Abstract
This study investigated the use of bi-exponential diffusion-weighted imaging (DWI) combined with structural features to differentiate high-grade glioma (HGG) from solitary brain metastasis (SBM). A total of 57 patients (31 HGG, 26 SBM) who underwent pre-surgical multi-b DWI and structural MRI (T1W, T2W, T1W + C) were included. Volumes of interest (VOI) in the peritumoral edema area (PTEA) and enhanced tumor area (ETA) were selected for analysis. Histogram features of slow diffusion coefficient (Dslow), fast diffusion coefficient (Dfast), and perfusion fraction (frac) were extracted. Results showed that HGG patients had higher skewness of Dfast (P = 0.022) and frac (P = 0.077), higher kurtosis of Dslow (P = 0.019) and frac (P = 0.025), and lower entropy of Dslow (P = 0.005) and frac (P = 0.001) within the ETA. Additionally, HGG exhibited lower mean frac in both ETA (P = 0.007) and PTEA (P = 0.017). Combining skewness of frac in ETA with clear tumor margin enhanced diagnostic performance, achieving an optimal AUC of 0.79. These findings suggest that histogram analysis of diffusion and perfusion characteristics in ETA and structural features can effectively differentiate HGG from SBM.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83452-x.
Keywords: Diffusion, Perfusion, Diffusion-weighted imaging, High-grade glioma, Solitary brain metastasis
Subject terms: CNS cancer, CNS cancer, Cancer imaging, Metastasis
Introduction
High-grade glioma (HGG) and brain metastasis are prevalent malignant neoplasms associated with substantial morbidity and mortality1–3. Differentiating a solitary brain metastasis (SBM) from an HGG has important prognostic and therapeutic implications.
HGG is commonly managed through surgical intervention followed by the Stupp protocol4. Brain metastases, which can present as single or multiple enhancing lesions, necessitate comprehensive whole-body staging5. Current treatment options for SBM include surgery or gamma-knife surgery and whole-brain radiation therapy6,7. Open surgical procedures are recommended for SBM patients with intractable intracranial hypertension, obstructive hydrocephalus, tumor apoplexy, and similar emergent situations8. However, considering that both HGG and SBM typically exhibit distinct imaging characteristics, including well-defined, ring-enhancing lesions in contrast-enhanced T1-weighted (T1W + C) imaging and high signal peritumoral edema in T2-weighted (T2W) imaging9, diagnosis may be challenging. Also, individuals with extracranial malignancies may develop gliomas10, which further complicates decision-making. Moreover, the incidence of geriatric glioma has been on the rise, challenging the notion that older individuals are more prone to developing brain metastases11. Furthermore, the clinical manifestations of HGG and SBM share similarities, such as secondary epilepsy, functional impairment, and intracranial hypertension, which contribute to the complexity of distinguishing between the two entities, both radiologically and clinically12.
Primary malignancy history, multiple lesions, and the combined localization of grey–white substances can help diagnose brain metastasis. Traditional morphological analyses, including assessments of tumor parenchymal or peritumoral edema volume, midline shift, and enhancement pattern in various conventional magnetic resonance sequences, have proven ineffective in differentiating HGG from SBM13,14. On the other hand, histogram and texture analysis techniques based on diffusion-weighted imaging (DWI) and diffusion kurtosis imaging have shown promising results in differentiating HGG from SBM. This method can reflect the distribution of diffusion within the tumor or edema area15,16. Furthermore, conventional perfusion MR studies utilizing dynamic susceptibility contrast (DSC), dynamic contrast-enhanced imaging, and arterial spin labeling have demonstrated excellent diagnostic performance in distinguishing these tumor types17,18. However, research focusing on analyzing perfusion histogram features is lacking.
Bi-exponential analysis of DWI data enables the simultaneous assessment of molecular water diffusion at high-b values and the microcirculation of blood capillaries associated with perfusion at low-b values (b < 200 s/mm2)19. Previous studies have suggested that this imaging approach can accurately differentiate high-grade from low-grade gliomas20. In this study, we explored the potential of bi-exponential analysis of DWI data in differentiating HGG and SBM in both enhanced tumor area (ETA) and peritumoral edema area (PTEA) through histogram analysis combined with structural features.
Results
Clinical characteristics and structural differences between HGG and SBM
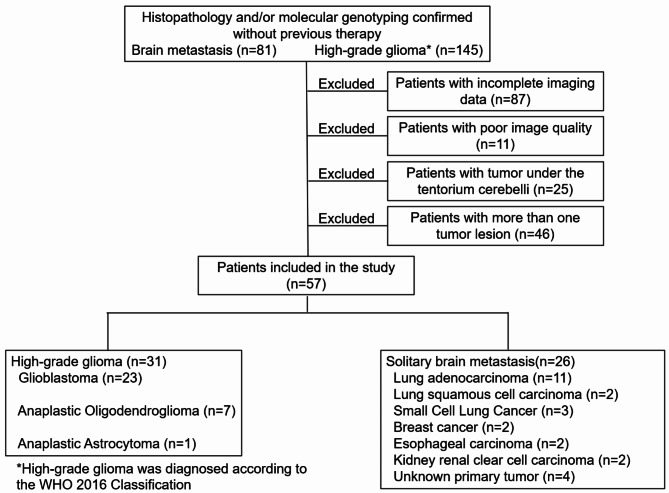
Among initially enrolled 81 patients with brain metastasis and 145 patients with HGG, 87 with incomplete image data, 11 with poor image quality, 25 with tumor tumors located below the tentorium cerebelli, and 46 with more than one tumor lesion above the tentorium cerebelli were excluded. The final cohort consisted of 31 patients with HGG (Table S1), with a mean age of 58.6 ± 19.37, and 26 patients with SBM (Table S2), with a mean age of 59.69 ± 11.59 (Fig. 1).
Fig. 1.
Study flow chart.
There were no significant differences in age or gender distribution between the HGG and SBM groups. However, SBM exhibited a significantly cleaner tumor margin than HGG (P = 0.017). No significant differences were observed between the two groups regarding tumor location, edema degree, or enhancement pattern. Detailed information is provided in Table 1.
Table 1.
Clinical characteristics and morphological features comparison between HGG and SBM.
| HGG (n = 31) | SBM (n = 26) | P | |
|---|---|---|---|
| Demographics | |||
| Age (year) | 58.61 ± 9.37 | 59.69 ± 11.59 | 0.356 |
| Gender (male/female) | 18/13 | 10/16 | 1 |
| Tumor location | 0.183 | ||
| Sub-cortex | 13 | 16 | |
| Deep white matter | 18 | 10 | |
| T2 margin | 0.017* | ||
| Blurred margin | 19 | 7 | |
| Well-defined margin | 12 | 19 | |
| Edema degree | 0.63 | ||
| Mild | 9 | 6 | |
| Moderate | 7 | 6 | |
| Severe | 15 | 14 | |
| Enhancement pattern | |||
| Non-enhanced | 1 | 0 | 0.901 |
| Focal | 5 | 0 | |
| Diffuse | 9 | 15 | |
| Ring-like | 16 | 11 | |
*P < 0.05.
Parametric value comparison between HGG and SBM
The histogram features of ETA and PTEA in patients with HGG) and solitary brain SBM are shown in Table 2. A significantly higher skewness of Dfast (1.1 ± 0.32 in HGG, 0.81 ± 0.54 in SBM, P = 0.022) and frac (1.02 ± 0.51 in HGG, 0.6 ± 0.61 in SBM, P = 0.077), as well as higher kurtosis of Dslow (3.9 ± 1.22 in HGG, 3.39 ± 1.29 in SBM, P = 0.019) and frac (5.39 ± 2.07 in HGG, 4.51 ± 2.72 in SBM, P = 0.025) were observed for patients with HGG compared to SBM. Additionally, significantly lower entropy of Dslow (3.86 ± 0.21 in HGG, 4.01 ± 0.22 in SBM, P = 0.005) and frac (3.51 ± 0.3 in HGG, 3.78 ± 0.28 in SBM, P = 0.001) were observed in ETA for HGG. In addition, the mean of frac in both ETA (21.29 ± 4.02 in HGG, 25.09 ± 6.03 in SBM, P = 0.007) and PTEA (25.47 ± 4.46 in HGG, 28.37 ± 4.22 in SBM, P = 0.017) was significantly lower in HGG compared to SBM. Furthermore, there was a significant difference between the groups in the 10th percentile of frac (10.99 ± 2.56 in HGG, 13.87 ± 4.66 in SBM, P = 0.009) in ETA. Representative images and histograms of Dfast, Dslow, and frac in the ETA volume of interest (VOI) for a patient with HGG (Fig. 2) and another with SBM (Fig. 3) are shown below.
Table 2.
Comparison of parametric values in the volume of interest between HGG and SBM.
| Mean | Entropy | Skewness | Kurtosis | 10th percentile |
90th percentile |
|
|---|---|---|---|---|---|---|
| ETA | ||||||
| Dfast (×10−4mm2/s) | ||||||
| HGG | 41.63 ± 3.34 | 3.62 ± 0.37 | 1.1 ± 0.32 | 5.48 ± 1.91 | 28.03 ± 3.71 | 58.54 ± 8.52 |
| SBM | 42.22 ± 5.73 | 3.8 ± 0.42 | 0.81 ± 0.54 | 4.78 ± 2.2 | 26.61 ± 6.12 | 59.99 ± 11.66 |
| P | 0.641 | 0.092 | 0.022* | 0.082 | 0.628 | 0.598 |
| Dslow (×10−4mm2/s) | ||||||
| HGG | 9.27 ± 1.1 | 3.86 ± 0.21 | 0.73 ± 0.43 | 3.9 ± 1.22 | 6.89 ± 0.89 | 11.96 ± 1.42 |
| SBM | 9.38 ± 1.37 | 4.01 ± 0.22 | 0.56 ± 0.53 | 3.39 ± 1.29 | 7.08 ± 1.13 | 11.94 ± 1.8 |
| P | 0.748 | 0.005* | 0.450* | 0.019* | 0.469 | 0.960 |
| frac (%) | ||||||
| HGG | 21.29 ± 4.02 | 3.51 ± 0.3 | 1.02 ± 0.51 | 5.39 ± 2.07 | 10.99 ± 2.56 | 33.08 ± 6.87 |
| SBM | 25.09 ± 6.03 | 3.78 ± 0.28 | 0.6 ± 0.61 | 4.51 ± 2.72 | 13.87 ± 4.66 | 36.7 ± 8.61 |
| P | 0.007* | 0.001* | 0.007* | 0.025* | 0.009* | 0.088 |
| PTEA | ||||||
| Dfast (×10−4mm2/s) | ||||||
| HGG | 37.73 ± 2.99 | 3.29 ± 0.5 | 1.69 ± 0.67 | 8.91 ± 4.75 | 26.73 ± 3 | 51.93 ± 9.11 |
| SBM | 37.9 ± 3.75 | 3.3 ± 0.66 | 1.58 ± 0.64 | 8.83 ± 4.63 | 25.82 ± 4.65 | 53.17 ± 12.56 |
| P | 0.793 | 0.769 | 0.530 | 0.943 | 0.685 | 0.943 |
| Dslow (×10−4mm2/s) | ||||||
| HGG | 11.08 ± 1.44 | 4.06 ± 0.32 | 0.13 ± 0.46 | 2.9 ± 1.79 | 7.93 ± 0.95 | 14.18 ± 1.86 |
| SBM | 11.32 ± 1.31 | 4.03 ± 0.23 | 0.03 ± 0.41 | 2.72 ± 1.05 | 8.25 ± 1.17 | 14.22 ± 1.68 |
| P | 0.709 | 0.254 | 0.377 | 0.981 | 0.264 | 0.88 |
| frac (%) | ||||||
| HGG | 25.47 ± 4.46 | 3.32 ± 0.25 | 0.55 ± 0.75 | 6.26 ± 3.62 | 14.99 ± 3.61 | 34.73 ± 5.47 |
| SBM | 28.37 ± 4.22 | 3.39 ± 0.36 | 0.51 ± 0.71 | 5.55 ± 2.55 | 17.59 ± 4.2 | 38.81 ± 7.36 |
| P | 0.017* | 0.327 | 0.864 | 0.377 | 0.059 | 0.059 |
ETA: enhanced tumor area; PTEA: peritumor edema area; * P < 0.05.
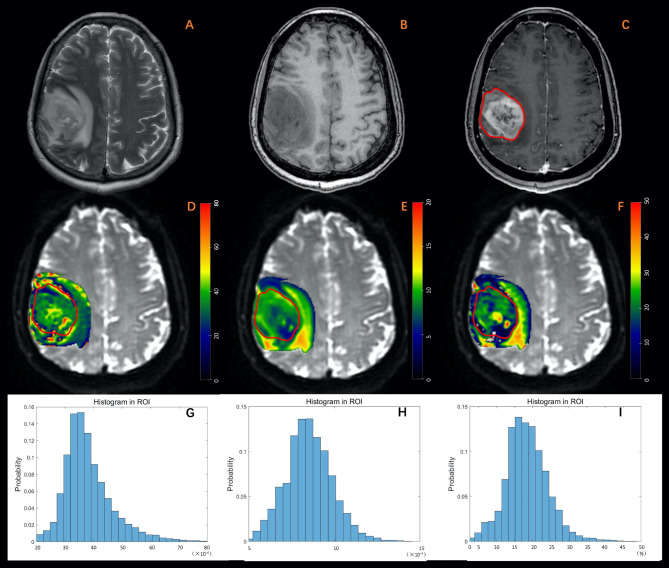
Fig. 2.
A 63-year-old male diagnosed with glioblastoma. (A) T2W imaging. (B) T1W imaging. (C) Contrast-enhanced T1W imaging. (D) Dfast (×10-4mm2/s). (E) Dslow (×10-4mm2/s). (F) frac (%). (G-I) Histogram plot of Dfast, Dslow, and frac of the enhanced tumor area. The MRI examination revealed an irregular lesion in the sub-cortex of the right frontal lobe, characterized by a blurred margin. The lesion exhibited diffuse enhancement, marked in red, along with hyper-intensity on T2-weighted imaging and hypo-intensity on T1-weighted imaging. Peritumoral edema was also observed. Parametric values obtained from histogram analysis of the enhanced tumor area are as follows: Dfast: mean: 37.98, entropy: 3.19, skewness: 1.15, kurtosis: 6.60. Dslow: mean: 8.52, entropy: 3.68. skewness: 0.33, kurtosis: 3.60, frac: mean: 18.46, entropy: 3.07, skewness: 0.68, kurtosis: 5.06.
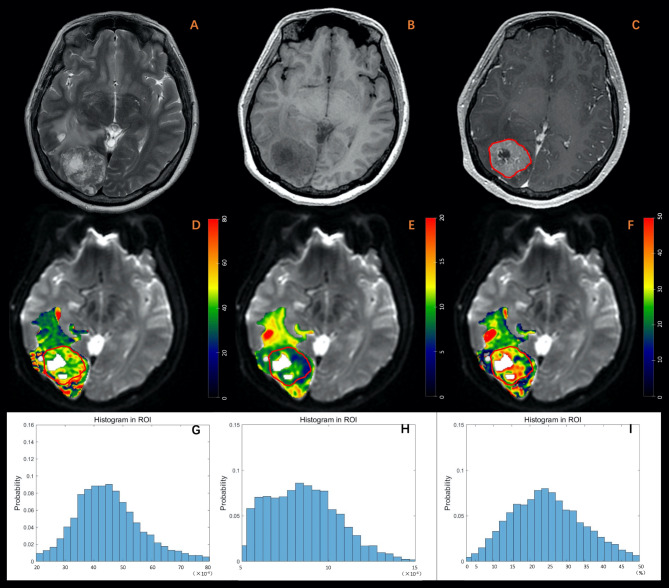
Fig. 3.
A 34-year-old female diagnosed with brain metastasis originating from breast cancer. (A) T2W imaging. (B) T1W imaging. (C) Contrast-enhanced T1W imaging. (D) Dfast (×10-4mm2/s). (E) Dslow (×10-4mm2/s). (F) frac (%). (G-I) Histogram plot of Dfast, Dslow, and frac of the enhanced tumor area. The MRI examination revealed an irregular lesion with a well-defined margin in the sub-cortex of the right occipital lobe. The lesion exhibited diffuse enhancement, marked in red, and appeared hyper-intense on T2-weighted imaging, hypo-intense on T1-weighted imaging, and was accompanied by peritumoral edema. Additionally, central necrosis was observed within the lesion. Parametric values obtained from histogram analysis of the enhanced tumor area are as follows: Dfast: mean: 45.38, entropy: 3.80, skewness: 0.70, kurtosis: 4.42; Dslow: mean: 8.55, entropy: 4.09, skewness: 0.47, kurtosis: 2.85; frac: mean: 25.15, entropy: 3.76, skewness: 0.49, kurtosis: 3.31.
Evaluating the performance with structural and histogram features
Through single-factor regression analysis, skewness of Dfast (OR = 0.22, 95%CI 0.06–0.80, P = 0.022) and frac (OR = 0.24, 95% CI 0.08–0.74, P = 0.013), the entropy of Dslow (OR = 26.20, 95% CI 1.71–402.6, P = 0.019) and frac (OR = 22.32, 95% CI 2.92–170.7, P = 0.003), and mean of frac (OR = 1.16, 95% CI 1.03–1.31, P = 0.014) in ETA, mean of frac (OR = 1.17, 95% CI 1.02–1.35, P = 0.025) in PTEA, and clear tumor margin on T2W (OR = 4.30, 95% CI 1.39–13.26, P = 0.011) were identified as associated factors for differentiating HGG from SBM (Table 3).
Table 3.
Univariate and binary logistic regression analysis.
| Univariate logistic regression | OR | (95% CI) | P | Binary logistic regression | OR | (95% CI) | P |
|---|---|---|---|---|---|---|---|
| ETA | |||||||
| Dfast_skewness | 0.22 | (0.06,0.80) | 0.022* | Dfast_skewness | 0.25 | (0.07,0.94) | 0.040* |
| +clear_t2_margin | 3.93 | (1.21,12.72) | 0.022* | ||||
| Dslow_entropy | 26.20 | (1.71,402.6) | 0.019* | Dslow_entropy | 21.77 | (1.21,392.7) | 0.037* |
| +clear_t2_margin | 3.91 | (1.20,12.75) | 0.024* | ||||
| Dslow_kurtosis | 0.71 | (0.44,1.13) | 0.146 | ||||
| frac_mean | 1.16 | (1.03,1.31) | 0.014* | frac_mean | 1.15 | (1.01,1.30) | 0.032* |
| +clear_t2_margin | 3.64 | (1.11,11.87) | 0.032* | ||||
| frac_entropy | 22.32 | (2.92,170.7) | 0.003* | frac_entropy | 17.44 | (2.14142.30) | 0.008* |
| +clear_t2_margin | 3.48 | (1.02,11.85) | 0.046* | ||||
| frac_skewness | 0.24 | (0.08,0.74) | 0.013* | frac_skewness | 0.21 | (0.06,0.68) | 0.009* |
| +clear_t2_margin | 5.43 | (1.55,18.97) | 0.008* | ||||
| frac_kurtosis | 0.84 | (0.65,1.09) | 0.190 | ||||
| PTEA | |||||||
| frac_mean | 1.17 | (1.02,1.35) | 0.025* | frac_mean | 1.18 | (1.01,1.37) | 0.034* |
| +clear_t2_margin | 4.34 | (1.31,14.31) | 0.016* | ||||
| clear_t2_margin | 4.30 | (1.39,13.28) | 0.011* | ||||
HGG: high-grade glioma; SBM: solitary brain metastasis; ETA: enhanced tumor area; PTEA: peritumor edema area. OR: odds ratio; 95% CI: 93% confidence interval; * P < 0.05.
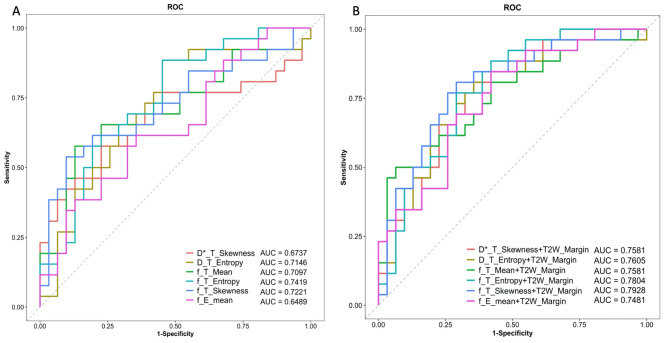
When combined with clear tumor margin on T2W imaging, the binary logistic regression analysis showed that skewness of Dfast (OR = 0.25, 95%CI 0.07–0.94, P = 0.040) and frac (OR = 0.21, 95% CI 0.06–0.68, P = 0.009), entropy of Dslow (OR = 21.77, 95% CI 1.21–392.7, P = 0.037) and frac (OR = 17.44, 95% CI 2.14–142.3, P = 0.008), and mean of frac (OR = 1.15, 95% CI 1.01–1.30, P = 0.032) in ETA, as well as mean of frac (OR = 1.18, 95% CI 1.01–1.37, P = 0.034) in PTEA, showed significant differences (Table 3). Among these factors, the entropy of frac in ETA exhibited the best performance for differentiation, with an AUC of 0.74 (sensitivity = 0.88, specificity = 0.55) in ROC analysis (Table 4; Fig. 4A). Furthermore, integrating clear tumor margin on T2W and skewness of frac in ETA improved the performance, with an optimal AUC of 0.79 (sensitivity = 0.81, specificity = 0.71) (Table 4; Fig. 4B). Moreover, calibration analysis using a bootstrap resampling method showed a good correlation between apparent and bias-corrected multivariate logistic regression in all the binary logistic regression models (Figure S1).
Table 4.
Univariate logistic regression and binary logistic regression performance in the differentiation of HGG and SBM.
| AUC (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
Accuracy (TN + TP)/(N + P) |
PPV (TP/P) |
NPV (TN/N) |
Threshold | |
|---|---|---|---|---|---|---|---|
| ETA | |||||||
| Dfast_skewness |
0.67 (0.52,0.83) |
0.58 (0.38.0.77) |
0.77 (0.61,0.90) |
0.68 (39/57) |
0.68 (15/22) |
0.69 (24/35) |
0.87 |
| Dslow_entropy |
0.71 (0.58,0.85) |
0.88 (0.77,1.00) |
0.55 (0.39,0.71) |
0.70 (40/57) |
0.62 (23/37) |
0.85 (17/20) |
3.85 |
| frac_mean |
0.71 (0.57,0.85) |
0.58 (0.38,0.77) |
0.87 (0.74,0.967) |
0.74 (42/57) |
0.79 (15/19) |
0.71 (27/38) |
24.74 |
| frac_entropy |
0.74 (0.61,0.87) |
0.88 (0.73.1.00) |
0.55 (0.39,0.71) |
0.70 (40/57) |
0.62 (23/37) |
0.85 (17/20) |
3.51 |
| frac_skewness |
0.72 (0.58,0.86) |
0.54 (0.35,0.73) |
0.90 (0.81,1.00) |
0.74 (42/57) |
0.82 (14/17) |
0.70 (28/40) |
0.52 |
| PTEA | |||||||
| frac_mean |
0.65 (0.50,0.79) |
0.62 (0.42,0.81) |
0.65 (0.48,0.81) |
0.63 (36/57) |
0.59 (16/27) |
0.67 (20/30) |
27.29 |
| clear_t2_margin |
0.67 (0.55.0.79) |
0.73 (0.54,0.88) |
0.61 (0.42,0.77) |
0.67 (38/57) |
0.61 (19/31) |
0.73 (19/26) |
0.44 |
| ETA | |||||||
|
Dfast_skewness +clear_t2_margin |
0.76 (0.63,0.89) |
0.77 (0.62,0.92) |
0.68 (0.48,0.84) |
0.72 (41/57) |
0.67 (20/30) |
0.78 (21/27) |
0.44 |
|
Dslow_entropy +clear_t2_margin |
0.76 (0.63,0.89) |
0.65 (0.46,0.85) |
0.81 (0.68,0.94) |
0.74 (42/57) |
0.74 (17/23) |
0.74 (25/34) |
0.54 |
|
frac_mean +clear_t2_margin |
0.76 (0.63,0.89) |
0.50 (0.31,0.69) |
0.94 (0.84,1.00) |
0.74 (42/57) |
0.87 (13/15) |
0.69 (29/42) |
0.61 |
|
frac_entropy +clear_t2_margin |
0.78 (0.66,0.90) |
0.77 (0.62,0.92) |
0.71 (0.55,0.87) |
0.74 (42/57) |
0.69 (20/29) |
0.79 (22/28) |
0.49 |
|
frac_skewness +clear_t2_margin |
0.79 (0.67,0.91) |
0.81 (0.65,0.92) |
0.71 (0.55,0.87) |
0.75 (43/57) |
0.70 (21/30) |
0.81 (22/27) |
0.42 |
| PTEA | |||||||
|
frac_mean +clear_t2_margin |
0.75 (0.62,0.88) |
0.85 (0.69,0.96) |
0.58 (0.42.0.74) |
0.70 (40/57) |
0.63 (22/35) |
0.82 (18/22) |
0.36 |
HGG: high-grade glioma; SBM: solitary brain metastasis; ETA: enhanced tumor area; PTEA: peritumor edema area. AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value. 95% CI: 95% confidence interval; TN: true negative; TP: true positive; N: negative; P: positive.
Fig. 4.
The ROC curves for univariate (A) and binary (B) logistic regression analysis.
Discussion
By employing histogram analysis in combination with the structural feature, we examined the accuracy of DWI in distinguishing HGG from SBM. The inclusion of DWI parametric values combined with the presence of a clear tumor margin in T2W imaging improved the differentiation process. The significant differences in histogram features of Dfast, Dslow, and frac within ETA suggested distinct diffusion and perfusion heterogeneity between HGG and SBM.
The accurate differentiation between HGG and SBM is crucial for patient management and can significantly impact clinical outcomes. While conventional structural MR imaging may not provide sufficient distinguishing features between HGG and SBM9, advanced diffusion, perfusion, and molecular MR techniques have been explored either individually or in combination with other models21–23. Previous studies have predominantly employed region of interest (ROI) based methods for analysis, with limited utilization of VOI-based approaches24,25. In this study, we adopted a VOI-based histogram analysis to investigate the diffusion and perfusion differences between HGG and SBM within the enhanced tumor area and the peritumoral edema area. This approach was superior to localized hotspot techniques regarding interobserver agreement and diagnostic accuracy, as supported by previous studies24.
In the ETA, HGG showed significantly higher skewness (Dfast, frac) and kurtosis (Dslow, frac) while demonstrating significantly lower entropy (Dslow, frac) and mean (frac) compared to SBM. The higher kurtosis indicated that the distribution of water diffusion and microvascular perfusion in the enhanced tumor region was more concentrated in HGG, and the lower entropy in HGG suggested a lower degree of molecular disorder in the diffusion and perfusion patterns, as shown in Fig. 2 (HGG) and Fig. 3 (SBM). Moreover, the significantly higher kurtosis and skewness in HGG indicated the presence of more extreme perfusion values, implying that micro-vessel characteristics influence microvascular perfusion in most ETA. A study conducted by Heynold et al. demonstrated that glioblastomas exhibit higher levels of neovascularization activity and metabolic rate of oxygen in the ETA compared to brain metastasis22. However, based on our findings of significantly lower mean and 10th percentile of frac in the ETA, we can conclude that HGG exhibits a predominantly poorer microvascular perfusion than SBM. This difference in perfusion could be due to the attenuation of blood supply caused by microscopic intravascular thrombosis26. HGG exhibits a pronounced capacity for neovascularization; nevertheless, most of these newly formed blood vessels are functionally inert26. These findings highlight the distinct diffusion and perfusion characteristics between HGG and SBM in ETA, shedding light on the underlying pathophysiological differences between the two tumor types.
In their recent study, Poulon et al.27 utilized real-time fresh human brain tumor samples imaging with endogenous fluorophores and observed higher microvascular density in metastasis compared to glioblastoma multiforme (GBM) and healthy tissues. They also found that the solid tumor component in GBM samples exhibited a highly disordered tumor cell architecture with microvascular growth, which could help explain the higher kurtosis values of Dslow and frac observed in our study. In the diffusion study of GBM and SBM differential diagnosis, Romano et al.25 found significantly lower mean apparent diffusion coefficient (ADC) values in GBM. Moreover, Chiang et al.28 reported higher ADC values in metastasis compared to HGG. On the other hand, Tsougos et al. found no significant difference in ADC and fractional anisotropy between the two tumor groups29. It is important to note that our study employed a VOI-based analysis and included HGGs in addition to GBM, whereas the mentioned studies utilized hotspot methods28,29, which could account for the observed discrepancies. In a DSC perfusion MRI study using histogram analysis, Qin et al. demonstrated that GBM exhibited a more heterogeneous distribution of blood perfusion in the enhancing tumor area than metastasis24. However, the mean value of absolute cerebral blood volume (CBV) did not show a significant difference, which aligns with previous research highlighting the limitations of relative CBV30. Our study focusing on micro-vessel perfusion revealed higher heterogeneity and significantly lower mean values in HGG compared to SBM. These findings suggest that micro-vessel perfusion parameters hold promise as potential discriminative factors warranting further investigation. Previous studies using DSC perfusion MRI have consistently shown higher perfusion values in the PTEA of GBM compared to brain metastasis28,29,31. Additionally, there is a decreasing gradient of relative CBV values from the region adjacent to the enhancing solid lesion to the normal white matter in GBM, whereas this gradient is less pronounced in brain metastasis25,32,33. The differential growth patterns of GBM and metastasis, with GBM exhibiting infiltrative growth beyond the boundaries of the enhancing tumor core34,35 and metastasis primarily expanding and causing vasogenic edema36, contribute to the distinct microenvironments associated with these tumors. These variations in microenvironments can lead to differences in diffusion and perfusion characteristics. However, in this study, only the mean value of the frac parameter showed a significant distinction between GBM and metastasis in the PTEA. Interestingly, this parameter did not outperform other measurements in the ETA. The presence of a perfusion gradient within the PTEA and the utilization of a VOI-based analysis in our study may account for these findings. It is worth noting that the distribution of Dfast, Dslow, and frac varies among different lobes, and the localization of the edema area also differs across lobes, which could potentially impact the observed outcomes37.
In this study, we included solitary brain metastases originating from various primary sites but mostly from lung, which is consistent with previous studies17,38,39. Among these, lung adenocarcinoma was the most prevalent histological subtype. In their analysis of the tumor and immune cell phenotypic composition in metastatic breast cancer, Kuett et al. highlighted significant heterogeneity both within individual patients and across different metastatic sites40. Research suggests that techniques like PET can assist in predicting the primary site of brain metastases38, which implies that brain metastases originating from different primary sites may exhibit distinct perfusion or metabolic patterns, reflecting their varied biological behaviors. However, a meta-analysis by Fioni et al. involving 399 HGG and 232 SBM cases found that the relative CBV values in the central area of the tumor did not significantly differ between HGG patients and SBM/controls.41 These findings highlight the need for further research with larger, more balanced cohorts to investigate whether metastases from different sites will lead to differences in imaging.
The DWI scanning protocol with 12 b-values ranging from 0 to 2000 s/mm2 was adopted in our study, while data analysis was performed with a bi-exponential fitting model using b-values of 200 to 1200 s/mm2. This range was selected because pseudo-diffusion from blood flow occurs at a faster rate than bulk-water diffusion, and diffusion signals below 200–400 s/mm2 are predominantly influenced by perfusion-diffusion effects42. Our initial analysis revealed poor fitting when data points at b = 1500 and 2000 s/mm2 were included (Figure S2). Currently, there is no universally standardized b-value protocol for multi-b DWI analysis, and different studies have used different b-value settings43,44. Han et al. reported significantly lower ADC values in the peritumoral region of HGG compared to SBM, with an AUC of 0.922 at b-values of 3000 s/mm2 and 0.886 at 1000 s/mm2 based on ROC analysis45. In our future work, we plan to investigate the potential of Cramer Rao Lower Bound analysis for selecting optimal b-values46,47.
Advanced magnetic resonance techniques offer valuable insights into the diffusion, perfusion, and metabolism of different lesions, while histogram analysis provides additional information for understanding tumor pathogenesis. Furthermore, radiomics, genomics, and neural network can deepen investigations, providing advanced tools for tumor differentiation. For example, the model of neurite orientation dispersion and density imaging radiomics within the solid tumor area showed promising performance for preoperative discrimination between glioblastoma and SBM (AUC = 0.904)48. GoogLeNet performed better than previous methods at differentiating HGG from SBM both in core (external dataset, accuracy of 86.76%, sensitivity of 81.82%, specificity of 83.33%, and AUC of 0.866) and peritumoral edema area (external dataset, accuracy of 79.31%, sensitivity of 81.82%, specificity of 77.78% and AUC of 0.826)49.
However, the higher-dimensional results from multi-omics can be challenging to interpret biologically, so there is a need for more practical and straightforward approaches that can be easily implemented in smaller medical facilities and time-limited clinical practice. In clinical settings, structural features are still widely utilized. Yamamoto et al.50 evaluated the efficacy of an extensive peritumor hyperintense rim in differentiating metastatic brain tumors from GBM using contrast-enhanced Fast imaging employing steady-state acquisition and reported high sensitivity (0.95), specificity (0.95), and accuracy (0.93) compared to conventional MR-based tumor shape analysis. However, they did not provide the AUC value. Herein, we identified a clear tumor margin on T2W imaging as the only structural feature distinguishing HGG from SBM. Further ROC analysis revealed that when combined with the skewness of frac in the enhanced tumor region, it significantly improved the differentiation performance, yielding the highest AUC of 0.79 (sensitivity = 0.81, specificity = 0.71). Moreover, they included metastasis cases with multiple lesions (32.6%), which may have potentially exaggerated their findings. Another efficient tool for tumor differential diagnosis is the Artificial Intelligence-Assisted Decision-Making System51; however, bridging the gap between experimental research and regulatory standards requires further validation, standardization, and extensive clinical trials.
The present study has some limitations. First, the number of patients included was relatively small, and there was variability in the original metastasis lesions. Second, external verification was lacking as all patients were from one medical center. Third, detailed pathological information, such as immunohistochemical staining, cell distribution density, and micro-vessel density, was not utilized in this study, and incorporating these factors in future research could provide valuable insights and help validate the findings. In our next study, we plan to compare diffusion kurtosis between HGG and SBM and analysis using the Cramer Rao Lower Bound method to determine the most suitable b-values.
Methods
Study population
Patients who underwent MR examination between December 2020 and June 2022 were included in this retrospective study. The inclusion criteria were: (1) patients with histopathologically or molecularly genotyped HGG (according to the WHO 2016 Classification52) or brain metastasis; (2) patients who received no radiotherapy or who did not undergo surgical treatment before diagnosis; (3) age > 18 years old. The exclusion criteria were: (1) tumors located below the tentorium cerebelli; (2) presence of more than one tumor lesion above the tentorium cerebelli; (3) incomplete imaging data; (4) poor imaging quality, such as severe motion artifacts, significant cystic, hemorrhagic, or extensive tumor necrosis. The pathology tissue was acquired through a craniotomy or robotic-assisted biopsy surgery about one week after the MR scanning. The patient enrollment process is depicted in Fig. 1.
This study was conducted in accordance with the guidelines outlined in the Declaration of Helsinki and was approved by the institutional review board of Fifth Hospital of Shanxi Medical University/Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, P. R. China. Informed consent was waived due to the retrospective nature of the study.
Imaging data acquisition
The imaging protocol was performed in the following order: T2W, T1W, DWI, contrast injection, and T1W + C. All magnetic resonance scans were conducted using a 3.0T MR scanner (Discovery MR 750 W, GE Healthcare, Waukesha, WI, USA) equipped with a 24-channel head-neck coil, ensuring consistent positioning. Axial DWI imaging utilized 12 b-values (0, 20, 40, 80, 110, 150, 200, 400, 800, 1200, 1500, and 2000 s/mm2) with corresponding acquisition times of 1, 1, 1, 1, 1, 1, 2, 2, 2, 2, 2, and 4 s. The following parameters were used: repetition time/echo time (TR/TE) = 5400/90 ms, field of view (FOV) = 220 × 220 mm2, matrix size = 110 × 110, slice thickness = 4.0 mm, number of slices = 24, gap = 1.0 mm, and acquisition time = 5 min and 28 s. Axial T2W imaging was performed using the following parameters: TR/TE = 7900/125 ms, FOV = 230 × 230 mm2, matrix size = 380 × 380, slice thickness = 4.5 mm, number of slices = 30, gap = 5 mm, flip angle = 140°, and acquisition time = 2 minutes. For axial T1W imaging, the following parameters were used: TR/TE = 2500/24 ms, FOV = 230 × 230 mm2, matrix size = 320 × 224, slice thickness = 4.0 mm, number of slices = 30, gap = 5 mm, flip angle = 110°, and acquisition time = 1 min and 19 s. T1W + C imaging employed the same parameters as T1W, and gadodiamide was power-injected at a rate of 2 ml/s, with dosages calibrated based on the patient’s body weight (0.2 ml/kg). The scan began 3–5 min after the injection. Images were acquired sequentially in the axial, sagittal, and coronal planes. The total imaging time was approximately 20 min.
Volume of interest (VOI)
VOI for the PTEA and ETA were delineated by two experienced neuroradiologists with 20 and 25 years of experience, respectively. Consensus was reached between the radiologists during the delineation process, which was performed on a slice-by-slice basis using 3D-slicer software (version 4.10, available at https://www.slicer.org) referring to the T2W and T1W + C images for PTEA and ETA, respectively. We especially made sure to minimize the inclusion of calcifications, intra-tumor hemorrhage, cysts, and hyperintensity surrounding the ventricular system and skull base, as suggested by previous studies53,54.
Bi-exponential analysis
For the calculation of the slow diffusion coefficient (Dslow), fast diffusion coefficient (Dfast), and perfusion fraction (frac) within the VOI, an in-house program was developed in MATLAB 2018b. The program utilized the bi-exponential fitting approach defined by:
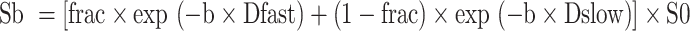 |
1 |
where Sb and S0 represents the signal intensity under b > 0 s/mm2 and b = 0 s/mm2, respectively. The diffusion data were used to fit the bi-exponential model using a bound-constrained optimization mini-search approach implemented in the MATLAB code55. Before the fitting process, a linear fitting was performed on the logarithm of the diffusion data with 1200 > b > 200 s/mm2 to generate the Dslow parametric map. Furthermore, another internally developed program was employed to extract histogram features of Dslow, frac, and Dfast from the parametric maps. These features included the 10th and 90th percentile, entropy (a measure of molecular disorder), kurtosis (a measure of the tailedness of a distribution), and skewness (a measure of the asymmetry of a distribution). The processing time per patient was approximately 10 min, including 3 min for ROI drawing in 3D Slicer, 5 min for bi-exponential analysis, and 1 min for histogram feature extraction in MATLAB.
Structural features evaluation
The structural characteristics were assessed by the same neuroradiologist, who was blinded to the patients’ initial diagnosis. The tumor site was divided into sub-cortex and deep white matter based on the distance between the tumor body and the cortex. Similar to the previously described method50, tumors were categorized based on the presence or absence of a peritumoral hyperintense rim on T2-weighted imaging into those with well-defined and blurred margins. The degree of edema was classified as mild, moderate, or severe, according to the criteria proposed in a previous study56. Mild edema was defined as hyperintensity on T2-weighted imaging within the radius of the tumor, while severe edema indicated edema extending beyond the diameter of the tumor. Additionally, the classification of enhancement patterns proposed by Wang et al.57 was used. Focal enhancement was defined as the absence of obvious hyperintensity on T1W + C imaging, with a smooth border and a maximal enhancing focal diameter of < 1.5 cm. Diffuse enhancement was characterized by enhancements with rough edges and maximal diameters > 1.5 cm. Ring-like enhancement was used to describe peripherally enhanced cystic necrosis. No obvious hyperintensity on T1W + C was considered evidence of non-enhancement.
Statistical analysis
Statistical analysis was conducted after confirming the normality and homogeneity of variance. Quantitative variables were presented as mean ± standard deviation and compared using either Student’s t-test or Wilcoxon test (Mann-Whitney U test). The Chi-square test was employed to compare age and gender. Subsequently, variables that exhibited significant differences were further analyzed using univariate logistic regression, and the best combination model was determined using binary logistic regression. The performance of logistic regression was evaluated using receiver operating characteristic (ROC) analysis, with calculations of the area under the ROC curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). All statistical analyses were performed using the R software (version 4.0.0). A P-value of < 0.05 was considered statistically significant.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ADC
Apparent diffusion coefficient
- CBV
Cerebral blood volume
- Dfast
Fast diffusion coefficient
- DWI
Diffusion-weighted imaging
- Dslow
Slow diffusion coefficient
- DSC
Dynamic susceptibility contrast
- ETA
Enhanced tumor area
- Frac
Perfusion fraction
- GBM
Glioblastoma multiforme
- HGG
High-grade glioma
- PTEA
Peritumoral edema area
- ROC
Receiver operator curves
- SBM
Solitary brain metastasis
- T1W
T1 weighted
- T1W + C
Contrast-enhanced T1 weighted
- T2W
T2 weighted
- VOI
Volume of interest
Author contributions
Conceptualization: Hongming Ji, Cheng Xu, and Rui Cheng. Methodology: Jinxia Guo and Yifei Su. Formal analysis and investigation: Yifei Su, Junhao Wang, and Jinxia Guo. Writing - original draft preparation: Yifei Su and Junhao Wang. Writing - review and editing: Jinxia Guo and Chunhong Wang. Funding acquisition: none. Resources: Yifei Su, Junhao Wang, Xuanchen Liu, and Xiaoxiong Yang. Supervision: Hongming Ji and Cheng Xu. All authors approved the version to be published and agreed to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
J. Guo is an employee of GE Healthcare. The other authors have no conflicts of interest to declare.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was reviewed and approved by the Ethics Committee of Shanxi Provincial People’s Hospital/Fifth Hospital of Shanxi Medical University (2022 Research Review No. 153).
Consent to participate
The informed consent was waived by the Ethics Committee of Shanxi Provincial People’s Hospital /Fifth Hospital of Shanxi Medical University (2022 Research Review No. 153).
Consent to publish
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller, K. D. et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin.71, 381–406. 10.3322/caac.21693 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Sacks, P. & Rahman, M. Epidemiology of brain metastases. Neurosurg. Clin. N. Am.31, 481–488. 10.1016/j.nec.2020.06.001 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol.23, 1231–1251. 10.1093/neuonc/noab106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horbinski, C. et al. NCCN Guidelines® insights: Central nervous system cancers, Version 2.2022. J. Natl. Compr. Canc. Netw.21, 12–20. 10.6004/jnccn.2023.0002 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Cha, S. et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am. J. Neuroradiol.28, 1078–1084. 10.3174/ajnr.A0484 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin, H. W., Rasp, G., Kim, J. & Hale, E. R. Role of radiosurgery in the treatment of brain metastasis. Fed Pract32, 32–37 (2015). [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai, A., Shibamoto, Y., Yoshida, M., Wakamatsu, K. & Kikuchi, Y. Treatment of single or multiple brain metastases by hypofractionated stereotactic radiotherapy using helical tomotherapy. Int. J. Mol. Sci.15, 6910–6924. 10.3390/ijms15046910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelbaum, M. A. et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J. Clin. Oncol.40, 492–516. 10.1200/JCO.21.02314 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Faehndrich, J. et al. Neuroradiological viewpoint on the diagnostics of space-occupying brain lesions. Clin. Neuroradiol.21, 123–139. 10.1007/s00062-011-0073-6 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Elmariah, S. B., Huse, J., Mason, B., Leroux, P. & Lustig, R. A. Multicentric glioblastoma multiforme in a patient with BRCA-1 invasive breast cancer. Breast J.12, 470–474. 10.1111/j.1075-122X.2006.00307.x (2006). [DOI] [PubMed] [Google Scholar]
- 11.Davis, F. G. et al. Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995–2015. Neuro Oncol.22, 301–302. 10.1093/neuonc/noz203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arévalo-Sáenz, A., Rodríguez-Boto Amago, G. & Pedrosa Sánchez, M. High-grade glioma and solitary metastasis: Differentiation by spectroscopy and advanced magnetic resonance techniques. Egypt. J. Neurosurg.37, 34. 10.1186/s41984-022-00172-y (2022). [Google Scholar]
- 13.Schwartz, K. M., Erickson, B. J. & Lucchinetti, C. Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology48, 143–149. 10.1007/s00234-005-0024-5 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Baris, M. M., Celik, A. O., Gezer, N. S. & Ada, E. Role of mass effect, tumor volume and peritumoral edema volume in the differential diagnosis of primary brain tumor and metastasis. Clin. Neurol. Neurosurg.148, 67–71. 10.1016/j.clineuro.2016.07.008 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Zhang, G. et al. Discrimination between solitary brain metastasis and glioblastoma multiforme by using ADC-based texture analysis: A comparison of two different ROI placements. Acad. Radiol.26, 1466–1472. 10.1016/j.acra.2019.01.010 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Gao, E. et al. Histogram analysis based on diffusion kurtosis imaging: Differentiating glioblastoma multiforme from single brain metastasis and comparing the diagnostic performance of two region of interest placements. Eur. J. Radiol.147, 110104. 10.1016/j.ejrad.2021.110104 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Voicu, I. P. et al. Differentiating solitary brain metastases from high-grade gliomas with MR: Comparing qualitative versus quantitative diagnostic strategies. Radiol. Med.127, 891–898. 10.1007/s11547-022-01516-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh, C. H., Kim, H. S., Jung, S. C., Choi, C. G. & Kim, S. J. Perfusion MRI as a diagnostic biomarker for differentiating glioma from brain metastasis: A systematic review and meta-analysis. Eur. Radiol.28, 3819–3831. 10.1007/s00330-018-5335-0 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Le Bihan, D. & Turner, R. The capillary network: A link between IVIM and classical perfusion. Magn. Reson. Med.27, 171–178. 10.1002/mrm.1910270116 (1992). [DOI] [PubMed] [Google Scholar]
- 20.Kusunoki, M. et al. Differentiation of high-grade from low-grade diffuse gliomas using diffusion-weighted imaging: A comparative study of mono-, bi-, and stretched-exponential diffusion models. Neuroradiology62, 815–823. 10.1007/s00234-020-02456-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen, D. H. et al. Discriminating glioblastoma from solitary brain metastases on 3 Tesla magnetic resonance imaging: The roles of fractional anisotropy and mean diffusivity. Eur. Rev. Med. Pharmacol. Sci.26, 8823–8831. 10.26355/eurrev_202212_30554 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Heynold, E. et al. Physiological MRI biomarkers in the differentiation between glioblastomas and solitary brain metastases. Mol. Imaging Biol.23, 787–795. 10.1007/s11307-021-01604-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, X. et al. Discrimination between glioblastoma and solitary brain metastasis: Comparison of inflow-based vascular-space-occupancy and dynamic susceptibility contrast MR imaging. AJNR Am J. Neuroradiol.41, 583–590. 10.3174/ajnr.A6466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin, J., Li, Y., Liang, D., Zhang, Y. & Yao, W. Histogram analysis of absolute cerebral blood volume map can distinguish glioblastoma from solitary brain metastasis. Medicine (Baltimore)98, e17515. 10.1097/md.0000000000017515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romano, A. et al. Single brain metastasis versus glioblastoma multiforme: A VOI-based multiparametric analysis for differential diagnosis. Radiol. Med.127, 490–497. 10.1007/s11547-022-01480-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markwell, S. M., Ross, J. L., Olson, C. L. & Brat, D. J. Necrotic reshaping of the glioma microenvironment drives disease progression. Acta Neuropathol.143, 291–310. 10.1007/s00401-021-02401-4 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Poulon, F. et al. Real-time Brain Tumor imaging with endogenous fluorophores: A diagnosis proof-of-concept study on fresh human samples. Sci. Rep.8, 14888. 10.1038/s41598-018-33134-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang, I. C. et al. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology46, 619–627. 10.1007/s00234-004-1246-7 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Tsougos, I. et al. Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging12, 423–436. 10.1102/1470-7330.2012.0038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha, S. et al. Intracranial mass lesions: Dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology223, 11–29. 10.1148/radiol.2231010594 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Pons-Escoda, A. et al. Voxel-level analysis of normalized DSC-PWI time-intensity curves: A potential generalizable approach and its proof of concept in discriminating glioblastoma and metastasis. Eur. Radiol.32, 3705–3715. 10.1007/s00330-021-08498-1 (2022). [DOI] [PubMed] [Google Scholar]
- 32.She, D., Xing, Z. & Cao, D. Differentiation of glioblastoma and solitary brain metastasis by gradient of relative cerebral blood volume in the peritumoral brain zone derived from dynamic susceptibility contrast perfusion magnetic resonance imaging. J. Comput. Assist. Tomogr.43, 13–17. 10.1097/rct.0000000000000771 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Aparici-Robles, F. et al. Glioblastoma versus solitary brain metastasis: MRI differentiation using the edema perfusion gradient. J. Neuroimaging32, 127–133. 10.1111/jon.12920 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Auffinger, B., Spencer, D., Pytel, P., Ahmed, A. U. & Lesniak, M. S. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert. Rev. Neurother.15, 741–752. 10.1586/14737175.2015.1051968 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson, M., Hassiotou, F. & Nowak, A. Glioblastoma stem-like cells: At the root of tumor recurrence and a therapeutic target. Carcinogenesis36, 177–185. 10.1093/carcin/bgu243 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Blecharz, K. G., Colla, R., Rohde, V. & Vajkoczy, P. Control of the blood-brain barrier function in cancer cell metastasis. Biol. Cell107, 342–371. 10.1111/boc.201500011 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Wang, C. et al. Distribution of intravoxel incoherent motion MRI-related parameters in the brain: evidence of interhemispheric asymmetry. Clin. Radiol.72(94), e91-94.e96. 10.1016/j.crad.2016.09.007 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Willemse, J. R. J. et al. Identifying the primary tumour in patients with cancer of unknown primary (CUP) using [(18)F]FDG PET/CT: A systematic review and individual patient data meta-analysis. Eur. J. Nucl. Med. Mol. Imaging10.1007/s00259-024-06860-1 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aslan, K., Gunbey, H. P., Tomak, L. & Incesu, L. Multiparametric MRI in differentiating solitary brain metastasis from high-grade glioma: Diagnostic value of the combined use of diffusion-weighted imaging, dynamic susceptibility contrast imaging, and magnetic resonance spectroscopy parameters. Neurol. Neurochir. Pol.53, 227–237. 10.5603/PJNNS.a2019.0024 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Kuett, L. et al. Distant metastases of breast cancer resemble primary tumors in cancer cell composition but differ in immune cell phenotypes. Cancer Res.10.1158/0008-5472.Can-24-1211 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fioni, F., Chen, S. J., Lister, I. N. E., Ghalwash, A. A. & Long, M. Z. Differentiation of high grade glioma and solitary brain metastases by measuring relative cerebral blood volume and fractional anisotropy: A systematic review and meta-analysis of MRI diagnostic test accuracy studies. Br. J. Radiol96, 20220052. 10.1259/bjr.20220052 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iima, M. & Le Bihan, D. Clinical intravoxel incoherent motion and diffusion MR imaging: Past, present, and future. Radiology278, 13–32. 10.1148/radiol.2015150244 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Chabert, S. et al. Impact of b-value sampling scheme on brain ivim parameter estimation in healthy subjects. Magn. Reson. Med. Sci.19, 216–226. 10.2463/mrms.mp.2019-0061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, C. et al. Distribution of intravoxel incoherent motion MRI-related parameters in the brain: evidence of interhemispheric asymmetry. Clin. Radiol.72, e91–e96. 10.1016/j.crad.2016.09.007 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Han, C., Huang, S., Guo, J., Zhuang, X. & Han, H. Use of a high b-value for diffusion weighted imaging of peritumoral regions to differentiate high-grade gliomas and solitary metastases. J. Magn. Reson. Imaging42, 80–86. 10.1002/jmri.24747 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Pavilla, A. et al. Intravoxel incoherent motion and diffusion kurtosis imaging at 3T MRI: Application to ischemic stroke. Magn. Reson. Imaging99, 73–80. 10.1016/j.mri.2023.01.018 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Pavilla, A. et al. Measuring cerebral hypoperfusion induced by hyperventilation challenge with intravoxel incoherent motion magnetic resonance imaging in healthy volunteers. J. Comput. Assist. Tomogr.42, 85–91. 10.1097/RCT.0000000000000640 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Bai, J. et al. High-performance presurgical differentiation of glioblastoma and metastasis by means of multiparametric neurite orientation dispersion and density imaging (NODDI) radiomics. Eur. Radiol.34, 6616–6628. 10.1007/s00330-024-10686-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong, Z. et al. Deep learning models for rapid discrimination of high-grade gliomas from solitary brain metastases using multi-plane T1-weighted contrast-enhanced (T1CE) images. Quant. Imaging Med. Surg.14, 5762–5773. 10.21037/qims-24-380 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto, J. et al. Evaluation of peritumoral brain parenchyma using contrast-enhanced 3D fast imaging employing steady-state acquisition at 3T for differentiating metastatic brain tumors and glioblastomas. World Neurosurg.120, e719–e729. 10.1016/j.wneu.2018.08.147 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Otman, H. Artificial Intelligence Improves Brain Tumor Diagnosis, https://www.michiganmedicine.org/health-lab/artificial-intelligence-improves-brain-tumor-diagnosis (2020).
- 52.Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol.131, 803–820. 10.1007/s00401-016-1545-1 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Yu, H. et al. Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur. Radiol.27, 4516–4524. 10.1007/s00330-017-4867-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim, M. et al. Diffusion- and perfusion-weighted MRI radiomics model may predict isocitrate dehydrogenase (IDH) mutation and tumor aggressiveness in diffuse lower grade glioma. Eur. Radiol.30, 2142–2151. 10.1007/s00330-019-06548-3 (2020). [DOI] [PubMed] [Google Scholar]
- 55.D’Errico, J. fminsearchbnd, fminsearchcon, https://www.mathworks.com/matlabcentral/fileexchange/8277-fminsearchbnd-fminsearchcon (2023).
- 56.Pope, W. B. et al. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am. J. Neuroradiol.26, 2466–2474 (2005). [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, Y. et al. Identifying the association between contrast enhancement pattern, surgical resection, and prognosis in anaplastic glioma patients. Neuroradiology58, 367–374. 10.1007/s00234-016-1640-y (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.