Abstract
Escherichia coli O78 strains are frequently associated with extraintestinal diseases, such as airsacculitis and septicemia, in poultry, livestock, and humans. To understand the influence of the pst operon in the virulence of E. coli, we introduced mutations into the pst genes of the avian pathogenic E. coli (APEC) O78:K80 strain χ7122 by allelic exchange. The mutation of pst genes led to the constitutive expression of the Pho regulon. Furthermore, the virulence of APEC strain χ7122 in a chicken infection model was attenuated by inactivation of the Pst system. The pst mutant caused significantly fewer extraintestinal lesions in infected chickens, and bacterial numbers isolated from different tissues after infection were significantly lower for the mutant than for the wild-type strain. Moreover, resistance to the bactericidal effects of rabbit serum and acid shock was impaired in the pst mutant, in contrast to the wild-type strain. In addition, the MIC of polymyxin was twofold lower for the mutant than for the wild-type strain. Although the pst mutant demonstrated an increased susceptibility to rabbit serum, this strain was not killed by chicken serum, suggesting the presence of differences in host innate immune defenses and complement-mediated killing. In APEC O78 strain χ7122, a functional Pst system is required for full virulence and resistance to acid shock and polymyxin. Our results suggest that the mutation of pst genes induces a deregulation of phosphate sensing and changes in the cell surface composition that lead to decreased virulence, indicating the importance of the Pst system for the virulence of pathogenic E. coli strains from different hosts.
Avian pathogenic Escherichia coli (APEC) is a frequent cause of extraintestinal infections, collectively called colibacillosis, in poultry. Each year, the poultry industry absorbs important financial losses due to the high morbidity and mortality rates caused by APEC. Housing conditions and coinfections by other microorganisms are major physical and biological risk factors that predispose poultry to colibacillosis. Most infections occur via aerosol transmission and colonization of air sacs (4). E. coli strains causing extraintestinal diseases have been termed extraintestinal pathogenic E. coli (ExPEC) (44). APEC O78:K80:H9 strain χ7122 possesses several ExPEC-associated attributes which are involved in the development of diseases such as septicemia, perihepatitis, and pericarditis, and it belongs to one of the most prevalent serogroups that cause avian colibacillosis (48). E. coli O78 strains can also cause diseases in hosts other than poultry, such as swine, sheep, and humans (2, 21). Research conducted on E. coli O78 strains from different hosts revealed that pathogenic strains are clonally related. Moreover, this phenomenon is host independent. Therefore, pathogenic O78 strains of animal origins represent a high risk of zoonotic infections (1, 11, 63, 64).
The ability to sense microenvironments encountered within the host is an essential virulence property of bacterial pathogens (35). Sensing is followed by the coordination of virulence factor expression, promoting bacterial survival and replication (24, 28). In E. coli, the expression of many proteins is induced by growth under limiting conditions. For example, the expression of the Pho regulon is activated during growth conditions of phosphate limitation, whereas its expression is inhibited when phosphate is in excess (60). The Pho regulon is controlled by the two-component regulatory system PhoB/PhoR, which responds to the phosphate concentration. The PhoR sensor protein, located in the inner membrane, transmits a signal by phosphorylating the response regulator protein PhoB, which in turn activates transcription by binding to the “Pho box” sequence of the targeted promoters. The PhoBR two-component regulatory system controls the expression of several genes, and currently at least 39 of these genes have been identified (7, 30). The Pho regulon could possibly include as many as 137 proteins (56). The specific phosphate transport system (Pst) belongs to the Pho regulon and is encoded by the pstSCAB-phoU (pst) operon. This system consists of a periplasmic Pi binding protein (PstS), two transmembrane proteins (PstA and PstC), an ATP binding protein (PstB), and PhoU (42, 49). The two transmembrane proteins and PstB mediate the uptake of phosphate from the outside to the inside of the cell, whereas the function of PhoU is not clear. PhoU probably plays a regulatory role in the negative control of the Pho regulon and is required to repress the Pho regulon under high-phosphate conditions (61). Interestingly, the Pst transport system itself appears to be directly involved in the regulatory cascade and might serve as the primary sensor of the extracellular phosphate concentration (59). An intact Pst system and the PhoR protein are necessary for Pho regulon repression, as constitutive expression of the Pho regulon, including phoA, was observed when genes of the pst-phoU operon and the phoR gene were mutated (62). Under phosphate-replete conditions, PhoB activation is blocked in a PhoR- and PhoU-dependent manner (23, 46, 47).
Several reports have described an association between the Pst system, the Pho regulon, and bacterial virulence (6, 12, 18, 34, 58). PhoB of APEC strain χ7122 was reported to be expressed during infection (14), as discovered by the use of a technique that selectively captures unique transcripts (selective capture of transcribed sequences [13, 22]). Moreover, other Pho-regulated genes were also shown to be expressed in vivo by ExPEC strains (3, 29). Inactivation of the pst operon in a porcine ExPEC strain resulted in constitutive expression of the Pho regulon and rendered the strain avirulent. APEC strain χ7122 (O78:K80:H9) (39) shares many virulence attributes with the porcine ExPEC strain, including the ability to persist systemically and resist serum (12, 15). Although the inactivation of pst has been shown to affect the virulence of several bacterial pathogens, the mechanisms underlying this attenuation have not been elucidated. One hypothesis to explain the decreased virulence due to abrogation of the Pst system is that the inactivation of pst genes causes an alteration in the bacterial cell surface composition.
For this study, we generated a Δpst mutant of APEC strain χ7122 and demonstrated that the abrogation of the Pst system influences multiple virulence attributes associated with the cell surface composition. Moreover, we demonstrate here that the deletion of pst genes significantly reduces the virulence of APEC strain χ7122. These results extend the importance of the Pst system in the virulence of pathogenic E. coli strains belonging to different serogroups and being pathogenic for different animal hosts.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strains and plasmids used for this study are listed in Table 1. In general, bacteria were grown in Luria-Bertani (LB) broth at 37°C. LB broth is a high-phosphate medium. Low-Pi (LP) broth was used for alkaline phosphatase assays and reverse transcription-PCR (RT-PCR) experiments [LP broth is composed of 50 mM Tris-HCl (pH 7.4), 10 mM KCl, 10 mM (NH4)2SO4, 0.4 mM MgSO4, 0.1% yeast extract, 20 mM glucose, and 1 mM methionine]. For experimental infections of chickens, beef heart infusion broth and MacConkey-lactose agar plates were used. Antibiotics or supplements were used at the following final concentrations, when required: ampicillin (Amp), 100 μg/ml; chloramphenicol, 12.5 μg/ml; kanamycin (Kan), 50 μg/ml; nalidixic acid, 40 μg/ml; streptomycin (Sm), 100 μg/ml; and 5-bromo-4-chloro-3-indolylphosphate (XP), 40 μg/ml.
TABLE 1.
Bacterial strains and plasmids used for this study
| Bacterial strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Bacterial strains | ||
| SM10λpir | thi-1 leu tonA lacY supE recA::RP4-2-Tc::Mu λpir Kmr | 37 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| χ7122 | Avian pathogenic strain, O78:K80:H9 gyrA, Nalr | 41 |
| χ7122(pAN92) | χ7122 + pAN92 | This work |
| K3 | χ7122 ΔpstCAB::kan | This work |
| CK3 | K3 + pAN92 | This work |
| χ7145 | χ7122, rfb deleted by replacement with E. coli K-12 region at 45 min | 9 |
| Plasmids | ||
| pAN92 | pACYC184::pst operon, Cmr | 26 |
| pCR2.1 | Cloning vector | Invitrogen |
| pKNG101 | Suicide vector, SmrsacB | 27 |
| pKNG800K | pKNG101, ΔpstCAB::kan sacB, Smr Kmr | This work |
| pUC71K | Contains aphT with transcription terminator, Kanr | 40, 57 |
Kmr, kanamycin resistant; Cmr, chloramphenicol resistant; Smr, streptomycin resistant; Nalr, nalidixic acid resistant; Tetr, tetracycline resistant.
Generation of pst mutant by allelic exchange.
E. coli strain XL1-Blue was used for cloning experiments. A 2,914-bp PCR fragment containing the pstSCAB genes was amplified from strain χ7122 (41) with the PstF (CTGCCGGATAGTGTAGT) and PstBrev (CATCATGTCCGTGCTCC) primers and then cloned into the pCR2.1 vector by use of an Original TA cloning kit (Invitrogen) according to the manufacturer's instructions. A deletion (ΔpstCAB) in the pst operon was obtained by removing an internal EcoRV fragment. A kanamycin resistance cassette, carried on a HincII fragment from pUC71K (40, 57), was then ligated to the EcoRV site, resulting in ΔpstCAB::kan. The construct was digested with XbaI and BamHI, and the ΔpstCAB::kan fragment was ligated to the pKNG101 suicide vector (27) cut with the same enzymes. The resulting construct, pKNG800K, was transferred to strain SM10λpir (37) and was then mobilized in χ7122 by conjugation. Single-crossover integrants of strain χ7122 were selected on M9 agar containing appropriate antibiotics (Amp, Kan, and Sm). Selection for double-crossover allele replacement was obtained by sacB counterselection on LB agar plates without NaCl but containing 5% sucrose (27) and XP. A χ7122 derivative, strain K3, was confirmed to contain a deletion in the pst operon, as determined by PCR amplification and Southern blot hybridization (data not shown). The pAN92 plasmid (26) carrying a functional pst operon was used to complement the ΔpstCAB::kan mutant K3 and the wild-type parent strain χ7122 to create strains CK3 and χ7122(pAN92), respectively. The pAN92 plasmid is a derivative of the medium-copy-number pACYC184 plasmid.
Alkaline phosphatase assay.
Alkaline phosphatase was measured as described previously (12, 52, 61). Briefly, cells grown under different conditions were adjusted to an optical density at 600 nm (OD600) of 1.0, and 4 μg/ml of p-nitrophenyl phosphate was added to cells permeabilized by 50 μl of 1% sodium dodecyl sulfate (SDS) and 50 μl of chloroform. Color development was monitored at 420 nm, and PhoA activity was expressed in enzyme units per minute, calculated as follows: 1,000 × [OD420 − (1.75 × OD550)]/T (min) × V (ml) × OD600.
RNA isolation and RT-PCR.
Strains were grown in high-phosphate (LB) or low-phosphate (LP) medium and were harvested during the mid-log growth phase. An RNeasy mini kit from QIAGEN was used for the isolation of total RNA from bacteria. Quantification of the total amount of RNA was achieved by spectrophotometry. A QIAGEN OneStep RT-PCR kit was used for RT-PCR, with 1 μg of RNA template used for each reaction. Two primer pairs were used to target pstA and phoB expression. The rpsL gene was used as a housekeeping control. The reverse transcription reaction was achieved at 50°C for 30 min. The DNA polymerase (HotStart Taq) was then activated at 95°C for 15 min as indicated by the manufacturer. For each sample, 25 cycles of PCR amplification were performed with each different primer pair. The annealing temperatures used were 67°C, 62°C, and 65°C for the pstA-, phoB-, and rpsL-specific primer pairs, respectively. The sequences of the pstA-specific forward and reverse primers were 5′-CAAACCACTGCGGCGCTGGCTGAATCTCG-3′ and 5′-GTGCTCCATCTGCGCCACCACAATGGTG-3′, respectively. The sequences of the phoB-specific forward and reverse primers were 5′-CGTATTCTGGTCGTAGAAGATGAAGCTC-3′ and 5′-CACGGTCTGCACCATGCGGTCA-3′, respectively. The sequences of the rpsL-specific forward and reverse primers were 5′-GTTAACCAGCTGGTACGCAAACCACGTGC-3′ and 5′-GGACGCTTCACGCCATACTTGGAACGAG-3′, respectively.
Experimental infection of chickens via air sacs.
Three groups of five 3-week-old White Leghorn specific-pathogen-free chickens (SPAFAS Farms) were reared in separate Horsfall isolator cages with food and water available ad libitum. Each group of chickens was inoculated in the right thoracic air sac with 0.1 ml (107 CFU) of a bacterial inoculum consisting of a diluted 24-h beef heart infusion broth culture of either E. coli χ7122, the pst mutant, or the complemented strain. Blood samples were collected aseptically from each chicken 6, 24, and 48 h following bacterial inoculation and were plated directly or diluted 1:4 in phosphate-buffered saline (pH 7.4), after which 0.1 ml was plated on MacConkey-lactose agar plates (Difco) supplemented with nalidixic acid (40 μg/ml) and appropriate antibiotics in the cases of the E. coli pst mutant and complemented strains. All birds were euthanized at 48 h postinfection and then necropsied. Macroscopic lesion scores for the air sacs and combined lesion scores for the internal organs (heart/pericardium and liver) were determined based on a scheme similar to that used by Melatta et al. (36). Lesions in the caudal thoracic air sacs were scored from 0 to 4 for each of the left and right air sacs based on the following criteria: 0, no lesions (normal); 1, slight opacity/edema; 2, mild diffuse thickening and fibrinous exudate, mild neovascularization; 3, moderate fibrinous exudate, opacity, thickening of membrane, and neovascularization; 4, severe extensive fibrinous exudate, opacity, and neovascularization of the air sac. The mean score value for the left and right air sacs was then used as the combined air sac lesion score for each individual. The maximal possible lesion score for the air sacs of an individual chicken was therefore 4. Combined lesion scores for pericarditis and perihepatitis were based on the following criteria. For the heart and pericardium, the following scores were used: 0, normal; 1, vascularization, opacity, and cloudy pericardial fluid; and 2, acute pericarditis (extensive fibrinous exudate covering the pericardium). For the liver, the following scores were used: 0, normal; 1, slight fibrinous exudate or decoloration of lobes; and 2, severe perihepatitis (extensive fibrinous exudate). The values for lesions of the heart/pericardium and liver were then added for each individual, giving a maximal possible combined individual score of 4 for lesions of the heart/pericardium and liver. The mean lesion scores ± standard deviations for either the air sacs or internal organs of individuals belonging to each of the infection groups were used for statistical analyses between groups. Organs were aseptically removed. The left lung, liver, and spleen of each animal were weighed, suspended in phosphate-buffered saline, and homogenized with an Omnimixer homogenizer. Dilutions of homogenates were plated onto MacConkey-lactose agar plates with appropriate antibiotics for bacterial quantification. Several randomly selected colonies per organ were verified by serotyping using O78-specific antiserum.
Serum bactericidal assay.
The serum bactericidal assay was adapted from the work of Taylor and Kroll (51). Briefly, bacteria were grown overnight in LB broth at 37°C. Bacterial cultures were then resuspended in fresh medium at a 10-fold dilution, incubated at 37°C, and harvested during the logarithmic growth phase. Bacteria were washed at room temperature with gelatin-Veronal-buffered saline pH 7.35 and then resuspended to a concentration of 107 CFU/ml. A volume of 0.1 ml of the bacterial suspension was added to 0.9 ml of normal rabbit serum or chicken serum and then incubated at 37°C. Viable cell counts were estimated at 0, 1, 2, and 3 h by spreading out dilutions of the suspension on LB agar plates. A strain was considered resistant if the bacterial count increased or did not change, intermediate if a decrease in the bacterial count of up to 2 orders of magnitude was observed, and sensitive if a decrease in the bacterial count of >2 orders of magnitude was observed. The survival rate was calculated as the CFU determined at each time point divided by the initial CFU present at time zero.
Polymyxin assay.
Strains were grown overnight and cultures were diluted in fresh LB medium to obtain exponential-phase cultures (OD600 = 0.7). Cultures were then diluted to obtain 106 CFU/ml. Microwell plates were loaded with 90 μl of the 106-CFU/ml cultures, and 10 μl of polymyxin at different concentrations was added to each well to obtain final concentrations of polymyxin between 0.5 μg/ml and 2.5 μg/ml. The plates were incubated overnight, and bacterial growth in the wells was evaluated by spectrophotometry. The MIC was considered the lowest drug concentration that reduced growth >50% compared with growth in the control well (19).
LPS analysis.
Two milligrams of whole bacterial cells were solubilized in Laemmli buffer (31), boiled, and treated for 1 h with proteinase K (0.5 mg/ml) at 60°C. Proteinase K was then inactivated by boiling the samples for 2 min (25). Classical SDS-polyacrylamide gel electrophoresis (SDS-PAGE) or Tricine SDS-PAGE (33) was used for lipopolysaccharide (LPS) analysis. A volume of 10 μl of each sample was added to each well. Silver staining was used to reveal LPS bands (54). Western blot analysis was performed according to a standard procedure (53). Briefly, LPS bands were transferred onto a 0.2-μm nitrocellulose sheet at 100 V for 1 h. The membrane was incubated with a rabbit anti-O78 antiserum. Anti-rabbit immunoglobulin conjugated to peroxidase was used as the secondary antibody. O-chain LPS bands were revealed with a 4-chloro-1-naphthol solution.
Acid shock.
Acid shock of the cell cultures was performed by using protocol A as described by Chang and Cronan (10). Since survival following acid shock is influenced by the growth phase, preliminary growth curves were done for all strains by growth of the strains in LB broth at 37°C with agitation (150 rpm). Growth was monitored spectrophotometrically at 600 nm. For the acid shock treatment, strains were grown to early stationary phase in LB broth. Prior to acid shock, viable cell counts were done by spreading out dilutions of the suspension on LB agar plates (pH 7). HCl (1.7 N) was then rapidly added to cultures grown in LB medium until a pH of 3.0 was reached. The pH values were monitored by pH measurements of identical but separate cultures. After the addition of acid, the cultures were shaken at 37°C, and at different times, aliquots were diluted and plated for colony formation on LB plates. The survival rate was calculated as the CFU determined at each time point divided by the initial CFU present at time zero.
Statistical analyses.
Statistical analyses were performed with the data analysis package SYSTAT 10.0. Analysis of variance followed by Tukey's multiple comparison test was used for the analysis of bacterial counts, lesion scores, and the results of the serum, polymyxin, and acid shock assays. For in vitro assays, mean values were obtained from a minimum of three independent values. Statistical significance was established at P values of <0.05.
RESULTS
To evaluate whether a pst mutation affects the virulence of an avian pathogenic O78 strain, we inactivated the pst genes in strain χ7122 by suicide vector-mediated allelic exchange (27). The inactivation of pst was confirmed by PCR and Southern blot analyses, and a confirmed mutant was named K3 (Table 1). Restoration of the Pst system in this strain was achieved by complementation with the plasmid pAN92, which contains a functional pst operon. This generated strain CK3. Mutations of the pst operon are known to deregulate the repressed state of the Pho regulon under high-phosphate conditions and to lead to the constitutive expression of Pho-regulated genes (47). Thus, an evaluation of the state of activity of the Pho regulon in strains grown in high-phosphate medium (LB) or low-phosphate medium (LP) was achieved by measurements of the alkaline phosphatase (AP) activity and by RT-PCR. These experiments revealed that the K3 pst mutant exhibits a constitutive Pho phenotype (2,948 AP units), in contrast to the wild-type χ7122 strain (0 AP units), the complemented strain CK3 (16 AP units), and the wild-type χ7122 strain carrying pAN92 (0 AP units). RT-PCR experiments were used to evaluate the transcriptional states of both the pst operon and the Pho regulon. As expected, the expression of the pst operon, as measured by pstA expression, was higher in the wild-type strain grown in low-phosphate medium than that in the same strain grown in high-phosphate medium (LB) (Fig. 1). The pstA expression level was also low in the wild-type strain and the pst mutant complemented with pAN92 (CK3) (Fig. 1). There was no pstA transcript detected from strain K3 since the pstCAB genes were absent from this strain. However, the Pho regulon was constitutive in the pst mutant strain K3, since phoB expression in this strain grown under high-phosphate conditions showed similar transcription levels to those of the wild-type strain grown under low-phosphate conditions (Fig. 1). In the complemented strain, the repression of transcription of phoB was restored in high-phosphate medium.
FIG. 1.
Transcriptional analysis of the pst operon and the Pho regulon in E. coli strain χ7122 and its mutants. RT-PCR was performed to detect the presence of pstA (A), phoB (B), and rpsL (C) mRNAs in strain χ7122 and its isogenic pst mutants. cDNAs from χ7122 (wild type) grown in low-phosphate (LP) medium and from χ7122, χ7122(pAN92), K3 (ΔpstCAB::kan), and CK3 [K3(pAN92)] grown in high-phosphate (LB) medium are shown in lanes 1 to 5, respectively. RNAs were isolated from mid-log-phase cells. There was no pstA transcript in K3 since the pstCAB genes were absent from this strain. The rpsL transcript was used as a housekeeping control.
Pathogenicity.
To characterize pst mutant strain K3, we first evaluated the virulence potential of this strain compared to that of the wild-type APEC strain χ7122 in a chicken experimental model. Colibacillosis lesions were present in the air sacs, livers, and hearts of chickens infected with APEC strain χ7122 at 48 h postinfection (Table 2). In contrast, such lesions were minimal or absent from the organs of chickens infected with pst mutant strain K3. Complementation of the pst mutation restored the severity of the colibacillosis lesions to a level similar to that observed for chickens infected with the wild-type parent strain χ7122. Also, for chickens infected with strain K3, fewer bacteria were isolated from the bloodstream and all tissues at 48 h postinfection than from those of either the wild-type parent or the complemented mutant (Fig. 2). Viable bacterial counts of the pst mutant were significantly reduced in blood, lungs, and spleens at 48 h postinfection. No significant differences in bacterial numbers were observed in tissues or blood from chickens infected with either the wild-type strain or the complemented pst mutant CK3.
TABLE 2.
Score-based evaluation of colibacillosis lesions
| Strain | Mean lesion score ± SD
|
|
|---|---|---|
| Airsacculitisa | Pericarditis/perihepatitisb | |
| χ7122 | 2.56 ± 0.25 | 2.43 ± 1.07 |
| K3 | 1.63 ± 0.50c | 1.06 ± 0.20d |
| CK3 | 2.29 ± 1.01 | 2.70 ± 0.90 |
Lesion scores for airsacculitis in both caudal thoracic air sacs. See Materials and Methods for scoring scheme.
Combined lesion scoring values for pericarditis and perihepatitis. See Materials and Methods for scoring scheme.
P = 0.046 compared to lesions observed in the wild-type strain-infected group.
P = 0.0017 compared to lesions observed in the wild-type strain-infected group.
FIG. 2.
Experimental infection of chickens with APEC strain χ7122 or derivatives via the air sacs. The results are reported as CFU per gram of tissue or milliliter of blood at 6 and 48 h postinfection. The strains used were χ7122 (wild type), K3 (ΔpstCAB::kan), and CK3 [K3(pAN92)]. Asterisks indicate significant differences observed between bacterial quantification of the wild-type χ7122 and the pst mutant strain K3 in blood (P = 0.0048), lungs (P = 0.0059), and spleens (P = 0.0003) at 48 h postinfection. No significant differences in bacterial numbers were observed between the wild-type χ7122 strain and the complemented strain CK3.
Resistance to serum, polymyxin, and acid shock.
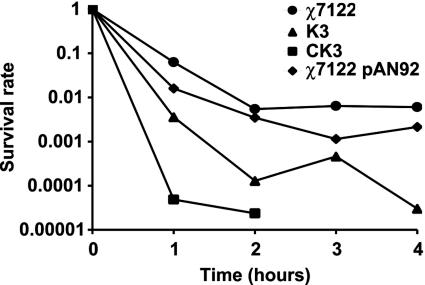
To provide further insight as to why the pst mutant of strain χ7122 was attenuated and to determine whether the inactivation of pst alters cell surface components, we investigated the capacity of the wild-type and mutant strains to resist serum, acid, and polymyxin. The K3 pst mutant was sensitive to the bactericidal effect of rabbit serum (Fig. 3A). In contrast, the wild-type strain χ7122 was resistant to the bactericidal effect of serum and was able to multiply in 90% rabbit serum after a 3-h period. Statistical analysis showed that the differences between the wild-type and K3 mutant strains were highly significant at 1, 2, and 3 h. There was no difference between the wild-type strain and CK3. In contrast to the results obtained for bacterial susceptibility to rabbit serum, the wild-type strain χ7122 and the pst mutant strain K3 were both resistant to the bactericidal effects of chicken serum (Fig. 3B). However, the O78-negative LPS mutant χ7145 (9) was rapidly killed by chicken serum and was not recovered from samples 1 h following serum exposure (data not shown).
FIG. 3.
Bacterial resistance to serum. The survival and growth of the E. coli wild-type strain χ7122 and the pst mutants were measured in 90% rabbit (A) or chicken (B) serum for various periods of time. •, strain χ7122 (wild type); ▴, strain K3 (ΔpstCAB::kan); ▪, strain CK3 [K3(pAN92)]. The results presented are means of three distinct tests. (A) The survival rate of the pst mutant strain K3 was significantly lower than the survival rates of both the wild-type strain χ7122 and the complemented strain CK3 at each time point. From 1 to 3 h of exposure to rabbit serum, the pst mutant K3 was significantly more sensitive to serum than either strain χ7122 (P < 0.006) or CK3 (P < 0.004). No significant differences were observed between the wild-type χ7122 strain and the complemented strain CK3. (B) No statistical differences were observed between the survival rates of strains χ7122, K3, and CK3 after exposure to chicken serum. The control strain χ7145 (χ7122 rfb−) did not survive after 1 h of exposure to 90% chicken serum (data not shown).
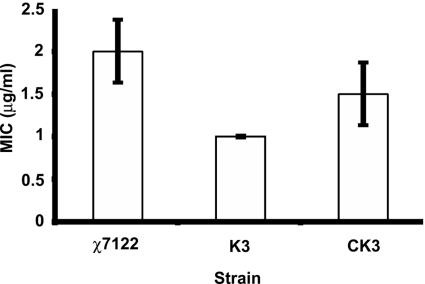
Strain K3 was more sensitive to polymyxin than the wild-type strain χ7122 since the MIC for the mutant strain was twofold lower (1 μg/ml compared to 2 μg/ml) than that for the wild-type strain (P < 0.001) (Fig. 4). Resistance to polymyxin was only partially restored in the complemented pst mutant strain (CK3). Since the O78 LPS antigen is an important factor that contributes to the resistance of strain χ7122 to serum (36) and since the modification of LPS can affect polymyxin resistance, we examined the LPS profiles of the strains. No differences were observed between strains regarding their LPS profiles, as assessed by silver staining (Fig. 5) or by Western blotting with an O78-specific antiserum (data not shown).
FIG. 4.
Polymyxin assay. The graph shows the MICs of polymyxin necessary to inhibit the growth of the different strains. The MIC was considered the lowest drug concentration that reduced growth >50% compared with the growth in the control well. The MICs of polymyxin for strains χ7122 (wild type), K3 (ΔpstCAB::kan), and CK3 [K3(pAN92)] are shown. The MIC for the pst mutant strain K3 was significantly lower than that for the wild-type χ7122 strain (P < 0.001). The MIC differences between the complemented strain CK3 and either strain χ7122 or strain K3 were significant (P = 0.044).
FIG. 5.
Analysis of LPS by Tricine SDS-PAGE (A) and SDS-PAGE (B). No differences in LPS profiles were observed among strains. Lanes 1, wild-type χ7122; lanes 2, pst mutant strain K3 (ΔpstCAB::kan); lanes 3, complemented strain CK3 [K3(pAN92)].
The pst mutant strain K3 clearly lost the ability to resist acid shock compared to the ability of the wild-type strain χ7122. Indeed, 2 h following acid shock, the viability of the K3 pst mutant cells was dramatically decreased (Fig. 6). In contrast, the wild-type strain χ7122 was more resistant to acid shock and the CFU stabilized after 2 to 4 h of exposure to acid. The differences between the wild-type and mutant strains were significant 1, 2, 3, and 4 h following acid shock. The complemented pst mutant strain CK3 was very sensitive to acid shock. One hour after acid exposure, CK3's viability was reduced >4 log, and no CFU were present after 2 h of acid-treated culture. The survival rate of the pst mutant strain K3 was significantly lower than that of the wild-type strain χ7122 at each time point (P < 0.035). However, the complementation of mutant K3 with pAN92 did not restore the wild-type survival rate. The presence of the pAN92 plasmid in the wild-type strain [χ7122(pAN92)] also decreased the survival rate at each time point compared to the wild-type survival rate.
FIG. 6.
Acid shock resistance experiment. The graph shows the survival rates of wild-type strain χ7122 and its mutant derivatives exposed to LB medium (pH 3) for various periods of time. •, strain χ7122 (wild type); ▴, strain K3 (ΔpstCAB::kan); ▪, strain CK3 [K3(pAN92)]; ⧫, strain χ7122(pAN92). In vitro acid shock tests were performed with early-stationary-phase cultures without habituation at 37°C.
DISCUSSION
APEC strain χ7122 is highly pathogenic for poultry and belongs to the O78 serogroup. This is one of the most prevalent serogroups associated with avian E. coli infections. In this study, we showed that a pst mutation reduces the virulence of APEC strain χ7122 and that certain virulence traits are modified. Indeed, in vivo experiments revealed that the ability of this strain to cause colibacillosis lesions and to survive systemically was significantly reduced in a chicken experimental infection model. Also, the resistance of APEC strain χ7122 to the bactericidal effect of rabbit serum and to acid shock was diminished in the pst mutant. In addition, sensitivity to the cationic peptide polymyxin was increased in the pst mutant strain K3. A decreased capacity to resist these conditions suggests a decrease in cell surface integrity and could explain, at least in part, the loss of virulence attributed to the deletion of pst genes.
Multiple virulence traits were affected in the pst mutant strain K3, and its virulence was significantly reduced. The pst mutant was present in the bloodstream at 6 h postinfection in experimentally infected chickens, but its numbers were considerably reduced in blood and tissues at 48 h postinfection. This is in contrast with the O78-negative LPS mutant strain χ7145 (9), which is rapidly cleared from the blood and extraintestinal organs of infected chickens (36). The capacity of the pst mutant strain K3 to survive in early stages of infection correlated with its ability to resist avian serum (Fig. 2B), since only serum-resistant APEC strains are normally able to invade and persist in the bloodstream early in the infectious process and then disseminate systemically (9, 36). Despite its ability to resist avian serum, the pst mutant strain K3 demonstrated a marked and significant reduction of virulence in chickens. The pst mutant strain K3 was clearly less able to persist and caused reduced lesions compared to the wild-type parent. Since the pst mutation did not contribute to decreased sensitivity to chicken serum or to early survival in the chicken infection model, the attenuation was likely due to other changes that resulted in decreased virulence. These may include changes in surface composition or, possibly, deregulated environmental sensing that may be linked to constitutive expression of the Pho regulon regardless of the environmental conditions encountered in host tissues.
In a porcine experimental infection model using an ExPEC strain, a pst mutation drastically affected the systemic survival of the mutant, as the strain was not able to colonize extraintestinal organs and was rapidly cleared from the bloodstream (12). The differences in the kinetics of infection and attenuation of the porcine and avian pathogenic E. coli strains in infection models are possibly due to intrinsic differences between the strains and/or differences between porcine and avian innate immune defenses. The clearly different results for serum susceptibility of the pst mutant when challenged with avian serum versus mammalian serum support a role for differences in host innate immune defenses. On the other hand, the pst mutants of the porcine and avian pathogenic E. coli strains were both less resistant to in vitro stresses, including acid shock and polymyxin (unpublished results), suggesting that the Pst system is equally important for the proper functioning of certain virulence traits in either of these strains.
There is a correlation between resistance to the bactericidal effects of serum and the capacity of APEC strains to cause septicemia and mortality (32, 36). For strain χ7122, this is exemplified by the O78-negative LPS mutant, which is serum sensitive and unable to persist in body fluids and internal organs of infected chickens (36). Resistance to the bactericidal effect of complement is a multifactorial phenomenon. Bactericidal resistance to serum can correlate with the expression of proteins and of certain capsular K antigens that in many cases can act in concert with the O polysaccharide (65). However, an analysis and comparison of the LPS O chains by SDS-PAGE and Tricine SDS-PAGE (Fig. 5) did not reveal important differences between the wild-type strain χ7122 and the pst mutant strain K3. Indeed, no apparent differences were seen in the pattern and length of the O chains or in the estimated molecular weight of lipid A. However, we cannot rule out the possibility that subtle modifications could occur in LPS molecules as consequences of the pst mutation. Thus, finer approaches are necessary to detect these kinds of modifications, and this will be the subject of future investigations. Strain χ7122 has a K80 capsular antigen which is not a typical capsular polysaccharide antigen, but is more similar to an O-antigen capsule. Atypical K antigens have been shown to be implicated in resistance to serum and are important for full virulence (20, 38, 39). The capsular contents of the strains were tested by a colorimetric assay (16) with strain χ7122 and the pst mutant and were found to be similar (data not shown). The K80 capsule has not been characterized yet. However, it is known that these types of KLPS are anchored to the cell surface by a lipid A moiety. Thus, a perturbation of the cell surface could also affect the proper anchoring of the K80 antigen.
The increased sensitivity of strain K3 to polymyxin further suggests that the mutation of pst causes some surface structure modification. Since polymyxin is a cationic peptide, the bacterial resistance to polymyxin is due, at least in part, to cell surface components. In gram-negative bacteria, some cationic peptides have low affinities for the K antigen and LPS, and these structures repel cationic peptides from the outer membrane. Changes of the bacterial surface net charge, such as modifications of lipid A, can confer sensitivity to polymyxin (8). In Rhizobium meliloti, mutations in the phoCDET genes, which are homologues of the E. coli pst genes, result in a significant reduction in the membrane phospholipid content (18). Perturbations of the cell surface, including an alteration of fluidity and modifications of some components, could explain, at least in part, the increased sensitivity of the pst mutant strain K3 to mechanisms affecting the cell membrane, such as those involved in resistance to serum, polymyxin, and acid.
Acid resistance is an important feature of pathogenic bacteria. The persistence of APEC in poultry facilities is associated with its capacity to resist acidic environments (32, 66). In addition, coprophagy of poultry leads to the ingestion of contaminated feces. Thus, bacterial cells must repetitively survive gastric challenge as well as membrane attack by volatile fatty acids in the less acidic environment of the intestine to persist in poultry facilities (43). The deletion of the pst operon significantly reduced the acid resistance of APEC strain χ7122. Indeed, the pst mutant K3 had a 3-log lower survival rate than that of the parent strain χ7122 after a 4-h challenge at pH 3 without habituation. The Pho regulatory system has been suggested to sense external acidity and to regulate the transcription of genes that are important for acid shock resistance. It was reported that the acid-inducible asr (acid shock RNA) gene in E. coli is under the transcriptional control of the phoBR operon (50, 55). Asr may play a role similar to that of the periplasmic protein HdeA by serving as a proton well or chaperone that protects other periplasmic proteins from the deleterious effects of low pH and/or by preventing the aggregation of denatured proteins (17, 45). Thus, it is possible that inactivation of the pst system contributes to sensitivity to acid shock in APEC strain χ7122 by affecting the regulation of such genes that are part of the Pho regulon and are implicated in acid resistance mechanisms. Complementation of the mutant strain did not restore the wild-type acid resistance phenotype (Fig. 6). The acid sensitivity of the complemented mutant could be due, at least in part, to gene dosage due to the use of a medium-copy-number plasmid.
Altogether, alterations of virulence traits in a pst mutant of APEC O78 strain χ7122 contributed to its reduced virulence in a chicken infection model. Since a constitutive expression of Pho is observed in pst mutants, the pleiotropic effects observed in these mutants could be indirectly due to the activation of the Pho network, directly due to effects on phosphate transport, or both. Our results showed that the deletion of the pst operon reduced the virulence of avian and porcine ExPEC strains and conferred an increased susceptibility to rabbit serum, acid, and polymyxin (this study; F. Daigle et al., unpublished data). These phenotypic changes suggest that alterations in the bacterial surface composition may occur in the absence of the pst operon. A more in-depth investigation needs to be done to identify the functions associated with E. coli virulence genes that are under the control of the Pho regulon. Functional genomics and proteomics could provide opportunities to advance such findings. The central control element of the Pho regulon is the PhoB-PhoR two-component system. It has been shown that some compounds are potent inhibitors of specific bacterial two-component systems and possess antibacterial activity (5). Drugs that induce the expression of the Pho regulon could have some utility as therapeutic agents that would compromise bacterial virulence and facilitate the elimination of the pathogen through host immune defenses. Thus, the Pst system and the associated Pho regulon could be potential drug targets for the treatment of extraintestinal diseases associated with pathogenic E. coli.
Acknowledgments
This work was supported in part by a grant to J.H. from the Fonds des Chercheurs et l'Aide à la Recherche (FCAR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). For this project, C.M.D. was funded by NSERC. Virulence studies were funded in part by USDA NRI grant 2002-35201-11607 (R.C.).
We are grateful to Melha Mellata and John M. Fairbrother for their early work on the chicken serum resistance assay. We thank Mahendrasingh Ramjeet, Josée Labrie, and Mario Jacques, Faculté de Médecine Vétérinaire, Université de Montréal, for their advice concerning LPS analysis and the polymyxin assay. We also thank Virginie E. Bérubé, Université du Québec à Montréal, TOXEN, for statistical assistance.
Editor: A. D. O’Brien
REFERENCES
- 1.Adiri, R. S., U. Gophna, and E. Z. Ron. 2003. Multilocus sequence typing (MLST) of Escherichia coli O78 strains. FEMS Microbiol. Lett. 222:199-203. [DOI] [PubMed] [Google Scholar]
- 2.Babai, R., G. Blum-Oehler, B. E. Stern, J. Hacker, and E. Z. Ron. 1997. Virulence patterns from septicemic Escherichia coli O78 strains. FEMS Microbiol. Lett. 149:99-105. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, H. J., J.-P. Vaillancourt, and W. B. Gross. 2003. Colibacillosis, p. 631-652. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry, 11th ed. Iowa State Press, Ames, Iowa.
- 5.Barrett, J. F., and J. A. Hoch. 1998. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42:1529-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batisson, I., M. P. Guimond, F. Girard, H. An, C. Zhu, E. Oswald, J. M. Fairbrother, M. Jacques, and J. Harel. 2003. Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect. Immun. 71:4516-4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco, A. G., M. Sola, F. X. Gomis-Ruth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure (Cambridge) 10:701-713. [DOI] [PubMed] [Google Scholar]
- 8.Breazeale, S. D., A. A. Ribeiro, and C. R. Raetz. 2003. Origin of lipid A species modified with 4-amino-4-deoxy-l-arabinose in polymyxin-resistant mutants of Escherichia coli. An aminotransferase (ArnB) that generates UDP-4-deoxyl-l-arabinose. J. Biol. Chem. 278:24731-24739. [DOI] [PubMed] [Google Scholar]
- 9.Brown, P. K., and R. Curtiss III. 1996. Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 93:11149-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. Y., and J. E. Cronan, Jr. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33:249-259. [DOI] [PubMed] [Google Scholar]
- 11.Cherifi, A., M. Contrepois, B. Picard, P. Goullet, I. Orskov, and F. Orskov. 1994. Clonal relationships among Escherichia coli serogroup O78 isolates from human and animal infections. J. Clin. Microbiol. 32:1197-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daigle, F., J. M. Fairbrother, and J. Harel. 1995. Identification of a mutation in the pst-phoU operon that reduces pathogenicity of an Escherichia coli strain causing septicemia in pigs. Infect. Immun. 63:4924-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daigle, F., J. Y. Hou, and J. E. Clark-Curtiss. 2002. Microbial gene expression elucidated by selective capture of transcribed sequences (SCOTS). Methods Enzymol. 358:108-122. [DOI] [PubMed] [Google Scholar]
- 14.Dozois, C. M., F. Daigle, and R. Curtiss III. 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 100:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois, M., K. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1951. A colorimetric method for the determination of sugars. Nature 168:167. [DOI] [PubMed] [Google Scholar]
- 17.Gajiwala, K. S., and S. K. Burley. 2000. HDEA, a periplasmic protein that supports acid resistance in pathogenic enteric bacteria. J. Mol. Biol. 295:605-612. [DOI] [PubMed] [Google Scholar]
- 18.Geiger, O., V. Rohrs, B. Weissenmayer, T. M. Finan, and J. E. Thomas-Oates. 1999. The regulator gene phoB mediates phosphate stress-controlled synthesis of the membrane lipid diacylglyceryl-N,N,N-trimethylhomoserine in Rhizobium (Sinorhizobium) meliloti. Mol. Microbiol. 32:63-73. [DOI] [PubMed] [Google Scholar]
- 19.Giacometti, A., O. Cirioni, R. Ghiselli, L. Goffi, F. Mocchegiani, A. Riva, G. Scalise, and V. Saba. 2000. Polycationic peptides as prophylactic agents against methicillin-susceptible or methicillin-resistant Staphylococcus epidermidis vascular graft infection. Antimicrob. Agents Chemother. 44:3306-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman, R. C., K. Joiner, and L. Leive. 1984. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J. Bacteriol. 159:877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gophna, U., T. A. Oelschlaeger, J. Hacker, and E. Z. Ron. 2001. Yersinia HPI in septicemic Escherichia coli strains isolated from diverse hosts. FEMS Microbiol. Lett. 196:57-60. [DOI] [PubMed] [Google Scholar]
- 22.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haldimann, A., L. L. Daniels, and B. L. Wanner. 1998. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J. Bacteriol. 180:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harel, J., and C. Martin. 1999. Virulence gene regulation in pathogenic Escherichia coli. Vet. Res. 30:131-155. [PubMed] [Google Scholar]
- 25.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jans, D. A., A. L. Fimmel, L. Langman, L. B. James, J. A. Downie, A. E. Senior, G. R. Ash, F. Gibson, and G. B. Cox. 1983. Mutations in the uncE gene affecting assembly of the c-subunit of the adenosine triphosphatase of Escherichia coli. Biochem. J. 211:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 28.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 29.Khan, M. A., and R. E. Isaacson. 2002. Identification of Escherichia coli genes that are specifically expressed in a murine model of septicemic infection. Infect. Immun. 70:3404-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, S. K., S. Kimura, H. Shinagawa, A. Nakata, K. S. Lee, B. L. Wanner, and K. Makino. 2000. Dual transcriptional regulation of the Escherichia coli phosphate-starvation-inducible psiE gene of the phosphate regulon by PhoB and the cyclic AMP (cAMP)-cAMP receptor protein complex. J. Bacteriol. 182:5596-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.La Ragione, R. M., and M. J. Woodward. 2002. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res. Vet. Sci. 73:27-35. [DOI] [PubMed] [Google Scholar]
- 33.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 34.Mantis, N. J., and S. C. Winans. 1993. The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J. Bacteriol. 175:6626-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellata, M., M. Dho-Moulin, C. M. Dozois, R. Curtiss III, P. K. Brown, P. Arne, A. Bree, C. Desautels, and J. M. Fairbrother. 2003. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect. Immun. 71:536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngeleka, M., J. Harel, M. Jacques, and J. M. Fairbrother. 1992. Characterization of a polysaccharide capsular antigen of septicemic Escherichia coli O115:K “V165”:F165 and evaluation of its role in pathogenicity. Infect. Immun. 60:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngeleka, M., M. Jacques, B. Martineau-Doize, F. Daigle, J. Harel, and J. M. Fairbrother. 1993. Pathogenicity of an Escherichia coli O115:K “V165” mutant negative for F165(1) fimbriae in septicemia of gnotobiotic pigs. Infect. Immun. 61:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka, A., H. Sugisaki, and M. Takanami. 1981. Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147:217-226. [DOI] [PubMed] [Google Scholar]
- 41.Provence, D. L., and R. Curtiss III. 1992. Role of crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronectin binding, or Curli production. Infect. Immun. 60:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao, N. N., and A. Torriani. 1990. Molecular aspects of phosphate transport in Escherichia coli. Mol. Microbiol. 4:1083-1090. [DOI] [PubMed] [Google Scholar]
- 43.Richard, H. T., and J. W. Foster. 2003. Acid resistance in Escherichia coli. Adv. Appl. Microbiol. 52:167-186. [DOI] [PubMed] [Google Scholar]
- 44.Russo, T. A., and J. R. Johnson. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. [DOI] [PubMed] [Google Scholar]
- 45.Seputiene, V., D. Motiejunas, K. Suziedelis, H. Tomenius, S. Normark, O. Melefors, and E. Suziedeliene. 2003. Molecular characterization of the acid-inducible asr gene of Escherichia coli and its role in acid stress response. J. Bacteriol. 185:2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spira, B., and E. Yagil. 1999. The integration host factor (IHF) affects the expression of the phosphate-binding protein and of alkaline phosphatase in Escherichia coli. Curr. Microbiol. 38:80-85. [DOI] [PubMed] [Google Scholar]
- 47.Steed, P. M., and B. L. Wanner. 1993. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J. Bacteriol. 175:6797-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stordeur, P., A. Brée, J. Mainil, and M. Moulin-Schouleur. 2004. Pathogenicity of pap-negative avian Escherichia coli isolated from septicaemic lesions. Microbes Infect. 6:637-645. [DOI] [PubMed] [Google Scholar]
- 49.Surin, B. P., H. Rosenberg, and G. B. Cox. 1985. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J. Bacteriol. 161:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suziedeliene, E., K. Suziedelis, V. Garbenciute, and S. Normark. 1999. The acid-inducible asr gene in Escherichia coli: transcriptional control by the phoBR operon. J. Bacteriol. 181:2084-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor, P. W., and H. P. Kroll. 1983. Killing of an encapsulated strain of Escherichia coli by human serum. Infect. Immun. 39:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 171:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 55.Tucker, D. L., N. Tucker, and T. Conway. 2002. Gene expression profiling of the pH response in Escherichia coli. J. Bacteriol. 184:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.VanBogelen, R. A., E. R. Olson, B. L. Wanner, and F. C. Neidhardt. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 178:4344-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 58.von Kruger, W. M., S. Humphreys, and J. M. Ketley. 1999. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate-limitation response and intestinal colonization. Microbiology 145:2463-2475. [DOI] [PubMed] [Google Scholar]
- 59.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 60.Wanner, B. L. 1992. Is cross regulation by phosphorylation of two-component response regulator proteins important in bacteria? J. Bacteriol. 174:2053-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wanner, B. L. 1996. Phosphorus assimilation and control of the phosphate regulon, p. 1357-1381. In R. C. I. Neidhardt, J. L. Ingraham, E. E. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbrager (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C.
- 62.Wanner, B. L., M. R. Wilmes, and D. C. Young. 1988. Control of bacterial alkaline phosphatase synthesis and variation in an Escherichia coli K-12 phoR mutant by adenyl cyclase, the cyclic AMP receptor protein, and the phoM operon. J. Bacteriol. 170:1092-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White, D. G., M. Dho-Moulin, R. A. Wilson, and T. S. Whittam. 1993. Clonal relationships and variation in virulence among Escherichia coli strains of avian origin. Microb. Pathog. 14:399-409. [DOI] [PubMed] [Google Scholar]
- 64.White, D. G., R. A. Wilson, D. A. Emery, K. V. Nagaraja, and T. S. Whittam. 1993. Clonal diversity among strains of Escherichia coli incriminated in turkey colisepticemia. Vet. Microbiol. 34:19-34. [DOI] [PubMed] [Google Scholar]
- 65.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 66.Zhu, C., M. Ngeleka, A. A. Potter, and B. J. Allan. 2002. Effect of fur mutation on acid-tolerance response and in vivo virulence of avian septicemic Escherichia coli. Can. J. Microbiol. 48:458-462. [DOI] [PubMed] [Google Scholar]