Abstract
The relationship between vitamin C nutritional status and inflammation has garnered increasing attention, but studies in younger populations are limited. This study aimed to investigate the association between serum vitamin C and high-sensitivity C-reactive protein (hs-CRP) levels in children and adolescents. A cross-sectional analysis was conducted using data from the National Health and Nutrition Examination Survey (NHANES). The demographic data of 1766 participants aged 6–19 years were analyzed using t-tests and chi-square tests. The relationship between serum vitamin C and hs-CRP levels was analyzed using logistic regression, trend tests, and smooth curve fitting. Subgroup analyses and interaction tests were performed to assess the stability of the relationship across different populations. Our findings indicated a negative correlation between serum vitamin C and hs-CRP levels. In the fully adjusted model, each unit increase in serum vitamin C was associated with a reduction of 0.84 mg/L in hs-CRP levels (β = -0.84, 95% confidence interval [CI]: -1.34, -0.35). The hs-CRP levels in the vitamin C saturating group were 3.04 mg/L lower than those in the deficiency group (β = -3.04, 95% CI: -4.99, -1.08). This correlation was more significant in males, individuals with a family income to poverty ratio of ≤ 1.3, and those with a body mass index of ≥ 30 kg/m2. Serum vitamin C levels were negatively correlated with hs-CRP levels in American children and adolescents aged 6–19 years. Males, individuals from low-income families, and those who are overweight derived greater benefits from higher serum vitamin C concentrations.
Keywords: Vitamin C, C-reactive protein, Adolescents, Children, Obesity
Subject terms: Nutrition, Weight management
Introduction
C-reactive protein (CRP) is an acute-phase reactant protein produced in the liver and secreted into the circulatory system in response to inflammation1. As the plasma concentration increases during inflammatory states, it is a commonly used marker of inflammation2,3. Furthermore, CRP levels rise during bacterial infections, positioning it as a marker for infection4–6. Additionally, elevated CRP is observed in autoimmune diseases such as rheumatoid arthritis, where it is frequently used to monitor treatment efficacy7,8. CRP has also been reported as a predictor of cardiovascular disease, aiding in the prediction of heart attack and stroke risk9,10. CRP can be detected at extremely low concentrations using more sensitive analytical methods, referred to as high-sensitivity CRP (hs-CRP)11.
Vitamin C, also known as L-ascorbic acid, is an essential nutrient that humans cannot synthesize endogenously. It acts as a powerful antioxidant that enhances immune defense through its support of diverse cellular functions within the immune system12–14. Research suggests that vitamin C deficiency ranks as the fourth most common nutrient deficiency in the United States and is prevalent among Western populations12. Vitamin C levels fluctuate with age, peaking between the ages of 6 to 11 years and gradually declining thereafter15. Plasma vitamin C levels may fairly reflect vitamin C intake. However, they can also be influenced by factors such as the bioavailability of the vitamin and conditions affecting its absorption16. The vitamin C intake requirements for children vary across different age groups. The average requirement is 25 mg/day for children aged 4–6 years, 40 mg/day for those aged 7–10 years, and 60 mg/day for those aged 11–14 years17. Additionally, vitamin C supplementation has beneficial therapeutic effects on recurrent respiratory infections, respiratory infections in preschool-aged children, and childhood depression18–20. The pharmacokinetics of vitamin C are complex and dose-dependent, with oral intake from food or supplements being the primary route of administration21. Even so, the use of vitamin C supplementation should be determined based on individual serum levels22.
Recently, the relationship between vitamin C nutritional status and inflammation has garnered increasing attention. Notably, studies investigating the association between serum vitamin C and hs-CRP have predominantly focused on adult populations, with a paucity of data available among children and adolescents23–25.
Given the critical growth and developmental stages in this younger demographic, the relationship between serum vitamin C status and hs-CRP levels in this age group concentrations requires additional study. Thus, we aimed to examine the relationship between serum vitamin C and hs-CRP levels in children and adolescents.
Methods
Study population
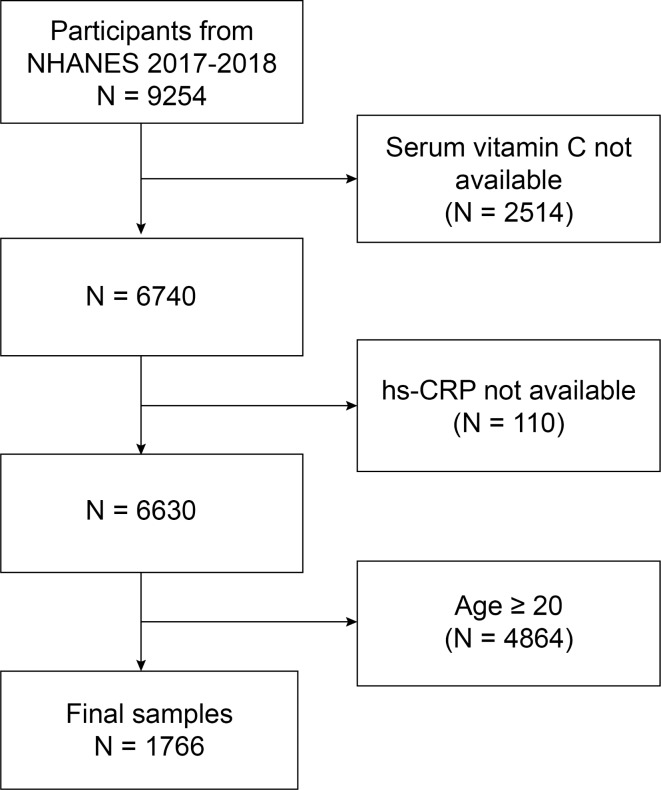
The NHANES is a population-based cross-sectional survey designed to collect information on the health and nutritional status of the United States household population. The study protocols for all NHANES received approval from the ethics review board, and written informed consent was obtained from all participants or from the parents/legal guardians of participants under the age of 16 years. All data used in this study are publicly available on the NHANES website. For our analysis, we utilized demographic data and relevant information on vitamin C and high-sensitivity C-reactive protein (hs-CRP) from the 2017–2018 NHANES cycle. We excluded participants with missing vitamin C data, followed by those with missing hs-CRP data, and finally those aged ≥ 20 years. This process resulted in a final sample of 1766 participants, of which 880 (49.83%) were male and 886 (50.17%) were female (Fig. 1).
Fig. 1.
Flow chart of participant selection NHANES, National Health and Nutrition Examination Survey.
Variables
Serum vitamin C levels were measured using isocratic ultra-high performance liquid chromatography with electrochemical detection at 450 mV (range 200 nA). Detailed laboratory method files, along with quality assurance and monitoring procedures, are available at https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/VIC_J.htm. For this study, serum vitamin C levels were categorized into five groups: deficiency (0–10.99 µmol/L), hypovitaminosis (11–23.99 µmol/L), inadequate (24–49.99 µmol/L), adequate (50–69.99 µmol/L), and saturating (≥ 70 µmol/L)26. Hs-CRP levels (mg/L) were measured using an immunoturbidimetric assay with a dual reagent system. Additional information on the assay methods is available on the NHANES website.
Covariates
The covariates considered in this study were gender (male/female), age (years), race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other races), the ratio of family income to poverty (family PIR), and body mass index (BMI, kg/m²). Family PIR was further categorized into three groups: low-income (< 1.3), middle-income (1.3–3.5), and high-income (> 3.5)27,28. Additionally, BMI was classified into three categories according to World Health Organization standards: normal weight (< 25), overweight (25–30), and obesity (≥ 30 kg/m2)29.
Statistical analysis
In the analysis of participants’ demographic characteristics, continuous variables were analyzed using t-tests, whereas categorical variables were examined using chi-square tests. Logistic regression analysis, trend tests, and smooth curve fitting were used to assess the relationship between serum vitamin C and hs-CRP levels. Subgroup analyses were conducted to assess the association between serum vitamin C and hs-CRP across different groups based on gender, age, BMI, and family PIR. The consistency of these associations across different subgroups was evaluated using interaction tests. All statistical analyses were performed using EmpowerStats (version 4.2). A two-sided P-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
There were 297 males with vitamin C levels below the adequate level, accounting for 16.82% of the total participants, and 260 females, accounting for 14.72%. Our findings indicated that as serum vitamin C levels increased, participant age, BMI, and hs-CRP levels decreased (P < 0.001; Table 1). However, the family PIR level increased with higher serum vitamin C levels (P < 0.01; Table 1). In the groups with higher vitamin C levels, the proportion of participants of non-Hispanic white, other Hispanic, and other races ethnicity decreased (P < 0.01), whereas the proportion of participants of non-Hispanic black and Mexican American ethnicity increased (P < 0.01; Table 1).
Table 1.
Basic characteristics of participants according to the serum vitamin C concentration among American children.
| Characteristics | Serum vitamin C | P-value | |||||
|---|---|---|---|---|---|---|---|
| Deficiency (< 11µmol/L, N = 33) | Hypovitaminosis (11-23.99µmol/L, N = 109) | Inadequate (24-49.99µmol/L, N = 415) | Adequate (50-69.99µmol/L, N = 518) | Saturating (≥ 70µmol/L, N = 691) |
|||
| Age (years) (mean ± SD) | 15.26 ± 2.70 | 14.88 ± 3.16 | 14.33 ± 3.41 | 13.22 ± 3.93 | 11.19 ± 3.81 | < 0.001 | |
| Gender (n) | 0.384 | ||||||
| Male | 22 (66.67%) | 55 (50.46%) | 220 (53.01%) | 262 (50.58%) | 344 (49.78%) | ||
| Female | 11 (33.33%) | 54 (49.54%) | 195 (46.99%) | 256 (49.42%) | 347 (50.22%) | ||
| Race/ethnicity (%) | 0.001 | ||||||
| Non-Hispanic White | 61.25 | 50.95 | 53.14 | 50.55 | 49.85 | ||
| Non-Hispanic Black | 5.24 | 7.13 | 13.42 | 13.63 | 13.17 | ||
| Mexican American | 65.55 | 50.95 | 53.14 | 50.55 | 49.85 | ||
| Other races | 18.26 | 13.02 | 10.63 | 12.20 | 12.80 | ||
|
Family PIR (mean ± SD) |
1.72 ± 1.45 | 2.58 ± 1.72 | 2.36 ± 1.64 | 2.44 ± 1.62 | 2.61 ± 1.64 | 0.008 | |
|
BMI (kg/m2) (mean ± SD) |
26.06 ± 8.26 | 24.04 ± 8.28 | 24.12 ± 6.23 | 22.66 ± 5.77 | 19.83 ± 4.72 | < 0.001 | |
| hs-CRP (mg/L) (mean ± SD) | 5.03 ± 15.23 | 2.32 ± 7.07 | 2.38 ± 5.26 | 2.22 ± 6.22 | 1.18 ± 2.32 | < 0.001 | |
Data are presented as the mean ± standard deviation for continuous variables: the P value was calculated by the weighted linear regression model; percentages for categorical variables: the P value was calculated by the weighted chi-square test. Abbreviation: PIR, ratio of income to poverty, BMI, body mass index; hs-CPR, high-sensitivity C-reactive protein.
Association between serum vitamin C and hs-CRP
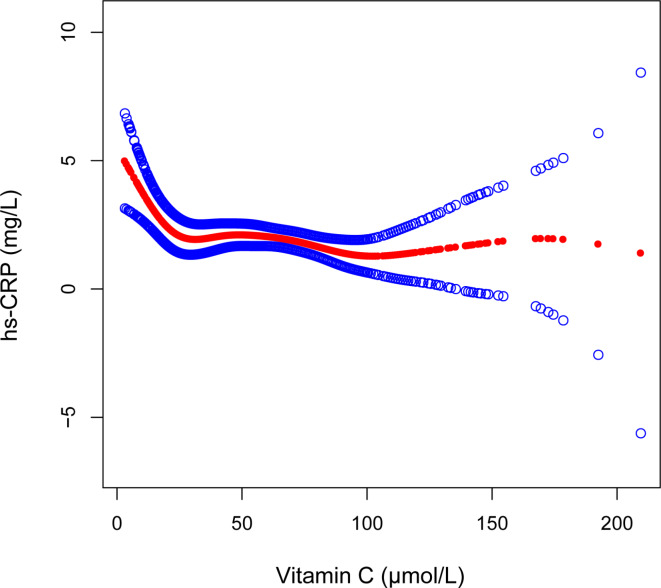
Serum vitamin C was significantly negatively correlated with hs-CRP levels across the crude, minimally adjusted, and fully adjusted models (Table 2). After adjusting for all covariates, each unit increase in serum vitamin C was associated with a reduction of 0.84 mg/L in hs-CRP levels (β = − 0.84, 95% CI: − 1.34, − 0.35). Further, when categorizing serum vitamin C levels, the association remained statistically significant (P < 0.05; Table 2). The hs-CRP levels were 3.04 mg/L lower in the vitamin C saturating group than in the deficiency group (β = -3.04, 95% CI: -4.99, -1.08; Table 2). Additionally, the results of the smooth curve fitting further validated the negative correlation between serum vitamin C and hs-CRP levels (Fig. 2).
Table 2.
Associations between serum vitamin C and hs-CRP levels in different models.
| Vitamin C | hs-CRP β (95% CI) |
|---|---|
| Crude model (Model 1) | |
| Continuous | − 1.17 (− 1.59, -0.74) |
| Categories | |
| Deficiency (< 11µmol/L) | 0 (ref) |
| Hypovitaminosis (11-23.99µmol/L) | − 2.71 (− 4.68, -0.73) |
| Inadequate (24-49.99µmol/L) | − 2.65 (− 4.46, − 0.84) |
| Adequate (50-69.99µmol/L) | − 2.81 (− 4.60, − 1.02) |
| Saturating (≥ 70µmol/L) | − 3.85 (− 5.63, − 2.07) |
| P for trend | < 0.001 |
| Minimally adjusted model (Model 2) | |
| Continuous | − 1.06 (− 1.51, − 0.62) |
| Categories | |
| Deficiency (< 11µmol/L) | 0 (ref) |
| Hypovitaminosis (11-23.99µmol/L) | − 2.83 (− 4.80, − 0.85) |
| Inadequate (24-49.99µmol/L) | − 2.72 (-4.53, − 0.91) |
| Adequate (50-69.99µmol/L) | − 2.82 (− 4.62, − 1.02) |
| Saturating (≥ 70µmol/L) | − 3.74 (− 5.54, − 1.94) |
| P for trend | < 0.001 |
| Fully adjusted model (Model 3) | |
| Continuous | − 0.84 (− 1.34, − 0.35) |
| Categories | |
| Deficiency (< 11µmol/L) | 0 (ref) |
| Hypovitaminosis (11-23.99µmol/L) | − 2.47 (− 4.60, − 0.34) |
| Inadequate (24-49.99µmol/L) | − 2.34 (− 4.28, − 0.39) |
| Adequate (50-69.99µmol/L) | − 2.33 (− 4.28, − 0.38) |
| Saturating (≥ 70µmol/L) | − 3.04 (− 4.99, − 1.08) |
| P for trend | 0.012 |
Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, the family PIR, and BMI were adjusted. Abbreviations: PIR, ratio of income to poverty, BMI, body mass index; hs-CPR, high-sensitivity C-reactive protein.
Fig. 2.
Associations between serum vitamin C and high sensitivity C-reactive protein, The solid red line indicates smooth curve fit between variables. Blue bands indicate the 95% confidence intervals of the fit. Age, gender, race, the ratio of family income to poverty, and body mass index were adjusted.
Subgroup analyses
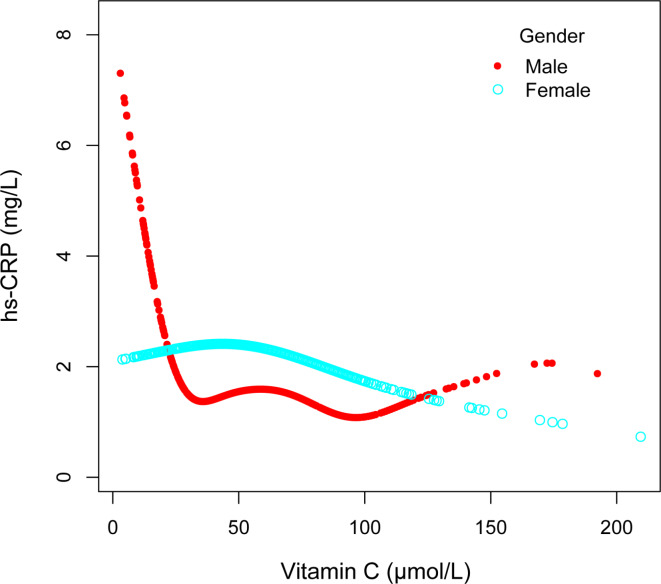
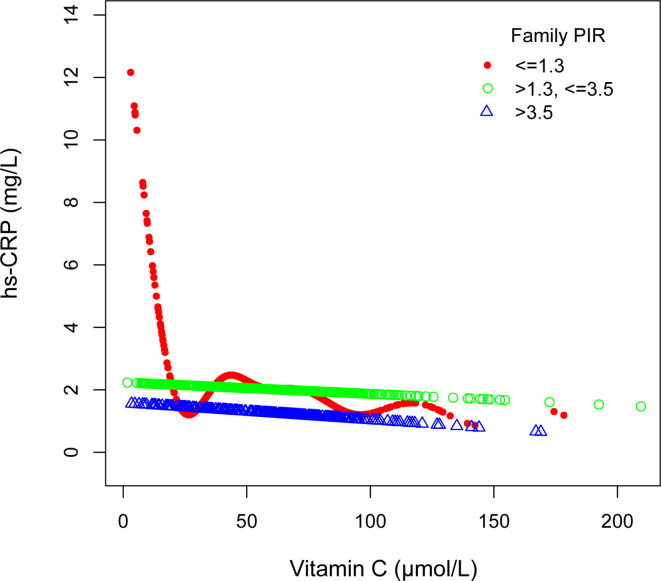
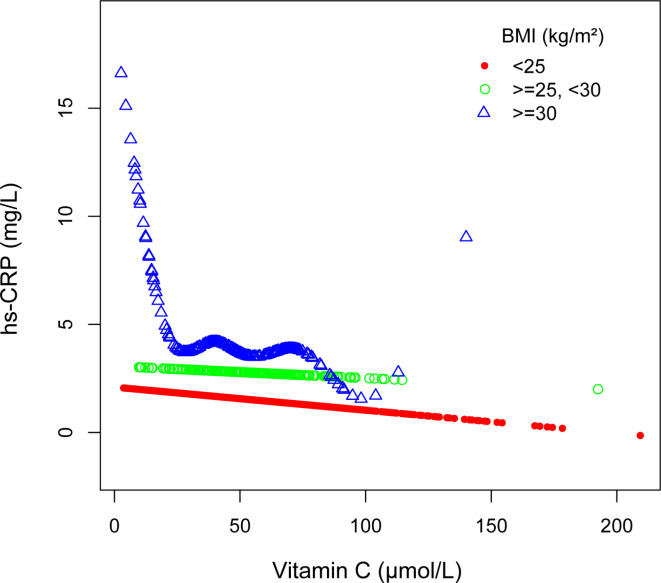
To assess whether the association between serum vitamin C and hs-CRP varies significantly across different subgroups, we performed subgroup analyses and interaction tests based on gender, age, the family PIR, and BMI (Table 3). We also utilized smooth curve fitting to conduct stratified analyses of the association between serum vitamin C and hs-CRP by gender, age, family PIR, and BMI (Figs. 3, 4, 5 and 6). The results demonstrated significant differences in the association between serum vitamin C and hs-CRP across gender, the family PIR, and BMI (P < 0.01; Table 3). In males, a significant negative correlation was observed between serum vitamin C and hs-CRP levels, with each unit increase in serum vitamin C associated with a reduction of 1.634 mg/L in hs-CRP levels. In contrast, females exhibited a non-significant positive correlation between serum vitamin C and hs-CRP. Additionally, when the family PIR was ≤ 1.3, a significant negative correlation was observed between serum vitamin C and hs-CRP, whereas a non-significant positive correlation was found when the family PIR was > 3.5. For participants with a BMI of ≥ 30 kg/m2, a significant negative correlation was noted, with each unit increase in serum vitamin C resulting in a reduction of 4.418 mg/L in hs-CRP levels.
Table 3.
Subgroup analysis of the association between serum vitamin C and hs-CRP.
| Subgroup | hs-CRP [β (95%CI)] | P for interaction |
|---|---|---|
| Gender | 0.001 | |
| Male | − 1.634 (− 2.300, − 0.967) | |
| Female | 0.088 (− 0.637, 0.813) | |
| Age | 0.150 | |
| < 12 years | − 1.627 (− 2.694, − 0.560) | |
| ≥ 12 years | − 0.751 (− 1.306, − 0.196) | |
| Family PIR | < 0.001 | |
| ≤ 1.3 | − 2.102 (− 2.857, − 1.347) | |
| 1.3–3.5 | − 0.041 (− 0.767, 0.685) | |
| > 3.5 | 0.148 (− 0.770, 1.066) | |
| BMI | < 0.001 | |
| < 24.9 kg/m2 | − 0.250 (− 0.822, 0.321) | |
| 25–29.9 kg/m2 | − 0.416 (− 1.813, 0.980) | |
| ≥ 30 kg/m2 | − 4.418 (− 5.679, − 3.156) |
Age, gender, race, the family PIR, and BMI were adjusted.
PIR, ratio of income to poverty, BMI, body mass index; hs-CPR, high-sensitivity C-reactive protein.
Fig. 3.
Associations between serum vitamin C and high sensitivity C-reactive protein stratified by gender, Age, race, the ratio of family income to poverty, and body mass index were adjusted.
Fig. 4.
Associations between serum vitamin C and high sensitivity C-reactive protein stratified by age, Gender, race, the ratio of family income to poverty, and body mass index were adjusted.
Fig. 5.
Associations between serum vitamin C and high sensitivity C-reactive protein stratified by the ratio of family income to poverty, Age, gender, race, and body mass index were adjusted.
Fig. 6.
Associations between serum vitamin C and high sensitivity C-reactive protein stratified by body mass index, Age, gender, race, and the ratio of family income to poverty were adjusted.
Discussion
Vitamin C possesses antioxidant properties and can reduce lipid peroxidation and alleviate inflammation30. The anti-inflammatory mechanisms of vitamin C can be attributed to its inhibition of NF-kappa B activation and downregulation of hepatic mRNA expression, thereby improving the ability to counteract pro-inflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-630–32. The influence of vitamin C on CRP could be mediated via its modulation of upstream factors, particularly the primary inducers of acute-phase response IL-6 and TNF-α33, as a major function of IL-6 is to stimulate the production of CRP and other acute-phase proteins in the liver2,34.
To our knowledge, this is the first large-scale study assessing the relationship between serum vitamin C and hs-CRP levels in children and adolescents in the United States. In a study involving 396 adults, vitamin C treatment reduced the levels of CRP in subjects at increased cardiovascular risk35. In another study, vitamin C supplementation was found to lower plasma CRP concentrations in smokers, with the effect of vitamin C alone being superior to the combination of multiple vitamins36. Church et al.37 investigated the use of multiple vitamins in reducing CRP levels, concluding that only vitamins B6 and C were negatively correlated with CRP levels in a population with an average age of 53 years. Recently, Talikoti et al.38 recruited 60 prehypertensive subjects aged 25–45 years and found that supplementation with water-soluble vitamins (with vitamin C being the most prominent) reduced their hs-CRP levels. These conclusions from small-scale adult studies are similar to ours in that serum vitamin C was negatively correlated with CRP levels.
Notably, our further subgroup analyses indicated gender disparities in the association between serum vitamin C and hs-CRP levels among individuals aged 6–19 years in the United States, wherein higher serum vitamin C levels were associated with lower hs-CRP levels in males but not in females. We analyzed the association between vitamin C intake and gender. The data on vitamin C intake were obtained from the total nutrient intake questionnaires, and the calculation was based on the average vitamin C intake from food on the first and second days of the participants. Our results showed a significant difference, with males consuming more vitamin C from their diet than females (P < 0.05; Supplementary Table 1). We speculate that this could be the reason for the significant negative correlation between serum vitamin C and hs-CRP observed in males. A study conducted by Oliveira et al.39 among Spanish adults found a similar association between higher vitamin C intake and lower levels of hs-CRP in males, which was not observed in females. Moreover, we found that the family PIR modified the association between serum vitamin C and hs-CRP, particularly in low-income families, where increasing serum levels of vitamin C were significantly linked to reduced hs-CRP levels. This suggests that low-income families benefit more from the increase in serum vitamin C. Additionally, we observed a significant negative correlation between serum vitamin C and hs-CRP among obese children and adolescents. Numerous studies have demonstrated that obesity is associated with chronic low-grade inflammation, characterized by elevated CRP levels in subjects with obesity and related diseases40–43. Mazaheri-Tehrani et al.44 demonstrated an inverse association between serum vitamin C levels and BMI. Vitamin C may be involved in the suppression of obesity-associated inflammatory states and play a beneficial role in the obese population by reducing NF-kappa B-mediated inflammatory responses32,45,46.
This study has several notable strengths. First, previous research on the relationship between vitamin C and hs-CRP has primarily focused on adults, while our study specifically targeted children and adolescents. Second, our data are nationally representative and our sample size was large. Third, we adjusted for confounding factors to improve the reliability of the study results. Finally, subgroup analyses were performed to assess the robustness of the association between serum vitamin C and hs-CRP across different populations. However, this study also has limitations that should be addressed. Due to the cross-sectional design, we were unable to establish a causal relationship between serum vitamin C and hs-CRP. Additionally, despite considering multiple covariates, we cannot completely eliminate the influence of all confounding variables.
Conclusions
In American children and adolescents aged 6–19 years, serum vitamin C is negatively associated with hs-CRP levels. This negative association is more pronounced in males, individuals from low-income families, and those classified as obese. Elevated serum vitamin C levels may offer protective effects by reducing inflammation within these groups. Nonetheless, further research is required to investigate the causal relationships and underlying mechanisms of these associations. Our results of the study open the perspectives of supplementing with vitamin C in certain categories of children. Such insights could inform clinical practices and nutritional guidelines to mitigate inflammation-related health risks in children and adolescents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all of the contributors for their valuable input on this paper.
Abbreviations
- NHANES
National health and nutrition examination survey
- hs-CRP
high-sensitivity C-reactive protein
- PIR
ratio of income to poverty
- BMI
body mass index
- CI
confidence interval
Author contributions
CL: Conceptualized the study, curated the data, and wrote the manuscript. ZWZ and SCJ: Conducted methodological analysis and operated the software. XF and LY: Validated the data and contributed to manuscript writing. KJG and JJN: Acquired resources. TWL and JMY: Conceptualized the study, and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Key Research, Development, and Promotion Projects of Henan Province (Scientific and Technological Tackling) [222102310328]; and the Medical Science and Technology (joint construction) Project of Henan Province [LHGJ20220750].
Data availability
The survey data were obtained from publicly available online sources(www.cdc.gov/nchs/nhanes/).
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All NHANES study protocols were approved by an ethics review board. Written informed consent was obtained from all participants or from the parents/legal guardians of participants under the age of 16. These details are publicly accessible on the NHANES website.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tiewei Li, Email: litieweind@163.com.
Junmei Yang, Email: yangjunmei7683@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81751-x.
References
- 1.Black, S., Kushner, I. & Samols, D. C-reactive protein. J. Biol. Chem.279 (47), 48487–48490 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Del Giudice, M. & Gangestad, S. W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain. Behav. Immun.70, 61–75 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Sproston, N. R. & Ashworth, J. J. Role of C-Reactive protein at sites of inflammation and infection. Front. Immunol.9, 754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li, S., Renick, P., Senkowsky, J., Nair, A. & Tang, L. Diagnostics for Wound infections. Adv. Wound care. 10 (6), 317–327 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Póvoa, P. et al. C-reactive protein as a marker of infection in critically ill patients. Clin. Microbiol. Infection: Official Publication Eur. Soc. Clin. Microbiol. Infect. Dis.11 (2), 101–108 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Du Clos, T. W. & Mold, C. The role of C-reactive protein in the resolution of bacterial infection. Curr. Opin. Infect. Dis.14 (3), 289–293 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Du Clos, T. W. & Mold, C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol. Res.30 (3), 261–277 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Du Clos, T. W. C-reactive protein as a regulator of autoimmunity and inflammation. Arthritis Rheum.48 (6), 1475–1477 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Ridker, P. M. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation108 (12), e81–85 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Stumpf, C. & Hilgers, K. F. C-reactive protein: more than just a marker of inflammation? J. Hypertens.27 (9), 1748–1749 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Moutachakkir, M., Lamrani Hanchi, A., Baraou, A., Boukhira, A. & Chellak, S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann. Biol. Clin.75 (2), 225–229 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Carr, A. C. & Maggini, S. Vitamin C and Immune function. Nutrients9(11). (2017). [DOI] [PMC free article] [PubMed]
- 13.Granger, M. & Eck, P. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res.83, 281–310 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Doseděl, M. et al. Vitamin C-Sources, physiological role, Kinetics, Deficiency, Use, Toxicity, and determination. Nutrients13(2). (2021). [DOI] [PMC free article] [PubMed]
- 15.Schleicher, R. L., Carroll, M. D., Ford, E. S. & Lacher, D. A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr.90 (5), 1252–1263 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Dehghan, M., Akhtar-Danesh, N., McMillan, C. R. & Thabane, L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr. J.6, 41 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EFSA Panel on Dietetic Products N & Allergies Scientific opinion on Dietary reference values for vitamin C. 11(11):3418. (2013).
- 18.Amr, M., El-Mogy, A., Shams, T., Vieira, K. & Lakhan, S. E. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutr. J.12, 31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara, P. et al. Beneficial therapeutic effects of vitamin C on recurrent respiratory tract infections in children: preliminary data. Minerva Pediatr.73 (1), 22–27 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Garaiova, I. et al. Probiotics and vitamin C for the prevention of respiratory tract infections in children attending preschool: a randomised controlled pilot study. Eur. J. Clin. Nutr.69 (3), 373–379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lykkesfeldt, J. & Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients11(10). (2019). [DOI] [PMC free article] [PubMed]
- 22.Martini, L. et al. Appropriate and inappropriate vitamin supplementation in children. J. Nutritional Sci.9, e20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding, N., Zeng, Z., Luo, J. & Li, K. The cross-sectional relationship between vitamin C and high-sensitivity C-reactive protein levels: insights from NHANES database. Front. Nutr.10, 1290749 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, K. et al. Cross-over study of influence of oral vitamin C supplementation on inflammatory status in maintenance hemodialysis patients. BMC Nephrol.14, 252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikirova, N., Casciari, J., Riordan, N. & Hunninghake, R. Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. J. Translational Med.11, 191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crook, J., Horgas, A., Yoon, S. J., Grundmann, O. & Johnson-Mallard, V. Insufficient Vitamin C Levels among adults in the United States: results from the NHANES surveys, 2003–2006. Nutrients13(11). (2021). [DOI] [PMC free article] [PubMed]
- 27.Ogden, C. L. et al. Prevalence of obesity among youths by Household Income and Education Level of Head of Household - United States 2011–2014. MMWR Morbidity Mortal. Wkly. Rep.67 (6), 186–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey, R. L. et al. Total usual intake of shortfall nutrients varies with poverty among US adults. J. Nutr. Educ. Behav.49 (8), 639–646e633 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 894:i-xii, 1-253. (2000). [PubMed]
- 30.Ellulu, M. S. Obesity, cardiovascular disease, and role of vitamin C on inflammation: a review of facts and underlying mechanisms. Inflammopharmacology25 (3), 313–328 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Bowie, A. G. & O’Neill, L. A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. Journal of immunology (Baltimore, Md: 2000, 165(12):7180–7188. (1950). [DOI] [PubMed]
- 32.Cárcamo, J. M., Pedraza, A., Bórquez-Ojeda, O. & Golde, D. W. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry41 (43), 12995–13002 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Härtel, C., Strunk, T., Bucsky, P. & Schultz, C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine27 (4–5), 101–106 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Wu, X. & Schauss, A. G. Mitigation of inflammation with foods. J. Agric. Food Chem.60 (27), 6703–6717 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Block, G. et al. Vitamin C treatment reduces elevated C-reactive protein. Free Radic. Biol. Med.46 (1), 70–77 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block, G. et al. Plasma C-reactive protein concentrations in active and passive smokers: influence of antioxidant supplementation. J. Am. Coll. Nutr.23 (2), 141–147 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Church, T. S., Earnest, C. P., Wood, K. A. & Kampert, J. B. Reduction of C-reactive protein levels through use of a multivitamin. Am. J. Med.115 (9), 702–707 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Talikoti, P., Bobby, Z. & Hamide, A. Supplementation of Water-Soluble vitamins reduces hyperhomocysteinemia, insulin resistance, and high-sensitivity C-reactive protein in prehypertension subjects. Cureus15 (1), e33481 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira, A., Rodríguez-Artalejo, F. & Lopes, C. The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur. J. Clin. Nutr.63 (11), 1345–1352 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Chudek, J. & Wiecek, A. Adipose tissue, inflammation and endothelial dysfunction. Pharmacol. Rep.58 Suppl, 81–88 (2006). [PubMed] [Google Scholar]
- 41.Ferrante, A. W. Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J. Intern. Med.262 (4), 408–414 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Kawai, T., Autieri, M. V. & Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell. Physiol.320 (3), C375–c391 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berg, A. H. & Scherer, P. E. Adipose tissue, inflammation, and cardiovascular disease. Circul. Res.96 (9), 939–949 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Mazaheri-Tehrani, S., Yazdi, M., Heidari-Beni, M., Yazdani, Z. & Kelishadi, R. The association between vitamin C dietary intake and its serum levels with anthropometric indices: a systematic review and meta-analysis. Complement. Ther. Clin. Pract.51, 101733 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Diaz, D. F. et al. Vitamin C modulates the interaction between adipocytes and macrophages. Mol. Nutr. Food Res.55 (Suppl 2), S257–263 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Diaz, D. F., Lopez-Legarrea, P., Quintero, P. & Martinez, J. A. Vitamin C in the treatment and/or prevention of obesity. J. Nutri. Sci. Vitaminol.60 (6), 367–379 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The survey data were obtained from publicly available online sources(www.cdc.gov/nchs/nhanes/).