMs). Archives bone Joint Surg.7, 303–306 (2019). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.