Abstract
Butyrate is one of the most abundant short-chain fatty acids (SCFAs), which are important metabolites of dietary fiber by fermentation of gut commensals, and has been shown to be vital in maintaining host health. The present study mainly investigated how sodium butyrate (NaB) supplementation in the diet with high proportion of soybean meal (SBM) affected turbot. Four experimental diets were formulated: (1) fish meal (FM) based diet (control group), (2) SBM protein replacing 45% FM protein in the diet (high SBM group), (3) 0.2% NaB supplementation in the high SBM diet (high SBM + 0.2% NaB group), and (4) 0.5% NaB supplementation in the high SBM diet (high SBM + 0.5% NaB group). The fish were fed four different diets for 8 weeks. The results showed that the high SBM diet significantly suppressed growth performance, induced typical enteritis symptoms and decreased resistance to bacterial infection. However, inclusion of 0.2% and 0.5% NaB in the high SBM diet both effectively increased the growth performance of turbot. Meanwhile, dietary NaB protected the intestinal morphology, and regulated the gene expression of inflammatory cytokines to relieve the inflammation of turbot, such as TNFα, IL-1β, NFκB and IL-10. Moreover, supplementation with NaB in the high SBM diet activated HIF-1α/IL-22/Lysozyme signaling pathway to against Edwardsiella tarda (E. tarda) infection, especially 0.5% NaB supplementation exerted more effectively to defence bacterial infection under inflammatory state. In conclusion, dietary NaB significantly promoted growth and gut health of turbot. Besides, it enhanced the resistance of fish to bacterial infection, especially dietary 0.5% NaB supplementation.
Keywords: Scophthalmus maximus L., Sodium butyrate (NaB), Soybean, Growth, Inflammatory cytokines, Signaling pathways
Subject terms: Antimicrobial responses, Animal physiology
Introduction
The gastrointestinal tract acts as a key organ, playing a role not only in digestion and absoption of nutrients, but also in fighting against pathogens. Short chain fatty acids (SCFAs) are mainly produced by gut microbiota through degradation and fermentation of non-digested carbohydrates1. Butyrate is one of the most abundant SCFAs metabolites in intestine. Being an important energy and signaling molecule, butyrate has favorable effects on various physiological processes2. Recently, butyrate has been widely used as a growth promoter and immunostimulant in aquaculture. For example, Aalamifar et al. found that dietary supplementation with butyric acid significantly enhanced the growth performance of barramundi (Lates calcarifer) in comparison with control group, containing of final body weight, specific growth rate (SGR) and protein efficiency ratio (PER)3. Additionally, sodium butyrate (NaB)-supplemented diet increased lysozyme activities, and elevated production of complement (ACH50) against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss)4. Moreover, butyrate plays a key role in regulating the intestinal health. Zhang et al. found that NaB supplementation in the diets effectively repaired the intestinal mucosal structure, increased the villi height and crypt depth ratio, and decreased the crypt depth of rice field eel (Monopterus albus)5. Furthermore, NaB also strengthened the intestinal tight junction and regulated the expression of immune genes and inflammatory cytokines to keep gut homeostatic in numerous fish, such as common carp (Cyprinus carpio), sea bream (Sparus aurata) and so on6,7. In addition to, Liu et al. reported that dietary NaB altered the gut microbial composition, contributing to be more beneficial for rainbow trout health8.
Due to the rapid development of intensive aquaculture, the need of fish meal (FM) in aquatic feeds continuously increases. It’s well known that commercial FM is mostly got from small, wild and caught sea fishes. However, the small pelagic fishes are vulnerable to the challenges of climate changes, resulting in the shortage of raw material to produce FM9. Moreover, extensive overfishing leads to a rapid decline in marine species that further limits FM production10. In addition to, the restrictions on fishing also contribute to decreasing the production of FM11. Due to all these factors, the availability of FM becomes constantly unstable in recent years. Therefore, it is imperative to find affordable and appropriate protein sources to replace FM. Plant protein are suitable sources, because they are wildly planted and low cost. Soybean meal (SBM) is the largest protein source among plant protein, and has a relatively balanced amino acid profile12. Moreover, it has been reported that SBM contains about 41% protein and 20% ether extract based on dry matter13. However, SBM is poor at palatability and the certain essential amino acids (EAAs), such as methionine and cystein, are inadequate for growth of aquacultural animals14. Additionally, SBM also has several anti-nutritional factors (ANFs), such asphytates, tannins, trypsin inhibitors, and oligosaccharides15. The ANFs affect the protein utilisation, digestion and mineral utilisation of fish, leading to the desreased growth performance, immune responses and increased the disease susceptibility16. A large number of studies have confirmed that high proportion of SBM in aquafeed decreases the growth performance, digestion, absorption, anti-oxidative capacity, and impaires the function of gut in aquatic animals17,18. These above adverse effects could be ameliorated by SCFAs to increase the growth, regulate intestinal health, enhance digestion and nutrient absorption, and promote antimicrobial ability of aquatic animals19–21.
As we all know, nuclear factor kappa B (NFκB) pathway is an important inflammatory signaling pathway that regulates the expression of various genes, such as cytokine production and cell survival22. NFκB pathway could be activated by different dietary factors to control inflammation. It has been reported that dietary NaB significantly downregulated the increased expression of NFκB caused by the plant protein replacement of FM to repair the intestinal tissue damage in largemouth bass (Micropterus salmoides)23. Moreover, NFκB regulates the pro-inflammatory response by the tumor necrosis factor alpha (TNFα) and interleukin-1 (IL-1) signaling pathway24.
Considered as a haemopoietic organ of teleost fish, the head kidney is consistent with the bone marrow of higher vertebrates, and it consists of various erythrocytes and leukocytes, such as macrophages, granulocytes and B lymphocytes25,26. Acting as innate immune cells, macrophages absorb and digest pathogens through phagocytosis, production of reactive oxygen (ROS) and nitrogen intermediates, and secretion of some antibacterial components, including lysozyme and antibacterial peptides (AMPs)27. Being a transcriptional factor, hypoxia-inducible factor 1 alpha (HIF-1α) is essential for myeloid cells to exert immune function and regulate inflammatory responses28. Our previous study demonstrated that butyrate could induce HIF-1α expression of head kidney macrophages (HKMs) from turbot, leading to the activation of innate immune effectors to prevent Edwardsiella tarda (E. tarda) infection, such as ROS, nitric oxide (NO) and lysozyme29. Interleukin 22 (IL-22) has been considered as a key cytokine, and is important to host defense by production of AMPs30. Our latest research found that butyrate activated HIF-1α/IL-22 signaling pathway to increase the expression of antibactericidal components, contributing to fighting against bacterial infection both in turbot and zebrafish31.
Turbot is an important commercial species in aquaculture, due to its high value and nutrition32. Liu et al. has already demonstrated that SBM protein replacing 40% FM protein in FM diet significantly reduced the growth and broke the intestinal morphology of turbot, leading to inflammation. And 0.2% dietary NaB alleviated the negative effects caused by high SBM diet21. Moreover, our previous study has also confirmed that supplementation with NaB in the FM diet promoted the immune response of turbot31. Unlikely, in our present study, we attempted to explore whether dietary NaB could alleviate adverse effects caused by the diet with high proportion of SBM (SBM replacing 45% FM protein in FM diet) in turbot, containing growth performance, inflammation and immune response. And this study would provide a theoretical basis for the use of NaB as a functional additive to reduce the reliance on FM, ameliorate the adverse effects caused by SBM diets, and create a more sustainable and efficient system in aquaculture.
Materials and methods
Diet formulation
Four isonitrogenous and isolipidic diets (52% crude protein and 12% crude lipid) were formulated in Table 1. The control was a FM-based diet, and the high SBM diet was soybean protein replacement of 45% FM protein. As for the other two diets, 0.2% or 0.5% NaB (Sigma, St. Louis, MO, USA) was added into the high SBM diet. All of dry ingredients in each diet were triturated into fine powder through a 180-μm mesh by a pulverizer (YongkangHongsun, Zhejiang, China). Thereafter, all the ingredients were thoroughly blended with fish oil and soy lecithin, up to the ingredients became yellow and gray powder. Then, added appropriate amount of distilled water to make stiff dough, according to the quality of granule feedstuff. This was a process of exploration, and needed constant adjustment to make successful granule feedstuff. Afterwards, the pellets were performed with a laboratory feed mill (South China University of Technology, Guangzhou, China) into 3 mm size of granule feedstuff. And then, the granule feedstuff was dried for about 12 h at 45 °C in a ventilated oven, and stored at − 20 ℃ for the feeding experiments. The proximate composition of experimental diets is showed in Table 1.
Table 1.
Formulation and proximate composition of the experimental diets (% dry matter).
| Ingredient | Control | High SBM | High SBM + 0.2%NaB |
High SBM + 0.5%NaB |
|---|---|---|---|---|
| Fish meala | 60.00 | 33.00 | 33.00 | 33.00 |
| Soybean mealb | 0.00 | 35.69 | 35.69 | 35.69 |
| Wheat gluten mealc | 4.46 | 8.75 | 8.75 | 8.75 |
| Wheat meald | 22.59 | 4.19 | 4.19 | 4.19 |
| Beer yeaste | 2.00 | 2.00 | 2.00 | 2.00 |
| Fish oil | 3.05 | 5.31 | 5.31 | 5.31 |
| Soy lecithin | 2.50 | 2.50 | 2.50 | 2.50 |
| Monocalcium phosphate | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin premixf | 1.50 | 1.50 | 1.50 | 1.50 |
| Mineral premixg | 1.50 | 1.50 | 1.50 | 1.50 |
| Choline chloride (99%) | 0.25 | 0.25 | 0.25 | 0.25 |
| Calcium propionate | 0.10 | 0.10 | 0.10 | 0.10 |
| Ethoxyquin | 0.05 | 0.05 | 0.05 | 0.05 |
| Attractantsh | 1.00 | 1.00 | 1.00 | 1.00 |
| Sodium alginate | 0.50 | 0.50 | 0.50 | 0.50 |
| Microcrystalline cellulose | 0.00 | 3.16 | 2.96 | 2.66 |
| Sodium butyratei | 0.00 | 0.00 | 0.20 | 0.50 |
| Proximate composition (dry matter basis) | ||||
| Dry matter content | 96.68 | 95.81 | 95.99 | 95.97 |
| Crude protein | 51.71 | 51.22 | 51.43 | 51.55 |
| Crude lipid | 11.92 | 11.81 | 11.80 | 12.16 |
| Crude ash | 12.94 | 10.37 | 10.70 | 10.62 |
Abbreviations: SBM, soybean meal diet; NaB, sodium butyrate.
aPurchased from Qingdao Seven Great Bio-tech Company Limited (Qingdao, China), crude protein: 711.6 g/kg, crude lipid: 103.4 g/kg (dry matter basis).
bPurchased from Qingdao Seven Great Bio-tech Company Limited (Qingdao, China), crude protein: 538.4 g/kg, crude lipid: 27.2 g/kg (dry matter basis).
cPurchased from Qingdao Fulin Company Limited (Qingdao, China), crude protein:858.9 g/kg, crude lipid: 22.5 g/kg (dry matter basis).
dPurchased from Qingdao Fulin Company Limited (Qingdao, China), crude protein:199.9 g/kg, crude lipid:29.0 g/kg (dry matter basis).
ePurchased from Qingdao Fulin Company Limited (Qingdao, China), crude protein:477.6 g/kg, crude lipid: 17.2 g/kg (dry matter basis).
fVitamin premix (mg/kg diet): retinyl acetate (500,000 IU/g), 32; thiamine HCl (98%), 25; riboflavin (80%), 45; niacin (99%), 200; calcium pantothenate (98%), 60; pyridoxine HCl (99%), 20; inositol (98%), 800; folic acid (98%), 20; cyanocobalamin (1%), 10; ascorbic acid (35%), 120; cholecalciferol (500,000 IU/g), 5; α-tocopheryl acetate (50%), 240; biotin (2%), 60; menadione sodium bisulphite (51%), 10; ethoxyquin (100%), 3; microcrystalline cellulose (100%), 11,470.
gMineral premix (mg/kg diet): CoCl2·6H2O (1%), 50; CuSO4·5H2O (25%), 10; FeSO4·H2O (30%), 80; ZnSO4·H2O (34.50%), 50; MnSO4·H2O (31.80%), 45; MgSO4·H2O (15%), 1200; Na2SeO3 (1%), 20; calcium iodine (1%), 60; zeolite powder, 13,512.
hBetaine: dimethyl-beta-propiothetin (DMPT): threonine: glycine: inosine-5-diphosphate trisodium salt = 4: 2:2: 1:1.
iPurchased from Sigma-Aldrich Co. (USA). The batch number was 303,410–500G, and the purity was > 98%.
Fish maintenance
Juvenile turbot were obtained from a private farm called Longhui Aquatic Product Co. Ltd. in Weihai, Shandong Province, China. The feeding trial was performed in a flowing water system, and fish were fed control diet and acclimated to experimental system for 14 days. Before the formal feeding trial, fish with initial weight of 22.0 ± 0.2 g fasted for 24 h, and then they were randomly allocated to 12 tanks (200 L). Each of diet was assigned for triplicate tanks (40 fish per tank). Fish were fed twice daily at 7:00 and 19:00 for eight weeks. After the end of feeding for 30 min, fish waste and half of the tank water was siphoned and supplementation with well-aerated and dechlorinated water. During the feeding trial, water quality parameters were measured as follows: water temperature 17.16 ± 0.65 °C, dissolved oxygen 7.93 ± 0.51 mg/L, salinity 28.23 ± 0.57‰, ammonia and nitrite 0.07 ± 0.02 mg/L, and pH 7.41 ± 0.26.
Sampling
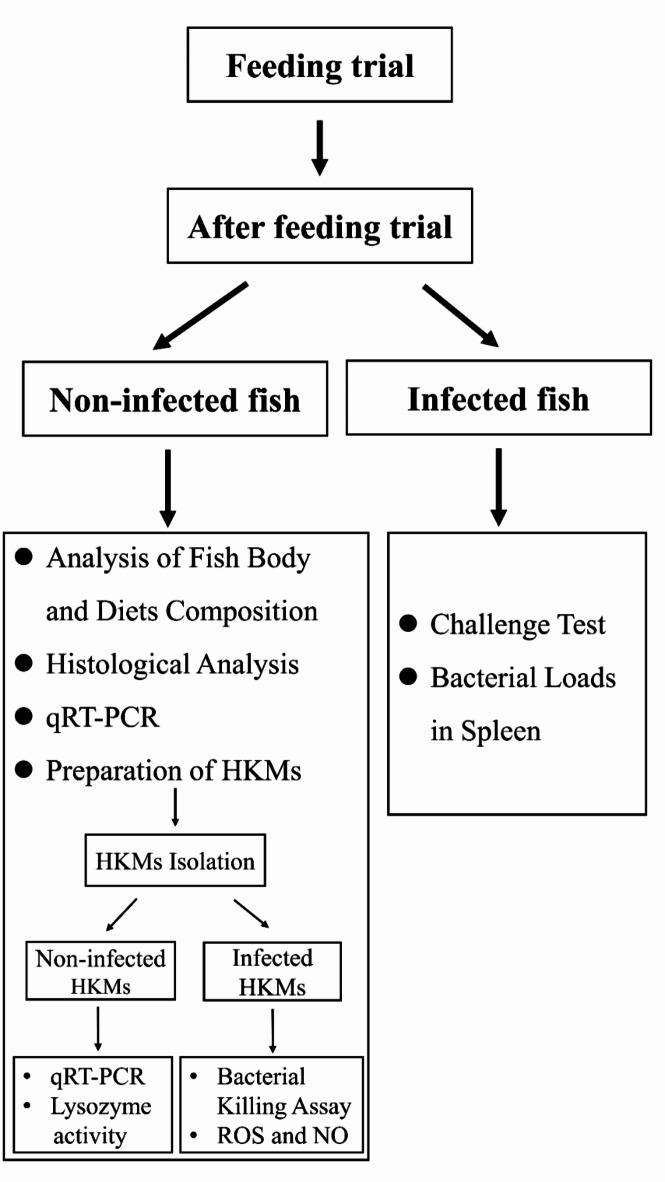
Turbot from each group were anesthetized with 20 mg/L tricaine after fasting for 24 h, which is commonly used in the fisheries industry33. After that, the body weight of each fish was measured before sampling, and four fish from per tank were selected and saved at -80°C for analysis of body composition. Additionally, liver samples from eight fish per tank were collected and weighted for the calculation of hepatosomatic index. Four distal intestine samples per tank were collected and fixed with 4% paraformaldehyde solution for further histological analysis. Meanwhile, four kidney and distal intestine samples per tank were frozen in liquid nitrogen immediately and stored at -80°C for further analysis. Forthermore, four fish per tank were selected for the isolation of HKMs. The key steps in this experimental protocol are summarized pictorially in Fig. 1.
Fig. 1.
Schematic representation of the key steps in the experimental protocol.
Analysis of fish body and diets composition
The composition of turbot and diets was detected through previously described methods34. Briefly, turbot in each group were dried about 48 h at 105°C in a ventilated oven to test the moisture contents. In addition, the crude protein and lipid of diets was individually tested by Kjeltec (TM 8400, FOSS, Sweden) and Soxhlet ether extraction (Buchi 36,680, Switzerland). According to the method of Thiex et al., the ash contents were measured by burning at 560°C in a muffle furnace for 10 h35.
Histological analysis
According to the standard histological procedures, the distal intestines from each group were firstly fixed with 4% paraformaldehyde solution for 24h. And then, the fixed distal intestines were dehydrated in ethanol, cleared in xylene, and embedded in paraffin36. Then, the intestines were cut into the thickness of 7 μm tissue segments by a rotary microtome (Lecia Jung RM 2016, Germany), placed on slides, and stained with hematoxylin and eosin (H&E). Finally, the intestinal histological preparation was observed by using eclipse Ti-S microscope (Nikon, Japan). The height of villus, the width of lamina propria, and the thickness of muscle layers was determined by analyzing micrographs with image analysis software, Image Pro Plus®6.0 (Media Cybernetics, Silver Spring, MD, USA).
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted by using Trizol reagent (Accurate Biology, Hunan, China). The quantity of RNA was detected by NanoDrop spectrophotometer (NanoDrop Technologies), and the quality was tested by agarose gel electrophoresis. Then, the cDNA was synthesized with PrimeScript RT reagent kit (Vazyme, China). qRT-PCR was performed on thermo-cycler CFX96TM Real Time System (Bio-Rad, USA) with a total volume of 25μl, containing 1μl cDNA, 1μl each primer, 12.5μl SYBR Green Premix Ex Taq™ II, and 9.5μl RNase-free water. The amplification program was as follows: 95 °C for 2 min, followed by 39 cycles of 95 °C for 10 s, 58 °C for 10 s, and 72 °C for 20 s. Primer of each gene for qRT-PCR designed based on mRNA sequences of turbot. And the amplification efficiencies of each pair of primer were ranged between 0.95 and 1.05. All of the primers were listed in Table 2. β-actin was chosen as the internal control. The expression level of genes was calculated and normalized via the 2-ΔΔCt method.
Table 2.
Primer sequences used for qRT-PCR.
| Target genes | Forward Primers (5′–3′) | Reverse primers (5′–3′) | Genebank accession number |
|---|---|---|---|
| TNFα | GGGTGGATGTGGAAGGTGAT | GGCCTCTGTTTGGCTTGACT | XM_035619868.2 |
| IL-1β | GGCAGACCCCTTGAAGAATA | TGGTGAACCCTTCCCATTAT | XM_035640817.2 |
| NFκB | CATCACGGTGCTGTCAAATCCA | CCGACGGTTATGGTCCAAAAAG | XM_035627239.2 |
| IL-10 | CCACGCCATGAACAGCATCCT | ACATCGGACTTGAGCTCGTCGAA | XM_035632547.2 |
| HIF-1α | CCACCACCACTGACGATTCA | GCTGGGGTAGCTGTTGACAT | XM_035610073.2 |
| IL-22 | GCTGCAGCGTGACTCACTA | CTGCAGGTACGTGAAGAGGA | JQ349070.1 |
| Lysozyme (LYZ) | GAGACTGGAACCCACACAGGAACG | CTGCTCTCCGCTCCAATCAGGAA | XM_035636032.2 |
| β-actin | GCGTGACATCAAGGAGAAGC | TGGAAGGTGGACAGGGAAGC | XM_035614479.1 |
Challenge test
At the end of feeding experiment, ten fish were randomly selected from each group and fasted for 24 h. Then, the fish were anesthetized with tricaine (20 mg/L), and injected intraperitoneally with E. tarda (1 × 107 CFU) per fish. The bacterial virulence of this concentration was able to induce the immune response of turbot based on previous studies37. During the infection, all of turbot were not fed in different four groups. The mortality of fish was recorded every 6 h.
Bacterial loads in spleen
At the end of feeding trial, twelve fish (four fish/tank) were randomly selected from four different groups. After infection with E. tarda for 12 h, all of fish were anaesthetized with tricaine (20 mg/ L). And then, the spleen was removed from each of fish and weighted. After that, the spleens were homogenized with ice cold PBS and the supernatants were coated on LB agar in incubator at 28°C overnight. Finally, the colony forming units (CFUs) were numbered. Bacterial load (BL) = CFUs in spleen/spleen weight.
Preparation of head kidney macrophages (HKMs)
HKMs isolation
Before infection, four fish per tank were selected to isolate HKMs. Macrophages were isolated according to the method described by our previous study29. In brief, the head kidney of fish was removed immediately and washed twice with PBS supplementation with antibiotic mixture (100 KU/ml penicillin, 10 mg/ml streptomycin and 25 μg/ml amphotericin B). After that, the head kidney was cut into small pieces and passed through a 100 μm nylon mesh, and the obtained cell suspension was washed twice in L-15 leibovitz cell culture medium with antibiotics and 2% fetal bovine serum (FBS), and centrifuged at 200 g for 5 min between washes. The obtained cell suspension was then separated in a 34/51% Percoll (Solarbio, China) by centrifugation at 400g for 30 min. Afterwards, the cells at interface were collected and washed twice by centrifugation at 200 g for 5 min. Then, HKMs were counted by cell counter (Bio-Rad, USA). After that, the cells were equally distributed to cell plates and cultured in incubator at 24°C. After 2 h, the non-adherent cells were removed, and the adherent macrophages were kept in complete L-15 leibovitz cell culture medium (2% FBS and 1% antibiotics) used for different tests.
Bacterial killing assay
HKMs were infected with equal number of E. tarda at 24°C for 2 h. Then, HKMs were broken up in ice-cold water, followed by the cell lysates were diluted and coated on LB agar overnight to calculate the survival rate of bacteria.
Measurement of ROS, Nitric Oxide (NO).
HKMs were infected with equal number of E. tarda at 24 °C for 2 h or 6 h. Then, the secretion of ROS and NO from HKMs was tested respectively through commercial kit from Beyotime (Shanghai, China). The analysis was conducted according to the manufacturers’ instruction.
Measurement of lysozyme activity
After isolation HKMs from turbot, the activity of lysozyme was measured by a commercial kit from Jiancheng (Nanjing, China). The analysis was conducted according to the manufacturers’ instruction.
Calculation and statistical method
The results are presented as means ± SEM. Raw data were analyzed by one-way ANOVA after normality and homogeneity of variance was verified. Multiple comparisons were conducted with Tukey’s test. Statistical analysis was performed by using GraphPad Prism 8.0, and P < 0.05 was considered as statistical significance.
Results
Growth performance and whole-body proximate composition
As shown in Table 3, no significance in survival was found among four different treatments. Compared to control group, the weight gain rate (WGR) and specific growth rate (SGR) of turbot fed with high SBM diet decreased significantly. Moreover, supplementation with NaB, no matter 0.2% or 0.5%, in high SBM diet both obviously elevated the WGR and SGR of turbot to similar levels as those in control group. However, the feed efficiency ratio (FER) and feed intake (FI) of turbot was not significantly affected by four different diets (Table 3). As for the body composition of juvenile turbot, there was no significance in four groups (Table 4).
Table 3.
Effects of dietary NaB on growth performance of juvenile turbot.
| Diet | Control | High SBM | High SBM + 0.2%NaB | High SBM + 0.5%NaB |
|---|---|---|---|---|
| Survival (%) | 97.50 ± 1.44 | 93.33 ± 0.83 | 95.83 ± 0.83 | 92.50 ± 3.82 |
| Final weight (g) | 76.34 ± 1.54b | 65.83 ± 1.44a | 76.00 ± 2.08b | 73.83 ± 2.20b |
| Weight gain rate (%) | 247.00 ± 7.00b | 199.20 ± 6.57a | 245.45 ± 9.46b | 235.61 ± 10.02b |
| Specific growth rate (%/d) | 2.22 ± 0.04b | 1.96 ± 0.04a | 2.21 ± 0.05b | 2.16 ± 0.05b |
| Feed efficiency ratio | 1.14 ± 0.03 | 1.05 ± 0.02 | 1.07 ± 0.02 | 1.08 ± 0.03 |
| Feed intake (%) | 1.73 ± 0.03 | 1.69 ± 0.03 | 1.84 ± 0.05 | 1.79 ± 0.06 |
| Hepatosomatic index (%) | 1.34 ± 0.07 | 1.33 ± 0.06 | 1.46 ± 0.05 | 1.41 ± 0.04 |
Survival (%) = 100 × (the final number of fish/the initial number of fish); Weight gain rate (WGR, %) = 100 × (final body weight-initial body weight) /initial body weight; Specific growth rate (SGR, %/d) = 100 × (Ln final body weight—Ln initial body weight)/days; Feed efficiency ratio, FER = weight gain (g)/total amount of feed consumption (g); Feed intake, FI (%BW/day) = 100 × feed consumption/(final body weight + initial body weight)/2)/day; Hepatosomatic index (HSI, %) = 100 × liver weight (g) of final individual fish/final individual weight (g).
*Values were presented as means ± SEM (n = 3 replicates). Multiple comparisons were conducted with Tukey’s test. And values in the same row with different letters were significantly different (P < 0.05).
Table 4.
Effects of dietary NaB on body composition of juvenile turbot (fresh weight, g/kg).
| Diet | Control | High SBM | High SBM + 0.2%NaB | High SBM + 0.5%NaB | P values |
|---|---|---|---|---|---|
| Moisture (%) | 77.42 ± 0.72 | 78.42 ± 0.42 | 78.59 ± 0.16 | 78.32 ± 0.18 | NS |
| Crude lipid (%) | 3.453 ± 0.13 | 3.457 ± 0.28 | 3.247 ± 0.08 | 3.347 ± 0.18 | NS |
| Crude protein (%) | 14.25 ± 0.38 | 14.32 ± 0.27 | 14.32 ± 0.27 | 13.68 ± 0.22 | NS |
| Crude Ash (%) | 3.79 ± 0.13 | 3.80 ± 0.07 | 3.93 ± 0.12 | 3.99 ± 0.09 | NS |
*Values were presented as mean ± SEM (n = 3). Multiple comparisons were conducted with Tukey’s test. NS means no significance (P > 0.05).
Histomorphology of distal intestine
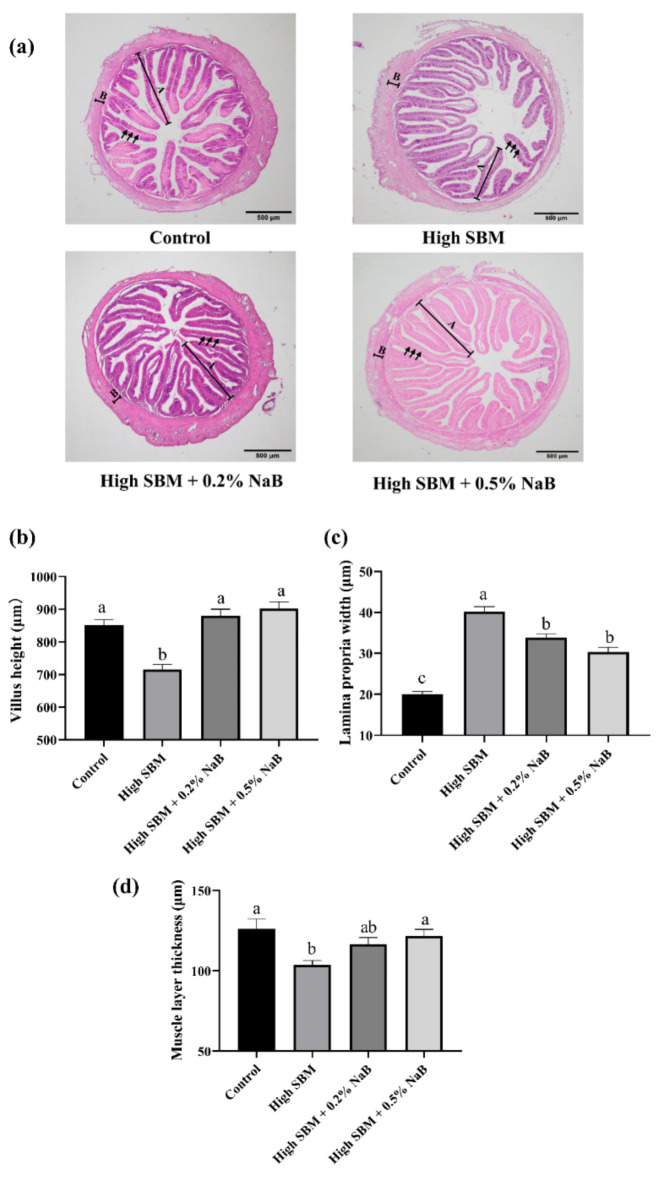
As the results showed in Fig. 2a, turbot fed with high SBM diet exhibited impaired intestinal histomorphology. Briefly, the intestinal villus height (Fig. 2b) and muscle layer thickness (Fig. 2d) of juvenile turbot was distinctly declined, while the intestinal lamina propria width was significantly elevated compared to the control group (Fig. 2c). However, inclusion of 0.2% or 0.5% NaB in high SBM diet both significantly ameliorated the impaired histomorphology of distal intestine caused by the high SBM diet.
Fig. 2.
Effects of NaB on intestinal histomorphology. (a) The intestines of turbot in different groups were collected and sectioned. Fixation with H&E, the morphology of the distal intestines was observed. The images were representative of at least three independent experiments, and scale bar indicates 500 μm; (A) and (B) in the images manifests villus height and muscle layer thickness, respectively; lamina propria width is indicated by arrows. (b–d) The micromorphology, including villus height (b), lamina propria width (c), and muscle layer thickness (d) of the intestine, was evaluated (n = 6). Values were presented as means ± SEM. Multiple comparisons were conducted with Tukey’s test. Different superscript letters indicated significant difference (P < 0.05).
Gene expression of inflammation-related factors
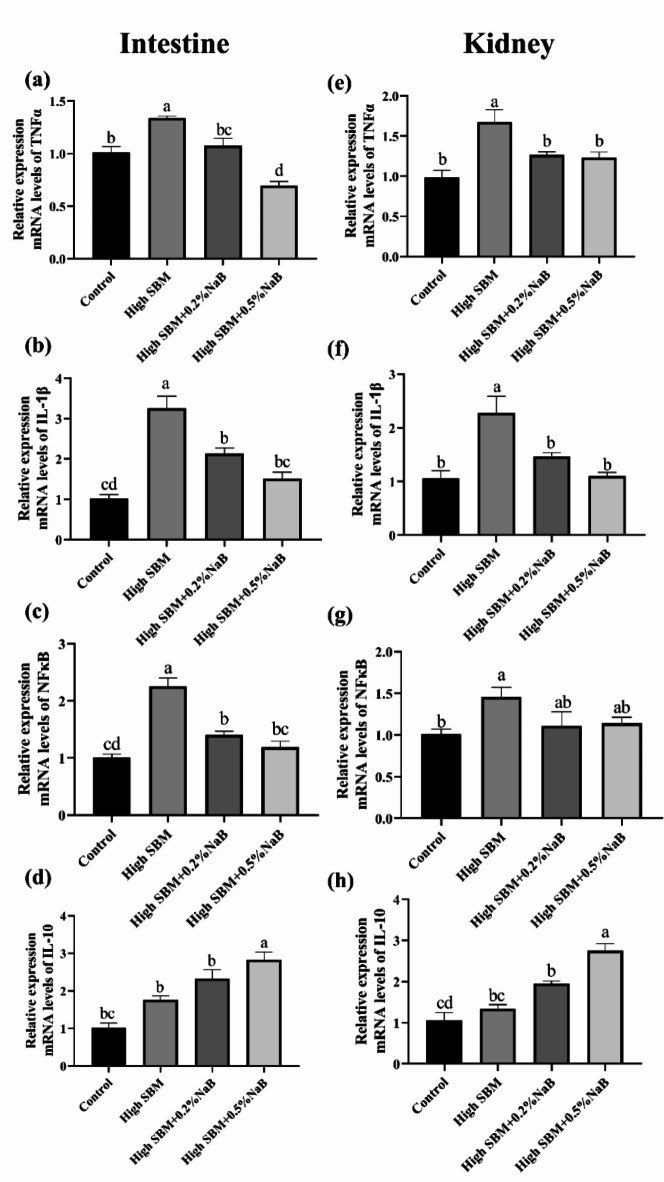
Next, we analyzed the gene expression of inflammation-related cytokines in distal intestine and kidney of turbot to further evaluate the effects of NaB supplementation on the inflammation caused by high SBM diet. As shown in Fig. 3, the gene expression of pro-inflammatory cytokines, such as TNFα and IL-1β, were significantly up-regulated in distal intestine and kidney of turbot in high SBM group in comparison with control group. And the expression of NFκB in mRNA level was also significantly increased in high SBM group. Moreover, inclusion of 0.2% or 0.5% NaB in high SBM diet significantly inhibited the increased expression of inflammation-related cytokines both in distal intestine and kidney.
Fig. 3.
Effects of dietary NaB on the gene expression of inflammatory cytokines and NF-κB in turbot intestine and kidney. The relative mRNA expressions of TNFα, IL-1β, NF-κB and IL-10 in the distal intestine (a–d) and kidney (e–h) of turbot in different groups were tested by qRT-PCR (n = 12). Values were presented as means ± SEM. Multiple comparisons were conducted with Tukey’s test. Different superscript letters indicated significant difference (P < 0.05).
Meanwhile, it was dietary supplementation with 0.5% not 0.2% NaB significantly improved the gene expression of anti-inflammatory cytokine IL-10, no matter in gut and kidney (Fig. 3d,h). And no significant difference was observed between control group and high SBM group (Fig. 3d,h).
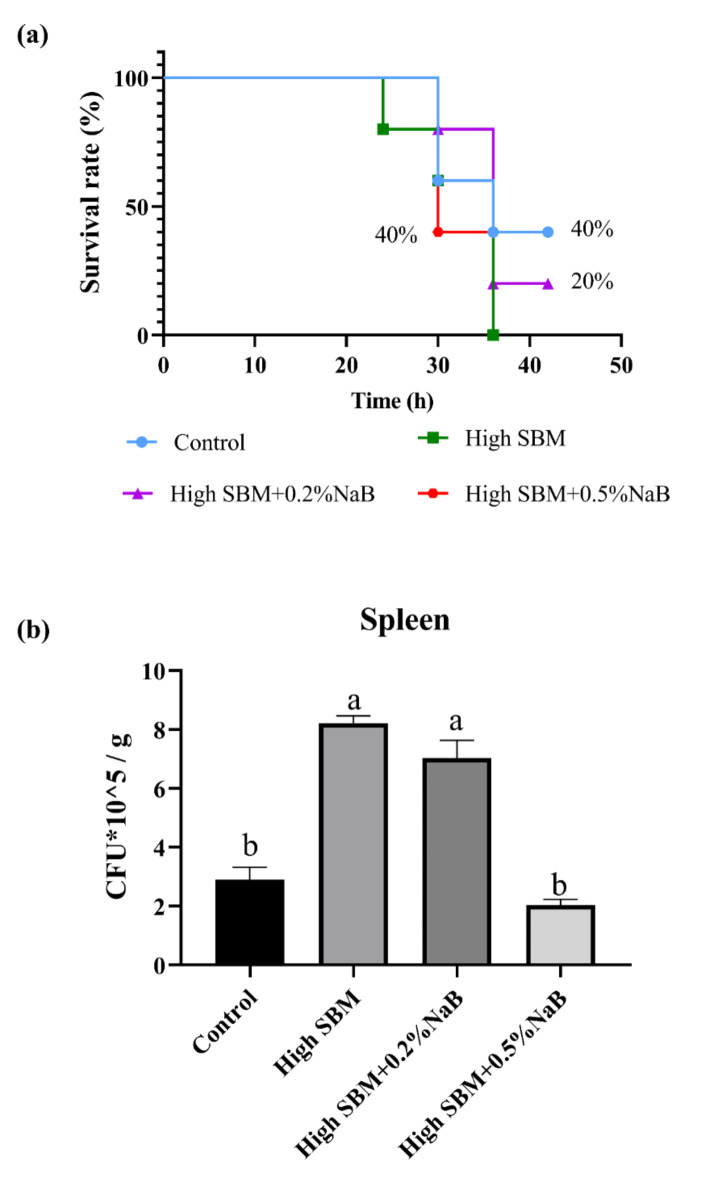
The mortality and bacterial loads after E. tarda infection
To further determine the effects of NaB supplementation on the anti-infectious ability of juvenile turbot under inflammatory state, the fish were intraperitoneally injected with E. tarda, and the mortality was recorded in different groups till 42 h. Our results showed that all fish died in high SBM group after infection with E. tarda for 36 h, while supplementation with NaB in high SBM diet increased the survival rate of turbot (Fig. 4a and Table 5). In brief, around 40% fish in high SBM + 0.5% NaB group still survived at 36 h post infection (hpi), consistently with control group (Fig. 4a and Table 5). And the relative protection score (RPS) reached 40% in high SBM + 0.5% NaB group, consistently with control group (Table 5). Although the fish exhibited a higher mortality in high SBM + 0.2% NaB group than that in control group and dietary 0.5% NaB group, the survival rate of turbot was also 20% higher than that in high SBM group (Fig. 4a and Table 5). The RPS in high SBM + 0.2% NaB group was also higher than high SBM group (Table 5).
Fig. 4.
Effects of NaB on the anti-bacterial ability in turbot. After the feeding trial, turbot from each group were infected with E. tarda (1 × 107 CFU/fish) by i.p. injection. (a) The mortality of the turbot was recorded every 6 h till 42 hpi (n = 10). (b) The viable bacteria in the spleen at 12 hpi were counted (n = 12). Values were presented as means ± SEM. Multiple comparisons were conducted with Tukey’s test. Different superscript letters indicated significant difference (P < 0.05).
Table 5.
The survival rate of turbot infected with E. tarda.
| Control | High SBM | High SBM + 0.2%NaB | High SBM + 0.5%NaB | |
|---|---|---|---|---|
| Total number for test | 10 | 10 | 10 | 10 |
| Number of death | 6 | 10 | 8 | 6 |
| Mortality rate (%) | 60 | 100 | 80 | 60 |
| Survival rate (%) | 40 | 0 | 20 | 40 |
| Relative protection score (%) | 40 | 0 | 20 | 40 |
Mortality Rate (%) = Number of death/Total number for test × 100%
Survival rate (%) = (1- number of death/total number for test) × 100%
Relative preotection score (RPS) = (1- number of death in different four groups/number of death in high SBM group) × 100%
Additionaly, we examined the bacterial loads in the spleen of turbot after infection with E. tarda for 12 h. As the results showed in Fig. 4b, significantly more numbers of E. tarda were detected in high SBM group than that in control group. Nonetheless, the bacterial loads were repressed significantly in spleen of fish fed with dietary 0.5% NaB supplementation diet. We also found that addition 0.2% NaB to high SBM diet couldn’t suppress the numbers of E. tarda in spleen when turbot were in the condition of inflammation.
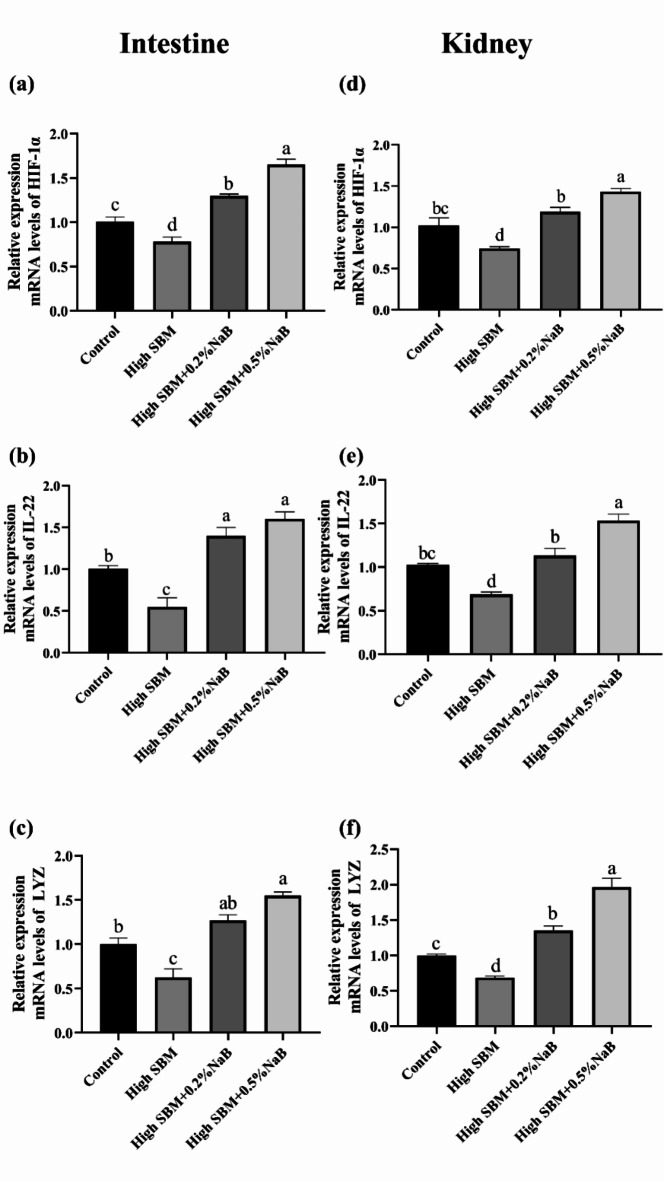
HIF-1α/IL-22/Lysozyme signaling pathway
To further investigate the effects of NaB supplementation on the bactericidal activity, we accessed whether HIF-1α/IL-22/Lysozyme signaling pathway was involved in the process. As Fig. 5 showed, in comparison with control group, the gene expression of HIF-1α, IL-22 and lysozyme was significantly inhibited in high SBM group both in gut and kidney. Nevertheless, supplementation with 0.2% or 0.5% NaB both obviously activated the HIF-1α/IL-22/Lysozyme signaling pathway and elevated the gene expression. It was worthy that the gene expression of antibacterial effectors in 0.5% NaB supplementation group was not only higher than high SBM group, but also higher than control group.
Fig. 5.
Effects of NaB on the gene expression of batericidal effectors in different groups. The relative mRNA expressions of HIF-1α, IL-22, and lysozyme in the distal intestine (a–c) and kidney (d–f) of turbot were analyzed by qRT-PCR (n = 12). Values were presented as means ± SEM. Multiple comparisons were conducted with Tukey’s test. Different superscript letters indicated significant difference (P < 0.05).
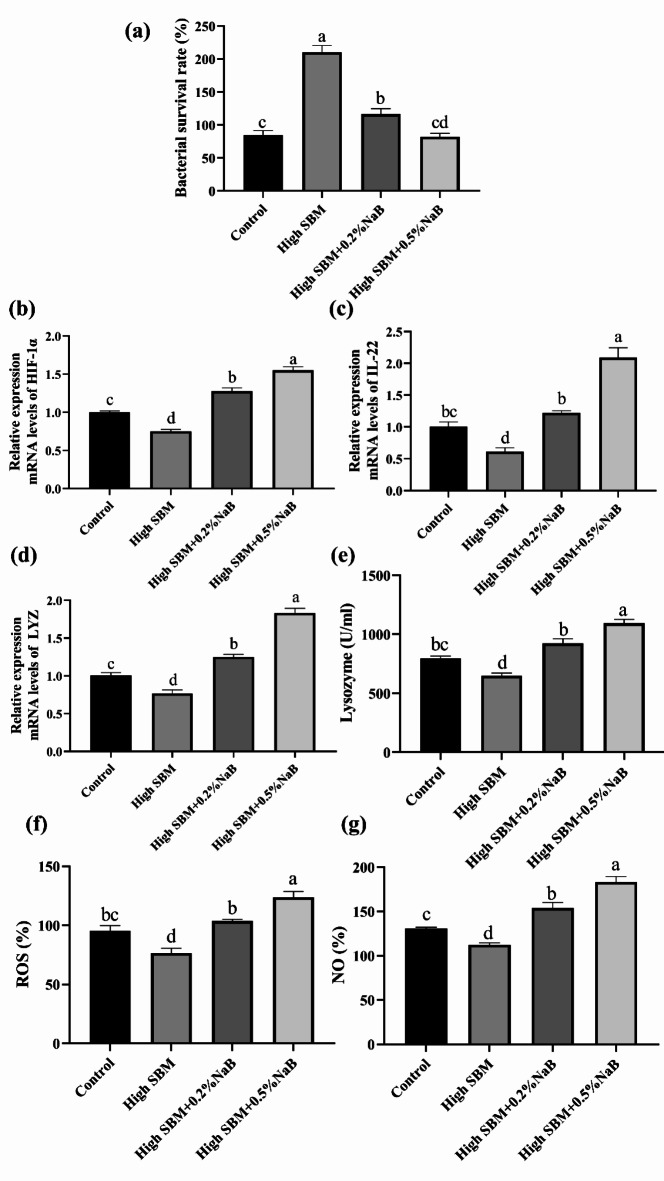
The bactericidal activity and the antibacterial effectors in HKMs
After the feeding trial, we isolated and cultured HKMs from turbot in four different groups, followed by coincubated with E. tarda for 2 h. The results showed that bacterial loads in HKMs from the fish in NaB-supplemented groups were significantly limited compared to high SBM group (Fig. 6a). Moreover, the numbers of E. tarda in dietary 0.5% NaB group were similar to control group.
Fig. 6.
Effects of NaB on the bactericidal activity and the production of antibacterial effectors in HKMs. After the feeding trial, HKMs were isolated and cultured. (a) The equal number of E. tarda was added and coincubated with HKMs for 2 h. Thereafter, the survival rate of ingested bacteria was calculated (n = 12). (b, c) The gene expression of HIF-1α and IL-22 was measured (n = 12). (d and e) The expression of lysozyme in mRNA level (d) and enzyme activity (c) in the cells was examined (n = 6). (f) E. tarda was added (cells: bacteria = 1:1) and coincubated for 2 h, followed by the production of ROS in the cells was analyzed (n = 12). (g) After the HKMs coincubation with E. tarda for 6 h, the production of NO in cells was investigated between different groups (n = 12). Values were presented as means ± SEM. Multiple comparisons were conducted with Tukey’s test. Different superscript letters indicated significant difference (P < 0.05).
Additionally, we examined the gene expression of HIF-1α, IL-22 and lysozyme in HKMs. The results demonstrated that the gene expression of HIF-1α, IL-22 and lysozyme in HKMs from high SBM group was significantly lower than control group (Fig. 6b–d). Compared to high SBM group, supplementation with 0.2% or 0.5% NaB significantly increased the gene expression of HIF-1α, IL-22 and lysozyme, respectively (Fig. 6b–d). What’s more, we accessed the lysozyme activity of HKMs from fish in different groups. The result exhibited that high SBM diet significantly suppressed the lysozyme activity of HKMs (Fig. 6e). In contrast, dietary NaB significantly ameliorated the lower lysozyme activity caused by high SBM (Fig. 6e).
Furthermore, after the HKMs isolated from the fish, they were infected with E. tarda for 2 h or 6 h to test the production of ROS and NO. We can see from the results, the production of ROS and NO in high SBM group was significantly lower than control group (Fig. 6f,g). However, supplementation with 0.2% or 0.5% NaB both significantly elevated the production of ROS and NO, compared to the high SBM group (Fig. 6f,g). Furthermore, HKMs isolated from 0.5% NaB supplementation group secreted more ROS and NO in comparison with 0.2% NaB supplementation group.
Discussion
Butyrate as a growth promoter and immunostimulant exerts several beneficial effects on farmed fish and shrimp2. In our study, we have confirmed that supplementation with NaB in the high SBM diet was beneficial to increase growth performance, ameliorate intestinal inflammation and promote the antibacterial activity of turbot.
Accumulating studies have demonstrated that dietary supplementation with butyric acid and NaB may affect the growth and body composition of several fish. Wu and Liu et al. reported that supplementation with NaB in the diets significantly improved SGR of grass carp in comparison with control group38,39. However, dietary NaB inclusion had no effect on the analysis of whole-body composition of grass carp38,39. Another research found that the inclusion of NaB in the diet apparently improved WG and SGR, with no effect on the analysis of body composition of Nile tilapia (Oreochromis niloticus)40. Consistently with aboved results, our results also showed that supplementation with 0.2% or 0.5% NaB both obviously increased the growth performance of turbot in comparison with high SBM group (Table 3). Moreover, the growth performance in NaB supplementation group was recovered to a similar level as that in control group (Table 3). In addition, there was also no significant difference in the proximate analysis of body composition of turbot between four different groups (Table 4).
Considerable studies have confirmed that high SBM diet can lead to intestinal inflammation in aquatic animals19,20. Our results found that high SBM diet caused damage to the gut of turbot, including villus height, muscle layer thickness and lamina propria width, and led to inflammation (Fig. 2). Butyrate is one of the most studied SCFAs that modulates a variety of processes, consisting of cell proliferation and differentiation, hormones secretion and activation of immune/inflammatory responses41,42. Inclusion of NaB in SBM diets inhibited the intestinal inflammation and increased gene expression of TNFα in European sea bass (Dicentrarchus labrax). Nevertheless, there was no difference in the gene expression of IL-1β, IL-8, and IL-1043. Our present study showed that a significant down-regulation of pro-inflammatory cytokines (e.g., TNFα, IL-1β) accompanied with up-regulation of the expression of anti-inflammatory cytokines (e.g., IL-10) of turbot fed with a diet inclusion of NaB in high SBM diet (Fig. 3). As a crucial transcription activator, NFκB regulates the inflammatory response and the gene expression of inflammatory cytokines44. Butyrate has an outstanding character of anti-inflammation through its inhibition of NFκB, as demonstrated in several in vitro and in vivo studies45. In the present study, fish fed NaB also inhibited the gene expression of NFκB (Fig. 3). When NFκB signaling pathway was inhibited by NaB, the expression of pro-inflammatory cytokines (e.g., TNFα, IL-1β) was also downregulated. The main mechanism described for significant in vivo changes in the expression of genes might be the attenuation of histone deacetylase (HDAC) activity2. Butyrate is the most potent inhibitor of HDAC46. By inhibition of the HDAC activity, butyrate increases the acetylation of histone and non-histone proteins to modulate the gene expression of inflammatory cytokines47.
Under inflammatory conditions, the immune homeostasis of fish is interfered. Therefore, resistance to the attack of pathogenic microorganisms becomes more difficult. Our study demonstrated that inclusion of NaB in the high SBM diet increased the survival rate of infected turbot and declined the E. tarda numbers in the spleen (Fig. 4). What’s more, the survival rate of turbot and the died number of E. tarda in the spleen isolated from 0.5% NaB supplementation group was higher than 0.2% NaB supplementation group (Fig. 4). Substantial studies have identified that HIF-1α plays an important role in host homeostasis. Fachi et al. demonstrated that butyrate protected the intestinal epithelium from the damage caused by Clostridium difficile toxins by stabilizing HIF-1, thus inhibiting bacterial translocation48. Moreover, commensal bacteria induced HIF-1α expression, and then activated the expression of innate immune effectors to prevent Candida albicans colonization49. In addition, our previous study found that butyrate triggered HIF-1α signaling to elevate IL-22 and lysozyme expression, contributing to the clearance of bacteria both in turbot and zebrafish31. And IL-22 induced the production of AMPs, such as β-defensins, leading to higher survival rate of infected zebrafish31. Considered as a component of innate immune effector, lysozyme palys a vital role in pathogen infections50. In our present study, we identified that both dietary 0.2% and 0.5% NaB supplementation activated the HIF-1α/IL-22/Lysozyme signaling pathway to against bacterial infection (Fig. 5). Furthermore, the gene expression of HIF-1α, IL-22 and lysozyme in 0.5% NaB supplementation group was much higher than in 0.2% NaB supplementation group.
In addition, head kidney as a key immune organ of teleost fish plays a vital role in bacterial infection. As we all know, macrophages are one of the most numerous immune cells in head kidney. It has been reported that fish macrophages have microbicidal activity, in part mediated by oxygen and nitrogen radicals. Macrophage ROS response is a character of these cells’ antimicrobial mechanism and the efficiency of this response often reflects on the ability of destroying internalized microorganisms51. It has been clarified that macrophages isolated from rainbow trout produced ROS to kill fish bacterial pathogen Aeromonas salmonicida52. The immune mechanism mediating the production of NO in teleost fish macrophages appears to be well conserved to those described in mammals53. NO production by fish macrophages is essential to antimicrobial immunity to a range of pathogens, such as Aeromonas salmonicida, Renibacterium salmoninarum and Yersinia ruckeri54. Our results showed that HKMs isolated from the fish fed with dietary NaB produced more ROS and NO to fight against bacterial infection than high SBM diet (Fig. 6f,g). Activated by NaB, HKMs immediately released a large amount of ROS and NO. Then, ROS and NO were both transformed into antimicrobial substances, like H2O2, peroxynitrate, to defense infection55. In consistent with the expression of HIF-1α, IL-22 and lysozyme, HKMs isolated from the fish fed with 0.5% NaB supplementation diet significantly produced more ROS and NO than in the group fed with 0.2% NaB supplementation diet. The above results might explain the differences that the survival rate of infected turbot, bacterial loads in the spleen and the bactericidal acitivity of HKMs between the two NaB supplementation groups.
Conclusion
This study has shown that NaB supplementation in high SBM diets significantly enhanced growth performance, intestinal health, and infection resistance in turbot. It was evidenced that both 0.2% and 0.5% NaB supplementation in high SBM diets had a similar effect on promoting growth rates and alleviating intestinal inflammation. Especially, the 0.5% NaB supplementation was more effective in enhancing the ability of turbot to defend against bacterial infection, especially under intestinal inflammatory situations. These results indicate that NaB, especially higher supplementation, supports not only better growth and gut health but also enhanced immune responses for a stronger defense against bacterial attacks under an inflammatory situation.
Acknowledgements
This study was supported by the Research Foundation for Talented Scholars, Weifang University of Science and Technology (Grant No. KJRC2023034).
Author contributions
J.Z. designed and performed the experiments, analyzed the data, and wrote the manuscript; C.J., J.D., J.W and M.L. designed the experiments and reviewed the manuscript; H.S. and C.Z performed the experiments and analyzed the data.
Data availability
The data used to support the findings of this study are included within the article.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the protocols for animal care, handling procedures and animal anesthesia, and approved by the Laboratory Animal Care Committee in Weifang University of Science and Technology.
ARRIVE statement
We followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (https://arriveguidelines.org).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martin-Gallausiaux, C. & Marinelli, L. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc.80, 37–49. 10.1017/s0029665120006916 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif, H. M., Abdel-Tawwab, M., Dawood, M. A., Menanteau-Ledouble, S. & El-Matbouli, M. Benefits of dietary butyric acid, sodium butyrate, and their protected forms in aquafeeds: A review. Rev. Fish. Sci. Aquac.28, 421–448. 10.1080/23308249.2020.1758899 (2020). [Google Scholar]
- 3.Aalamifar, H. et al. Dietary butyric acid improved growth, digestive enzyme activities and humoral immune parameters in Barramundi (Lates calcarifer). Aquac. Nutr.26, 156–164. 10.1111/anu.12977 (2020). [Google Scholar]
- 4.Mirghaed, A. T., Yarahmadi, P., Soltani, M., Paknejad, H. & Hoseini, S. M. Dietary sodium butyrate (Butirex(®) C4) supplementation modulates intestinal transcriptomic responses and augments disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol.92, 621–628. 10.1016/j.fsi.2019.06.046 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Zhang, J. et al. Sodium butyrate supplementation in high-soybean meal diets for juvenile rice field eel (Monopterus albus): Effects on growth, immune response and intestinal health. Aquaculture.520, 734952. 10.1016/j.aquaculture.2020.734952 (2020). [Google Scholar]
- 6.Liu, W. et al. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr.112, 15–29. 10.1017/S0007114514000610 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Estensoro, I., et al. Histopathological and transcriptional scoring of intestinal traits in gilthead sea bream (Sparus aurata) fed low fish meal and fish oil diets with butyrate supplementation. Aquaculture Europehttp://hdl.handle.net/10261/143764 (2014).
- 8.Liu, S. et al. Dietary sodium butyrate improves intestinal health of triploid Oncorhynchus mykiss fed a low fish meal diet. Biology.12, 145. 10.3390/biology12020145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman, N. F., Yacout, D. M. & Hassaan, M. A. Responsible fishmeal consumption and alternatives in the face of climate changes. J. Mar. Sci.10.5376/ijms.2017.07.0015 (2017). [Google Scholar]
- 10.Zhou, S., Smith, A. D. & Knudsen, E. E. Ending overfishing while catching more fish. Fish. Fish.16, 716–722. 10.1111/faf.12077 (2015). [Google Scholar]
- 11.Hussain, S. M. et al. Substitution of fishmeal: Highlights of potential plant protein sources for aquaculture sustainability. Heliyon.10, e26573. 10.1016/j.heliyon.2024.e26573 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urán, P., Aydin, R., Schrama, J., Verreth, J. & Rombout, J. Soybean meal-induced uptake block in the distal enterocytes of Atlantic salmon (Salmo salar L.). J. Fish. Biol.73, 2571–2579. 10.1111/j.1095-8649.2008.02091.x (2008). [Google Scholar]
- 13.Potter, L., & Potchanakorn, M., Digestibility of the carbohydrate fraction of soybean meal by poultry. In World Soybean Research Conference III, 218–224 (CRC Press, 2022).
- 14.Krishnan, H. B. & Jez, J. M. The promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Plant Sci.272, 14–21. 10.1016/j.plantsci.2018.03.030 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Francis, G., Makkar, H. P. & Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture.199, 197–227. 10.1016/S0044-8486(01)00526-9 (2001). [Google Scholar]
- 16.Krogdahl, Å., & Bakke, A.M. Antinutrients. In Dietary Nutrients, Additives, and Fish Health. Vol. 10, 211–235. 10.1002/9781119005568.ch10 (2015).
- 17.Mollaei Berenti, A. et al. Effect of extrusion of soybean meal on feed spectroscopic molecular structures and on performance, blood metabolites and nutrient digestibility of Holstein dairy calves. Anim. Biosci.34, 855–866. 10.5713/ajas.19.0899 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlader, S. et al. Effects of dietary replacement of fish meal by soybean meal on growth, feed utilization, and health condition of stinging catfish, Heteropneustes fossilis. Saudi. J. Biol. Sci.30, 103601. 10.1016/j.sjbs.2023.103601 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, W. et al. Sodium acetate alleviates adverse effects caused by the diet with high proportion of soybean meal in turbot (Scophthalmus maximus L.). Aquaculture.566, 739163. 10.1016/j.aquaculture.2022.739163 (2023). [Google Scholar]
- 20.Sun, H. et al. The effects of sodium propionate supplementation in the diet with high soybean meal on growth performance, intestinal health, and immune resistance to bacterial infection in turbot (Scophthalmus maximus L.). Aquac. Nutr.10.1155/2022/8952755 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Y. et al. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol.88, 65–75. 10.1016/j.fsi.2019.02.064 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Blaj, L. A. & Cucu, A. I. The role of the NF-kB pathway in intracranial aneurysms. Brain Sci.13, 1660. 10.3390/brainsci13121660 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge, Y., Yao, S., Shi, Y. & Cai, C. Effects of low or high dosages of dietary sodium butyrate on the growth and health of the liver and intestine of largemouth bass Micropterus salmoides. Aquac. Nutr.2022, 6173245. 10.1155/2022/6173245 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas, R. & Bagchi, A. NFkB pathway and inhibition: An overview. Comput. Mol. Biol.10.5376/cmb.2016.06.0001 (2016). [Google Scholar]
- 25.Abdel-Aziz, E. H., Abdu, S. B. S., Ali, T. E. & Fouad, H. F. Haemopoiesis in the head kidney of tilapia, Oreochromis niloticus (Teleostei: Cichlidae): A morphological (optical and ultrastructural) study. Fish. Physiol. Biochem.36, 323–336. 10.1007/s10695-008-9297-z (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondera, E. Haematopoiesis in the head kidney of common carp (Cyprinus carpio L.): A morphological study. Fish. Physiol. Biochem.37, 355–362. 10.1007/s10695-010-9432-5 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Van den Bossche, J., O’Neill, L. A. & Menon, D. Macrophage Immunometabolism: Where are we (going)?. Trends Immunol.38, 395–406. 10.1016/j.it.2017.03.001 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Kim, Y. E. & Lee, M. HIF-1α activation in myeloid cells accelerates dextran sodium sulfate-induced colitis progression in mice. Dis. Models Mech.11, dmm033241. 10.1242/dmm.033241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, J. et al. Short-chain fatty acids promote intracellular bactericidal activity in head kidney macrophages from turbot (Scophthalmus maximus L.) via hypoxia inducible factor-1α. Front. Immunol.11, 615536. 10.3389/fimmu.2020.615536 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenberg, G. F., Fouser, L. A. & Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol.12, 383–390. 10.1038/ni.2025 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Zhang, J. et al. Butyrate-induced IL-22 expression in fish macrophages contributes to bacterial clearance. Fish Shellfish Immunol.133, 108545. 10.1016/j.fsi.2023.108545 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Hoerterer, C. et al. Sustainable fish feeds: Potential of emerging protein sources in diets for juvenile turbot (Scophthalmus maximus) in RAS. Aquacult Int.30, 1481–1504. 10.1007/s10499-022-00859-x (2022). [Google Scholar]
- 33.Carter, K. M., Woodley, C. M. & Brown, R. S. A review of tricaine methanesulfonate for anesthesia of fish. Rev. Fish. Biol. Fisher.21, 51–59. 10.1007/s11160-010-9188-0 (2011). [Google Scholar]
- 34.Horwitz, W., & Latimer, G.W., Official methods of analysis of AOAC International. In AOAC International Gaithersburg (2000).
- 35.Thiex, N., Novotny, L. & Crawford, A. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int.95, 1392–1397 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Hu, H. et al. Safety level evaluation of dietary 2-hydroxy-4-(methylthio) butanoic acid (HMTBa) for turbot Scophthalmus maximus based on growth performances, anti-oxidative responses, and liver and intestine conditions. Aquaculture.444, 13–20. 10.1016/j.aquaculture.2015.03.008 (2015). [Google Scholar]
- 37.Liu, J. et al. Vitamin D(3) protects turbot (Scophthalmus maximus L.) from bacterial infection. Fish Shellfish Immunol.118, 25–33. 10.1016/j.fsi.2021.08.024 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Wu, F. Effects of dietary gamma aminobutyric acid and sodium butyrate on growth performance, antioxidant status and intestinal structure of grass carp (Ctenopharyngodon idellus), Master Thesis, China. https://www.globethesis.com (2017).
- 39.Liu, M. et al. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquacu. Res.48, 4102–4111. 10.1111/jwas.12327 (2017). [Google Scholar]
- 40.Ali, T.E.-S., El-Sayed, A. M., Eissa, M.A.-R. & Hanafi, H. M. Effect of dietary supplementation of sodium butyrate on growth performance and feed utilization of Nile tilapia (Oreochromis niloticus) fries. Indian J. Geo-marine Sci.47, 2071–2076 (2018). [Google Scholar]
- 41.Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature.461, 1282–1286. 10.1038/nature08530 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinolo, M. A. et al. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem.22, 849–855. 10.1016/j.jnutbio.2010.07.009 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Rimoldi, S. et al. Butyrate and taurine exert a mitigating effect on the inflamed distal intestine of European sea bass fed with a high percentage of soybean meal. Fish. Aquatic Sci.19, 40 (2016). [Google Scholar]
- 44.Nennig, S. E. & Schank, J. R. The role of NFkB in drug addiction: Beyond inflammation. Alcohol. Alcohol.52, 172–179. 10.1093/alcalc/agw098 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siddiqui, M. T. & Cresci, G. A. The immunomodulatory functions of butyrate. J. Inflamm. Res.10.2147/JIR.S300989 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, H. et al. Butyrate: A double-edged sword for health?. Adv. Nutr.9, 21–29. 10.1093/advances/nmx009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinolo, M. A., Rodrigues, H. G., Nachbar, R. T. & Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients.3, 858–876. 10.3390/nu3100858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fachi, J. L. et al. Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep.27, 750-761.e7. 10.1016/j.celrep.2019.03.054 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Fan, D. et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med.21, 808–814. 10.1038/nm.3871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saurabh, S. & Sahoo, P. K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res.39, 223–239. 10.1111/j.1365-2109.2007.01883.x (2008). [Google Scholar]
- 51.Grayfer, L. et al. Mechanisms of fish macrophage antimicrobial immunity. Front. Immunol.9, 1105. 10.3389/fimmu.2018.01105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharp, G. & Secombes, C. The role of reactive oxygen species in the killing of the bacterial fish pathogen Aeromonas salmonicida by rainbow trout macrophages. Fish Shellfish Immunol.3, 119–129. 10.1006/fsim.1993.1013 (1993). [Google Scholar]
- 53.Hodgkinson, J. W., Grayfer, L. & Belosevic, M. Biology of bony fish macrophages. Biology.4, 881–906. 10.3390/biology4040881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos-Pérez, J. J., Ellis, A. E. & Secombes, C. J. Toxicity of nitric oxide and peroxynitrite to bacterial pathogens of fish. Dis. Aquat. Organ.43, 109–115. 10.3354/dao043109 (2000). [DOI] [PubMed] [Google Scholar]
- 55.El-Benna, J., Dang, P. M. & Gougerot-Pocidalo, M. A. Priming of the neutrophil NADPH oxidase activation: Role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol.30, 279–289. 10.1007/s00281-008-0118-3 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.