Abstract
The type III secretion system (TTSS) is a specialized cytotoxin-translocating apparatus of gram-negative bacteria which is involved in lung injury, septic shock, and a poor patient outcome. Recent studies have attributed these effects mainly to the ExoU effector protein. However, few studies have focused on the ExoU-independent pathogenicity of the TTSS. For the present study, we compared the pathogenicities of two strains of Pseudomonas aeruginosa in a murine model of acute lung injury. We compared the CHA strain, which has a functional TTSS producing ExoS and ExoT but not ExoU, to an isogenic mutant with an inactivated exsA gene, CHA-D1, which does not express the TTSS at all. Rats challenged with CHA had significantly increased lung injury, as assessed by the wet/dry weight ratio for the lungs and the protein level in bronchoalveolar lavage fluid (BALF) at 12 h, compared to those challenged with CHA-D1. Consistent with these findings, the CHA strain was associated with increased in vitro cytotoxicity on A549 cells, as assessed by the release of lactate dehydrogenase. CHA was also associated at 12 h with a major decrease in polymorphonuclear neutrophils in BALF, with a proinflammatory response, as assessed by the amounts of tumor necrosis factor alpha and interleukin-1β, and with decreased bacterial clearance from the lungs, ultimately leading to an increased mortality rate. These results demonstrate that the TTSS has a major role in P. aeruginosa pathogenicity independent of the role of ExoU. This report underscores the crucial roles of ExoS and ExoT or other TTSS-related virulence factors in addition to ExoU.
Pseudomonas aeruginosa is a frequent cause of life-threatening infections. Patients requiring mechanical ventilation especially have a high risk of developing P. aeruginosa pneumonia (3, 14). Moreover, patients with ventilator-acquired P. aeruginosa pneumonia are more likely to develop bacteremia, septic shock, and multiple organ failure and have a higher mortality rate than patients with pneumonia due to other infectious agents (2).
Genetic pathogen-related factors underlie the severity of P. aeruginosa infection. The recently sequenced (38) P. aeruginosa genome (PAO1 strain) has the largest percentage of genes (among other sequenced bacterial genomes) which are dedicated to controlling systems that allow the modulation of genetic and biochemical capabilities geared towards survival. Among these are systems allowing adaptation to host conditions, such as quorum sensing or resistance to antimicrobials (20). In addition to numerous extracellularly secreted virulence determinants, most clinical isolates of P. aeruginosa possess a specialized apparatus to translocate cytotoxins into eukaryotic cells, namely, the type III secretion system (TTSS). The TTSS is composed of 20 proteins which, after assembling into a syringe-like apparatus, can inject cytotoxins directly into the target cell. This system requires direct contact between the bacterium and the host cell to deliver toxic bacterial effector proteins directly into the cytosol of the host cell. P. aeruginosa secretes at least four effector proteins through its TTSS, namely, ExoS, ExoT, ExoY, and ExoU (12, 42, 43). This system has been found to be a critical virulence factor in animal models as well as in human diseases. Indeed, although P. aeruginosa TTSS genes are present in nearly all clinical and environmental isolates (11), TTSS expression is associated with more severe infections (32). In a study of 108 clinical isolates of P. aeruginosa, the prevalence of the TTSS-expressing phenotype was significantly higher among acutely infected patients than among chronically infected cystic fibrosis patients and was linked to a higher mortality rate (29). Consistent with these data, in a retrospective clinical study of patients with ventilator-associated P. aeruginosa pneumonia, TTSS-positive strains were associated with poor clinical outcomes (13). Although early studies found that ExoS has a role in mediating injury to the alveolar epithelial barrier during acute P. aeruginosa pneumonia in rabbits (17, 21, 41), more recent studies established that ExoU has a crucial role in P. aeruginosa virulence (1, 12, 26, 30, 31, 33). Kurahashi et al. showed that ExoU is responsible for the decompartmentalization of inflammatory mediators (18).
Several studies evaluated the inflammatory response within the alveoli during P. aeruginosa-induced lung injury: this response includes the role of inflammatory cells (polymorphonuclear neutrophils and alveolar macrophages), cytokine production, and the role of the pathogen itself. While the role of alveolar macrophages remains controversial (4, 16), polymorphonuclear neutrophils have been associated with bacterial clearance (22), with the accumulation of these cells being under the control of tumor necrosis factor alpha (TNF-α) production (37). Finally, it was demonstrated in vitro that the TTSS is also associated with a toxic role toward polymorphonuclear neutrophils (5) and macrophages (8).
Therefore, our main objective was to establish the role of the TTSS, independent of that of ExoU, in the pathogenesis of P. aeruginosa-mediated acute lung injury. Specifically, our aims were to assess the ExoU-independent role of the TTSS on mortality, lung injury, bacterial clearance from the lungs, polymorphonuclear neutrophil recruitment to the lungs, lung and systemic inflammation, and cytotoxicity during P. aeruginosa-mediated acute lung injury. Using an experimental model of murine pneumonia, we compared two strains of P. aeruginosa, namely, CHA, a cystic fibrosis clinical isolate which produces the type III effectors ExoS and ExoT but not ExoU, and CHA-D1 (5), an isogenic mutant of CHA in which the exsA gene, which encodes the ExsA transcriptional factor necessary for type III system synthesis, has been inactivated (6). We assessed mortality at 72 h following P. aeruginosa instillation. Lung injury, as assessed by lung permeability and extravascular lung water, was evaluated sequentially between 2 and 12 h. Bacterial clearance from the lungs was evaluated with cultures of lung homogenates. Polymorphonuclear neutrophil (PMN) recruitment to the lungs was assessed by the PMN count in bronchoalveolar lavage fluid (BALF). The inflammatory response was studied with cytokine assays using BALF, lung tissue, and serum. In addition, cytotoxicity was evaluated in vitro on A549 cells by use of a lactate dehydrogenase (LDH) assay.
MATERIALS AND METHODS
Animals.
Pathogen-free Sprague-Dawley rats (n = 105; 280 to 320 g) (Charles River Laboratories France, St. Germain/l'Arbresle, France) were housed in the Lille University Animal Care Facility and allowed food and water ad libitum. All experiments were performed with the approval of the Lille Institutional Animal Care and Use Committee.
P. aeruginosa-induced lung injury.
The P. aeruginosa strains used for this study included CHA, a bronchopulmonary isolate from a cystic fibrosis patient, and CHA-D1, an isogenic mutant of CHA in which the exsA gene, which encodes the ExsA transcriptional factor necessary for type III system synthesis, has been inactivated (5). P. aeruginosa was incubated in 125 ml of tryptic soy broth at 37°C in a rotating shaking water bath for 8 h. Cultures were centrifuged at 1,000 × g for 10 min, and the bacterial pellets were washed twice and diluted in phosphate-buffered saline to reach 109 CFU/ml, as evaluated by spectrophotometry. Acute lung injury was produced according to the method described by Pennington and Ehrie (25). Mice were put under short-duration anesthesia, and a small midline incision was made on the ventral surface of the neck after swabbing it with ethanol. The trachea was exposed by blunt dissection. Using a 28-gauge needle, we instilled 0.5 ml of bacterial suspension/kg of body weight into the trachea, followed by the injection of 0.5 ml of air. The animals were studied 2, 4, 12, 24, or 72 h after instillation of the bacteria, depending on the group.
Bacterial culture from the lungs.
The lungs were homogenized in sterile containers with sterile water. Lung homogenates were sequentially diluted and cultured on bromocresol purple agar plates for 24 h for assessments of the bacterial load.
In vitro cytotoxicity.
A human lung epithelial cell line (A549) was cultured to confluence in modified Eagle's medium with Earle's salts and l-glutamine (Invitrogen, Cergy Pontoise, France) supplemented with 10% heat-inactivated fetal bovine serum (Dustcher, Vilmorin, France) and a penicillin-streptomycin association (1%). The cells were cultured at 37°C with 5% CO2. When the cells reached confluence, 2 × 104 cells were transferred to 96-well tissue culture plates and incubated overnight. The following day, 10 μl of each of the four different P. aeruginosa strains (PAO1, PA103, CHA, and CHA-D1 at 2 × 107 CFU/ml) was mixed with medium (not containing antibiotics) and applied to the cells for 4 or 6 h. PA103, the positive control for major cytotoxicity, and PAO1, the positive control for minor cytotoxicity, were used as references. Cytotoxicity was quantitated by measuring the lactate dehydrogenase (LDH) in the culture supernatants at 4 and 6 h, using a cytotoxicity assay kit (Cytotox 96; Promega, Charbonnieres, France). The 100% value represented the amount of LDH released from cells lysed by 0.8% Triton X-100 for 45 min.
BAL.
Bronchoalveolar lavage (BAL) was performed by cannulating the trachea of each mouse. Lungs from each experimental group were lavaged with a total of 20 ml in 5-ml aliquots of phosphate-buffered saline with 3 mM EDTA. The recovered fluid was pooled and centrifuged (200 × g for 10 min), and the cellular pellet was washed twice with phosphate-buffered saline. BAL fluid (BALF) samples were filtered and immediately frozen at −80°C after collection. Cell counts were performed directly with a hemocytometer. Cellular monolayers were prepared with a cytocentrifuge and stained with Wright-Giemsa stain. Cellular morphotype differentials were obtained by counting 200 cells/sample and expressing each type of cell as a percentage of the total number counted. The protein concentration in the BALF was measured with an automated analyzer (Hitachi 917).
Wet-to-dry weight ratio for the lungs.
The wet-to-dry weight ratio (W/D) for the lungs was determined by removing the lungs at the end of the experiment and recording the wet weight. The lungs were then placed in a 37°C incubator for 7 days, at which time the dry weight was recorded. The W/D weight ratio was then calculated for each pair of lungs.
Cytokine assays.
The levels of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-10, and cytokine-induced neutrophil chemoattractant 3 (CINC-3) were determined by the use of commercial immunoassay kits specific for rat cytokines (Quantikine rat TNF-α, IL-1β, IL-10, and CINC-3 kits; R&D Systems, Abingdon, Oxford, United Kingdom). Readings were performed with a Digiscan microplate reader (Spectracount Packard Instrument Company, Meriden, Conn.).
Levels were measured in the sera, lung parenchyma, and BALF of animals from each group at 2, 4, and 12 h post-instillation of bacteria.
Experimental groups.
Animals were randomly assigned to the following two groups, with a minimum sample size of five animals per group, for each series of experiments: the P. aeruginosa CHA strain instillation group (CHA) and the P. aeruginosa CHA-D1 strain instillation group (CHA-D1). Each animal received a 0.5-ml/kg intratracheal injection of the calibrated inoculum.
In a first series of experiments, we studied the natural evolution of the infections induced by the two strains for 72 h. With a different subgroup, the protein concentrations in BALF and blood cultures were evaluated at 24 h.
Animals were then studied at 2, 4, and 12 h for extravascular lung water measurements and assessments of the inflammatory response.
Statistical analysis.
The data were analyzed by use of the Kruskal-Wallis test and the Wilcoxon test where appropriate. P values of <0.05 were regarded as statistically significant. R software was used (The R Foundation for Statistical Computing, Vienna, Austria). A Holm correction was used for multiple comparisons (15).
RESULTS
A functional TTSS increases mortality rate at 72 h.
Instillation of the CHA strain, a clinical isolate which produces the type III effectors ExoS and ExoT but not ExoU, was associated with a 100% mortality rate at 72 h, with most of the animals dying within the first 36 h. In contrast, an isogenic mutant of CHA, CHA-D1, was associated with no mortality during the study period. Consistent with these results, an analysis of the protein concentration in BALF at 24 h showed a marked increase for the CHA-infected group (CHA group) compared to the CHA-D1-instilled animals (CHA-D1 group), reflecting the major acute lung injury in the first group. These results are summarized in Table 1.
TABLE 1.
Natural evolution of infection observed 72 h after CHA or CHA-D1 instillationa
| Strain | % Mortality at 72 h | Protein level in BALF (g/dl) | No. of positive blood cultures/total no. of cultures |
|---|---|---|---|
| CHA | 100b | 3 ± 0.2b | 2/5 |
| CHA-D1 | 0 | 0.2 ± 0.1 | 0/5 |
Protein levels in the BAL fluid and positive blood culture as were measured 24 h after instillation.
Significantly different from values for the CHA-D1 group (P < 0.05). Values are means ± standard deviations.
From these results, in accordance with the work of others (18), we predicted a major role of the TTSS in P. aeruginosa pneumonia-induced mortality.
A functional TTSS increases lung injury.
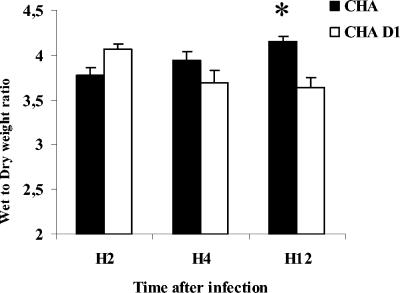
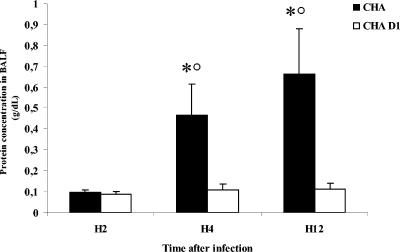
An analysis of the wet-to-dry weight ratio of the lungs showed a significant increase for the CHA group compared to the CHA-D1 group at 12 h (Fig. 1). Moreover, the amount of proteins recovered in BALF was significantly higher for the CHA group than for the CHA-D1 group at 4 and 12 h. Furthermore, the protein increase occurring from 2 to 4 h was larger than that occurring from 4 to 12 h, underscoring the rapid occurrence of injury in the CHA group (Fig. 2).
FIG. 1.
Wet-to-dry lung weight ratios (W/D) 2 hours (H2), 4 hours (H4), and 12 hours (H12) after Pseudomonas aeruginosa CHA and CHA-D1 intratracheal instillations. The data are means + standard errors (SE) (indicated by error bars). *, statistically different from the CHA-D1 group (P < 0.05).
FIG. 2.
Protein concentrations (g/dl) in bronchoalveolar lavage fluid (BALF) 2 hours (H2), 4 hours (H4), and 12 hours (H12) after Pseudomonas aeruginosa CHA and CHA-D1 intratracheal instillations. The data are means + SE (indicated by error bars). *, statistically different from the CHA-D1 group (P < 0.05); ○, statistically different from the previous time point with the same strain (CHA) (P < 0.05).
A functional TTSS increases in vitro cytotoxicity for A549 cells.
To further document the direct cytotoxicity of P. aeruginosa on the alveolar barrier, we tested the cytotoxicity of each strain in vitro on A549 cells. Cytotoxicity was evaluated by measuring the LDH release 4 and 6 h after infection. The amount of LDH release was statistically higher for CHA-infected cells than for CHA-D1-infected cells at 4 and 6 h. The values for CHA-D1 were similar to those for the minor cytotoxicity reference strain, PAO1 (28 and 26%, respectively, over a 6-h incubation period). The values for CHA were closer to those for the major cytotoxic reference strain, PA103 (85 and 100%, respectively, after 6 h of coculture) (Table 2).
TABLE 2.
Cytotoxicity of P. aeruginosa strains (CHA, CHA-D1, PAO1, and PA103) to lung epithelial cellsa
| Strain | % Cytotoxicity
|
|
|---|---|---|
| H4 | H6 | |
| CHA | 26 ± 1b | 85 ± 4b |
| CHA-D1 | 0 ± 0 | 28 ± 13 |
| PA103 | 66 ± 18c | 100 ± 0c |
| PAO1 | 0 ± 0 | 26 ± 3 |
Cytotoxicity to lung epithelial cells was evaluated by measuring the LDH release. A549 (2 × 104) cells were cocultured with each strain at a multiplicity of infection of 10. LDH release was measured at 4 and 6 h (H4 and H6) in triplicate. The values shown are means ± standard deviations.
Significantly different from values for the CHA-D1 group (P < 0.05).
Significantly different from values for the PAO1 group (P < 0.05).
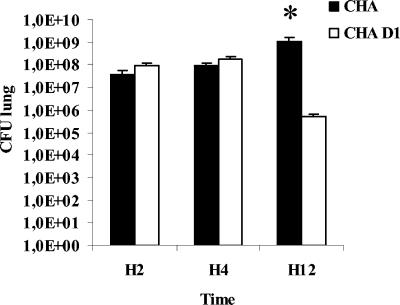
Bacterial loads in the lungs of TTSS-exposed animals do not decrease after 12 h.
We quantified the ability of animals to clear bacteria from their infected lungs. Two and 4 h after the instillation of bacteria, the bacterial loads in the lungs were not significantly different between the two groups (Fig. 3). After 12 h, animals infected with CHA showed a significantly higher bacterial burden in the lungs than that observed for the CHA-D1 group. This result implies that animals infected with the CHA strain could not clear the bacteria efficiently from their infected lungs 12 h after the infection.
FIG. 3.
Bacteriology. Rats were infected with 0.5 ml/kg of CHA or CHA-D1 at 109 CFU/ml. The number of viable bacteria remaining in the infected lungs was counted 2, 4, and 12 h after the instillation. Lung homogenates were sequentially diluted with sterile water and placed on bromocresol purple agar plates for 24 h at 37°C. n = 5 rats per group. The data are means + SE. *, statistically different from the CHA-D1 group (P < 0.05).
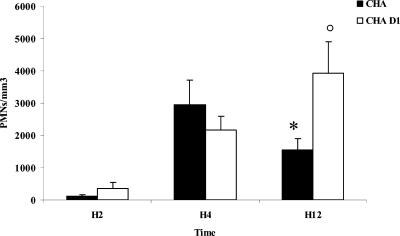
Alveolar polymorphonuclear neutrophils are significantly decreased in bronchoalveolar lavage fluid after 4 h in TTSS-exposed animals.
To explain the lack of bacterial clearance in the animals infected by the CHA strain, we studied the number of polymorphonuclear neutrophils in BALF. For the CHA-D1 group, there was a time-dependent increase in the number of polymorphonuclear neutrophils in BALF. In contrast, for the CHA group there was an initial increase in neutrophils at 4 h followed by a decline at 12 h. The number of cells was significantly lower for the CHA group than for the CHA-D1 group at 12 h (Fig. 4). Thus, the instillation of P. aeruginosa induced a neutrophil influx into the BALF. However, the number of neutrophils increased in the CHA-D1 group after 4 h. The lack of polymorphonuclear neutrophils in the BALF could explain why the CHA group could not clear the bacteria efficiently from their infected lungs 12 h after infection. Therefore, a functional TTSS-producing strain influences inflammatory cell recruitment or eliminates recruited leukocytes by its direct or indirect cytotoxicity.
FIG. 4.
Numbers of polymorphonuclear neutrophils/mm3 in bronchoalveolar lavage fluid (BALF) 2 hours (H2), 4 hours (H4), and 12 hours (H12) after Pseudomonas aeruginosa CHA and CHA-D1 intratracheal instillations. The data are means + SE (indicated by error bars). *, statistically different from the CHA group (P < 0.05); ○, significantly different from the H2 value for the same strain (CHA-D1) (P < 0.05).
A functional TTSS is associated with a major increase in the proinflammatory response in BALF and lung tissue.
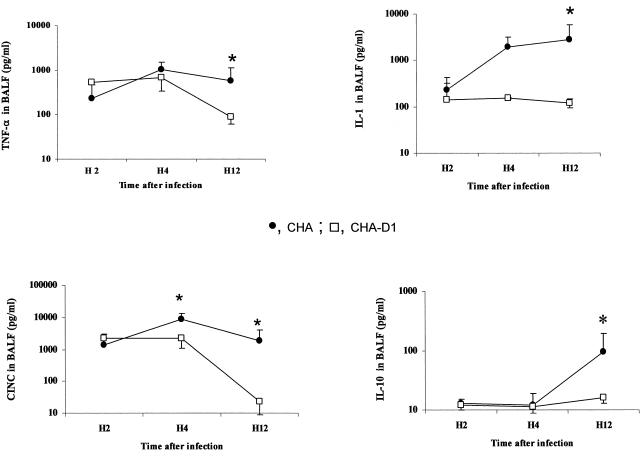
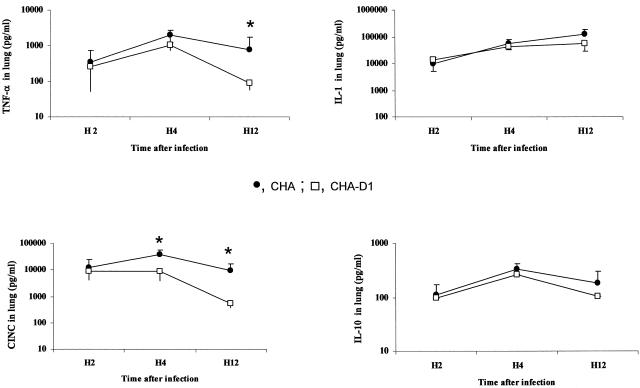
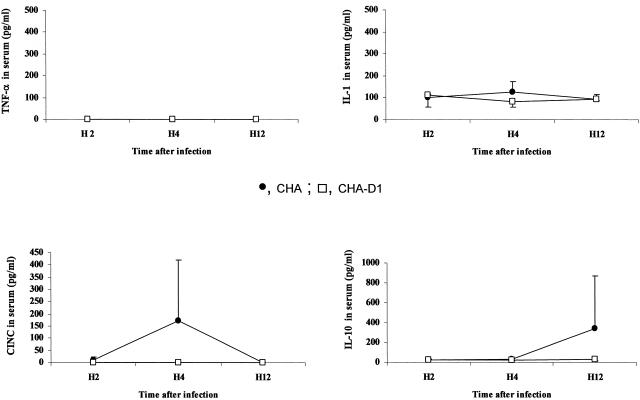
In BALF, a significant release of TNF-α and IL-1β was observed for rats receiving either strain of P. aeruginosa. This increase was significantly higher for the CHA group than for the CHA-D1 group (Fig. 5). In lung homogenates, only TNF-α production followed a similar increase over time, reaching significantly higher values for the CHA group at 4 and 12 h (Fig. 6). The levels in sera remained undetectable for TNF-α and low for IL-1β for both groups, suggesting the absence of decompartmentalization of the inflammatory response in our model (Fig. 7). The secretion of CINC-3 was also observed in both BALF and lung tissue after instillation of both strains of P. aeruginosa (Fig. 5 and 6). For both groups, CINC-3 levels reached their maxima at 4 h, and the increase was significantly higher for the CHA group at 4 h. This increase at 4 h was followed by a decrease at 12 h. Whereas the CINC-3 levels remained high in the CHA group at 12 h, a marked decrease was observed for the CHA-D1 group, and the difference at 12 h was statistically significant between the two groups both in the BALF and in the lung parenchyma. In sera, a transient secretion of CINC-3 was detected at 4 h in rats exposed to CHA (Fig. 7).
FIG. 5.
Concentrations of TNF-α, IL-1, IL-10, and CINC-3 (pg/ml) in bronchoalveolar lavage fluid (BALF) 2 hours (H2), 4 hours (H4), and 12 hours (H12) after Pseudomonas aeruginosa CHA and CHA-D1 intratracheal instillations. The data are means ± SE (indicated by error bars). *, statistically different from the CHA-D1 group (P < 0.05).
FIG. 6.
TNF-α, IL-1, IL-10, and CINC-3 levels (pg/ml) in lung homogenates 2 hours (H2), 4 hours (H4), and 12 hours (H12) after Pseudomonas aeruginosa CHA and CHA-D1 intratracheal instillations. The data are means ± SE (indicated by error bars). *, statistically different from the CHA-D1 group (P < 0.05).
FIG. 7.
Concentrations of TNF-α, IL-1, IL-10, and CINC-3 (pg/ml) in sera 2 hours (H2), 4 hours (H4), and 12 hours (H12) after Pseudomonas aeruginosa CHA and CHA-D1 intratracheal instillations. The data are means ± SE (indicated by error bars). *, statistically different from the CHA-D1 group (P < 0.05).
The anti-inflammatory response, as evaluated by the production of interleukin-10 (IL-10), followed an interesting pattern. The response was delayed, increasing in BALF at 12 h for the CHA group but not for the CHA-D1 group (Fig. 5). Unlike the proinflammatory cytokine levels, IL-10 levels increased in the systemic compartment at 12 h in the CHA group but not in the CHA-D1 group (Fig. 7). However, the differences did not reach statistical significance for any of these compartments.
These data show that a functional TTSS in P. aeruginosa is responsible for a larger and prolonged release of inflammatory cytokines in the lung.
DISCUSSION
This study was designed to determine the contribution of a functional type III secretion system (TTSS), independent of ExoU production, to the pathogenesis of acute lung injury.
Using a model of experimental murine pneumonia, we compared the effects of the following two strains of P. aeruginosa: CHA, which produces the type III effectors ExoS and ExoT but not ExoU, and its isogenic mutant, CHA-D1, in which the exsA gene, which encodes a transcriptional activator of TTSS, has been inactivated. Our results demonstrate that, independently of ExoU, there exists a relationship between the TTSS and an increased pathogenicity of P. aeruginosa.
TTSS and mortality.
The most robust evidence for an ExoU-independent role of the TTSS in P. aeruginosa pathogenicity is that the mortality rate at 72 h was 100% for the group infected by the strain expressing the TTSS but not ExoU, whereas the mortality rate was 0% for the group infected by the non-TTSS-expressing strain. This increase in mortality due to the TTSS has been observed in animal models of P. aeruginosa acute lung infections (10) and in clinical studies of lower respiratory tract infections and ventilator-acquired pneumonia (13, 29). However, few studies have differentiated such a high mortality rate imputable to the TTSS independent of ExoU. Our observation is supported in part by the fact that Shaver and Hauser found a significant mortality rate linked to a mutant strain expressing only ExoS by using a similar model (35). Our larger difference in mortality rates may be explained by the fact that we compared a TTSS-expressing (producing all TTSS effectors except ExoU) strain instead of a strain solely expressing ExoS.
TTSS and lung injury.
Further evidence of the role of TTSS in P. aeruginosa pathogenicity is derived from our finding of an ExoU-independent/TTSS-mediated increase in lung injury, as assessed by functional markers of alveolar-capillary barrier damage such as the alveolar protein concentration and the amount of extravascular lung water. This increase in lung injury is consistent with the pioneer study of Wiener-Kronish et al., who showed that the TTSS, through ExoS production, could injure the alveolar-capillary barrier (41). Conversely, our TTSS-deficient/exsA mutant strain led to less injury, which is consistent with the findings of Kudoh et al., who showed that an exsA mutant strain resulted in significantly less lung injury than its parental PAK strain at 8 h postinfection (17). Interestingly, exotoxin A had a minor role, but the authors raised the hypothesis that other proteins may be involved in lung injury pathophysiology. In fact, a genetic analysis identified two other exoproducts, ExoT and ExoU, which are also secreted through the TTSS (12, 43). Many studies since have highlighted the prominent role of the TTSS in P. aeruginosa-induced lung injury; however, research concerning TTSS regulation is still ongoing. Recently, cyclic AMP was shown to be involved in pathogenicity. Smith et al. observed a decreased virulence of PAK strains with a cyclic AMP deletion, with lower bacterial loads of the mutants in the lungs than those of the parental strain (36).
None of these studies provided a dynamic analysis of P. aeruginosa pathogenicity in the lungs over time. In our study, the exsA mutant strain showed results consistent with those of previous studies with a clinical strain which did not produce ExoU. We emphasized the critical roles of ExoS and -T in lung injury and described the inflammatory response in the alveolar as well as the systemic compartments in a dynamic analysis over 12 h.
The analysis of functional variables reflecting alveolar-capillary barrier damage, such as the alveolar protein concentration and the amount of extravascular lung water, confirmed the hypothesis of ExoU-independent pathogenicity. In CHA-infected animals, there was a progressive increase from 2 to 12 h in the amount of proteins recovered from BALF, showing an increase in lung alveolar permeability. After CHA-D1 instillation, the protein levels remained significantly lower throughout the experiment.
The wet-to-dry weight ratio of the lungs initially increased for both groups. This period was followed by a trend toward a decrease for the CHA-D1 group. This trend could be related to a resolution of the injury, with a progressive elimination of the water excess through a noninjured alveolar-capillary membrane. In contrast, in CHA-infected animals from 4 to 12 h, there was a progressive increase in the wet-to-dry weight ratio of the lungs. Such an accumulation of water in the lungs may have been related to a decrease in the liquid clearance from the lungs, as we have previously shown (19, 39). This is consistent with data published by other authors, who also described an increase in permeability or extravascular lung water (17, 27, 28, 41). From these data, we conclude that lung injury is dependent on a functional TTSS, even in the absence of ExoU production, since the only difference between the two strains was the inactivation of the exsA gene in CHA-D1.
TTSS effect on alveolar epithelial cells.
Further evidence of the pathogenicity of the TTSS, independent of ExoU, was obtained by the difference in the cytotoxicities of the strains (CHA and CHA-D1) tested in vitro on A549 cells and compared to laboratory strains with known different cytotoxicities, namely, PAO1 and PA103. A control group with PA103 showed massive destruction at 4 and 6 h, and this wild-type cytotoxic strain produces ExoU. In contrast, with PAO1, a well-characterized noncytotoxic strain which possesses the TTSS (5), the release of LDH was significantly lower. We observed a major release of LDH (26 and 85% at 4 and 6 h, respectively) with the CHA strain which was statistically higher than the values observed with CHA-D1-exposed cells (0 and 28% at 4 and 6 h, respectively). CHA has a functional TTSS which can be activated by calcium depletion or eukaryotic cell contact (5). The values obtained with CHA-D1 are comparable to the results with PAO1. These results suggest that a functional type III secretion system producing ExoS and ExoT is highly involved in the injury observed in our model, with destruction of the epithelium in the CHA group associated with both increased lung injury and increased mortality compared to the CHA-D1 group. This result can further explain the improvement of fluid transport in the CHA-D1 group. Although we did not explore CHA-related A549 cytotoxic mechanisms, Pederson et al. used a different cell line to show that the ExoS carboxyl-terminal domain has a FAS-dependent ADP-ribosyltransferase activity which is cytotoxic to Chinese hamster ovary cells (23, 24).
TTSS and polymorphonuclear neutrophils.
We demonstrated that animals infected with a functional TTSS-producing strain could not clear bacteria efficiently from their infected lungs 12 h after instillation. Because bacterial clearance from the lungs is mainly the result of macrophage and polymorphonuclear neutrophil functions, bacterial clearance can only be interpreted in conjunction with polymorphonuclear neutrophil recruitment. Therefore, to assess polymorphonuclear neutrophil recruitment, we studied the number of polymorphonuclear neutrophils (PMN) in BALF. We observed an initial rapid increase in PMN counts in BALF which was common to both the CHA and CHA-D1 groups 4 h after infection. This initially observed PMN recruitment is in accordance with the work of Kooguchi et al., who observed a rapid increase in the amount of polymorphonuclear neutrophils recovered from the BALF after a P. aeruginosa challenge. Reported PMN recruitment during P. aeruginosa lung injury starts at 4 h postinfection, reaching a peak at 8 (22), 16 (16), or 24 h (4). Likewise, in our study, for the CHA-D1 group the PMN count continued to increase at 12 h. However, for the CHA group, PMN recruitment was halved at 12 h. Furthermore, the amount of bacterial clearance was parallel to the amount of neutrophil recruitment; when the CHA-D1 group partially lost its neutrophil recruitment ability at 12 h, it also lost its bacterial clearance ability and the bacterial load increased markedly compared to that of the CHA group. Therefore, a loss of PMN recruitment due to the TTSS, independent of ExoU, is probably responsible for the lack of bacterial clearance. Such an association between the TTSS and a major drop in the number of polymorphonuclear neutrophils has already been reported for mice (10). Sixteen hours after the intratracheal instillation of PA103, significantly fewer leukocytes were detected in BALF than the number observed with the isogenic mutant PA103ΔUT, which was missing both the ExoT and ExoU toxins.
Two hypotheses may explain why neutrophil recruitment was transitory in the TTSS-expressing CHA group. The TTSS may either suppress inflammatory chemotactic factors or have a direct cytotoxic effect, eliminating recruited neutrophils. To explore the first hypothesis, we measured CINC-3, a chemotactic factor known to be increased in BALF, lung homogenates, and serum during lipopolysaccharide-induced acute lung injury (44). The CINC-3 level in BALF was significantly higher for the CHA group, thus invalidating an antichemotactic effect of the TTSS (Fig. 5). Although we did not experimentally explore the second hypothesis, P. aeruginosa TTSS-dependent neutrophil cytotoxicity has been previously reported. Indeed, Dacheux et al. demonstrated in vitro that the cell death of human polymorphonuclear neutrophils requires a functional TTSS (5, 7, 8). Furthermore, using the same strains as those used for our study, Dacheux et al. showed that 70% of polymorphonuclear neutrophil cell lysis occurred after 3 h of coincubation with the CHA strain, in contrast to only 20% with CHA-D1. Although the complete mechanism by which polymorphonuclear neutrophils are killed by the CHA strain is unknown, CHA-induced macrophage death results from a pore-forming activity of TTSS-secreted proteins (8). We observed in vivo a similar major decrease in polymorphonuclear neutrophils in the BALF from the CHA group which was probably related to TTSS-induced neutrophil cytotoxicity independent of ExoU.
TTSS and cytokines.
There is a strong body of evidence to support the hypothesis that the lung inflammatory response is an indirect cause of alveolar injury. The recruitment of polymorphonuclear neutrophils into the alveolar compartment associated with activated macrophages leads to lung injury via the production of oxidant products such as superoxide anions and nitric oxide and via the release of proteinases (e.g., elastase and collagenase) (9, 40). Stimulated pulmonary macrophages also appear to be a potent source of proinflammatory cytokines, including TNF-α and IL-1β (37). In our study, the release of TNF-α and IL-1β was increased in the CHA group compared to the CHA-D1 group. These data suggest that the proinflammatory reaction was rapidly controlled by the CHA-D1 group, which was not the case for the CHA group. This is at least partly related to the lack of bacterial clearance in the CHA group (Fig. 3). We demonstrated that animals infected with a functional TTSS strain could not clear bacteria efficiently from their infected lungs 12 h after the instillation. This difference between the two strains in bacterial loads in the lungs indicates that the decreased cytokine levels observed with CHA-D1 could be related to the clearance of bacteria from the lungs and the resolution of infection.
An increase in TNF-α in BALF was also observed by Kurahashi et al., but in their study they showed an increase in TNF-α at 7 h in rabbits which was more important for a strain with a TTSS deletion than for the potent parental strain (18). These contradicting results could be related to the model or the strain used in their study.
The anti-inflammatory response was delayed and appeared only at 12 h with the CHA strain in both BALF and serum. We did not observe any production of IL-10 after the CHA-D1 challenge. This result is consistent with the literature; the instillation of the cytotoxic strain PA103 into mice led to IL-10 up-regulation in the lung parenchyma and to increased concentrations of IL-10 in the blood (34). In contrast, the noncytotoxic strain, CHA-D1, did not lead to any increase in IL-10 in the lung parenchyma or in the blood. Therefore, IL-10 production appears to be strictly controlled in the lungs since the acute inflammatory reaction observed with CHA-D1 was not associated with its secretion.
Conclusions.
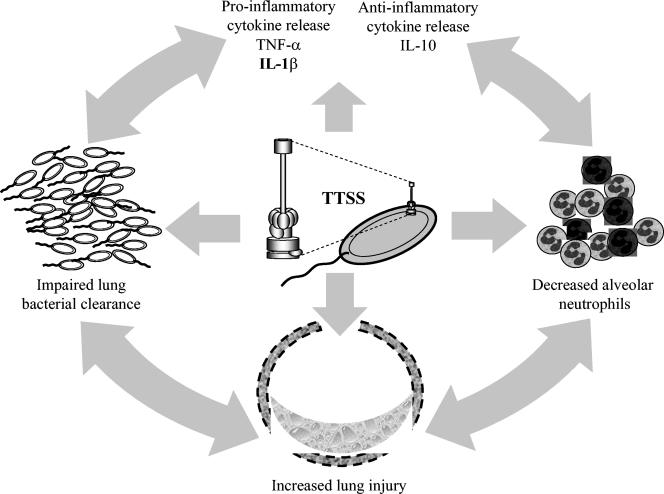
In summary, using an experimental murine model of P. aeruginosa-induced acute lung injury, we demonstrated a relationship between the type III secretion system and pathogenicity which was independent of ExoU. We demonstrated that the TTSS, independent of ExoU production, is associated with increased lung injury, an increased proinflammatory response, decreased polymorphonuclear neutrophil recruitment, and decreased bacterial clearance, ultimately resulting in an increased mortality rate at 72 h (Fig. 8). Such a major role of non-ExoU TTSS factors in P. aeruginosa pathogenicity should be taken into account for future antipseudomonal therapies. Our results also suggest that neutrophil recruitment and the resulting bacterial clearance are the keys to ExoU-independent TTSS pathogenicity.
FIG. 8.
Schematic involvement of the TTSS in a self-propagating pathogenic cycle. The TTSS is associated with acute lung injury through different mechanisms, including an enhancement of the proinflammatory response and a decrease in the number of alveolar neutrophils, both of which lead to impaired bacterial clearance from the lungs and a higher mortality rate. The darkly stained neutrophils may have been killed through a TTSS-dependent mechanism.
Editor: J. B. Bliska
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brun-Buisson, C., F. Doyon, J. Carlet, P. Dellamonica, F. Gouin, A. Lepoutre, J. C. Mercier, G. Offenstadt, and B. Regnier. 1995. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 274:968-974. [PubMed] [Google Scholar]
- 3.Chastre, J., and J. Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 4.Cheung, D. O., K. Halsey, and D. P. Speert. 2000. Role of Pulmonary Alveolar Macrophages in Defense of the Lung against Pseudomonas aeruginosa. Infect. Immun. 68:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dacheux, D., I. Attree, C. Schneider, and B. Toussaint. 1999. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect. Immun. 67:6164-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacheux, D., B. Toussaint, M. Richard, G. Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils [In Process Citation]. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacheux, D., J. Goure, J. Chabert, Y. Usson, and I. Attree. 2001. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 40:76-85. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, M. M., M. Dunne, and D. W. Kanys. 1990. Polymorphonuclear leukocyte and P. aeruginosa-induced damage to a human pulmonary epithelial cell line. J Infect. Dis. 162:172-177. [DOI] [PubMed] [Google Scholar]
- 10.Faure, K., T. Sawa, T. Ajayi, J. Fujimoto, K. Moriyama, N. Shime, and J. P. Wiener-Kronish. 2004. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir. Res. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 12.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 13.Hauser, A. R., E. Cobb, M. Bodi, D. Mariscal, J. Valles, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 14.Heyland, D. K., D. J. Cook, L. Griffith, S. P. Keenan, and C. Brun-Buisson. 1999. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am. J. Respir. Crit Care Med. 159:1249-1256. [DOI] [PubMed] [Google Scholar]
- 15.Ihaka, R., and R. R. Gentleman. 1996. A language for data analysis and graphics. J. of Comput and Graph. 5:299-314. [Google Scholar]
- 16.Kooguchi, K., S. Hashimoto, A. Kobayashi, Y. Kitamura, I. Kudoh, J. P. Wiener-Kronish, and T. Sawa. 1998. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect. Immun. 66:3164-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudoh, I., J. P. Wiener-Kronish, S. Hashimoto, J. Pittet, and D. Frank. 1994. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am. J. Physiol. 267:L551-L556. [DOI] [PubMed] [Google Scholar]
- 18.Kurahashi, K., O. Kajikawa, T. Sawa, M. Ohara, M. A. Gropper, D. W. Frank, T. R. Martin, and J. P. Wiener-Kronish. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Invest. 104:743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Berre, R., K. Faure, H. Fauvel, N. B. Viget, F. Ader, T. Prangere, A. M. Thomas, X. Leroy, J. F. Pittet, P. Marchetti, and B. P. Guery. 2004. Apoptosis inhibition in P. aeruginosa-induced lung injury influences lung fluid balance. Intensive Care Med. 30:1204-1211. [DOI] [PubMed] [Google Scholar]
- 20.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 21.McElroy, M. C., J. Pittet, S. Hashimoto, L. Allen, J. P. Wiener-Kronish, and L. G. Dobbs. 1995. A type I cell-specific protein is a biochemical marker of epithelial injury in a rat model of pneumonia. Am. J. Physiol. 268:L181-L186. [DOI] [PubMed] [Google Scholar]
- 22.Morissette, C., C. Francoeur, C. Darmond-Zwaig, and F. Gervais. 1996. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect. Immun. 64:4984-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pederson, K. J., R. Krall, M. J. Riese, and J. T. Barbieri. 2002. Intracellular localization modulates targeting of ExoS, a type III cytotoxin, to eukaryotic signalling proteins. Mol. Microbiol. 46:1381-1390. [DOI] [PubMed] [Google Scholar]
- 24.Pederson, K. J., A. J. Vallis, K. Aktories, D. W. Frank, and J. T. Barbieri. 1999. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol. Microbiol. 32:393-401. [DOI] [PubMed] [Google Scholar]
- 25.Pennington, J. E., and M. G. Ehrie. 1978. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J. Infect. Dis. 137:764-774. [DOI] [PubMed] [Google Scholar]
- 26.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 27.Pittet, J. F., M. A. Matthay, G. Pier, M. Grady, and J. P. Wiener-Kronish. 1993. Pseudomonas aeruginosa-induced lung and pleural injury in sheep. Differential protective effect of circulating versus alveolar immunoglobulin G antibody. J. Clinical Investigation. 92:1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaiguia, S., C. Garat, C. Delclaux, J. Fleury, P. Legrand, M. A. Matthay, and C. Jayr. 1997. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J. Clinical Investigation. 99:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 30.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53:1279-1290. [DOI] [PubMed] [Google Scholar]
- 31.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawa, T., M. Ohara, K. Kurahashi, S. S. Twining, D. Frank, D. B. Doroques, T. Long, M. Gropper, and J. P. Wiener-Kronish. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 34.Sawa, T., D. B. Corry, M. A. Gropper, M. Ohara, K. Kurahashi, and J. P. Wiener-Kronish. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 159:2858-2866. [PubMed] [Google Scholar]
- 35.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, R. S., M. C. Wolfgang, and S. Lory. 2004. An adenylate cyclase-controlled signaling network regulates Pseudomonas aeruginosa virulence in a mouse model of acute pneumonia. Infect. Immun. 72:1677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonoda, F., K. Oishi, A. Iwagaki, and K. Matsumoto. 1997. Endogenous tumor necrosis factor α mediates neutrophil accumulation at the mid-phase of a murine model of Pseudomonas aeruginosa pneumonia. Microbiol Immunol. 41:601-608. [DOI] [PubMed] [Google Scholar]
- 38.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 39.Viget, N., B. Guery, F. Ader, R. Nevière, S. Alfandari, C. Creusy, M. Roussel-Delvallez, C. Foucher, C. M. Mason, G. Beaucaire, and J. F. Pittet. 2000. Keratinocyte Growth Factor protects against Pseudomonas aeruginosa-induced lung injury. Am. J. Physiol Lung Cell Mol. Physiol. 279:L1199-L1209. [DOI] [PubMed] [Google Scholar]
- 40.Weiss, S. J. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320:365-376. [DOI] [PubMed] [Google Scholar]
- 41.Wiener-Kronish, J. P., T. Sakuma, I. Kudoh, J. Pittet, D. Frank, L. Dobbs, M. L. Vasil, and M. A. Matthay. 1993. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J. Applied Physiology. 75:1661-1669. [DOI] [PubMed] [Google Scholar]
- 42.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasawa, H., Y. Ishii, and S. Kitamura. 1999. Cytokine-induced neutrophil chemoattractant in a rat model of lipopolysaccharide-induced acute lung injury. Inflammation 23:263-274. [DOI] [PubMed] [Google Scholar]