Abstract
The parasitic wasp, Cotesia congregata, manipulates the behaviour of its host, the caterpillar Manduca sexta. The female wasp injects her eggs and a symbiotic virus (i.e. bracovirus, CcBV) into the body of its host. The host’s behaviour remains unchanged until the wasps exit the caterpillar, and then the caterpillar becomes a non-feeding “bodyguard” for the wasp cocoons. Using proteomic, transcriptomic and qPCR studies, we discovered an increase in antimicrobial peptide gene expression and protein abundance in the host central nervous system at the time of wasp emergence, correlating with the change in host behaviour. These results support the hypothesis that the wasps hyperactivate an immune-neural connection to help create the change in behaviour. At the time of wasp emergence, there was also an increase in bracoviral gene expression and proteins in the host brain, suggesting that the bracovirus may also be involved in altering host behaviour. Other changes in gene expression and protein abundance suggest that synaptic transmission may be altered after wasp emergence, and a reduction in descending neural activity from the host’s brain provides indirect support for this hypothesis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82506-4.
Keywords: Neuroinflammation, Polydnavirus, Neural activity, Parasitic manipulation, Feeding, Neuroimmunology, Cytoskeleton, Extracellular matrix
Subject terms: Neuroscience, Zoology
Introduction
Parasitic manipulators are parasites that enhance their fitness by altering their hosts’ behaviour1. Our understanding of how parasitic manipulators alter host behaviour has progressed rapidly in the last few years2–4. Parasitic manipulators can exploit existing neuroregulatory networks in their host5–7. They can remodel these networks by secreting chemicals, e.g. venoms8,9or toxins3,10and/or by inducing changes in gene transcription within the host’s neurons and/or glia6,11. Immune-neural connections may be especially prone to exploitation because parasites are pre-adapted to manipulate immune signaling to survive within the host5. Parasitic manipulators typically impact multiple neuromodulatory systems simultaneously8,12across a range of neural networks1. Using this multi-targeted approach, parasitic manipulators produce reliable changes in host behaviour13, although the details remain unclear.
The interactions between the parasitic wasp Cotesia congregata and its caterpillar host, Manduca sexta, have been studied for decades14,15. However, the effect of the wasp on the host’s central nervous system (CNS) is still poorly understood, despite the pronounced changes in host behaviour16. Given the extensive background information available for this host-parasite system, as well as on the neurobiology of the host17, a more detailed examination of the effects of wasp parasitism on the host’s CNS promises to provide important insights into how the wasp exerts control over its host’s neural function.
Female C. congregata wasps co-inject venom, a polydnavirus, and wasp eggs into the body of M. sexta14. The venom is not necessary for wasp development18or altering host behaviour14. The polydnavirus of C. congregata (C. congregataBracovirus, CcBV) is a domesticated virus that has become incorporated into the wasp’s genome19. This non-replicating virus is made in the wasp’s ovaries20and acts as a gene delivery agent, inserting its genes into the host’s genome19. CcBV attacks host tissues such as the fat body21, allowing the wasp to alter host physiology, optimizing the caterpillar host for wasp larval development14. CcBV gene expression can also be found within the host CNS soon after wasp oviposition22, and at least some of these genes are translated into CcBV proteins23. The genes of other polydnaviruses (e.g. Microplitis demolitor bracovirus) also show expression in the brains of their host (e.g. Pseudoplusia includens(Lepidoptera)24). However, whether polydnaviruses promote parasitic manipulation of behaviour is unknown. M. sexta caterpillars show normal feeding and locomotion behaviour during wasp larval development, despite CcBV activity and the numerous physiological and endocrinological changes that occur within the host14. However, approximately 1 day prior to the wasps’ exit from the host, host feeding and spontaneous locomotion decline dramatically, never to recover16. Once the 50–80 wasp larvae scrape their way through the host’s body wall, the wasps spin cocoons that remain attached to the cuticle of the caterpillar, and eclose as adult wasps 4 to 5 days later14. The caterpillar loses all self-generated behaviours after the wasps emerge; however, it retains its defensive behaviours16,25. Host defensive behaviours appear to be crucial for wasp survival26,27. In the field, cocoons have a much lower survival without their host26,27 and M. sexta defensive behaviours are thought to to repel predator attacks on the cocoons27. However, a living host is also a threat. M. sexta caterpillars will eat wasp cocoons16,26. The wasp larvae avoid this problem by inducing anorexia in caterpillars during their emergence25,28, and the host never feeds again16. The host eventually dies of starvation several days after the wasps emerge from its body16. In essence, the caterpillar becomes a non-feeding (i.e. anorexic) “bodyguard” for the wasps, able to defend itself, and, by extension, the cocoons, but unable to feed on them. In other words, the “bodyguard” phenotype is a non-feeding caterpillar with intact defensive behaviours.
Transforming an actively feeding caterpillar into an anorexic “bodyguard” requires changes in multiple behaviours, while at the same time ensuring that the mechanisms needed for survival and defensive behaviour remain functional. As expected, during the this phase the host’s sensory and motor systems remain operational16,29–32. However, the ability of the host to initiate feeding and spontaneous locomotion is greatly reduced32. One of the most parsimonious ways for the wasp to induce the “bodyguard” phenotype is to exploit an existing host network that naturally produces a similar behavioural phenotype. For example, reduced feeding, decreased locomotion and heightened defensive behaviours are observed in M. sexta during an immune response, and these changes are thought to benefit the caterpillar16,28,33,34. The details of how sickness behaviours are activated and maintained in insects is poorly understood, however immune-neural connections appear to be involved35. During an immune response, insects release immunomodulators (e.g. octopamine36, cytokines35). These immunomodulators can activate receptors in the brain37, and this activation is thought to induce sickness behaviours such as illness-induced anorexia38, a phylogenetically conserved host behaviour39 that promotes recovery in M. sexta40,41. The wasp larvae activate a massive systemic immune response as they scrape their way through the host’s bodywall28. Therefore, wasps could exploit an immune-neural connection to create an anorexic bodyguard16,25. However, there is no direct evidence that systemic immune activation in the body of the M. sexta caterpillar has an impact on the caterpillar brain, although nematode infections can increase the expression of antimicrobial peptide (AMP) genes in the brain of another lepidopteran, Galleria mellonella42. We use qPCR to test whether an immune response in the body of M. sexta also produces an increase in immune gene expression in its brain. Such an activation would help explain how the wasp larvae could alter the neural function of the caterpillar without physically contacting its CNS. We also perform proteomics and transcriptomics analyses of the M. sexta CNS to help determine whether neuroinflammation (i.e. excessive immune activation within the brain43) occurs in the host concomitant with the change in host behaviour. Neuroinflammation is known to alter synaptic transmission44. Neuroinflammation is common in the hosts of parasitic manipulators and may be critical for host manipulation in some systems45–48.
CcBV activity within the CNS could also activate neuroimmunological responses and potentially cause neuroinflammation. If viral genes and proteins increase in the brain at the time of wasp emergence from the host, CcBV could also play a role in altering host behaviour. We examine whether there is a burst of CcBV gene expression and/or protein abundance at the time of host behavioural change. We further examine whether the expression of specific CcBV genes, and/or the presence of certain CcBV proteins, within the caterpillar CNS correlates with the host’s change in behaviour. Such a temporal correlation would suggest that specific CcBV genes and/or proteins play a role in mediating host behavioural change49. CcBV gene expression remains measurable within the CNS for at least six days after wasp emergence50, and, therefore, appears to persist for the duration of “bodyguard” behaviour.
Finally, changes in neural activity are required to produce changes in behaviour. Transcriptomics and proteomics alone cannot demonstrate that neural activity has been altered. Nervous systems have powerful homeostatic mechanisms to maintain neural circuit function despite perturbations in ion channel performance or neurotransmitter abundance51,52. Therefore, we assessed neural activity descending from the brain during different stages of parasitism, allowing us to correlate transcriptomic, proteomic and electrophysiological changes within the CNS of the host with the expression of the “bodyguard” phenotype. We predict that: (1) systemic immune activity results in increased immune activity in the brain of M. sexta, (2) immune activity within the brain increases dramatically with the change in host behaviour; (3) CcBV gene expression and protein abundance increase when host behaviour changes, and (4) the change in host behaviour correlates with decreases in descending neural activity from the brain. Examination of the correlations across the different measures will provide insight into how the wasps alter the host’s brain to create the “bodyguard” phenotype.
Results and discussion
Overview
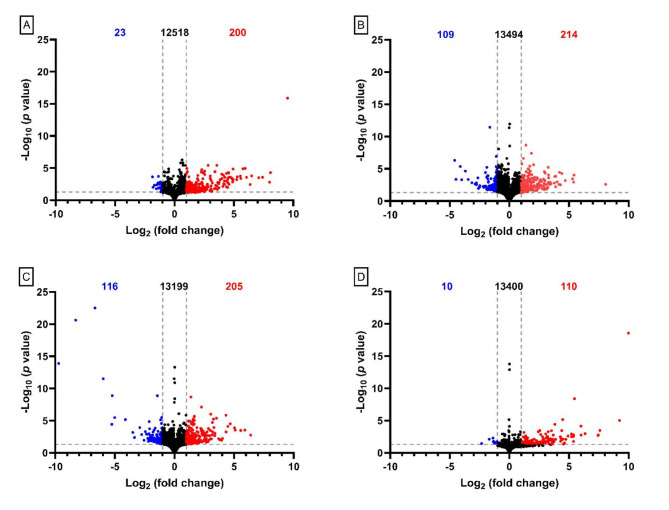
Changes in protein abundance (Table S1, Table S4) and gene expression (Fig. 1) within the CNS of parasitized M. sexta correlated with the stage of wasp development. Using the brains of parasitized caterpillars prior to the change in host behaviour (i.e. pre-emergent brains) as a baseline to remove the effects of parasitism without behavioural change, we found over 200 genes changed in expression as hosts adopted the “bodyguard” phenotype (Fig. 1), although the number of changes declined by three days after the wasps had emerged from the caterpillar (i.e. 3-Days Post emergence, Fig. 1). Interestingly, most of the changes in gene expression were an upregulation (Fig. 1), even though the host suffers a dramatic decline in its resting metabolic rate during the “bodyguard” phase (approximately 40%53). The change in host behaviour also correlated with the change in abundance of over 100 proteins within the CNS (Table S4). However, it should be noted that given the total number of genes and proteins (Fig. 1, Table S1), these products represent less than 5% of M. sexta genes and proteins. Unfortunately some of the genes showing the largest changes in expression could not be identified.
Fig. 1.
Overall change in gene expression in the supraesophageal ganglion (i.e. brain) of M. sexta caterpillars. (A) Gene expression levels of Pre-emergence caterpillars compared to Unparasitized caterpillars. (B) Gene expression pattern of Emergent caterpillars compared to Pre-emergent caterpillars. (C) Gene expression pattern in 1-Day Post-emergent caterpillars compared to Pre-emergent caterpillars. (D) Gene expression pattern of 3-Days Post-emergent caterpillars compared to Pre-emergent. In all graphs the number of downregulated genes is indicated in blue on the top left of the graph, the number of upregulated genes is indicated by the red number in top right of each graph, and the number of unchanged genes is indicated in black in the top center. A grey horizontal dashed line indicates the significance cut-off for the false discovery rate of 0.05. Two grey vertical dashed lines indicate a 2-fold change, which was the chosen cut-off for significance.
The burst in gene expression and change in protein abundance that occurs during wasp emergence probably requires a trigger from the exiting larvae. This trigger could be the massive immune response that occurs during wasp emergence (i.e. an immune-neural signal28,31), and/or the ecdysteroid pulse that occurs 1 day prior to host emergence14, and/or secretions from the wasp larvae31themselves. There are receptors for immune signaling molecules34and ecdysteroids54,55in the M. sexta brain.
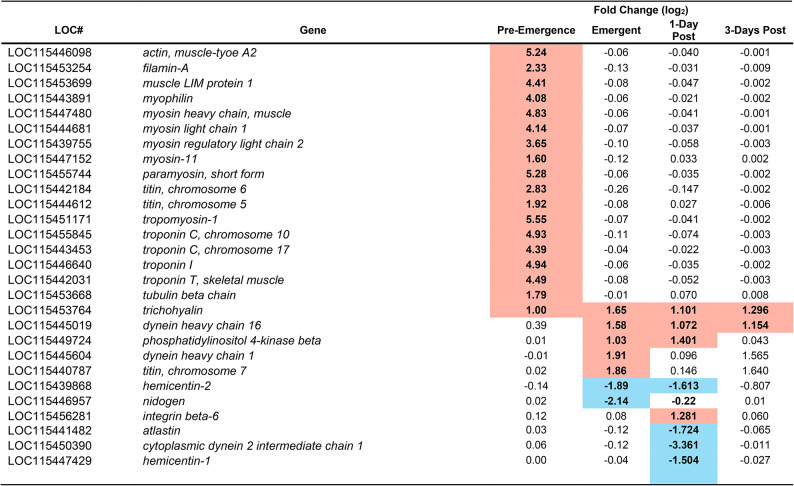
Parasitism resulted in changes in the expression of genes and in the abundance of proteins related to gene transcription, cytoskeleton architecture, intracellular transport, the extracellular matrix and immune defense (Table S3, Table S4, S5, Figs. S1 – S23). These changes began prior to the change in host behaviour. However, there is a deepening in many of these changes as the caterpillar transitions into the “bodyguard” phenotype (e.g. Tables 1, 2, 3, 4, 5, 6, 7, 8). We focus on the changes in three physiological networks that we believe are most likely to be involved in creating the this phenotype.
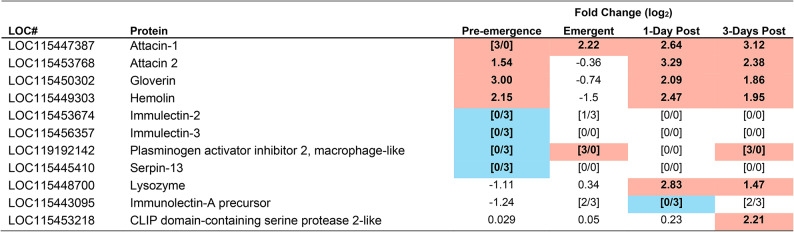
Table 1.
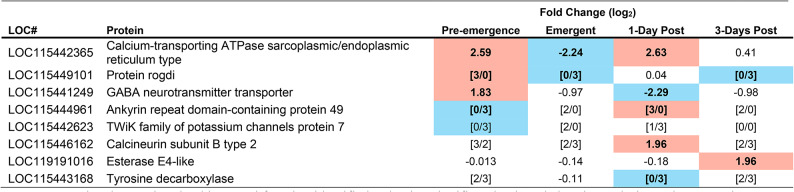
Immune proteins identified as having significantly altered abundance in the brain of M. sexta during at least one time point during parasitism. Highlighted in red indicates an increased abundance at that timepoint, highlighted in blue indicates a reduced abundance at that timepoint. To be considered significant the Fold change must have been a minimum of 2 and a p-value of less than 0.001 must have been achieved (false discovery rate correction). p-values can be found in table S4. Pre-emergence groups were contrasted with unparasitized caterpillars as a baseline, whereas Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline. [#/#] Indicate incidents in which a protein was below detection limit in either the experimental group (numerator), or the baseline group (denominator), e.g. 0/3 in the pre-emergence column means that 0 of the 3 biological replicates from pre-emergent caterpillars had detectable amounts of a particular protein, but 3/3 of the control samples did.
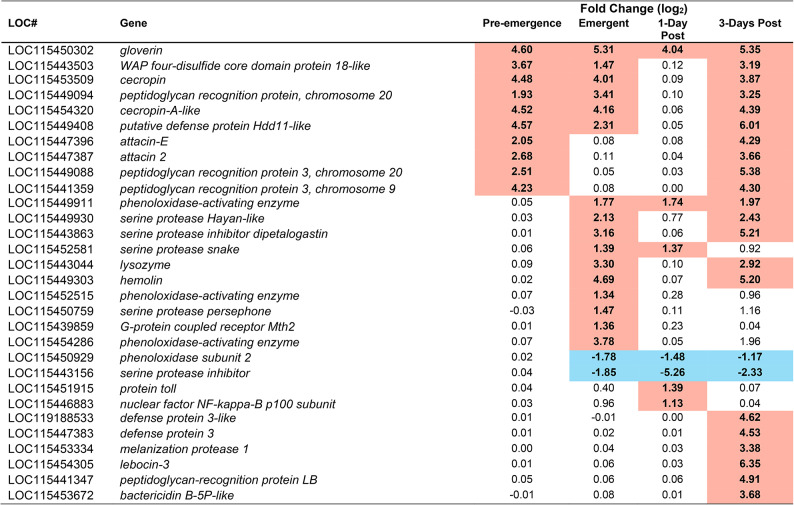
Table 2.
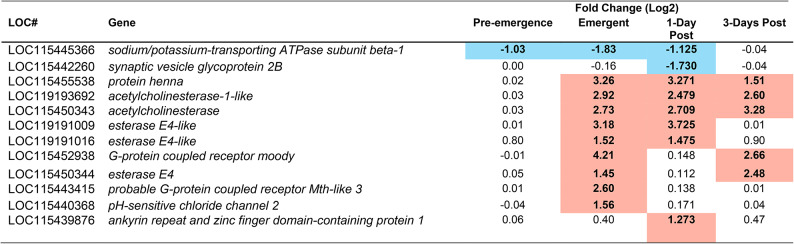
Immune gene mRNA transcripts from the brain of M. sexta identified as having significantly altered abundance during at least one time point during parasitism. Highlighted in red indicates an upregulation at that timepoint, highlighted in blue indicates a downregulation at that timepoint. To be considered significant, a p-value < 0.05 must have been achieved, and a Fold change of at least 2. p-values can be found in table S3. Pre-emergence groups were contrasted with unparasitized caterpillars as a baseline, whereas Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline.
Table 3.
CcBV proteins found in the supraesophageal ganglion (i.e. brain) during at least one of the sampled timepoints. ‘+’ indicates that the protein was found to be present at that time point. ‘-‘ indicates that it was absent, or below detection threshold at that time point.
| LOC# | Protein | Pre-Emergent | Emergent | 1-Day Post | 3-Days Post |
|---|---|---|---|---|---|
| CcBV_30.1 | Hypothetical protein | + | + | + | + |
| CcBV_13.4 | EP2-1 | + | + | - | + |
| CcBV_36.2 | BV7-6 | + | + | + | + |
| CcPL9.004 | CcPL9.004 | + | + | + | + |
| CcBV_1.5 | EP1-3 | + | + | - | + |
| CcBV_5–13 | CcV1 | + | + | + | + |
| CcBV_31.9 | Hypothetical protein | - | + | - | - |
| CcBV_19.2 | Cystatin 1 | + | + | + | + |
| CcBV_22.1 | BV7-1 | - | + | + | + |
| CcPL4.001 | CcPL4.001 | - | + | + | + |
Table 4.
CcBV mRNA transcripts from the brain of M. sexta identified as having significantly altered abundance during at least one time point during parasitism. To be considered significant a p-value of less than 0.05 must have been achieved, and a Fold change of at least 2. Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline.
| Fold Change (Log2) | |||||||
|---|---|---|---|---|---|---|---|
| LOC# | Gene | Emergent | p | 1-Day Post | p | 3-Days Post | p |
| CcBV_26.5 | hypothetical Protein | 2.20 | < 0.05 | 2.75 | < 0.01 | 2.58 | < 0.01 |
| CcBV_14.6 | protein-Tyrosine Phosphatase W | 3.41 | < 0.001 | 2.85 | < 0.05 | - | - |
| CcBV_17.2 | protein-Tyrosine Phophatase Y | 4.47 | < 0.001 | - | - | 3.41 | < 0.01 |
| CcBV_31.5 | hypothetical Protein | 4.04 | < 0.001 | - | - | - | - |
| CcBV_3.4 | hypothetical Protein | 3.02 | < 0.001 | - | - | - | - |
| CCBV_32.1 | hypothetical Protein | 2.27 | < 0.05 | - | - | - | - |
| CcBV_32.9 | hypothetical Protein | 2.06 | < 0.05 | - | - | - | - |
| CcBV_3.3 | hypothetical Protein | 1.95 | < 0.05 | - | - | - | - |
| CcBV_7.5 | EP1-5 | 1.89 | < 0.05 | - | - | - | - |
| CcBV_8.2 | EP1 | 1.81 | < 0.05 | - | - | - | - |
| CcBV_26.4 | hypothetical Protein | 1.71 | < 0.05 | - | - | - | - |
| CcBV_19.5 | cystatin 2 | 1.47 | < 0.05 | - | - | - | - |
| CcBV_31.2 | hypothetical Protein | - | - | 4.82 | < 0.001 | - | - |
| CcBV_31.3 | hypothetical Protein | - | - | 3.25 | < 0.05 | - | - |
| CcBV_26.3 | ankyrin-6 | - | - | 1.91 | < 0.01 | - | - |
| CcBV_10.5 | hypothetical Protein | - | - | 1.83 | < 0.05 | 2.85 | < 0.01 |
| CcBV_10.1 | protein-Tyrosine Phosphatase E | - | - | - | - | 4.63 | < 0.0001 |
| CcBV_1.9 | protein-Tyrosine Phosphatase P | - | - | - | - | 4.54 | < 0.0001 |
| CcBV_1.3 | EP1-1 | - | - | - | - | 4.21 | < 0.001 |
| CcBV_10.2 | protein-Tyrosine Phosphatase S | - | - | - | - | 4.17 | < 0.0001 |
| CcBV_26.1 | protein-Tyrosine Phosphatase Δ | - | - | - | - | 4.06 | < 0.001 |
| CcBV_26.7 | protein-Tyrosine Phosphatase ε | - | - | - | - | 3.76 | < 0.001 |
| CcBV_1.5 | EP1-3 | - | - | - | - | 3.72 | < 0.001 |
| CcBV_1.8 | protein-Tyrosine Phosphatase M | - | - | - | - | 3.33 | < 0.001 |
| CcBV_18.7 | hypothetical Protein | - | - | - | - | 3.27 | < 0.001 |
| CcBV_1.4 | EP1-2 | - | - | - | - | 3.12 | < 0.01 |
| CcBV_18.5 | CRP2 | - | - | - | - | 2.92 | < 0.01 |
| CcBV_1.7 | protein-Tyrosine Phosphatase L | - | - | - | - | 2.88 | < 0.01 |
| CcBV_1.2 | protein-Tyrosine Phosphatase I | - | - | - | - | 2.63 | < 0.01 |
| CcBV_26.6 | protein-Tyrosine Phosphatase A | - | - | - | - | 2.6 | < 0.01 |
| CcBV_1.10 | protein-Tyrosine Phosphatase Q | - | - | - | - | 2.21 | < 0.01 |
| CcBV_10.3 | protein-Tyrosine Phosphatase T | - | - | - | - | 2.02 | < 0.05 |
| CcBV_1.11 | protein-Tyrosine Phosphatase D | - | - | - | - | 2.00 | < 0.05 |
| CcBV_7.3 | histone 4 | - | - | - | - | 1.87 | < 0.05 |
| CcBV_7.4 | protein-Tyrosine Phosphatase R | - | - | - | - | 1.61 | < 0.05 |
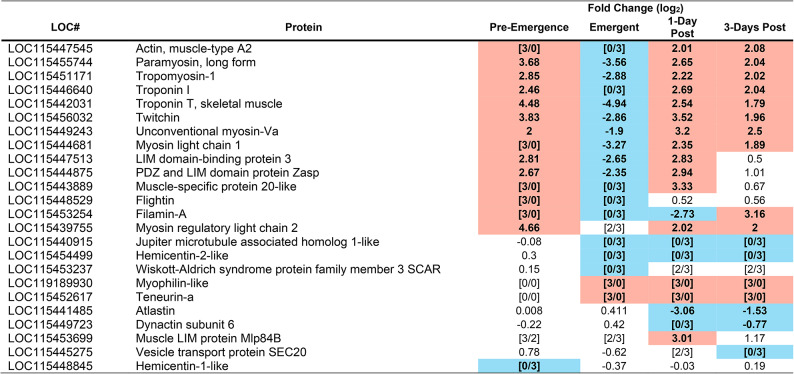
Table 5.
Cytoskeleton and Extracellular Matrix (ECM) proteins identified as having significantly altered abundance in the brain of M. sexta during at least one time point during parasitism. Highlighted in red indicates an increased abundance at that timepoint, highlighted in blue indicates a reduced abundance at that timepoint. To be considered significant a p-value of less than 0.001 must have been achieved. P-values can be found in table S4. Pre-emergence groups were contrasted with unparasitized caterpillars as a baseline, whereas Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline. [#/#] Indicate incidents in which a protein was below detection limit in either the experimental group (numerator), or the baseline group (denominator), e.g. 0/3 in the pre-emergence column means that 0 of the 3 biological replicates from pre-emergent caterpillars had detectable amounts of a particular protein, but 3/3 of the control samples did.
Table 6.
Cytoskeleton and Extracellular Matrix (ECM) related mRNA transcripts from the brain of M. sexta identified as having significantly altered abundance at a minimum of one time point during parasitism. Highlighted in red indicates an upregulation at that timepoint, highlighted in blue indicates a downregulation at that timepoint. To be considered significant a p-value < 0.05 must have been achieved, and a Fold change of at least 2. p-values can be found in Table S3. Pre-emergence groups were contrasted with unparasitized caterpillars as a baseline, whereas Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline.
Table 7.
Proteins that are involved in neural function identified as having significantly altered abundance during at least one time point during parasitism in the brain of M. sexta. Highlighted in red indicates an increased abundance at that timepoint, highlighted in blue indicates a reduced abundance at that timepoint. To be considered significant the Fold change must have been a minimum of 2 and a p-value of less than 0.001 must have been achieved. P-values can be found in table S4. Pre-emergence groups were contrasted with unparasitized caterpillars as a baseline, whereas Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline. [#/#] Indicate incidents in which a protein was below detection limit in either the experimental group (numerator), or the baseline group (denominator), e.g. 0/3 in the pre-emergence column means that 0 of the 3 biological replicates from pre-emergent caterpillars had detectable amounts of a particular protein, but 3/3 of the control samples did.
Table 8.
mRNA transcripts from the brain of M. sexta involved in neural function identified as having significantly altered abundance at a minimum of one time point during parasitism. Highlighted in red indicates an upregulation at that timepoint, highlighted in blue indicates a downregulation at that timepoint. To be considered significant a p-value of less than 0.05 must have been achieved, and a Fold change of at least 2. P-values can be found in table S3. Pre-emergence groups were contrasted with unparasitized caterpillars as a baseline, whereas Emergent, 1-Day post, and 3-Days Post, were contrasted with the pre-emergence condition as baseline.
Immune activity in the brain
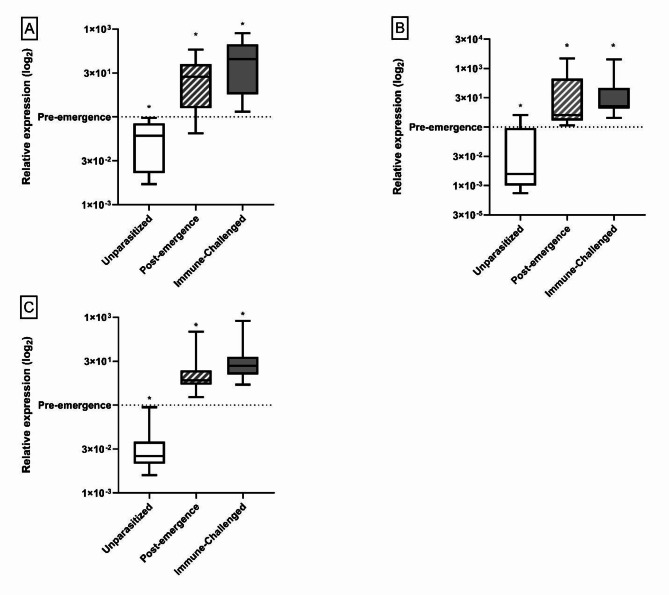
Our results support our hypothesis that the “bodyguard” phenotype is produced, at least in part, by activating immune-neural connections. We confirmed that a systemic immune challenge in unparasitized M. sexta induced an increase in gene expression for 2 different antimicrobial peptides (gloverin and attacin-1) and a pattern recognition molecule (hemolin) in the brain (Fig. 2), correlating with the expression of illness-induced anorexia28. This result demonstrates that immune activity in the brain correlates with the expression of sickness behaviour. AMPs themselves may act as neural signals, although the details remain unclear56,57.
Fig. 2.
Targeted qPCR for immune gene expression in the brains of unparasitized, Post-emergence parasitized, and Immune challenged M. sexta compared to Pre-emergence parasitized caterpillars. (A) Relative expression of attacin-1 (B) Relative expression of gloverin (C) Relative expression of hemolin. All groups compared to the pre-emergence group whose expression has been normalized to 1 (Indicated by dashed line). Immune challenged M. sexta have been injected with an inert (heat-killed) challenge. * Denotes a significant change p < 0.001.
The caterpillar exhibits a massive systemic immune response at wasp emergence28,31 and, as predicted, parasitized caterpillar brains showed proteomic (Table 1), transcriptomic (Table 2) and qPCR (Fig. 2) evidence of increased immune activity at this time, compared with parasitized caterpillars prior to wasp emergence. Moreover, expression of CcBV genes (Table 3) and the abundance of CcBV proteins (Table 4) increased after the wasps emerge, and this increase could also initiate immune activity in the brain as a host response. Although there was also an increase in immune activity in the brain before the wasps emerge, compared with unparasitized controls (Fig. 2; Tables 1 and 2), caterpillar behaviour remained normal31 during this smaller increase in immune activity within the CNS.
The large and long-lasting increase in immune activity that occurs in the brain of M. sexta after the wasps emerge (Tables 1 and 2) could be producing neuroinflammation. In mammals, increased immune-neural activity causes neuroinflammation58, resulting in altered neural function59. Such hyperactivation of immune-neural connections could help produce this behavioural phenotype by heightening illness-induced anorexia and other sickness behaviours (e.g. lack of locomotion and enhanced defensive behaviours)34. In D. melanogaster, increased AMP production in the brain60and hyperactivation of immune pathways61 also leads to neural damage. Neuroinflammatory damage is key to creating a bodyguard host in the Dinocampus coccinellae-Coleomegilla maculata wasp-ladybug system46. The exiting wasp larva induces an increase in the production of the wasp’s symbiotic RNA virus within the host’s brain46 Electron microscopy of the C. maculata brain suggest that the increase in viral production damages the host brain, leading to a partially paralyzed, trembling host that sits on top of the wasp cocoon46. This behaviour is sufficient to repel arthropod predators, increasing wasp reproductive success62. However, such uncoordinated behaviour would not produce the bodyguard phenotype observed in M. sexta. M. sexta defends the cocoons using behaviours such as the defensive strike, that requires a coordinated motor response63. Moreover, the polydnavirus in C. congregata (CcBV) is non-replicating, and, therefore, cannot damage the brain in the same way as D. coccinellae’s RNA virus. Further studies are needed to determine the extent (if any) of neuroinflammatory damage and its location within the M. sexta CNS.
Cytoskeleton and extracellular matrix (ECM)
Although not part of our original hypothesis, both the proteomic (Table 5) and transcriptomic analyses (Table 6) showed strong changes in the protein abundance and gene expression of molecules related to the cytoskeleton (e.g. actin, tropomyosin, troponin-1) and extracellular matrix (e.g. hemicentin, nidogen, integrin beta 6, teneurin), including those thought to be directly involved in synaptic transmission (e.g. filamin A64, tropomyosin65and teneurin66). Changes began before the wasps emerged, when the caterpillar still has normal behaviour. There was a pronounced upregulation of gene expression, and an increase in protein abundance, of molecules that are key for intracellular transport (e.g. actin, paramyosin, troponin 1, Tables 5 and 6). However, at the time of wasp emergence, these proteins declined in abundance, compared with their pre-emergence amounts. Once the wasps had emerged from the host, these proteins then surged in abundance to levels higher than that observed prior to wasp emergence (Table 5). Furthermore, after wasp emergence, additional proteins involved in axonal transport (e.g. Atlastin, Dynactin subunit 6, Vesicle transport protein Sect. 20) changed in abundance, and additional genes (atlastin, dynein heavy chain 1) changed in expression (Table 6). The increasingly large changes in genes and proteins related to intracellular transport suggest that axonal transport is probably disrupted after the wasps emerge. Disruption in axonal transport leads to a decline in synaptic transmission in D. melanogaster67 Also correlating with the change in host behaviour, were changes in gene expression (hemicentin-1 and 2, nidogen and integrin beta 6) and protein abundance (Hemicentin-2-like and Teneurin-A) of molecules that are important in the ECM (Tables 5 and 6). These changes are also likely to depress synaptic transmission68.
Viruses commonly alter cellular architectural proteins, and, therefore, some change in cytoskeleton proteins are expected in virally infected insect cells69. Moreover, the CcBV virus appears to target the cytoskeleton, at least in M. sexta hemocytes70. CcBV-induced changes in hemocyte cytoarchitecture contribute to the survival of the wasp larvae by reducing host hemocyte activity71. If the virus produces similar changes in the microglia of the M. sextabrain, it could alter the functioning of these cells67. Such changes could also impact immune-neural signaling.
Neural function
Even before the wasps emerge from the host, both the transcriptomics and proteomics results showed changes in the gene expression and protein abundance of molecules important for neuronal function (e.g. potassium channels, Table 7). Although these changes were insufficient to produce a change in host behaviour, they could facilitate the production of the “bodyguard” phenotype, when combined with the additional changes that occur after wasp emergence (Tables 7 and 8). However, the dynamic nature of neural networks52makes interpreting these changes challenging. Moderate changes in neurotransmitter synthesis, or the number of receptors, can be compensated for by the brain’s homeostatic mechanisms51. Similarly, different ion channel subtypes can often compensate for each other52,72. Additionally, synaptic mechanisms tend to keep firing rates within a set-point range, despite perturbations73. Therefore, changes in proteomics and transcriptomics of the host brain could be a direct effect of parasitism, but could also be a sign of the activation of host homeostatic mechanisms (also see discussion in52,74).
CcBV
CcBV is known to rapidly enter host cells and is transcribed within an hour of wasp oviposition in fat body and hemocytes21. We found that bracovirus gene expression occurs in every region of the CNS within 24 h (Fig S24). How CcBV enters the brain this quickly remains unknown, and whether it enters all brain areas simultaneously is also unknown. Once in the cell, CcBV genes appear to make a number of proteins that could interfere with intracellular signaling pathways75, and have been shown to be immunosuppressive when expressed in immune tissues76. Unfortunately, nothing is known about their effects in the CNS.
Some CcBV proteins could be found in all regions of the CNS at all time points (e.g. CcV1, Table 4, S24), however two proteins occurred in the brain only during and after wasp emergence (BV7-1 and CcPL4.001) or in the ventral nerve cord at emergence (Table 4, S24). The timing of the appearance of these two proteins makes them good candidates for further studies on the possible involvement of CcBV proteins in changing host behaviour.
Transcripts for CcBV genes could be detected at all late-stage parasitism timepoints measured (Pre-emergent, Emergent, 1-Day Post, and 3-Days Post) (Table 4).
Likely overall effect of neuroparasitic changes
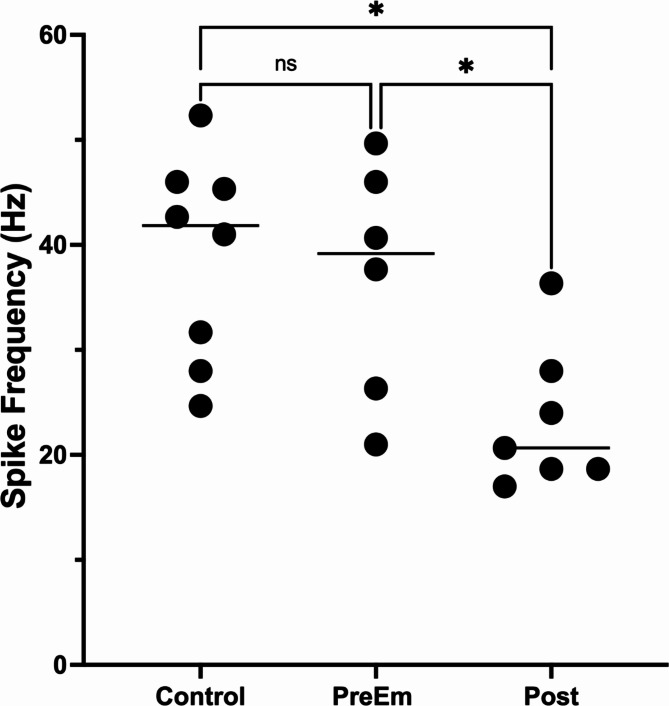
The cytoskeleton and ECM results suggest that synaptic transmission may be reduced in parasitized caterpillars after the wasps emerge. Such an effect would be consistent with previous research that showed that immunohistochemical staining for multiple neuropeptides increases after wasp emergence, likely caused by a build-up of these neuropeptides12. Similarly, at least one biogenic amine (octopamine) increases in abundance in the brain and CNS after wasp emergence77 as would be expected if release was reduced. We also found a decline in neural activity descending from the brain (both spontaneous and evoked activity) in caterpillars after wasp emergence (n = 7) compared to controls (n = 8) or to parasitized caterpillars prior to wasp emergence (Fig. 3, spontaneous: F(2, 16) = 6.8, p = 0.007. Tukey’s multiple comparison test, Control vs. Pre-emergent caterpillars (n = 4), p = 0.72; Control vs. Post-emergent caterpillars, p = 0.006; Evoked: F(2, 18) = 5.89, p = 0.01; Control (n = 8) vs. Pre-Emergent caterpillars (n = 6), p = 0.91; Control vs. Post-Emergent caterpillars (n = 8), p = 0.01, Pre-Emergent vs. Post-emergent, p = 0.04). These results are consistent with a reduction in synaptic release in post-emergent caterpillars (i.e. during the “bodyguard” phase); however, more studies are required to determine whether synaptic transmission is actually reduced. Further studies are also required to determine whether neuroinflammation, bracoviral effects, and/or other factors are causally linked to thes decline in descending neural activity.
Fig. 3.
Descending activity in the supraesophageal connective contralateral to the stimulus. After the wasps emerge (Post, n = 7) there is a decline in evoked neural activity compared with that of controls (Control, n = 8). A significant decline was not observed prior to wasp emergence (PreEm, n = 6). The horizontal lines denote the median, and each circle represents an individual data point. The asterisk denotes statistically significant differences (p < 0.05).
A decline in synaptic transmission throughout the CNS could produce the “bodyguard” phenotype. Neural circuits mediating defensive behaviours tend to have few synaptic connections (e.g. M. sexta78,79) and would probably be minimally affected by a small decline in synaptic transmission. However, motivated behaviours, that rely on more complex multisynaptic circuits80, would be disproportionately reduced. This differential impact on neural circuits could produce a caterpillar with robust defensive reflexes, but one that lacks motivated behaviours – i.e. a “bodyguard”. This hypothesis will provide the basis for future studies.
Limitations
Changes in host behaviour can reflect parasitic manipulation or host responses (e.g. see discussion Bernardo and Singer81). The large number of changes that occur within the host during parasitism make it difficult to determine which of our reported changes are directly caused by the parasite (e.g. via CcBV) and whether any of these changes are directly responsible for producing the “bodyguard” phenotype. Future studies (e.g. suppressing the production of CcBV proteins BV7-1 and CcPL4.001 at the time of wasp emergence) are needed. A further difficulty is that our data likely underestimate the number of molecular changes occurring during host behavioural change.
Conclusions
Proteomics and transcriptomics are complementary techniques82. Changes in immune, cytoskeleton and ECM molecules were found in both the proteomics and transcriptomics results using two different methods of assessing network connections (e.g. Tables 1, 2, 3, 4, 5, 6, 7 and 8, and S5), giving us confidence that these networks are altered during parasitism.
As predicted, we found that: (1) systemic immune activity increased immune activity in the brain of M. sexta, and (2) immune activity within the brain increased dramatically with the change in host behaviour. These results support the hypothesis that enhanced immune activity in the caterpillar brain is playing a role in the change in host behaviour. (3) Changes in CcBV gene expression and protein abundance correlated with the appearance of the “bodyguard” phenotype. This correlation suggests that polydnaviruses may play a role in parasitic manipulation of host behaviour. (4) Changes in genes and proteins important for intracellular transport and the ECM suggested that synaptic transmission could be reduced in “bodyguards”. This hypothesis was consistent with our finding of a reduction in descending neural activity from the brain after wasp emergence.
Controlling the ECM and cytoskeleton may be important for parasitic manipulators. The ECM83and cytoskeleton84 contribute to neuronal homeostasis. Manipulating the ECM and neuronal cytoskeleton may be a novel method of circumventing neuronal homeostasis, leading to long term changes of neural circuits. Many manipulated hosts from different parasite-host systems exhibit altered cytoskeleton and/or ECM dynamics in the host’s brain (e.g. jewel wasp/cockroach9; trematode/crustacean85; gordian worm/crustacean86; Hairworm/grasshopper87, cestode/ant88). The need to overcome homeostatic mechanisms will be especially important for parasitic manipulators that require their hosts to survive for several days in the altered state. Multi-targeted interventions appear to allow parasitic manipulators to achieve effective, predictable and long-lasting control of the host’s brain.
Methods
Animals
All studies were performed on Manduca sextalarvae obtained from our in-house colony, and were maintained as described previously31.
Cotesia congregata were obtained from an in-house colony. Mated adults were given 3rd instar M.sexta (Proteomics, transcriptomics, late-stage qPCR, and electrophysiological studies) or 4th instar (early-stage qPCR) in which to lay their eggs. Parasitized M. sexta were reared on lab-made high nutrition diet (Frontier Agricultural Sciences, (#F9783B Neward, DE) until tissue extraction.
All studies were approved by the University Committee on Laboratory Animals (Dalhousie University, I-11–025) and were in accordance with the Canadian Council on Animal Care.
Sample sizes followed minimum guidelines for proteomics and transcriptomics68.
A Benjamini-Hochberg correction was applied when multiple tests were performed on the same data set89.
Tissue extraction
Caterpillars were cooled to induce a chill coma90and dissected over ice.
Methods for proteomics, transcriptomics, immune and bracovirus qPCR
Final instar (5th ) M. sexta caterpillars were sorted into five groups based on stage of parasitism: Unparasitized (Control), Pre-emergent (3 days prior to emergence of wasp larvae), Emergent (tissue collected during the emergence of wasp larvae), 1 day post emergence (1 day after emergence of wasp larvae), and 3 days post emergence (3 days after emergence of wasp larvae).
The supraesophageal ganglion or subesophageal ganglion or ventral nerve cord (thoracic ganglia + abdominal ganglia) were extracted and washed briefly in PBS (< 10 s). For transcriptomics, the tissue was placed into an empty tube and flash frozen with liquid nitrogen. For proteomics, tissue was added to a tube containing protease inhibitor and then flash frozen using liquid nitrogen (cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail, Roche, Switzerland). For the qPCR assays, tissues were added to tubes containing RNAlater (qPCR) (Invitrogen, MA, USA) and flash frozen in liquid nitrogen. All tissues were kept at −80 °C until use.
Methods for early-stage RT-qPCR
Fourth instar M. sexta caterpillars were sorted into two groups: Unparasitized (control), or 24 h post-parasitism (i.e. after wasp oviposition).
Tissues were collected as described above.
Proteomics
Extraction and quantification
Due to protein concentration required, 10 individuals per group were pooled to create 3 replicates per group (i.e. CNS samples from 30 individuals in total). The tissues (supraesophageal ganglia, subesophageal ganglia, or ventral nerve cords) were suspended in 30 µl of extraction buffer containing Urea 7 M, Thiourea 2 M, TrisHCl 40 mM, CHAPS 4%, DTT 1% and protease inhibitor. Samples were then mechanically homogenized on ice using a micro pestle. Samples were further disrupted using a micro-sonicator on maximum for 3 cycles of 10 s, followed by 20 s on ice. Following homogenization, samples were centrifuged at 15 800 g at 4 °C for 12 min, after which the supernatant was collected for protein quantification.
A Bradford test for total protein was conducted on the samples followed by a 1D SDS PAGE on a 10% pre-cast acrylamide gel (Biorad). Gels were stained overnight using Sypro ruby protein stain (Invitrogen, Massachusetts, USA) before being visualized under UV light.
Protein identification
Extracted protein samples were denatured and digested in a trypsin solution. The resulting peptide solution was suspended in 10 µl of a 0.1% formic acid solution and injected into an HPLC nano debit (RSLC U3000, Thermo Fisher Scientific) coupled with a nanoelectrospray mass spectrometer (Q Exactive HF, Thermo Fisher Scientific). Peptides were separated on a C18 reverse-phase capillary column (0.075 mm x 500 mm, Acclaim Pepmap 100, NanoViper, Thermo Fisher Scientific), using a gradient of 0.1% formic acid: acetonitrile, 2–40%:98 − 60%, at a flow rate of 300 nL/min.
Resulting mass spectrographs were recorded using Xcalibur 4 software (Thermo Fisher Scientific). These spectrographs were then analyzed using MaxQuant v1650 and Perseus v1.6.10.43 programs using the script leading FPP v3.2. As a template for protein comparison, we created four protein databases that encompassed known proteins from the family braconidae (NCBI txid 7402), the polydnavirus genus bracoviriform (NCBI txid 2946836), the species C. congregata (NCBI txid51543) and the species M. sexta(NCBI txid 7130). These datasets have been made publicly available91. A MaxQuant database was also used to reduce proteins being identified due to contamination (contaminants_fpp_180320.FASTA)92. Protein validation was conducted with a 1% false discovery rate filter at both the peptide and the protein level.
Ratio analysis
Normalization of protein intensity signals was required to analyze the data. Individual proteins had to have been detected in all samples (all three replicates of parasitized caterpillars per group, and all three replicates of control caterpillars) to be included in the analysis. Preliminary examination of the data found that the raw intensity values were not normally distributed, therefore these values were log2 transformed prior to statistical analysis. Furthermore, the intensity values per replicate were centered using the following procedure. For each sample, the median intensity value was established taking all detectable protein intensities into account. This overall median was then subtracted from each individual protein intensity value within that sample. This process was repeated for each of the three replicates. Once the data was centered, a ratio was calculated for each protein intensity by subtracting the control group intensities from the treatment group intensities for each protein. The median ratio of the three replicates was then Z-Scored. These Z-scores were converted to p-values, and a Benjamini-Hochberg procedure was used to determine statistical significance89.
qPCR
RNA extraction
RNA extraction was performed using a RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany). All steps adhered to the manufacturer’s instructions and included a DNase 1 treatment (RNase-free DNaset, Qiagen) step to remove genomic DNA. The integrity of total RNA samples was assessed using a Bioanalyzer (Agilent, California, USA). The purity of extracted RNA was determined using an EPOCH spectrophotometer (BioTek, Vermont, USA) using the 260/280 ratio. Only samples with a 260/280 between 1.8 and 2.4 were used in accordance with the MIQE guidelines93. The concentration of total RNA was determined using a Qubit Fluorometer (Q32857, Invitrogen, California, USA) using a HS RNA quantification kit. cDNA was synthesized using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad, California, USA) and samples were stored in −80 °C until use. Samples sizes are given in Table 9.
Table 9.
Sample Group information for RT-QPCR studies. Each sample consists of CNS from a single caterpillar (i.e. CNS tissue was not pooled).
| Experiment | Groups | N per group |
|---|---|---|
| Early parasitism |
Unparasitized, 24-hours post parasitism (2 groups, total of 16 samples) |
8 |
| Bracovirus PCR | Unparasitized, Pre-emergence, emergence, post-emergence (4 groups, total of 20 samples) | 5 |
| RNA-Seq immune validation | Unparasitized, Pre-emergence, post-emergence, Immune challenged (4 groups, total of 48 samples) | 12 |
Primers
Primer efficiency (E) and correlation coefficient (R2) were estimated from a standard curve generated with 10-fold dilutions of mixed cDNA samples (Table 10).
Table 10.
Forward and reverse gene primer sequences for target bracovirus genes, M. Sexta immune genes and reference genes.
| Forward Primer (5’ to 3’) | Reverse Primer (5’ to 3’) | % Efficiency | |
|---|---|---|---|
| Ubiquitin 94 | AAAGCCAAGATTCAAGATAAG | TTGTAGTCGGATAGCGTGCG | 94 |
| RpL17a 95 | TCCGCATCTCACTGGGTCT | CACGGCAATCACATACAGGTT | 98 |
| Cystatin-1 22 | TCGAGCGGCCGCAATGGGCAAGGAATATCGAG | TGGCGCGGCCGCTTAACAATTTTCATATTCCCAAC | 98 |
| EP-1 96 | GCGCCCGTAGTGTCATTAATG | CCCAGTACTTGATGCGCTTG | 101 |
| CcV1 | ATTCCTGGGCACCTCCAAG | TGCAACGATCGATCCAGGTC | 104 |
| Hemolin 97 | CAACCAAGCAACAACACAGG | CAGCACAGGCATCTTCTCC | 96 |
| Gloverin | CCCGCAATACGCTCAGATA | TGCTGGAAGAGACCTTGGA | 91 |
| Attacin-1 97 | GCAGGCGACGACAAGAAC | ATGCGTGTTGGTAAGAGTAGC | 94 |
Primer specificity was checked by running endpoint PCR products for each primer on a 1.5% acrylamide gel. The resulting bands were excited and sent out for Sanger sequencing (Genewiz, NJ, USA). The resulting sequences were put through a BLAST search on NCBI to confirm fragment identity.
Reference gene selection
Six candidate reference genes were investigated to select two of the most stable reference genes in our tissues of interest. These genes were used in previous studies in M. sexta caterpillars: RpL17a95 (Rewitz et al., 2006), actin98, glycerol-3-phosphate dehydrogenase (G3PDH)99, beta-FTZ-F1100, ubiquitin94, and ribosomal protein S3101. We used NormFinder for R (http://moma.dk/normfinder-software) to determine the stability of pairs of reference genes (Andersen et al., 2004), using Cq values of five biological samples per treatment for each candidate reference gene. RpL17a and ubiquitin were found to be the most stable across treatment groups and were used as reference genes in further qPCR analysis.
RT-qPCR
RT-qPCR was run using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) on a CFX96 real-time system (Bio-rad) with the following parameters: 40 cycles of (95℃ for 10 s; 55℃ for 30 s; 60℃ for 45 s) followed by a final extension of 60℃ for 10 min. After the qPCR a melt curve analysis was run to assess the specificity of the qPCR product. For each biological sample, qPCR reactions were performed in duplicate, and for each gene no-template controls were run. Cq values for each sample and gene target were calculated in CFX Maestro (BioRad).
The qPCR data were analysed using the REST program102 (http://rest.genequantification.info).
Transcriptomics
Sample Group Information
For the transcriptomic analysis there were five groups of interest: Unparasitized caterpillars, pre-emergent caterpillars, emergent caterpillars, 1-day post emergence, and 3-days post emergence (see Tissue Extraction for more details). There were 6 biological replicates per group.
RNA extraction
RNA extraction was performed using a RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany). All steps adhered to the manufacturer’s instructions and included a DNase 1 treatment (RNase-free DNaset, Qiagen) step to remove genomic DNA. The integrity of total RNA samples was assessed using a Bioanalyzer (Agilent, California, USA). The purity of extracted RNA was determined using an EPOCH spectrophotometer (BioTek, Vermont, USA) using the 260/280 ratio. Only samples with a 260/280 between 1.8 and 2.4 were used in accordance with the MIQE guidelines93. The concentration of total RNA was determined using a Qubit Fluorometer (Q32857, Invitrogen, California, USA) using a HS RNA quantification kit. All RNA samples were stored at −80 °C until library preparation.
Library preparation and transcriptome sequencing
Library construction and sequencing were performed at Farncombe Metagenomics Facility (McMaster University, ON, Canada). The RNA-Seq libraries were prepared using Illumina TruSeq RNA sample preparation kit (Illumina, CA, USA). RNA-Seq libraries were sequenced on an Illumina NextSeq for 2 × 50 bp paired-end reads.
Trimming, mapping of sequences, and differential gene analysis
Raw sequencing reads were trimmed using Trimmomatic software103(v0.39), removing low quality leading and tailing bases (Q < 3) and reads below 30 bases long. Trimmed reads were aligned using STAR (v.2.7.5a)104, to either the M. sextagenome105 (JHU_Msex_v1.0), the C. congregatagenome106, or the Bracoviriform congregataegenome107(ViralMultiSegProj14556. Uniquely mapped reads with a maximum of four mismatches were counted to genes using FeatureCounts from the Rsubread R package108 (v2.4.2). First, reads were counted to features in the M. sexta genome, which resulted in an average 32 million counted reads per sample. Next, reads were counted to features in the C. congregata genome. For the C. congregata analysis, some M. sexta reads mapped to the C. congregata genome, aligning to a handful of genes in controls (e.g. LOCUS4576). To exclude these reads, C. congregata was aligned simultaneously with M. sexta and then separated during counting. We effectively found no C. congregata reads in control samples (an average of 66 counted reads), and in parasitized samples there was an average of 420,000 reads. Finally, we counted reads to features in the Bracovirusgenomes, where control samples had on average 0 counted reads, and parasitized samples had on average 9000 counted reads. Genes were pre-filtered before differential expression analysis by removing genes with < 10 counts across all samples. Differential expression analysis was performed using the R package DESeq2 (v1.40.2)109, in 4 pairwise comparisons, either using the pre-emergent condition as the reference level (Pre-emergent vs. Emergent, Pre-Emergent vs. 1-Day Post, and Pre-Emergent vs. 3-Days Post), or the control (Pre-emergent vs. Control). Statistical significance was determined using a Wald test and corrected using a Benjamini–Hochberg correction for multiple comparisons89. Differentially expressed genes were considered for further analysis with a cut-off of an FDR adjusted p-value < 0.05 and a fold change in expression > 2.0110.
To test the robustness of the results for the both the proteomics and transcriptomics analyses, we performed a second analysis using the STRING database (https://string-db.org) to identify significantly enriched terms within the protein-protein interaction network111 (Yuan et al., 2021).
Validation of gene expression profiles using RT-qPCR of select immune genes
RT-qPCR was used to validate the expression profiles of select immune genes from RNA-seq results. For more information, please see RT-qPCR section. An additional group was added “Immune Challenge”. This group allowed us to test whether a systemic infection also increased gene expression for antimicrobial peptides (AMPs) in the brain. This group was reared on high nutrition diet and were unparasitized. On day 1 of their fifth instar this group was given a 40 µl injection of heat-killed mix of Serratia marcescens (Gram-negative bacterium, Microkwik culture, Carolina Biological, 1/10 LD50), Bacillus cereus (Gram-positive bacterium, Microkwik culture, Carolina Biological, 1/10 LD50), Beauveria bassiana(strain GHA, fungus, 1/10 LD50, BotaniGard 22WP; Laverlam, Butte, MT, USA) into the hemocoel. Post injection, these caterpillars had their food removed for 24 h to mimic the large systemic immune response and lack of feeding observed in Post 1 caterpillars28,31. The supraesophageal ganglia was collected 24 h after the injection using the same methods listed above.
The Effect of Parasitism on the Descending Neural Activity from the Supraesophageal Ganglion.
Caterpillars were cooled for approximately 10 min to induce chill coma and then the gut and a section of the body wall (between the second and fifth abdominal ganglia) was removed without disturbing the ventral nerve cord. The physiological saline for M. sextawas modified from Miyzaki112by Trimmer and Weeks78 and contained 140 mM NaCl, 5 mM KCl, 4 mM CaCl2, 29 mM glucose and 5 mM HEPES adjusted to pH 7.4 using NaOH (all chemicals were from Sigma-Aldrich (St Louis, MO). The posterior and anterior ends of the caterpillar were pinned to an elastomer-covered dish. A suction electrode (Bipolar Suction Electrode, A-M Systems, Carlsberg, WA) was connected to a differential amplifier (A-M Systems, Model 3000), and digitized using a PowerLab SP4 (ADInstruments, Colorado Springs, CO). The output was collected and analyzed using Lab Chart (ver. 7.3.8, ADInstruments). The suction electrode was used to make en passant recordings of the connective between the subesophageal ganglion and the supraesophageal ganglion. Spontaneous activity was collected for 3 min and then the posterior end of the animal was electrically stimulated by a Grass S9 stimulator. Low voltage was applied to the dorsal nerve root of the sixth abdominal ganglion that was ipsilateral to the suction electrode. The voltage was increased until evoked potentials were visible in the en passant recordings. The suction electrode was then moved to the opposite supraesophageal – subesophageal connective. The connective was cut near the subesophageal ganglion, and recordings of the descending activity in the contralateral connective recorded for 3 min using the suction electrode. The stimulus to the dorsal root of the contralateral sixth abdominal ganglion was re-applied as described above. If no evoked potentials were observed, the voltage was increased until evoked potentials were visible. Recordings were made from control caterpillars (5th instar day 2 to day 3, n = 8), pre-emergent caterpillars (estimated as 3 days prior to emergence, n = 4–6, see Results and Discussion section); and post emergent caterpillars (1 to 3 days after wasp emergence, n = 7)). The number of potentials at least two times above noise were counted during spontaneous recordings, as well as after the stimulus artifact. Using this threshold meant that only the largest spikes were counted (i.e. greater than 30 µV in amplitude and 2 ms in duration). We used the response of control caterpillars to determine the time frame for counting the evoked potentials (i.e. for 30 s after the stimulus artifact).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
L.M. Study design, Data collection, Data curation, Analysis, Writing of manuscript; R.H. Analysis; Data collection, Manuscript review; D.B. Conceptualization of the study, Study Design, Analysis; A.B. Data collection, Manuscript review; D.M. Analysis, Manuscript review; NR: Study design, Analysis, Manuscript Review; S.A.: Conceptualization of the study, Analysis, Interpretation of Results, Writing of manuscript.
Data Availability
Data are available in the supplementary file. Complete transcriptomic and proteomic datasets are available for download at: McMillan, L., Herbison, R., Raun, N. & Adamo, S. Datasets for “The multiple effects of the wasp Cotesia congregata, a parasitic manipulator, on the brain of its host, the caterpillar Manduca sexta” https://doi.org/10.5683/SP3/FRJDPT , Borealis, V1.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Biron DG is deceased
References
- 1.Hughes, D. P. & Libersat, F. Neuroparasitology of parasite–insect associations. Ann. Rev. Entomol.63, 471–487 (2018). [DOI] [PubMed] [Google Scholar]
- 2.de Bekker, C., Beckerson, W. C. & Elya, C. Mechanisms behind the madness: how do zombie-making fungal entomopathogens affect host behavior to increase transmission? MBio12, 101128mbio01872–101128mbio01821 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elya, C. et al. Neural mechanisms of parasite-induced summiting behavior in ‘zombie’ Drosophila. Elife12, e85410 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rana, A., Adams, M. E. & Libersat, F. Parasitoid wasp venom re-programs host behavior through downmodulation of brain central complex activity and motor output. J. Exp. Biol.226, jeb245252 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamo, S. A. Parasites: evolution’s neurobiologists. J. Exp. Biol.216, 3–10 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Shi, M. et al. The endoparasitoid, Cotesia vestalis, regulates host physiology by reprogramming the neuropeptide transcriptional network. Sci. Rep.5, 8173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanuel, S. & Libersat, F. Do quiescence and wasp venom-induced lethargy share common neuronal mechanisms in cockroaches? Plos One. 12, e0168032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvidson, R. et al. Parasitoid Jewel Wasp mounts multipronged neurochemical attack to hijack a host Brain*[S]. Mol. Cell. Proteom.18, 99–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser, M., Arvidson, R., Zarivach, R., Adams, M. E. & Libersat, F. Molecular cross-talk in a unique parasitoid manipulation strategy. Insect Biochem. Mol. Biol.106, 64–78 (2019). [DOI] [PubMed] [Google Scholar]
- 10.de Bekker, C. & Das, B. Hijacking time: how ophiocordyceps fungi could be using ant host clocks to manipulate behavior. Parasite Immunol.44, e12909 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, X. et al. Baculoviruses hijack the visual perception of their caterpillar hosts to induce climbing behaviour thus promoting virus dispersal. Mol. Ecol.31, 2752–2765 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Žitňan, D., Kingan, T. G., Kramer, S. J. & Beckage, N. E. Accumulation of neuropeptides in the cerebral neurosecretory system of Manduca sexta larvae parasitized by the braconid wasp Cotesia congregata. J. Comp. Neurol.356, 83–100 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Moore, J. Parasites and the Behavior of Animals (Oxford University Press, 2002). [Google Scholar]
- 14.Beckage, N. & Gelman, D. Parasitism of Manduca sexta by Cotesia congregata: A multitude of disruptive endocrine effects. In Endocrine Interactions of Parasites and Pathogens (ed. J. P. Edwards and R. J. Weaver), pp. 59–81. Oxford: Bios Scientific Publishers Ltd. (2001).
- 15.Thompson, S. & Redak, R. Parasitism of an insect Manduca sexta L. alters feeding behaviour and nutrient utilization to influence developmental success of a parasitoid. J. Comp. Physiol. B.178, 515–527 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Adamo, S. A., Linn, C. E. & Beckage, N. E. Correlation between changes in host behaviour and octopamine levels in the tobacco hornworm Manduca sexta parasitized by the gregarious braconid parasitoid wasp Cotesia congregata. J. Exp. Biol.200, 117–127 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Griss, C., Simpson, S., Rohrbacher, J. & Rowell, C. Localization in the central nervous system of larval Manduca sexta (Lepidoptera: Sphingidae) of areas responsible for aspects of feeding behaviour. J. Insect. Physiol.37, 477–482 (1991). [Google Scholar]
- 18.Beckage, N. E., Tan, F. F., Schleifer, K. W., Lane, R. D. & Cherubin, L. L. Characterization and biological effects of Cotesia congregata polydnavirus on host larvae of the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol.26, 165–195 (1994). [Google Scholar]
- 19.Chevignon, G. et al. Cotesia congregata Bracovirus circles encoding PTP and Ankyrin genes integrate into the DNA of parasitized Manduca sexta hemocytes. J. Virol.92, 101128jvi00438–101128jvi00418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckage, N. E. & Gelman, D. B. Wasp parasitoid disruption of host development: implications for new biologically based strategies for insect control. Annual Reviews Entomol.49, 299–330 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Chevignon, G. et al. Transcriptomic response of Manduca sexta immune tissues to parasitization by the bracovirus associated wasp Cotesia congregata. Insect Biochem. Mol. Biol.62, 86–99 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Espagne, E. et al. A virus essential for insect host-parasite interactions encodes cystatins. J. Virol.79, 9765–9776 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwood, S. H. & Beckage, N. E. Purification and characterization of an early-expressed polydnavirus-induced protein from the hemolymph of Manduca sexta larvae parasitized by Cotesia congregata. Insect Biochem. Mol. Biol.24, 685–698. 10.1016/0965-1748(94)90056-6 (1994). [Google Scholar]
- 24.Bitra, K., Zhang, S. & Strand, M. R. Transcriptomic profiling of Microplitis demolitor bracovirus reveals host, tissue and stage-specific patterns of activity. J. Gen. Virol.92, 2060–2071 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Adamo, S. A., Kovalko, I., Turnbull, K. F., Easy, R. H. & Miles, C. I. The parasitic wasp Cotesia congregata uses multiple mechanisms to control host (Manduca sexta) behaviour. J. Exp. Biol.219, 3750–3758 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Adamo, S. A. Feeding suppression in the tobacco hornworm, Manduca sexta: costs and benefits to the parasitic wasp Cotesia congregata. Can. J. Zool.76, 1634–1640. 10.1139/z98-105 (1998). [Google Scholar]
- 27.Kester, K. M. & Jackson, D. M. When good bugs go bad: intraguild predation by Jalysus wickhami on the parasitoid, Cotesia congregata. Entomol. Exp. Appl.81, 271–276 (1996). [Google Scholar]
- 28.Adamo, S. A. Parasitic suppression of feeding in the tobacco hornworm, Manduca sexta: parallels with feeding depression after an immune challenge. Arch. Insect Biochem. Physiol.60, 185–197 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Beckage, N. E. & Templeton, T. J. Physiological effects of parasitism by Apanteles congregatus in terminal stage tobacco hornworm larvae. J. Insect. Physiol.32, 299–314 (1986). [Google Scholar]
- 30.Miles, C. I. & Booker, R. Octopamine mimics the effects of parasitism on the foregut of the tobacco hornworm Manduca sexta. J. Exp. Biol.203, 1689–1700 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Adamo, S. A., Davies, G., Easy, R., Kovalko, I. & Turnbull, K. F. Reconfiguration of the immune system network during food limitation in the Caterpillar Manduca sexta. J. Exp. Biol.219, 706–718 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Miles, C. I., Chen, W. P., Adamo, S. A., Kester, K. M. & Miller, D. W. Manduca sexta caterpillars parasitized by the wasp Cotesia congregata stop chewing despite an intact motor system. J. Exp. Biol.226, jeb245716 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Dunn, P. E., Bohnert, T. J. & Russell, V. Regulation of Antibacterial protein synthesis following infection and during metamorphosis. Annals New. York Acad. Sci. 117 (1994). [DOI] [PubMed]
- 34.Adamo, S. A. & McMillan, L. E. Listening to your gut: immune challenge to the gut sensitized body wall nociception of the Caterpillar Manduca sexta. Philosophical Transactions: Biol. Sci.374, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanha-Aho, L. M., Valanne, S. & Rämet, M. Cytokines in Drosophila immunity. Immunol. Lett.170, 42–51 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Adamo, S. Why should an immune response activate the stress response? Insights from the insects (the cricket Gryllus texensis). Brain. Behav. Immun.24, 194–200 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Sung, E. J. et al. Cytokine signaling through Drosophila Mthl10 ties lifespan to environmental stress. Proceedings of the National Academy of Sciences 114, 13786–13791 (2017). [DOI] [PMC free article] [PubMed]
- 38.Adamo, S. A. Bidirectional connections between the immune system and the nervous system in insects. Insect Immunol., 129–149 (2008).
- 39.Sullivan, K., Fairn, E. & Adamo, S. A. Sickness behaviour in the cricket Gryllus texensis: comparison with animals across phyla. Behav. Process.128, 134–143 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Adamo, S. A., Fidler, T. L. & Forestell, C. A. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain. Behav. Immun.21, 292–300 (2007). [DOI] [PubMed] [Google Scholar]
- 41.McMillan, L. E., Miller, D. W. & Adamo, S. A. Eating when ill is risky: immune defense impairs food detoxification in the Caterpillar Manduca sexta. J. Exp. Biol.221, jeb173336 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Shaik, H. A., Mishra, A., Sehadová, H. & Kodrík, D. Responses of sericotropin to toxic and pathogenic challenges: possible role in defense of the wax moth Galleria mellonella. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.227, 108633 (2020). [DOI] [PubMed] [Google Scholar]
- 43.DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem.139, 136–153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarkson, B. D., Kahoud, R. J., McCarthy, C. B. & Howe, C. L. Inflammatory cytokine-induced changes in neural network activity measured by waveform analysis of high-content calcium imaging in murine cortical neurons. Sci. Rep.7, 9037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helluy, S. & Thomas, F. Parasitic manipulation and neuroinflammation: evidence from the system Microphallus Papillorobustus (Trematoda)-Gammarus (Crustacea). Parasites Vectors. 3, 1–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dheilly, N. M. et al. Who is the puppet master? Replication of a parasitic wasp-associated virus correlates with host behaviour manipulation. Proceedings of the Royal Society B: Biological Sciences 282, 20142773 (2015). [DOI] [PMC free article] [PubMed]
- 47.Wang, T. et al. From inflammatory reactions to neurotransmitter changes: implications for understanding the neurobehavioral changes in mice chronically infected with Toxoplasma Gondii. Behav. Brain. Res.359, 737–748 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Mangold, C. A. & Hughes, D. P. Insect behavioral change and the potential contributions of neuroinflammation—a call for future research. Genes12, 465 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biron, D. G. & Loxdale, H. D. Host–parasite molecular cross-talk during the manipulative process of a host by its parasite. J. Exp. Biol.216, 148–160 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Le, N., Asgari, S., Amaya, K., Tan, F. & Beckage, N. E. Persistence and expression of Cotesia congregata polydnavirus in host larvae of the tobacco hornworm, Manduca sexta. J. Insect. Physiol.49, 533–543 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Zang, Y. & Marder, E. Neuronal morphology enhances robustness to perturbations of channel densities. Proceedings of the National Academy of Sciences 120, e2219049120 (2023). [DOI] [PMC free article] [PubMed]
- 52.Marom, S. & Marder, E. A biophysical perspective on the resilience of neuronal excitability across timescales. Nat. Rev. Neurosci.24, 640–652 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Alleyne, M., Chappell, M. A., Gelman, D. B. & Beckage, N. E. Effects of parasitism by the braconid wasp Cotesia congregata on metabolic rate in host larvae of the tobacco hornworm, Manduca sexta. J. Insect. Physiol.43, 143–154 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Truman, J. W., Talbot, W. S., Fahrbach, S. E. & Hogness, D. S. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development120, 219–234 (1994). [DOI] [PubMed] [Google Scholar]
- 55.Fahrbach, S. E., Klukas, K. A. & Mesce, K. A. Steroid regulation of cell populations in the insect central nervous system. Neuroplasticity, development, and steroid hormone action. Boca Raton, FL: CRC Press. p, 31–44 (2001).
- 56.Hanson, M. A. & Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol.62, 22–30 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Stączek, S., Cytryńska, M. & Zdybicka-Barabas, A. Unraveling the role of antimicrobial peptides in insects. Int. J. Mol. Sci.24, 5753 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvador, A. F., de Lima, K. A. & Kipnis, J. Neuromodulation by the immune system: a focus on cytokines. Nat. Rev. Immunol.21, 526–541 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Düsedau, H. P. et al. Influenza a virus (H1N1) infection induces microglial activation and temporal dysbalance in glutamatergic synaptic transmission. MBio12, 101128mbio01776–101128mbio01721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao, Y., Chtarbanova, S., J Petersen, A. & Ganetzky, B. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proc. Natl. Acad. Sci.110, E1752–E1760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shukla, A. K., Spurrier, J., Kuzina, I. & Giniger, E. Hyperactive innate immunity causes degeneration of dopamine neurons upon altering activity of Cdk5. Cell. Rep.26, 131–144 (2019). e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maure, F., Brodeur, J., Droit, A., Doyon, J. & Thomas, F. Bodyguard manipulation in a multipredator context: different processes, same effect. Behav. Process.99, 81–86 (2013). [DOI] [PubMed] [Google Scholar]
- 63.van Griethuijsen, L. I., Banks, K. M. & Trimmer, B. A. Spatial accuracy of a rapid defense behavior in caterpillars. J. Exp. Biol.216, 379–387 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Lee, G. & Schwarz, T. L. Filamin, a synaptic organizer in Drosophila, determines glutamate receptor composition and membrane growth. Elife5, e19991 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schevzov, G., Curthoys, N. M., Gunning, P. W. & Fath, T. Functional diversity of actin cytoskeleton in neurons and its regulation by tropomyosin. Int. Rev. cell. Mol. Biology. 298, 33–94 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Mosca, T. J. On the Teneurin track: a new synaptic organization molecule emerges. Front. Cell. Neurosci.9, 204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cafferty, P. & Auld, V. J. No pun intended: future directions in invertebrate glial cell migration studies. Neuron Glia Biol.3, 45–54 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Torson, A. S., Dong, Y. & Sinclair, B. J. Help, there are ‘omics’ in my comparative physiology! J. Exp. Biol.223, jeb191262 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Khorramnejad, A., Perdomo, H. D., Palatini, U., Bonizzoni, M. & Gasmi, L. Cross talk between viruses and insect cells cytoskeleton. Viruses13, 1658 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amaya, K. E., Asgari, S., Jung, R., Hongskula, M. & Beckage, N. E. Parasitization of Manduca sexta larvae by the parasitoid wasp Cotesia congregata induces an impaired host immune response. J. Insect. Physiol.51, 505–512 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Lovallo, N. & Cox-Foster, D. L. Alteration in FAD–glucose dehydrogenase activity and hemocyte behavior contribute to initial disruption of Manduca sexta immune response to Cotesia congregata parasitoids. J. Insect. Physiol.45, 1037–1048 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Goaillard, J. M. & Marder, E. Ion channel degeneracy, variability, and covariation in neuron and circuit resilience. Annu. Rev. Neurosci.44, 335–357 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Turrigiano, G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci.34, 89–103 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Will, I., Attardo, G. & de Bekker, C. Multiomic interpretation of fungus-infected ant metabolomes during manipulated summit disease. Sci. Rep.13, 14363 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bézier, A. et al. Bracovirus gene products are highly divergent from insect proteins. Archives Insect Biochem. Physiology: Published Collab. Entomol. Soc. Am.67, 172–187 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Bitra, K., Suderman, R. J. & Strand, M. R. Polydnavirus Ank proteins bind NF-κB homodimers and inhibit processing of Relish. PLoS Pathog.8, e1002722 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adamo, S. A. & Shoemaker, K. L. Effects of parasitism on the octopamine content of the central nervous system of Manduca sexta: a possible mechanism underlying host behavioural change. Can. J. Zool.78, 1580–1587 (2000). [Google Scholar]
- 78.Trimmer, B. A. & Weeks, J. C. Effects of nicotinic and muscarinic agents on an identified motoneurone and its direct afferent inputs in larval Manduca sexta. J. Exp. Biol.144, 303–337 (1989). [Google Scholar]
- 79.Tabuena, D. R., Solis, A., Geraldi, K., Moffatt, C. A. & Fuse, M. Central neural alterations predominate in an insect model of nociceptive sensitization. J. Comp. Neurol.525, 1176–1191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emanuel, S., Kaiser, M., Pflueger, H. J. & Libersat, F. On the role of the head ganglia in posture and walking in insects. Front. Physiol.11, 135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernardo, M. A. & Singer, M. S. Parasite-altered feeding behavior in insects: integrating functional and mechanistic research frontiers. J. Exp. Biol.220, 2848–2857 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Zapalska-Sozoniuk, M., Chrobak, L., Kowalczyk, K. & Kankofer, M. Is it useful to use several omics for obtaining valuable results? Mol. Biol. Rep.46, 3597–3606 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Dzyubenko, E. et al. Inhibitory control in neuronal networks relies on the extracellular matrix integrity. Cell. Mol. Life Sci.78, 5647–5663 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alberti, P., Semperboni, S., Cavaletti, G. & Scuteri, A. Neurons: the interplay between cytoskeleton, ion channels/transporters and mitochondria. Cells11, 2499 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ponton, F. et al. Do distantly related parasites rely on the same proximate factors to alter the behaviour of their hosts? Proc. Royal Soc. B: Biol. Sci.273, 2869–2877 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biron, D. G. et al. First analysis of the proteome in two nematomorph species, Paragordius tricuspidatus (Chordodidae) and Spinochordodes tellinii (Spinochordodidae). Infect. Genet. Evol.5, 167–175 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Biron, D. et al. Suicide’of crickets harbouring hairworms: a proteomics investigation. Insect Mol. Biol.15, 731–742 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Feldmeyer, B. et al. Gene expression patterns underlying parasite-induced alterations in host behaviour and life history. Mol. Ecol.25, 648–660 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc.: Ser. B (Methodol.). 57, 289–300 (1995). [Google Scholar]
- 90.Robertson, R. M., Spong, K. E. & Srithiphaphirom, P. Chill coma in the Locust, Locusta Migratoria, is initiated by spreading depolarization in the central nervous system. Sci. Rep.7, 10297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laura, M. & Mendeley 10.5683/SP3/FRJDPT, Borealis, (2022). V1.
- 92.Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol.26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 93.Taylor, S., Wakem, M., Dijkman, G., Alsarraj, M. & Nguyen, M. A practical approach to RT-qPCR—publishing data that conform to the MIQE guidelines. Methods50, S1–S5 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Kumar, P., Pandit, S. S. & Baldwin, I. T. Tobacco rattle virus vector: a rapid and transient means of silencing Manduca sexta genes by plant mediated RNA interference. PloS One. 7, e31347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rewitz, K. F., Rybczynski, R., Warren, J. T. & Gilbert, L. I. Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol.36, 188–199 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Gad, W. & Kim, Y. A viral histone H4 encoded by Cotesia plutellae bracovirus inhibits haemocyte-spreading behaviour of the diamondback moth, Plutella Xylostella. J. Gen. Virol.89, 931–938 (2008). [DOI] [PubMed] [Google Scholar]
- 97.An, C., Jiang, H. & Kanost, M. R. Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J.277, 148–162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwartz, L. M., Jones, M. E., Kosz, L. & Kuah, K. Selective repression of actin and myosin heavy chain expression during the programmed death of insect skeletal muscle. Dev. Biol.158, 448–455 (1993). [DOI] [PubMed] [Google Scholar]
- 99.Mészáros, M. & Morton, D. B. Comparison of the expression patterns of five developmentally regulated genes in Manduca sexta and their regulation by 20-hydroxyecdysone in vitro. J. Exp. Biol.199, 1555–1561 (1996). [DOI] [PubMed] [Google Scholar]
- 100.MacWilliam, D., Arensburger, P., Higa, J., Cui, X. & Adams, M. E. Behavioral and genomic characterization of molt-sleep in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol.62, 154–167 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Zhu, Y., Wang, Y., Gorman, M. J., Jiang, H. & Kanost, M. R. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J. Biol. Chem.278, 46556–46564 (2003). [DOI] [PubMed] [Google Scholar]
- 102.Pfaffl, M. W., Horgan, G. W. & Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res.30, e36–e36 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21. 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gershman, A. et al. De novo genome assembly of the tobacco hornworm moth (Manduca sexta). G3 (Bethesda). 1110.1093/g3journal/jkaa047 (2021). [DOI] [PMC free article] [PubMed]
- 106.Gauthier, J. et al. Chromosomal scale assembly of parasitic wasp genome reveals symbiotic virus colonization. Commun. Biol.4, 104. 10.1038/s42003-020-01623-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lentz, B. R., McIntyre, G. F., Parks, D. J., Yates, J. C. & Massenburg, D. Bilayer curvature and certain amphipaths promote poly(ethylene glycol)-induced fusion of dipalmitoylphosphatidylcholine unilamellar vesicles. Biochemistry31, 2643–2653. 10.1021/bi00125a003 (1992). [DOI] [PubMed] [Google Scholar]
- 108.Liao, Y., Smyth, G. K. & Shi, W. Feature counts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30, 923–930. 10.1093/bioinformatics/btt656 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Love, M. I., Huber, W. & Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550. 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martyniuk, C. J. Perspectives on transcriptomics in animal physiology studies. Comp. Biochem. Physiol. B: Biochem. Mol. Biol.250, 110490. 10.1016/j.cbpb.2020.110490 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Yuan, Y., Xiao, R., Ge, Q., Taha, R. H. & Chen, K. Complementary transcriptomic and proteomic analysis of Bombyx mori middle silk glands reveals a predominant ribosome-biogenesis regulating network during silkworm yellow-cocoon color formation. J. Asia. Pac. Entomol.24, 260–270 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the supplementary file. Complete transcriptomic and proteomic datasets are available for download at: McMillan, L., Herbison, R., Raun, N. & Adamo, S. Datasets for “The multiple effects of the wasp Cotesia congregata, a parasitic manipulator, on the brain of its host, the caterpillar Manduca sexta” https://doi.org/10.5683/SP3/FRJDPT , Borealis, V1.