Abstract
Infection of BALB/c mice with Brugia pahangi third-stage larvae (L3) results in the production of interleukin-4 (IL-4), IL-5, and IL-10 with a resultant down-regulation in Th1 responses. Previously, this was thought to reflect a skewing of immune responses towards a Th2 phenotype by the infective stage of the parasite. In this study, we show that exposure to the L3 of Brugia also induces the expansion of a population of CD4 cells that express CD25 and cytotoxic-T-lymphocyte-associated antigen 4 in an IL-4-independent fashion. By quantitative reverse transcription-PCR, we show that the CD25+ population is highly enriched in mRNA for the Foxp3 transcription factor and that these cells express significantly more IL-10 mRNA than the CD25− population, suggesting a likely regulatory phenotype. The functional capacity of these cells was demonstrated using a neutralizing CD25 monoclonal antibody (MAb). Mice treated with this MAb demonstrated elevated levels of antigen (Ag)-specific proliferation in vitro, and levels of Ag-specific Th2 cytokines were significantly increased. These results suggest a complex network of regulation in L3-infected mice with Th2 cells limiting the Th1 response, while T-regulatory cells modulate Th2 responses.
T-regulatory (Treg) cells are a family of CD4+ cells that are antiinflammatory and profoundly suppressive. Three populations of Treg cells have been characterized to date: Th3, Tr1, and CD4+ CD25+ cells. Each of these populations differs in specific details such as the profile of cytokines secreted, the expression of cell surface markers, and their probable mode of action. Perhaps the best characterized of these populations are the naturally occurring CD4+ CD25+ cells (36), which differ from Tr1 and Th3 in that they preexist in the thymus as suppressor cells, while Th3/Tr1 cells can arise from naïve peripheral CD4+ cells (35). Tr1 and Th3 cells have the capacity to inhibit potentially harmful immune responses via the secretion of cytokines such as transforming growth factor β (TGF-β) and interleukin-10 (IL-10), while the role of secreted cytokines is more controversial in the activity of CD4+ CD25+ T cells. This latter population is thought to mediate suppression of CD25− cells via a direct T-cell-T-cell interaction, a mechanism associated with surface-bound TGF-β (27) and/or cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) (14, 34), that results in inhibition of the IL-2 receptor α-chain.
The original studies of Sakaguchi et al. (37) demonstrated the function of CD4+ CD25+ cells in tolerance to self, and subsequent studies have implicated these cells in the prevention of inflammatory bowel disease in mice (34) and the inhibition of autoimmune diabetes in rats (40) and have defined their role in transplant tolerance (reviewed in reference 43). In contrast, their role in regulating immune responses to infectious agents is less well understood. However, recent studies in the mouse model of Leishmania major have demonstrated that CD4+ CD25+ cells regulate the production of early IL-4, a key cytokine determining whether an infected animal will progress towards a resistant (Th1) or a susceptible (Th2) phenotype (1). In an additional study, parasite-specific CD4+ CD25+ cells were shown to accumulate at the site of L. major infection in the dermis of infected mice, where they suppressed the ability of effector T cells (CD4+ CD25−) to eliminate the parasite (2). In that study, depletion of the CD4+ CD25+ population resulted in total eradication of the parasite from the site of infection and the loss of concomitant immunity, demonstrating that the regulatory subset also functions in the maintenance of memory.
Helminth parasites typically produce chronic infections in their natural hosts and are frequently associated with a profound skewing of the immune response (reviewed in reference 24). Human filariasis, caused by vector-borne nematode parasites, is typical in this respect, with the adult worms surviving for several years while producing many millions of microfilariae (MF) (first-stage larvae), which circulate in the bloodstream or the skin. Peripheral blood mononuclear cells from individuals actively infected with the lymphatic filarial parasite Wuchereria bancrofti or Brugia malayi exhibit suppressed antigen (Ag)-specific T-cell-proliferative responses while secreting little or no gamma interferon (IFN-γ) and elevated levels of IL-4 and IL-10 (44). This antiinflammatory/antiproliferative response appears to enhance survival of the parasite while protecting the host from the tissue destruction that may occur due to an acute inflammatory response. TGF-β and IL-10 have been shown to be important antiproliferative cytokines in both lymphatic filariasis (17, 22, 23) and infection with the related parasite Onchocerca volvulus (7). Indeed, Tr1 cells, which produced no IL-2 and high levels of IL-10 and TGF-β, were cloned from individuals with generalized onchocerciasis and were proposed to be involved in maintaining the hyporesponsive state (7). These cells displayed elevated levels of CTLA-4 after Ag stimulation and, importantly, were able to inhibit the proliferation of other T cells in coculture (38).
In the studies referred to above, in filaria-infected humans, the presence of a Treg population has been associated with active infection with adult parasites and/or MF, consistent with the concept that these cells are important in regulating immune responses in chronic conditions. In this study, we investigated the potential of the infective third-stage larvae (L3) of Brugia pahangi to elicit a Treg population in a well-characterized murine model of infection. L3 are known to have the capacity to modulate immune responses in both exposed humans (16) and mice (32) at least in part through the induction of IL-4 and IL-10. Here, we show that infection with L3 specifically elicits a population of CD4+ CD25+ CTLA-4+ cells that are IL-4 independent. The CD25+ population expresses high levels of Foxp3 mRNA and significantly increased levels of IL-10 mRNA compared to those of the CD25− population. The regulatory function of these cells was demonstrated by in vivo depletion, which resulted in a severely dysregulated immune response to parasite Ag.
MATERIALS AND METHODS
Animals and infection protocols.
B. pahangi L3 were harvested from infected Aedes aegyptii mosquitoes at day 9 postinfection (p.i.) as described previously (5). Six-week-old male BALB/c mice were purchased from Harlan-Olac (Bicester, United Kingdom), while IL-4−/− mice on the BALB/c background were bred at the University of Glasgow. Animals were housed in filter-topped cages and maintained in accordance with local and Home Office regulations. Groups of 5 to 10 BALB/c or IL-4−/− mice were infected subcutaneously (s.c.) with 50 L3 of B. pahangi or an equivalent volume of Hanks balanced salt solution in the scruff of the neck. In some experiments, a further group of five mice was infected with 105 MF by the s.c. route. At day 12 p.i., the mice were killed by CO2 inhalation and spleens were removed under asceptic conditions.
Preparation of spleen cells, proliferation, and cytokine assays.
Single-cell suspensions were prepared in RPMI medium by homogenization through Nytex mesh (Cadish and Sons, London, United Kingdom) using a syringe barrel exactly as described previously (28). The cells were resuspended at 1 × 107 cells per ml (for proliferation assays) or 2 × 107 cells per ml (for cytokine assays) in RPMI 1640 medium (Dutch modification, containing 5 mM glutamine, 5 mM HEPES, 100 U per ml penicillin, and 100 μg per ml streptomycin [all from Invitrogen]) and 20% heat-inactivated fetal calf serum (FCS) (Invitrogen) to give a final concentration of 10%. Soluble extract of B. pahangi adult worms for use in cell culture was prepared by extensive homogenization of frozen mixed-sex adult worms on ice exactly as described previously (28).
Proliferation of splenocytes was measured by the incorporation of [3H]thymidine. Triplicate 100-μl cultures (5 × 105 cells per well) in half-area 96-well flat-bottomed plates (CoStar) were incubated in the presence or absence of 10 μg antigen per ml. Cells were cultured at 37°C in an atmosphere of 5% CO2 and pulsed with 0.5 μCi of [3H]thymidine/well (Amersham) during the last 16 h of incubation. The cells were harvested, and radioactivity was measured in a “Topcount” Microplate scintillation counter (Canberra Packard Instrument Co.).
For cytokine assays, spleen cells were incubated at 1 × 107 cells per ml in 1-ml cultures in 24-well flat-bottomed plates (Co-star) in the presence of Ag at 10 μg per ml or medium only. Supernatants were harvested at 48 h, unless otherwise stated, and levels of IL-2, IL-4, IL-5, IL-10, and IFN-γ were determined by two-site enzyme-linked immunosorbent assay using antibody (Ab) pairs purchased from Pharmingen, as described previously (28). Results are expressed as picograms per milliliter in reference to commercially available standards (IL-12, IL-4, IL-5, and IL-10 from Pharmingen and IFN-γ from R & D Systems). The sensitivity of the assay was determined as the mean plus three standard deviations (SD) of the 16 wells containing medium only (RPMI-10% FCS).
Cell depletions and purifications.
Splenocytes from L3-infected animals were depleted of either CD4+ or B220+ cells by magnetic separation prior to in vitro culture. In brief, splenocytes pooled from 10 infected mice were washed in phosphate-buffered saline (PBS) and stained with either fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (L3T4) or anti-B220 (RM4-5) monoclonal Ab (MAb) (both from Pharmingen) in 500 μl of PBS on ice for 20 min. Both Abs were used at 1 μg per 2 × 106 cells. Prior to depletion, unseparated cells were plated out and 5 × 106 cells were removed for fluorescence-activated cell sorter (FACS) analysis and stained with an isotype control antibody (KLH/G2a-1-1; Pharmingen). The cells were then resuspended in 90 μl degassed buffer per 107 cells (prepared to MACS [Miltenyi Biotech Ltd.] specifications) with anti-FITC beads (10 μl of beads per 107 cells) and incubated on ice for 15 min, and then ∼2.5 × 108 cells were loaded onto the column. The flowthrough cells were washed in PBS, and 5 × 107 cells were removed for FACS analysis to determine the efficiency of the depletion. The remaining cells were then resuspended in RPMI-20% FCS and plated out as described previously. For purification, bound cells were flushed from the column using 5 ml of buffer, following the manufacturers recommendation. Purified CD4+ and B220+ cells were stored in liquid nitrogen prior to analysis. Purification of CD25+ cells was carried out using the same method as described above with cells stained with CD25 (IL-2 receptor α-chain p55) (7D4) (Pharmingen).
RT-PCR detection of IL-10, TGF-β, and Foxp3 mRNA by TaqMan.
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's protocol. RNA was stored in diethyl pyrocarbonate-treated H2O at −70°C until use. Levels of mRNA for IL-10 and TGF-β were quantified from CD4 or B220 populations purified as described above, while levels of Foxp3 mRNA were quantified in CD25+ and CD25− populations. Reverse transcription (RT) was carried out in a total of 20 μl using 2 μl of random primers (Promega) and 2 μg of total RNA in a volume of 10 μl. The mix was heated to 70°C for 10 min and then cooled on ice for 10 min. Four microliters of first-strand buffer (Invitrogen), 2 μl dithiothreitol (Invitrogen), and 1 μl 10 μM deoxynucleoside triphosphate mix were added and incubated at 42°C. One microliter of Superscript was added to the mix, which was heated to 42°C for 50 min with a final extension of 70°C. One microliter of RNase was added, and the reaction was incubated at 37°C for 20 min.
TaqMan quantitative reverse transcriptase PCR used gene-specific primers and internal probes for IL-10, TGF-β, and hypoxanthine phosphoribosyltransferase (HPRT) (Table 1) (Cruachem) designed to specifically amplify the target cDNA. For the TaqMan protocol, probes were used at a concentration of 5 μM and primers were used at 10 μM. Each 25-μl reaction mixture contained 12.5 μl TaqMan Universal PCR Master Mix (Applied Biosystems), 1 μl probe, 0.75 μl each primer, and 9 μl H2O with 1 μl cDNA. TaqMan cycling conditions were 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. Results are expressed relative to the level of HPRT mRNA.
TABLE 1.
Primer and probe sequences for amplification of IL-10, TGF-β, and Foxp3
| Gene | Sequencea |
|---|---|
| HPRT | Forward, 5′-GCA GTA CAG CCC CAA AAT GG-3′ |
| Reverse, 5′-AAC AAA GTC TGG CCT GTA TCC AA-3′ | |
| Probe, 5′-FAM-TAA GGT TGC AAG CTT GCT GGT GAA AAG GA-TAMRA-3′ | |
| IL-10 | Forward, 5′-ACA ACA TAC TGC TAA CCG ACT CCT T-3′ |
| Reverse, 5′-AGG TAA AAC TGG ATC ATT TCC GAT A-3′ | |
| Probe, 5′-FAM-TGG CAA CCC AAG TAA CCC TTA AAG TCC TG-TAMRA-3′ | |
| TGF-β | Forward, 5′-CCC CAC TGA TAC GCC TGA GT-3′ |
| Reverse, 5′-ACA AGA GCA GTG AGC GCT GA-3′ | |
| Probe, 5′-FAM-TGA ACC AAG GAG ACG GA-TAMRA-3′ | |
| Foxp3 | Forward, 5′-CCC AGG AAA GAC AGC AAC CTT-3′ |
| Reverse, 5′-TTC TCA CAA CCA GGC CAC TTG-3′ | |
| Probe, 5′-FAM-ATC CTA CCC ACT GCT GGC AAA TGG AGT C-TAMRA-3′ |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
In vivo administration of PC61 and GL113.
Five mice were injected intraperitoneally with 0.5 mg (total protein) of neutralizing CD25 MAb (PC61) (21, 30) or isotype-matched control (GL113) on days −4 and −2 prior to infection. Both Abs were prepared by ammonium sulfate precipitation of hybridoma supernatant and subsequent dialysis against PBS. The Ab was sterilized by passage through a 0.45-μm filter and stored at 4°C. Proliferation assays, cytokine assays, and FACS analysis were carried out on the splenocytes from each group of animals.
Three-color FACS analysis.
Cells to be stained were removed from culture, transferred to FACS tubes (Falcon), and washed twice in 200 μl of staining buffer by centrifugation at 1,000 rpm for 5 min. Cells were stained with 2 μg/test of fluorochrome-conjugated antibody in staining buffer or staining buffer only (100 μl/sample) on ice for 20 min. Cells were washed twice in staining buffer as before and resuspended in 300 μl of fixation buffer if not analyzed immediately. Stained samples were stored in the dark at 4°C. Samples were gated on lymphocytes, as determined by size and granularity (forward and side scatter). The following monoclonal antibodies were used: FITC-labeled anti-mouse CD4 (L3T4), phycoerythrin (PE)-labeled anti-mouse CD152 (CTLA-4, UC10-4F10-11), and antigen-presenting cell (APC)-labeled anti-mouse CD25 (PC61) (all Pharmingen). Fc block (Pharmingen) was used at a concentration of 1 μg per test to reduce nonspecific binding. Isotype controls were used in all experiments and included the following: FITC-labeled rat immunoglobulin G2a (IgG2a) (KLH/G2a-1-1; Southern Biotechnology Associates), APC-labeled rat IgG2a (R35-95), and PE-labeled rat IgG1 (both from Pharmingen).
Statistical analysis.
The Mann-Whitney U test was used to determine the statistical significance of differences between groups. A P value of ≤ 0.05 was considered to be significant.
RESULTS
Depletion of CD4+ cells from spleens of L3-infected mice results in decreased levels of IL-10.
Splenocytes from L3-infected mice are known to produce high levels of IL-10 when restimulated with parasite Ag in vitro. To characterize the cellular source of IL-10, selected populations were depleted from the spleens of L3-infected BALB/c mice using magnetic beads and the relevant cell surface MAb. Depletions were monitored by FACS analysis and were ≥95% efficient in all experiments. Depletion of CD4+ cells resulted in a dramatic decrease in Ag-stimulated IL-10 relative to unseparated splenocytes from the same animals, although levels of IL-10 were never reduced to background. The results of three separate experiments are shown in Table 2 and demonstrate a 75% reduction in IL-10 in response to Ag stimulation when CD4+ cells were removed. In addition, levels of both IL-4 and IL-5 were also decreased (data not shown). No Th1 cytokines were detected in supernatants from CD4+-depleted cultures or whole-splenocyte cultures.
TABLE 2.
CD4+ cells are the major source of IL-10 in L3-infected micea
| Expt | IL-10 (pg per ml) in whole splenocytes | IL-10 (pg per ml) in splenocyte pools depleted of CD4+ or B+ cells | % Reduction | Avg % reduction |
|---|---|---|---|---|
| 1 | 14,535 | 2,892 | 80 | |
| 2 | 16,070 | 4,376 | 73 | 75 |
| 3 | 19,805 | 5,440 | 73 | |
| 4 | 28,434 | 16,218 | 43 | |
| 5 | 10,834 | 6,975 | 36 | 39 |
| 6 | 31,559 | 19,343 | 39 |
Mice were infected s.c. with 50 L3 of B. pahangi or an equal volume of HBSS only. At 12 d.p.i., splenocytes pooled from 10 L3-infected mice were depleted of CD4+ cells (experiments 1 to 3) or B220+ cells (experiments 4 to 6) by magnetic separation. Pooled cells prior to separation or purified populations were plated out at 1 × 107 cell per ml and cultured in the presence of Brugia Ag at 10 μg per ml. IL-10 levels were measured in 48-h supernatants by two-site enzyme-linked immunosorbent assay.
Depletion of B220+ cells resulted in a more modest effect on Ag-specific IL-10 production relative to unseparated splenocytes from the same animals. Table 2 shows the results of three separate experiments in which B-cell depletion resulted in ∼39% decrease in IL-10 production compared to that of whole-splenocyte cultures.
CD4+ cells from L3-infected mice express IL-10 and TGF-β mRNA in an IL-4-independent fashion.
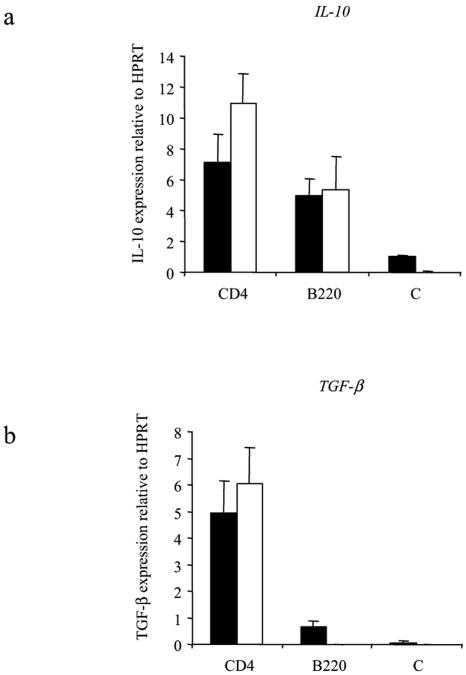
While the results of the depletion experiments suggested that CD4+ cells were the major producers of IL-10, it was not possible to rule out indirect effects of depletion; e.g., the depletion of one cell type could affect the ability of another cell to produce IL-10. Therefore, levels of expression of IL-10 mRNA were assessed in purified populations by quantitative RT-PCR. These experiments demonstrated that CD4+ cells express approximately twofold more IL-10 mRNA than do B cells relative to HPRT (Fig. 1a), similar to results from cell depletion experiments. In the same experiments, levels of TGF-β mRNA were also determined and the CD4+ population was shown to be the source of TGF-β mRNA (Fig. 1b). As both IL-10 and TGF-β can be expressed by Th2 cells, which are also elicited in response to L3 infection, expression of IL-10 and TGF-β mRNA was investigated in IL-4−/− or wild-type (WT) mice. However, both cytokines were expressed at approximately equal levels in CD4+ T cells from L3-infected IL-4−/− mice and their wild-type counterparts (Fig. 1).
FIG. 1.
IL-4−/− mice express levels of IL-10 and TGF-β mRNA equivalent to those of BALB/c WT mice following L3 infection. Ten IL-4−/− (black bars) or WT (white bars) mice were infected s.c. with 50 L3 of B. pahangi or were given an equal volume of HBSS only (C). At day 12 p.i., splenocytes from L3-infected mice were pooled and separated into purified CD4+ and B220+ cells using magnetic beads. The efficiency of the purification was analyzed by FACS at >95%. Quantitative RT-PCR was carried out on the separated populations using primers specific for IL-10, TGF-β, or HPRT (constitutive gene). Expression of IL-10 mRNA (a) and TGF-β mRNA (b) was measured relative to HPRT in CD4+ and B220+ cells from BALB/c WT mice or IL-4−/− mice, immediately ex vivo. Graphs show mean values of triplicate wells ± SD.
Foxp3 is expressed only in the CD25+ population.
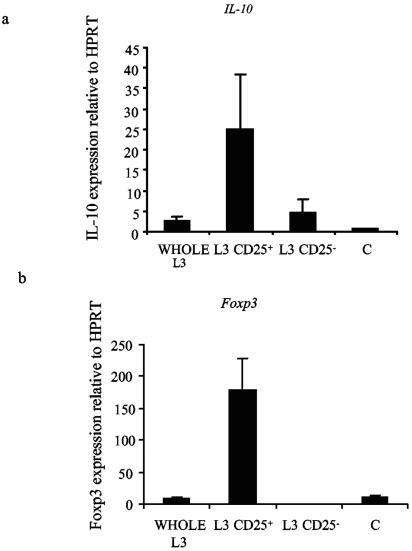
In order to further characterize the cellular source of IL-10, splenocytes from L3-infected mice were separated into CD25+ and CD25− fractions directly ex vivo. TaqMan RT-PCR was then carried out on cDNA prepared from RNA from each population using primers designed to amplify IL-10 or Foxp3, a transcription factor expressed in regulatory T cells. These experiments showed that the CD25+ population expressed the majority of IL-10 mRNA (Fig. 2a). Moreover, selection of the CD25+ population resulted in very high levels of Foxp3 expression, indicating the significant enrichment of regulatory T cells within the CD25+ population (Fig. 2b).
FIG. 2.
Foxp3 and IL-10 mRNA is enriched within the CD25+ population in splenocytes from L3-infected mice. Ten mice were infected s.c. with 50 L3 of B. pahangi or an equal volume of HBSS only (C). At 12 days p.i. (d.p.i.), splenocytes from L3-infected mice were pooled and magnetically separated into purified CD25+ and CD25− cells. Quantitative RT-PCR was carried out on these cells and whole unseparated splenocytes from L3-infected (WHOLE L3) or naïve mice (C) using the TaqMan method. Levels of IL-10 (a) and Foxp3 (b) mRNA were expressed relative to the constitutive gene HPRT. Graphs show mean values of triplicate wells ± SD.
CD4+ CD25+ CTLA-4+ cells are expanded in cultures from L3-infected BALB/c WT and IL-4−/− mice.
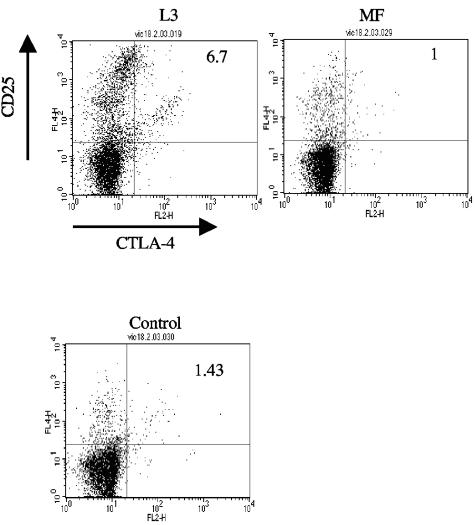
An alternative means of identifying Treg cells is to analyze cell surface markers associated with this population. Such markers include coexpression of CD4 with one or more of the following: CD25, CTLA-4 (CD152), glucocorticoid-induced tumor necrosis factor receptor, OX40 (CD134), CD103, and Ly6 (25). Three-color FACS analysis was carried out on splenocytes from L3-infected, MF-infected, or control uninfected mice using anti-CD4 MAb (FITC), anti-CD25 MAb (APC), and anti-CTLA-4 MAb (PE). Mice infected with MF were included in these experiments, as splenocytes from these animals display a Th1-type cytokine profile, and it was of interest to determine whether a Treg population would be expanded in these animals. In these experiments, cells were analyzed directly ex vivo or following culture with Ag for 72 h prior to labeling and flow cytometry. There was no difference in the percentage of triple-positive cells obtained from any group of mice directly ex vivo. However, following in vitro restimulation with Ag, CD4+ CD25+ CTLA-4+ cells were expanded only in cultures from L3-infected mice, where they comprised 7% ± 0.9% of total CD4+ cells. In these experiments, the CTLA-4+ population could be easily distinguished from activated CD25hi T cells (Fig. 3). In contrast, these cells were not expanded in culture from control mice or MF-infected animals, where this population accounted for only 1 to 2% of total CD4+ cells (L3 versus MF or control; P = 0.034).
FIG. 3.
Three-color FACS analysis demonstrates that a population of CD4+ cells which coexpress CD25 and CTLA-4 are expanded only in culture from L3-infected mice. Mice were infected s.c. with 50 L3 or 1 × 105 MF of B. pahangi or were given an equal volume of HBSS only. At day 12 p.i., splenocytes from L3-infected, MF-infected, or control mice were cultured with 10 μg B. pahangi Ag per ml for 72 h. Cells were harvested and stained with FITC anti-mouse CD4 MAb, PE anti-mouse CD25 MAb, and APC anti-mouse CTLA-4 MAb. Cells were analyzed by flow cytometry using gates set with isotype-matched control MAb. Panels show the staining profile of an individual mouse from each group. The numbers at the top right-hand corner of each panel indicate the percentage of CD4+ cells that coexpress CD25+ and CTLA-4+. These figures are representative of the responses of five animals per group.
Further analysis of these data (Table 3) demonstrated that the percentage of lymphocytes expressing CD4+ was equivalent in the three groups of mice. Approximately 25% of CD4+ cells from L3-infected mice expressed CD25, while CD4+ cells from control uninfected or MF-infected mice expressed lower levels of CD25 (∼6 to 7%). Similarly, CTLA-4 was expressed only on a significant percentage of cells from L3-infected mice. Although CD25 is a marker of T-cell activation, the coexpression of CTLA-4 on a proportion of the CD25+ population indicates that L3, but not MF, elicit a population of cells with the characteristics of Treg cells.
TABLE 3.
Expression of cell surface markers on CD4+ cells from L3-infected, MF-infected, and control micea
| Mouse group | % CD4 | % CD4 coexpressing CD25 | % CD4 coexpressing CTLA-4 | % CD4 coexpressing CD25 and CTLA-4 |
|---|---|---|---|---|
| L3 | 17.7 ± 1.8 | 24.6 ± 1 | 7.6 ± 0.6 | 6.9 ± 0.4 |
| MF | 23 ± 2.7 | 7.93 ± 0.7 | 1.82 ± 0.2 | 1.32 ± 0.2 |
| C | 20 ± 0.9 | 6.77 ± 0.3 | 3.34 ± 0.5 | 2.1 ± 0.2 |
Mice were infected s.c. with 50 L3 or 1 × 105 MF of B. pahangi or an equal volume of HBSS only. At 12 d.p.i., splenocytes from L3-infected (L3), MF-infected (MF), and HBSS control (C) mice were cultured with 10 μg B. pahangi Ag per ml for 72 hours. Cells were harvested and stained with anti-CD4 MAb and CD25 MAb and CTLA-4 MAb, each bound to a different flurochrome (CD4-FITC, CD25-PE, and CTLA-4-APC). Figures show the percentage of splenocytes which express CD4+, the percentage of CD4+ cells which express CD25+ alone or CTLA-4 alone, and the percentage of CD4+ cells which coexpress both of these cell surface markers.
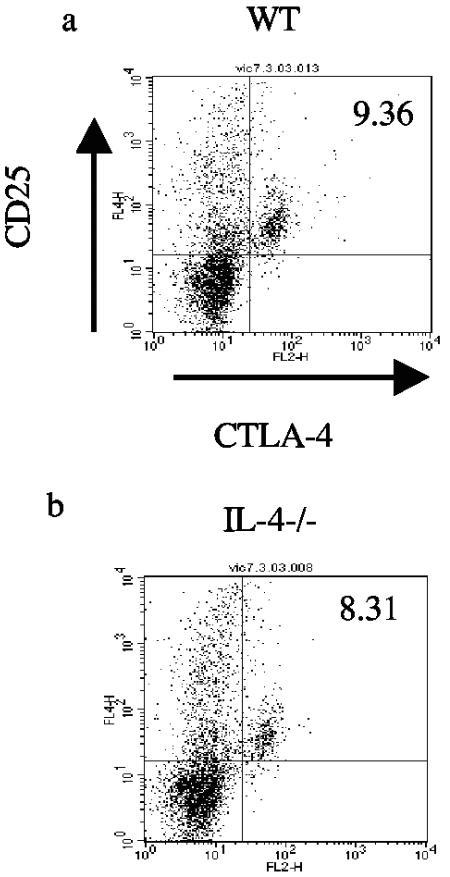
A similar analysis was also carried out in which IL-4−/− mice or wild-type BALB/c mice were infected with L3 and CD4+ CD25+ CTLA-4+ cells were quantified by three-color FACS following restimulation in vitro with Brugia Ag. These experiments confirmed the significant expansion of CD4+ CD25+ CTLA-4+ cells in L3-infected mice compared to that of uninfected controls (data not shown), but no difference was observed in the percentage of these cells between L3-infected BALB/c mice and IL-4−/− mice (P = 1) (Fig. 4).
FIG. 4.
CD4+ cells coexpressing CD25 and CTLA-4 are expanded in splenocyte cultures from L3-infected IL-4−/− mice. Splenocytes from L3-infected WT (a) and IL-4−/− (b) mice were cultured with 10 μg B. pahangi Ag per ml for 72 h. Cells were harvested and stained with FITC anti-mouse CD4 MAb, PE anti-mouse CD25 MAb, and APC anti-mouse CTLA-4 MAb and analyzed by flow cytometry. Gates were set on CD4+ cells, and these cells were analyzed for the expression of CD25 and CTLA-4. Each panel shows the staining profile of an individual mouse from each group. The numbers at the top right-hand corner of each panel indicate the percentages of CD4+ cells that coexpress CD25+ and CTLA-4+. These figures are representative of the responses of five animals per group.
CD25+ cells play a regulatory role in vivo.
The experiments described above demonstrate that a population of T cells with some of the characteristics of regulatory T cells was expanded only in cultures from L3-infected mice. In order to determine whether these cells were present in vivo and to investigate their likely function, further experiments were carried out using a neutralizing Ab (PC61) to deplete CD25+ cells in vivo, prior to L3 infection.
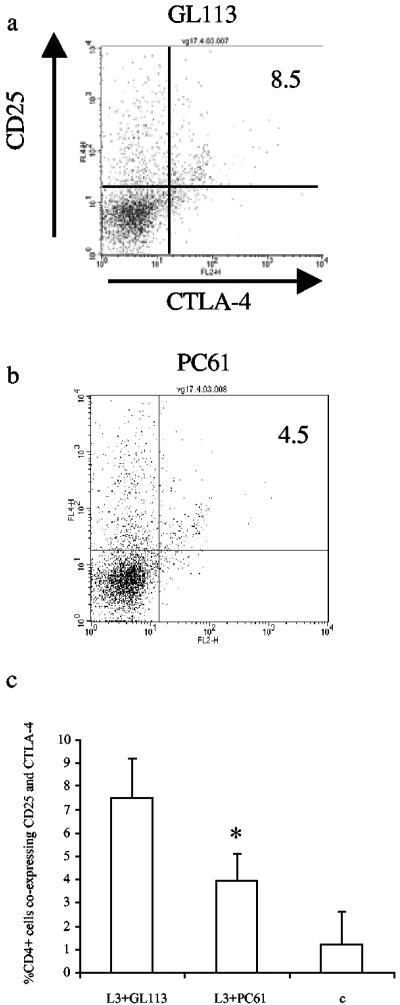
In these experiments, mice were treated with PC61 (anti-CD25 MAb) or GL113 (isotype-matched control MAb) on days −4 and −2 prior to s.c. infection with 50 L3 of B. pahangi. Administration of the MAb prior to infection is important to ensure that T cells activated by infection are not targeted (30). On day 12 p.i., spleens were harvested and splenocytes were cultured in vitro for 72 h with 10 μg B. pahangi Ag per ml. Three-color FACS analysis was then carried on splenocytes from L3-infected mice that were given either PC61 or GL113, as described previously. In mice that received PC61 MAb, there was a significant reduction in the percentage of CD4+ CD25+ CTLA-4+ cells compared to those given the isotype control (P = 0.0122), indicating that the Ab was effectively depleting these cells (compare Fig. 5a and b). However, at the concentration administered, the PC61 Ab did not completely ablate the CD25+ CTLA-4+ population. In most experiments, a ∼50% reduction in the numbers of these cells was observed (Fig. 5c). Importantly, analysis of these data also showed that the number of CD4+ cells expressing CD25 but not CTLA-4 did not decrease in mice that were given PC61, demonstrating that T cells could still be activated to a similar extent despite the previous treatment (11.6% of CD4+ cells from GL113-treated mice expressed CD25 but not CTLA-4, while 14% of CD4+ cells from PC61-treated mice expressed CD25 but not CTLA-4 [P = 0.6756]).
FIG. 5.
Administration of PC61 MAb results in decreased expansion of CD4+ cells coexpressing CD25 and CTLA-4. Splenocytes from two groups of mice given GL113 (a) or PC61 (b) prior to infection with 50 L3 of B. pahangi were cultured with 10 μg/ml B. pahangi Ag for 72 h. Cells were harvested and stained with FITC anti-mouse CD4 MAb, PE anti-mouse CD25 MAb, and APC anti-mouse CTLA-4 MAb and analyzed by flow cytometry. Each panel shows the staining profile of an individual mouse from each group. The numbers at the top right-hand corner of each panel indicate the percentage of CD4+ cells that coexpress CD25+ and CTLA-4+. These figures are representative of the responses of five animals per group. Panel c shows mean values ± SD of CD4+ cells coexpressing CD25 and CTLA-4 in mice given PC61 or GL113 MAb or in naïve mice (C). *, significant difference between L3-infected mice given PC61 and isotype control (P = 0.0122) and control (P = 0.0369) animals.
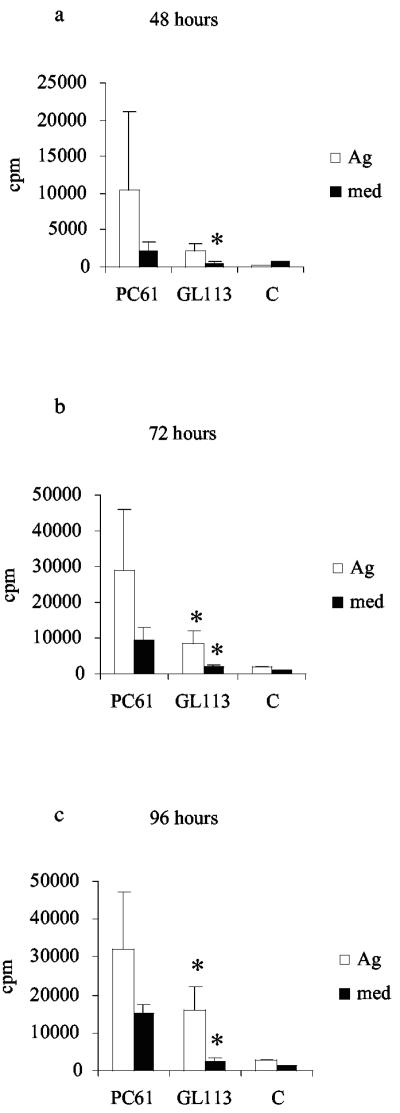
To determine whether the removal of a significant proportion of the CD25+ CTLA-4+ population affected immune responses, proliferation and cytokine assays were carried out. Proliferative responses of splenocytes from L3-infected mice that were given either PC61 or GL113 were measured over a time course of in vitro culture with medium only or 10 μg B. pahangi Ag per ml. Control mice received HBSS alone. Three experiments were carried out, each of which gave similar results (see Fig. 6 for a representative example). Two points are immediately obvious from these experiments: first, levels of Ag-specific proliferation were significantly higher in splenocytes from mice given PC61 than in those from mice given GL113 after 72 and 96 h incubation (P = 0.0122 and P = 0.0367, respectively). Second, it was notable that levels of proliferation in medium alone were significantly increased in cultures from PC61-treated mice at all time points (at 48 h, P = 0.0216 and at 72 and 96 h, P = 0.0122 for PC61-treated versus GL113-treated mice).
FIG. 6.
Ag-specific and spontaneous proliferation is increased in splenocyte culture from mice given PC61 MAb. Five mice were given PC61 or GL113 prior to s.c. infection with 50 L3 of B. pahangi. Control animals (C) received no treatment or infection. At day 12 p.i., splenocytes from the three groups of mice were cultured with medium only or restimulated with 10 μg B. pahangi Ag per ml for 48 h (a), 72 h (b), and 96 h (c), and proliferative responses were measured. Results are expressed as counts per minute (cpm). *, significant difference between PC61 and GL113 in medium-only cultures at all three time points and significant difference in Ag-stimulated cultures at 72 and 96 h.
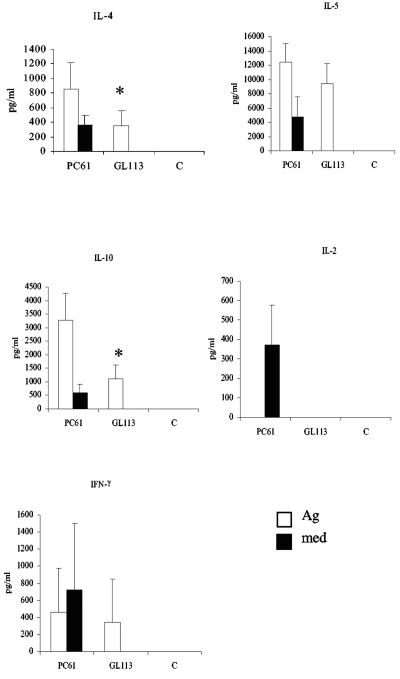
Ag-specific cytokine responses were measured in splenocyte culture from L3-infected mice that were given either PC61 or GL113 or from naïve animals after 72 h of in vitro culture. The results of a representative experiment are presented in Fig. 7. Mice depleted of CD25+ cells in vivo produced significantly more IL-10 (PC61 versus GL113, P = 0.0216) and IL-4 (PC61 versus GL113, P = 0.0367) than animals given the isotype control MAb or mice given no treatment. There was also a trend for levels of IL-5 to increase, but this did not reach statistical significance in any experiment (P = 0.0947 in the experiment shown). Interestingly, cells from PC61-treated mice also produced large amounts of cytokines in medium-only cultures, including IL-2 and IFN-γ. In contrast, splenocytes from PC61-treated mice cultured with Ag did not secrete IL-2.
FIG. 7.
Administration of PC61 MAb results in increased Ag-specific and spontaneous cytokine production. Mice were given either PC61 or GL113 prior to s.c. infection with 50 L3 of B. pahangi. Control animals (C) received no treatment or infection. At day 12 p.i., splenocytes from the three groups of mice were cultured with 10 μg B. pahangi Ag per ml or medium only. Ag-specific and spontaneous cytokine responses were measured at 72 h. Results are expressed as picograms per milliliter, and all values represent the means and standard deviations of five mice per group. *, significant differences in levels of IL-4 and IL-10 in mice given PC61 or GL113.
DISCUSSION
Filarial infections are characterized by profoundly suppressed T-cell-proliferative and IFN-γ responses, which at one time were interpreted as being a result of a Th2 bias. While these infections undoubtedly elicit Th2 cells, more recent thinking proposes a role for regulatory T cells in maintaining the hyporesponsive state (7, 24, 38). In this study, we show that exposure to the infective form of filarial nematodes, the L3, results in the expansion of a regulatory T-cell population. Previous results in the B. pahangi mouse model had demonstrated that infection with L3 induces elevated levels of IL-10 that functions to suppress Th1 responses (31). By cell depletion experiments and quantitative RT-PCR on purified populations, we now show that CD4+ cells are the primary source of IL-10 in the B. pahangi mouse model, with the CD25+ population expressing the most IL-10 mRNA. In addition, the CD4+ population was also shown to express TGF-β mRNA, indicating the presence of a possible regulatory population (reviewed in reference 35). CD4+ cells from L3-infected IL-4−/− mice expressed levels of IL-10 and TGF-β mRNA similar to those of WT mice, supporting the hypothesis that these suppressive cytokines may be produced by T cells other than Th2 cells. However, as IL-4-independent pathways of Th2 differentiation have been reported (3, 12, 18), additional studies will be required to definitively prove the cellular source of these cytokines.
Until recently, little was known about the molecular mechanism of development of CD25+ regulatory cells, but several studies have now demonstrated that Foxp3, a fork head transcription factor, is highly expressed in naturally arising CD25+ Treg cells (11). Purification of CD25+ cells from L3-infected mice confirmed this finding and showed a significant enrichment of Foxp3 mRNA in the CD25+ population. In an additional experiment, CD25+ cells were purified from control or L3-infected mice and Foxp3 expression was investigated by RT-PCR. Foxp3 levels were twofold higher in CD25+ cells from L3-infected mice than in those from control animals (data not shown), supporting the concept that regulatory T cells are induced by infection with the L3 of Brugia.
The identification of CD25+ regulatory T cells has been the source of some controversy, as specific markers for this population are not readily available. For example, two commonly used cell surface markers, CD25, the α-chain of the high-affinity IL-2 region, and glucocorticoid-induced tumor necrosis factor receptor-related protein, are also up-regulated upon activation of conventional T cells (8). In this study, we concentrated on the CD4+ CD25+ CTLA-4+ population, as many studies have now demonstrated the regulatory function of these cells (10, 19, 20, 26, 27, 41) and CTLA-4 has been implicated in immune regulation in human filarial infection (7, 38, 39). This population was expanded only in splenocyte culture from L3-infected animals restimulated with parasite Ag. Indeed, essentially all the CTLA-4+ cells in these cultures were also CD25+ (Table 3). The regulatory population was expanded in splenocyte cultures from IL-4−/− mice to a similar extent as those from WT mice, confirming that their differentiation is IL-4 independent. In contrast, these cells were not observed in cultures from MF-infected animals or in control cultures restimulated with parasite Ag. However, cells from MF-infected mice are clearly activated as demonstrated by the production of Ag-specific IFN-γ and the expression of CD44 and high levels of CD4 (29). These observations are intriguing, as the most significant suppression in human studies is observed in the MF-positive (MF+) population, and a recent study showed that MF+ patients had a higher frequency of CD4+ cells coexpressing CTLA-4+ than MF− patients. In that study, the greatest intensity of CTLA-4 expression was on CD4+ CD25+ cells. Blocking CTLA-4 interactions in vitro resulted in increased production of IL-5 and decreased levels of IFN-γ, indicating that CTLA-4 expression affects the Th1/Th2 balance (39). Our preliminary data from L3-infected mice show that blockade of CTLA-4 results in increased levels of both IL-2 and IL-5, while other type 2 cytokines were unaffected (our unpublished observations).
To determine whether the CD25+ population had the functional characteristics of regulatory T cells, depletion experiments were carried out in L3-infected mice using a MAb (PC61) that eliminates CD25+ cells. This MAb was administered prior to infection to exclude the possibility of depleting activated T cells (37). FACS analysis of splenocyte cultures from animals that were given PC61 revealed a significant reduction (approximately 50%) in the expansion of CD4+ CD25+ CTLA4+ cells. However, there was no significant difference in the percentage of CD4+ cells coexpressing CD25 but not CTLA-4, suggesting that activated T cells were not targeted by the MAb. Depletion of Treg cells had a profound effect on Ag-specific responses. Splenocytes from mice given PC61 showed significantly higher levels of proliferation than those from isotype control-treated mice, demonstrating dysregulation of immune responses following removal of ∼50% of the Treg population. Broadly similar results were observed with Ag-specific cytokine responses, with elevated levels of IL-4 and IL-10 in splenocyte cultures from mice given PC61. The increased levels of IL-10 secreted by splenocytes depleted of Treg cells in vivo might at first glance appear contradictory, as the CD25+ population appears to be the major source of IL-10 in these animals (Fig. 2a). However, as activated T cells (CD25+ CTLA4−) are not depleted from these cultures, this population may have an increased capacity for cytokine production in the absence of regulatory T cells.
A notable feature of these results was an increase in the background level of proliferation (medium only) in mice depleted of Tregs. Levels of proliferation in medium alone are always negligible in splenocyte culture from L3-infected mice, and these animals never secrete detectable levels of any cytokine, demonstrating the suppressive capacity of regulatory T cells in intact mice. However, further studies will be required to define the mechanism by which CD25+ T cells regulate responses in L3-infected mice. For example, do regulatory T cells directly silence Ag-primed T cells or do they act indirectly via an APC population?
CD25+ T-regulatory cells account for a minor proportion of the peripheral T-cell pool (5 to 10%) and act via a cell contact-dependent mechanism, yet they exert a major effect on conventional T cells. Several recent studies have demonstrated that naturally arising CD25+ Treg cells may promote the differentiation of other suppressive regulatory T-cell populations, such as Tr1 or Th3, which act more broadly via secretion of suppressive cytokines (15). For example, coculture of CD25+ and CD25− T cells was shown to induce high levels of IL-10 in the CD25− population, which then gave rise to a population of suppressive CD25− T cells that were anergic (6). APC can also influence the generation of regulatory T cells as shown by recent studies on the trematode parasite Schistosoma mansoni. Exposure of dendritic cells to a lipid molecule, lyso-phosphatidylserine, derived from S. mansoni induced the differentiation of naïve Th cells down the Treg pathway in a Toll-like-receptor-2-dependent fashion (42). Whether a similar scenario is involved in the initiation of responses that result in the expansion of regulatory T cells in the L3-infected BALB/c mouse awaits further study.
The L3 of Brugia are known to prime early IL-4 responses, resulting in the induction of Th2 cells and the down-regulation of IFN-γ production (32). Now we show that exposure to this life cycle stage also induces regulatory T cells. While Treg cells are best characterized for their ability to suppress potentially damaging Th1 inflammatory responses, they can also suppress Th2 responses (4). The elevated levels of proliferation observed in cultures from PC61-treated mice presumably reflect increased proliferation of Th2 cells when Treg cells are depleted, as demonstrated by the abundant secretion of IL-4 and IL-10 in these cultures. In our model system, we observed no effects of PC61 depletion on Ag-specific Th1 responses, but this likely reflects the intensity of the Th2 response elicited by the L3 (33). Interestingly, the presence of parasite Ag in cultures from L3-infected mice given PC61 appeared to suppress the production of type 1 cytokines, with levels of IFN-γ and particularly IL-2 being elevated in medium only but not Ag-stimulated cultures. These results indicate a complex network of regulation in L3-infected mice in which Th1 responses are limited by Th2 cells, with Treg cells preventing an overwhelming Th2 response, which can also be damaging to the host (24). The complexities of the regulatory network are well exemplified by studies in other mouse models of parasite infection. For example in L. major and Plasmodium infection, Treg cells are required for persistence of the low-level parasitemia, which is essential for the maintenance of immunity (2, 9). In the context of lymphatic filariasis, there are many outstanding questions: does constant exposure to L3 in areas of endemicity elicit a Treg population in humans? Do these cells regulate immune responses that might otherwise kill adults and MF? How does immunity arise in this situation?
In conclusion, our results have shown that infection with the L3 of Brugia drives the expansion of CD25+ T-regulatory cells and that depletion of this population affects Ag-specific Th2 responses. By a process of “infectious tolerance,” these cells may then prime the expansion of CD25− Tr1 cells, resulting in the profound hyporesponsiveness observed in lymphatic filarial infection.
Acknowledgments
This study was supported by a Ph.D. studentship from the MRC.
We thank Kerry O'Neill, Margaret McFadyen, and Neil Bennett for help with the parasite life cycle; F. Y. Liew, Department of Immunology, University of Glasgow, for access to the FACS; and Helen Goodridge for assistance with setting up the TaqMan RT-PCR.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aseffa, A., A. Gumy, P. Launois, H. R. MacDonald, J. A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 169:3232-3241. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 3.Bird, J. J., D. R. Brown, A. C. Mullen, N. H. Moskowitz, M. A. Mahowald, J. R. Sider, T. F. Gajewski, C. R. Wang, and S. L. Reiner. 1998. Helper T cell differentiation is controlled by the cell cycle. Immunity 9:229-237. [DOI] [PubMed] [Google Scholar]
- 4.Cosmi, L., F. Liotta, R. Angeli, B. Mazzinghi, V. Santarlasci, R. Manetti, L. Lasagni, V. Vanini, P. Romagnani, E. Maggi, F. Annunziato, and S. Romagnani. 2003. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood 103:3117-3121. [DOI] [PubMed] [Google Scholar]
- 5.Devaney, E., and R. M. Jecock. 1991. The expression of the Mr 30,000 antigen in the third stage larvae of Brugia pahangi. Parasite Immunol. 13:75-87. [DOI] [PubMed] [Google Scholar]
- 6.Dieckmann, D., C. H. Bruett, H. Ploettner, M. B. Lutz, and G. Schuler. 2002. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J. Exp. Med. 196:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Loliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int. Immunol. 12:623-630. [DOI] [PubMed] [Google Scholar]
- 8.Ermann, J., and C. G. Fathman. 2003. Costimulatory signals controlling regulatory T cells. Proc. Natl. Acad. Sci. USA 100:15292-15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisaeda, H., Y. Maekawa, D. Iwakawa, and H. Okada. 2004. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat. Med. 10:29-30. [DOI] [PubMed] [Google Scholar]
- 10.Hori, S., T. L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282-1291. [DOI] [PubMed] [Google Scholar]
- 11.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 12.Huang, H., J. Hu-Li, H. Chen, S. Ben-Sasson, and W. Paul. 1997. IL-4 and IL-13 production in differentiated T helper type 2 cells is not IL-4 dependent. J. Immunol. 159:3731-3738. [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Jarvinen, L. Z., B. R. Blazar, O. A. Adeyi, T. B. Strom, and R. J. Noelle. 2003. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation 76:1375-1379. [DOI] [PubMed] [Google Scholar]
- 15.Jonuleit, H., E. Schmitt, H. Kakirman, M. Stassen, J. Knop, and A. H. Enk. 2002. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196:255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King, C. L., M. Connelly, M. P. Alpers, M. Bockarie, and J. W. Kazura. 2001. Transmission intensity determines lymphocyte responsiveness and cytokine bias in human lymphatic filariasis. J. Immunol. 166:7427-7436. [DOI] [PubMed] [Google Scholar]
- 17.King, C. L., S. Mahanty, V. Kumaraswami, J. S. Abrams, J. Regunathan, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 92:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kropf, P., L. R. Schopf, C. L. Chung, D. Xu, F. Y. Liew, J. P. Sypek, and I. Muller. 1999. Expression of Th2 cytokines and the stable Th2 marker ST2L in the absence of IL-4 during Leishmania major infection. Eur. J. Immunol. 29:3621-3628. [DOI] [PubMed] [Google Scholar]
- 19.Liu, H., B. Hu, D. Xu, and F. Y. Liew. 2003. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-β, and CTLA4. J. Immunol. 171:5012-5017. [DOI] [PubMed] [Google Scholar]
- 20.Long, T. T. A., S. Nakazawa, S. Onizuka, M. C. Huaman, and H. Kanbara. 2003. Influence of CD4+CD25+ T cells on Plasmodium berghei NK65 infection in BALB/c mice. Int. J. Parasitol. 33:175-183. [DOI] [PubMed] [Google Scholar]
- 21.Lowenthal, J., P. Corthesy, C. Tougne, R. Lees, H. MacDonald, and M. Nabholz. 1985. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J. Immunol. 135:3988-3994. [PubMed] [Google Scholar]
- 22.Mahanty, S., S. N. Mollis, M. Ravichandran, J. S. Abrams, V. Kumaraswami, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1996. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J. Infect. Dis. 173:769-773. [DOI] [PubMed] [Google Scholar]
- 23.Mahanty, S., M. Ravichandran, U. Raman, K. Jayaraman, V. Kumaraswami, and T. B. Nutman. 1997. Regulation of parasite antigen-driven immune responses by interleukin-10 (IL-10) and IL-12 in lymphatic filariasis. Infect. Immun. 65:1742-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 25.McHugh, R. S., M. J. Whitters, C. A. Piccirillo, D. A. Young, E. M. Shevach, M. Collins, and M. C. Byrne. 2002. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the gluccocorticoid-induced TNF receptor. Immunity 16:311-323. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, K., A. Kitani, I. Fuss, A. Pedersen, N. Harada, H. Nawata, and W. Strober. 2004. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 172:834-842. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor, R. A., J. S. Jenson, and E. Devaney. 2000. NO contributes to proliferative suppression in a murine model of filariasis. Infect. Immun. 68:6101-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor, R. A., and E. Devaney. 2000. 2. Nitric oxide limits the expansion of antigen-specific T cells in mice infected with the microfilariae of Brugia pahangi. Infect. Immun. 70:5997-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onizuka, S., I. Tawara, J. Shimizu, S. Sakaguchi, T. Fujita, and E. Nakayama. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128-3133. [PubMed] [Google Scholar]
- 31.Osborne, J., and E. Devaney. 1999. Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi. Infect. Immun. 67:1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborne, J., and E. Devaney. 1998. The L3 of Brugia induces a Th2-polarized response following activation of an IL-4-producing CD4−CD8− alpha beta T cell population. Int. Immunol. 10:1583-1590. [DOI] [PubMed] [Google Scholar]
- 33.Osborne, J., S. Hunter, and E. Devaney. 1996. Anti-interleukin-4 modulation of the Th2 polarized response to the parasitic nematode Brugia pahangi. Infect. Immun. 64:3461-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read, S., and F. Powrie. 2001. CD4+ regulatory T cells. Curr. Opin. Immunol. 13:644-649. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531-562. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 38.Satoguina, J., M. Mempel, J. Larbi, M. Badusche, C. Loliger, O. Adjei, G. Gachelin, B. Fleischer, and A. Hoerauf. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291-1300. [DOI] [PubMed] [Google Scholar]
- 39.Steel, C., and T. B. Nutman. 2003. CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. J. Immunol. 170:1930-1938. [DOI] [PubMed] [Google Scholar]
- 40.Stephens, L. A., and D. Mason. 2000. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J. Immunol. 165:3105-3110. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:969-1980. [DOI] [PubMed] [Google Scholar]
- 42.van der Kleij, D., E. Latz, J. F. H. M. Brouwers, Y. C. M. Kruize, M. Schmitz, E. A. Kurt-Jones, T. Espevik, E. C. de Jong, M. L. Kapsenberg, D. T. Golenbock, A. G. M. Tielens, and M. Yazdanbakhsh. 2002. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidyl serine activates toll-like receptor 2 and effects immune polarization. J. Biol. Chem. 277:8122-48129. [DOI] [PubMed] [Google Scholar]
- 43.Wood, K. J., and S. Sakaguchi. 2003. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 3:99-210. [DOI] [PubMed] [Google Scholar]
- 44.Yazdanbakhsh, M., E. Sartono, Y. C. Kruize, A. Kurniawan, T. van der Pouw-Kraan, P. H. van der Meide, M. E. Selkirk, F. Partono, R. Q. Hintzen, and R. A. van Lier. 1993. Elevated levels of T cell activation antigen CD27 and increased interleukin-4 production in human lymphatic filariasis. Eur. J. Immunol. 23:312-3317. [DOI] [PubMed] [Google Scholar]