Abstract
Dopamine (DA) plays important roles in various behaviors, including learning and motivation. Recently, THOC5 was identified as an important regulator in the development of dopaminergic neurons. However, how THOC5 is regulated has not been explored. In this study, we found an interaction between THOC5 and necdin, which is encoded by a gene located in the chromosome deletion region of Prader-Willi syndrome (PWS), by using a yeast two-hybrid assay. Necdin affects the mRNA export function of THOC5 by regulating its nucleocytoplasmic localization. As a result, the expression of a few DA neuronal development-related genes, such as Mef2c, Lef1 and Prkcg, is altered in necdin-deficient mice. We also found neurodegeneration of dopaminergic neurons and an increase of glial cells in necdin-deficient mice, which may underlie the dyspraxia behaviors in these mice. Our results thus identified necdin as a novel regulator for THOC5, which may underlie, at least partly, the abnormal DA neuron development in necdin-deficient mice.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76981-y.
Subject terms: Developmental biology, Molecular biology, Neuroscience, Neurodegeneration
Introduction
Dopamine is a catecholamine neurotransmitter that plays important roles in neuromodulation, such as motor control, motivation, reward, cognitive function, maternal behavior, and reproductive behavior1. Dopaminergic signaling pathways are essential for the maintenance of physiological processes, and deficiency in dopamine contributes to the typic symptoms in neurodegenerative diseases such as Parkinson disease2.
In mammals, the dopaminergic neurons are mainly located in the ventral tegmental area (VTA) and substantia nigra (SN) of the midbrain. Dopaminergic neurons project from the VTA to the prefrontal cortex through the mesolimbic pathway and to the nucleus accumbens through the mesocortical pathway, which together form mesocorticolimbic system and play a role in reward and motivation3,4. In addition, the dopamine neurons projecting to the striatum in the substantia nigra form the nigra-striatal pathway, which controls motor function and learning ability5. SN, together with VTA, plays a key role in regulating motor response through the basal ganglia. The degeneration of dopaminergic neurons in SNC and VTA lead to a variety of motor and non-motor behavioral disorders, such as Parkinson’s disease6.
THOC5 protein7 is a member of the THO nuclear export complex (THOC)8, which is a conserved RNA binding complex involved in the transportation of mRNA out of the nuclei9. In c. elegans mutants with defective THOC, synaptic messenger RNAs are sequestered in the nucleus, causing a marked decrease in synaptic protein expression, almost complete loss of synapses, and compromised dopamine function10. Deletion of Thoc5in mouse dopaminergic neurons induces severe impairments in synaptic maintenance, leading to subsequent neuronal death in the substantia nigra compacta10. These cellular anomalies result in disrupted dopamine release, ataxia, and eventual death of the animals. Nevertheless, how THOC5 is regulated is still elusive.
In this study, we identified THOC5 as one of the interactive proteins for Necdin, a Prader Willi syndrome protein. We further demonstrated that necdin regulates the nucleocytoplasmic distribution of THOC5. Necdin-deficiency leads to retain of THOC5 in the cytoplasm, impairing the export of mRNA into the cytoplasm, which may partly underlie the degeneration of dopaminergic neurons.
Methods
Animals
Necdin mice were generated by using CRISPR-Cas9 technology. Cas9 mRNA and two guide RNAs (gRNA) targeting the upstream and downstream regions of the mouse necdin gene were injected into C57BL/6 mouse oocytes, and a mouse with deletion of the entire necdin gene was used as a founder. Before behavioral tests, mice of the same sex were group-housed (3–5 animals per cage) under controlled conditions [temperature, 20 ± 2◦C; relative humidity, 50– 60%; 12:12-h light–dark (LD) cycle, lights on at 7:00 AM and lights off at 7:00 PM] and had free access to food and water. All procedures regarding the care and use of animals were approved by the Institutional Animal Care and Use Committee of Central South University of China. All experiments were carried out according to the relevant guidelines and regulations. This study is reported in accordance with the ARRIVE guidelines.
Plasmids and siRNAs
pcDNA3.1-Myc-THOC5 and pcDNA3.1-FLAG-NDN vectors were constructed by insertion of the coding region of the indicated mouse Thoc5 and necdin into pcDNA3.1-Myc and pcDNA3.1-FLAG. The siRNA sequence of Negative Control was UUCUCCGAACGUGUCACGUTT. The sense strand sequences of siRNA were CCUGC-ACACCAUGGAGUUUTT(#1), UCAUGAUCCUGAGCCUCAUTT(#2), and GGA-AGAAGCACUCCACCUUTT(#3) for necdin.
Culture of KO mouse embryonic fibroblast cells
Mouse embryonic fibroblast (MEF) cells derived from female WT mice crossed with male KO mice. The female mouse was transferred to a new cage, and the presence of a vaginal plug is considered embryonic day 0.5 euthanized the pregnant female mouse while the embryos developed for 13.5–14.5 days and transferred each embryo carefully to a fresh dish. Then, we dissected the embryos and removed their head, hearts, and limbs, using the head for genotyping. The remaining tissues were minced into small fragments and digested with 0.05% trypsin-EDTA at 37℃ for 15 min. The suspension was transferred to a 15 ml conical tube and terminated digestion with an equal volume medium. The cells were spun down at 1000 × g for 3 min. Cell pellets from the tube were resuspended in a complete culture medium. Allow cells to adhere and grow in a 37 °C incubator with 5% CO2.
Cell culture and transfection
SH-SY5Y and HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin at 37◦C in 5% CO2 incubators. According to the manufacturer’s protocol, Plasmid and siRNA transfections were performed with Lipofectamine 2000 (Invitrogen) reagents.
Co-immunoprecipitation
Cells were homogenized in ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.5, 0.3% Triton X-100, 100 mM NaCl, five mM EDTA, 10% glycerol and protease inhibitor cocktail, and then the cell extracts were incubated with antibody overnight at 4◦C. 30 µl protein G agarose bead slurry (Sigma; P3296) was added to pull down the immunocomplexes. The beads were collected by centrifugation, washed extensively with lysis buffer 4–5 times, mixed with SDS loading buffer, and subjected to the standard SDS–polyacrylamide gel electrophoresis and Western blotting procedures.
Yeast two-hybrid screening assay
Necdin cDNA was cloned into a pGBKT7 plasmid and used as bait to screen a mouse embryo 11-day library (Clontech/Takara). Interacting clones were grown on a selection medium lacking the amino acids Leu and Trp and containing Aureobasidin A and X-α-Gal. Auxotrophic marker genes and the β-galactosidase assay were used to screen for positive clones. Plasmids of positive clones were isolated, amplified, and sequenced. Interactions were further confirmed by retransforming the identified plasmids together with the bait. The identified DNA sequences were further characterized by BLAST analysis of the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the identity of potential necdin-interacting proteins.
Immunofluorescence staining
For immunofluorescent microscopy, cells, or brain slices (The mice were deeply anesthetized by sodium pentobarbital, 50 mg/kg i.p., and perfused intracardially with 4% paraformaldehyde) were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100 in PBS, and then incubated with primary antibodies to necdin (diluted 1:100, Abcam, ab227908), THOC5 (diluted 1:100, Abcam, ab86070), GFAP (diluted 1:500, Millipore, MAB360), IBA1 (diluted 1:500, Wako, 019-19741) or TH (diluted 1:500, Millipore, AB152) overnight. The slices/cells were then incubated in the dark with Alexa-conjugated labeled secondary antibodies. Nuclei were stained with DAPI. Images were captured using a confocal microscope (Zeiss LSM880 Airyscan), and immunostaining density was quantified using fluorescence image analysis software.
Western blotting
Cells or tissue samples were lysed in 2× SDS lysis buffer (2% SDS, 63 mM Tris–HCl, and 10% glycerol). SDS-PAGE separated proteins in lysates, transferred to nitrocellulose membranes (PVDF), and immunoblotted with the corresponding antibodies overnight at 4◦C. Membranes were then washed and incubated with horseradish peroxidase conjugate secondary antibodies. The proteins were visualized using the Pierce™ ECL Western Blotting Substrate kit (Thermo Scientific; 32106). ImageJ quantified band intensities.
Subcellular fractionation
The subcellular fractionation assay was isolated from cells using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, P0028). Cells grown in 10 cm dishes were digested with 200 µl Cytoplasmic protein extraction reagents A (with Protease or RNase inhibitors) on ice for 10–15 min, after vortexing for 5s. Then, the suspension was mixed with 10 µl reagents B on ice for 1 min, after vortexed for 5s. In a moment, the resuspension was centrifuged at 12000rcf for 5 min at 4 °C after vortexed for 5s. The supernatant, which was the cytoplasmic fraction, was carefully removed without disturbing the pellet. The remaining supernatant was carefully removed, and the pellet was washed with 1×PBS. The sample was centrifuged at 12000rcf for 5 min at 4 °C, and the entire supernatant was discarded. The pellet constituted the nuclear fraction.
RNA extraction and analysis
According to the manufacturer’s instructions, RNA was extracted using the TRIZOL method (Invitrogen, CA). In brief, for every 150 µl of cytoplasmic fraction or each nuclear pellet, 350 µl (or 500 µl) of TRIZOL reagent was added, followed by vortexing. One micrograms of total RNA were reversed transcribed using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific; K1622), and 10 ng of total cDNA equivalents were then analyzed using Fast SYBR™ Green Master Mix (Thermo Scientific; 4385612) according to the manufacturer’s instructions using a C1000 touch Thermal Cycler.
Open field test
The open-field experimental device is a plexiglass box with an open top. The specific parameters are 72 cm×72 cm×40 cm. The bottom of the open field is divided into 16 square grids on average. The four inner squares are called the central area. Male mice at the age of 3 or 13 months were removed from the breeding cage and placed gently in the central area of the open-field device. At the same time, the video tracking software was turned on for 10 min of video recording to record the total distance of the experimental mice and the time and distance they walked in the central area.
Rotarod test
Male mice at the age of 3 or 13 months were placed on a rotating rod with rotation speed gradually increasing from 5 to 20 RPM using a programmable acceleration paradigm (Harvard Apparatus; 76–0770). Before the start of the formal experiment, the mice were trained for one week, and the speed was increased from 5 to 20 rpm during the training until the mice could maintain the movement at the 20-rpm speed. The time mice fell from the rotating rod was measured, with 200 s being the maximal time measured on the rod. The rotarod test was performed on three consecutive days for each testing session.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.01. All experiments were repeated at least three times, and the distribution of data points is presented as mean ± SEM. Student’s t-test for comparison of two conditions or ANOVAs was utilized with post hoc Bonferroni multiple comparisons test for three or more conditions. P < 0.05 was considered significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Results
Necdin interacts with THOC5
In a previous study, we performed a yeast two hybrid screening by using necdin as a bait11. As a result, we identified THOC5 as a potential necdin-interactive protein. As THOC5 plays an important role in the RNA export and development of dopaminergic neuron, we further confirmed the necdin-THOC5 interaction with a series of biochemical approaches.
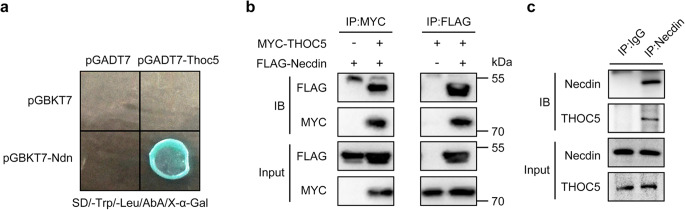
First, we confirmed the necdin-THOC5 interaction with a yeast two hybrid assay. As shown in Fig. 1a, only the yeast colonies co-transformed with the pGBKT-necdin and pGADT7-Thoc5 plasmids grew blue on the high-stringent plate (SD/-Trp/-Leu/AbA/X-β-Gal). No colonies grew when transforming yeast with pGBKT7-necdin and pGADT7 or pGBKT7 and pGADT7-Thoc5.
Figure 1.
Necdin interacts with THOC5. (a) Interaction of necdin and THOC5 as assayed with a yeast two-hybrid assay. pGBKT7-Ndn denotes necdin fused to a DNA-binding domain, whereas pGADT7-Thoc5 denotes THOC5 fused to an activation domain. Only yeast colonies co-transformed with pGADT7-Thoc5 and pGBKT7-Ndn can grow and turn blue on an SD/-Leu/-Trp/AbA/X-α-Gal plate. (b) Interaction of necdin and THOC5 as assayed by a co-immunoprecipitation (co-IP) assay in HEK293T cells expressing FLAG-necdin and MYC-THOC5. Antibodies against MYC or FLAG were used to immunoprecipitate (IP) the cell lysate, and the immunocomplex was blotted with antibodies against MYC or FLAG, respectively. (c) Interaction of necdin and THOC5 as assayed by a co-IP assay in SH-SY5Y cells. The cell lysate was immunoprecipitated with an antibody against necdin or control IgG, and the immune complex was blotted with antibodies against necdin or THOC5, respectively. Equal amounts of protein from whole cell lysates were used in immunoblotting for the indicated proteins.
We then investigated the necdin-Thoc5 interaction with coimmunoprecipitation (co-IP). Only when MYC-tagged THOC5 and FLAG-tagged necdin were co-expressed in HEK293T cells, the FLAG antibody was able to pulldown MYC -tagged THOC5 and the MYC antibody was able to pulldown FLAG-tagged necdin (Fig. 1b). We also performed co-IP in SH-SY5Y cells with an antibody against necdin or control IgG. As shown in Fig. 1c, THOC5 was detected in the immunocomplex pulled down by necdin antibody, but not by control IgG, indicating that necdin interacts with THOC5 endogenously.
Depletion of necdin alters the nucleocytoplasmic distribution of THOC5
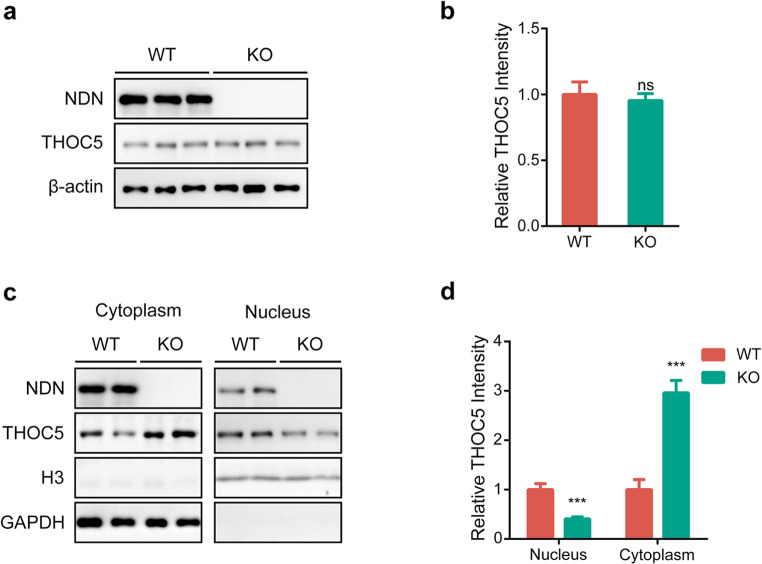
It has been reported that necdin is able to regulate the stability of its interactive proteins, we therefore sought to investigate the effect of overexpression and depletion of necdin on the level of THOC5 protein. As shown in Figure S1a-d, overexpression, or RNA-interference of necdin have no effect on the protein level of endogenous THOC5 protein in SH-SY5Y cells. Furthermore, we did not see significant changes of THOC5 protein levels in the mouse embryonic fibroblasts (MEFs) from wild-type (WT) or necdin-deficient (KO) mice (Fig. 2a-b). Collectively, our results indicated that necdin has no effect on the stability of THOC5 protein.
Figure 2.
Necdin regulates the nucleocytoplasmic distribution of THOC5. (a, b) Representative immunoblots (a) and statistics data of three independent experiments (b) from WT and necdin KO MEF cells. (c, d) The nuclear form of THOC5 was significantly reduced in the MEF from necdin KO mice, while the cytoplasm form of THOC5 was significantly increased. Data were mean ± SEM, n = 5, *p < 0.05, **p < 0.01, unpaired t-test. Equal amounts of protein from whole cell lysates were used in immunoblotting for the indicated proteins.
Considering that THOC5 is a nucleocytoplasmic shuttle protein, we sought to investigate if necdin affects the subcellular distribution of THOC5. To this end, we isolated and purified the nucleus and cytoplasm of primary MEF cells and detected their protein and mRNA levels (Figure S1e), respectively. As shown in Fig. 2c-d, the expression of THOC5 in the nucleus was significantly increased in necdin-deficient MEF cells, as compared with that in WT, while the expression in the cytoplasm was decreased considerably. This data thus suggests that necdin is involved in the nucleocytoplasmic shuttling of THOC5. Nevertheless, depletion of necdin did not alter the nucleocytoplasmic distribution of Thoc5 mRNA (Figure S1f).
Depletion of necdin influences the THOC5-mediated RNA export
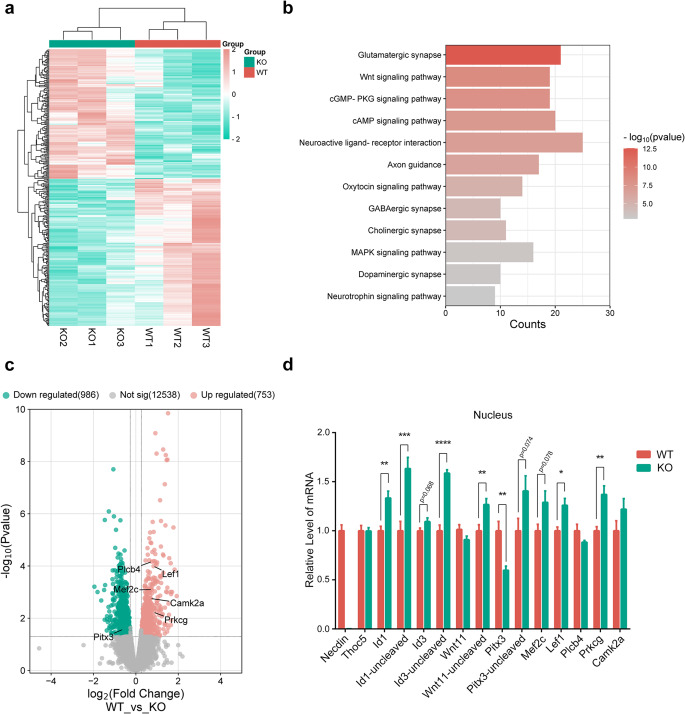
It has been shown that THOC5 is involved in mRNA maturation, nuclear export, and the formation of messenger ribonucleoprotein (mRNP)8. As necdin regulates the nucleocytoplasmic shuttling of THOC5, we speculate that depletion of necdin may influence the mRNA export. To this end, we performed an RNA-sequencing analysis on the nucleus extracted from the ventral tegmental area (VTA) of 3-month-old WT and necdin KO mice (Fig. 3a). As a result (Fig. 3c), we identified 753 upregulated genes (fold change ≥ 1.2 and p < 0.05), and 986 down-regulated genes (fold change ≤-1.2 and p < 0.05). Gene enrichment analysis revealed that the upregulated genes are highly enriched in synaptic development and related signaling pathways (Fig. 3b). Downregulated genes do not show a solid neuron-specific functional enrichment but are associated with various cellular structures (Figure S2a).
Figure 3.
Necdin affects mRNA export by regulating THOC5. (a) Gene expression in the nuclear from VTA of WT and KO mice as assayed by RNA-Seq. (b) KEGG pathway analysis on differentially expressed genes. (c) Volcano plot depicting the relative mRNA abundance in the nuclear from VTA of KO mice compared with WT controls. Upregulated synaptic development and related signaling pathways are indicated. (d) qPCR quantification of DA neuronal development-related genes and THOC5 target genes, and control transcripts in WT and KO mice. Data were mean ± SEM, n = 6, *p < 0.05, **p < 0.01, unpaired t-test.
To verify the RNA-seq data, we analyzed the mRNA levels of some THOC5 target genes, including some DA neuronal development-related genes, in the nucleus by qPCR. As shown in Fig. 3d, the expression of genes related to DA neuron development in the nucleus was increased after necdin deletion, such as Mef2c, Lef1, Prkcg. Furthermore, the mRNAs of immediate early genes, such as Id1, Id3 and Wnt11, were retained in the nucleus after necdin deletion (Fig. 3d, S2b), consistent with the previous report that deletion of THOC5 impairs the transport of these mRNAs from the nucleus to the cytoplasm12.
The degeneration of dopaminergic neurons in necdin-deficient mice
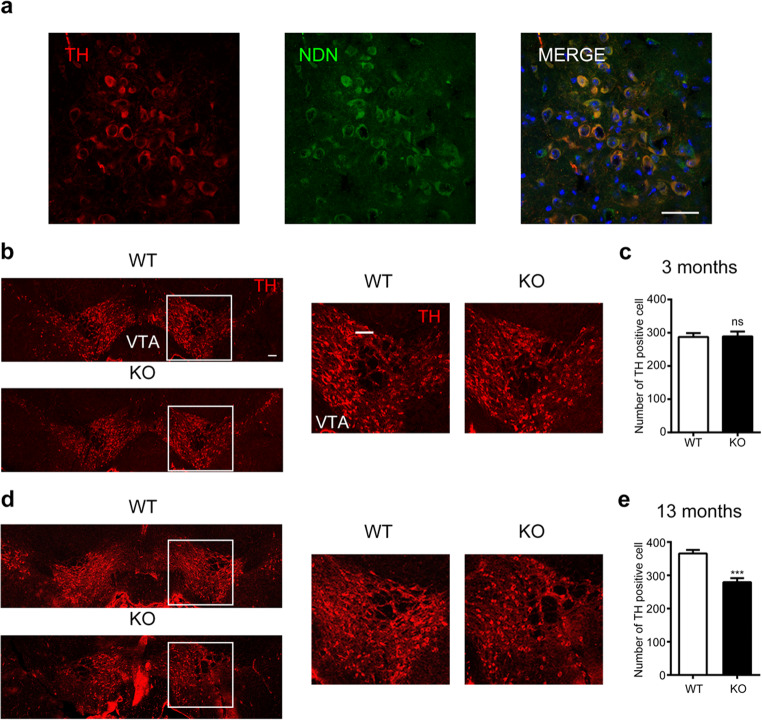
As developmental dysfunctions were reported in the dopaminergic neurons in THOC5-deficient mice10, we therefore sought to compare the development of dopaminergic neurons in WT and necdin KO mice. As shown in Fig. 4a and S3a, necdin was expressed in tyrosine hydroxylase (TH)-positive dopamine neurons. Although there was no significantly genotypic difference in the number of dopamine neurons in the 3-month-old mice (Fig. 4b-c); the number of dopaminergic neurons decreased significantly in 13-month-old necdin KO mice as compared to age-matched WT controls (Fig. 4d-e).
Figure 4.
Phenotype of dopaminergic neurons in necdin deficient mice. (a) Expression of necdin (green) and TH (red) in the VTA using immunofluorescence staining. Scale bar, 50 μm. (b) Representative images of midbrain TH immunofluorescence in 3-month-old WT and KO mice. Scale bar, 100 μm. (c) Quantification of DA cell number in VTA. (d) Representative images of midbrain TH immunofluorescence in 13-month-old WT and KO mice. Scale bar, 100 μm. (e) Quantification of DA cell number in VTA. Data were mean ± SEM, n = 5, *p < 0.05, **p < 0.01, unpaired t-test.
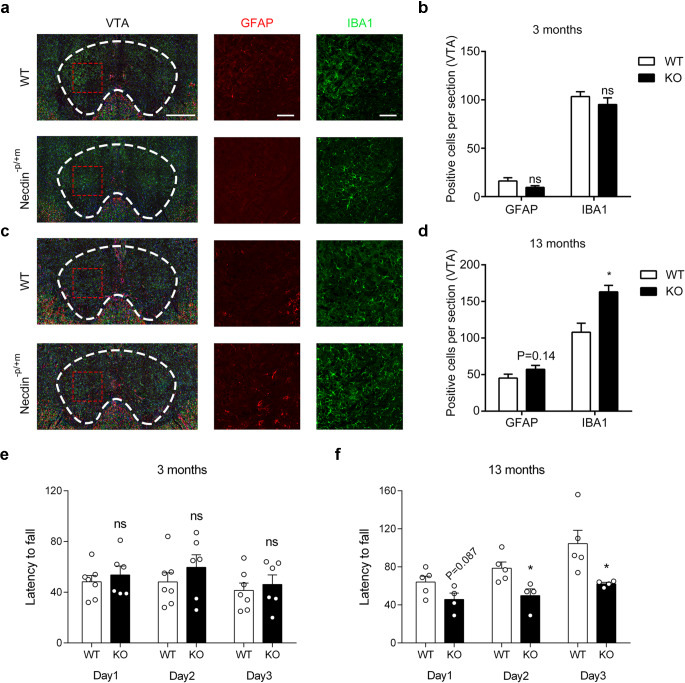
Effect of necdin deficiency on the activation of glial cells
Neuroinflammation, involving the activation of microglia and astrocytes within the central nervous system, has been identified as a feature of neurodegenerative diseases13. Therefore, we analyzed the number of astrocytes by using immunoreactivity of glial fibrillary acidic protein (GFAP), and microglia by using the immunoreactivity of ion-calcium binding connector molecule 1 (IBA1). The number of both astrocytes and microglial cells did not show any significant difference in the VTA brain region between 3-month-old WT and necdin-KO mice (Fig. 5a-b). Nevertheless, the number of glial neurons of necdin-KO mice was significantly higher than that of the WT (Fig. 5c-d). Therefore, this suggests that the degeneration of DA neurons in the VTA brain region of necdin-KO mice may be related to the increase of microglia and astrocytes.
Figure 5.
Effect of necdin deficiency on activation of glial cells. (a, b) Double-labeling for IBA-1 and GFAP in brain sections containing the VTA from 3-month-old mice. Scale bar, 500 μm and 100 μm. The bar plots represent the mean number of IBA-1 or GFAP-positive cells per section in the indicated areas. (c, d) Double-labeling for IBA-1 and GFAP in brain sections containing the VTA from 13-month-old mice. The bar plots represent the mean number of IBA-1 or GFAP-positive cells per section in the indicated areas. (e, f) The mean latency fall over three consecutive days of training on the rotarod in 3 and 13-month-old WT and KO mice. Data were mean ± SEM, n = 4–7 mice/genotype, *p < 0.05, **p < 0.01, unpaired t-test.
Motor coordination ability of necdin-deficient mice
As dopaminergic neurons are involved in the motor control1, we then measured the motor ability of WT and necdin KO mice by using open field and rotarod tests. As shown in Figure S3b and S3c, the spontaneous motor ability, as measured with the open field test, was not different between the two genotypes. However, the 13-month-old necdin-KO mice were more likely to fall from the rotarod than their age-matched WT controls, although no difference was observed in the 3-month-old mice (Fig. 5e-f). These data suggest that necdin-deficiency caused an age-dependent decline in the motor coordination and balance ability.
Discussion
In this study, we demonstrated that necdin interacts with THOC5 and regulates its nucleocytoplasmic distribution. Depletion of necdin results in the sequestration of THOC5 in the cytoplasm, leading to retain of its target mRNA in the nucleus. Furthermore, we illustrated that necdin deficiency induces age-dependent degeneration of dopaminergic neurons, activation of glial cells, and decline in motor coordination in mice. Thus, our findings suggest an important role for necdin in maintaining the homeostasis of dopaminergic neurons.
The mechanism by which necdin affects the function of its interactive proteins
Necdin itself lacks functional domains, such as enzymes or transcription factors. However, it participates in various pathophysiological pathways by interacting with other proteins. Numerous proteins that interact with necdin have been identified, and necdin may influence the function of its interactive proteins at multiple levels.
Previous reports have indicated that necdin can modulate the stability of target proteins in a ubiquitin-dependent pathway. Specifically, necdin exhibits a dual function: it inhibits the ubiquitination of BMAL1, PGC-1α, and LepR, while promoting the deubiquitination of PIAS1, HIF-1, and CCAR111,14–18. Our recently research demonstrated that necdin regulates BMAL1 stability through the SGT1-HSP90 chaperone machinery11. However, Wijesuriya et al. demonstrated that necdin stabilizes the leptin receptor by interacting with MAGEL2, along with RNF41 and USP818.
Additionally, necdin can downregulate the acetylation levels of target proteins, such as p53 and Foxo1, through Sirtuin1, thereby maintaining their transcriptional activity19,20. Moreover, necdin can impact three epigenetic signatures implicated in expression of PPARγ: increased MeCP2 (methyl CpG binding protein 2) and HP-1 co-repressor recruitments to Pparγ promoter and enhanced H3K27 dimethylation at the exon 5 locus, again in a manner dependent on suppressed canonical Wnt21.
On the other hand, it has been reported that necdin can enhance the cytoplasmic retention of its target proteins such as EID-1 and NEFA22. Interestingly, our results suggest that necdin may enhance the nucleic retention of THOC5, as its depletion leads to the sequestration of THOC5 in the cytoplasm. It will be intriguing to study how necdin regulates the nucleocytoplamic distribution in a protein-dependent manner.
Regulation of THOC5 shuttling
THOC5 is a nuclear-cytoplasmic shuttle protein, with its nuclear localization being crucial for its function. Extracellular stimuli can phosphorylate THOC5 at multiple sites, prompting its translocation into the nucleus and facilitating the export of target mRNA from the nuclei8. For instance, protein kinase C phosphorylates THOC5 at serine sites 5 and 6 near the nuclear localization signal (NLS), thus modulating its nuclear import23. It is conceivable that necdin recruits certain kinases or phosphatases to THOC5, thereby impacting its phosphorylation levels and consequently influencing its nuclear-cytoplasmic distribution.
Possible mechanism by which necdin affects dopamine neurons
Necdin is widely expressed in postmitotic neurons throughout the brain and plays a critical role in various aspects of neuronal development and maintenance, including the proliferation of neuronal stem cells, the promotion of neuronal survival, neuronal migration, and axon growth24,25. In necdin-deficient mouse embryos (E15.5), abnormalities in TH-positive axon growth and severe disruptions in TH-positive projections within the nigrostriatal pathway and caudal nucleus were observed26, indicating a role for necdin in the development of dopaminergic neurons. Additionally, necdin has been reported to enhance mitochondrial biogenesis, thus supporting neuronal survival by stabilizing PGC-1a17. Consequently, overexpression of necdin in the substantia nigra has been shown to protect dopaminergic neurons from degeneration in mice.
In conclusion, our study suggests that necdin regulates the maturation of mRNAs involved in dopaminergic neuron development by influencing the nucleocytoplasmic distribution of Thoc5. We provide new evidence that Necdin affects dopaminergic neurodevelopment, suggesting that necdin plays an important role in nervous system development. Given that THOC5 is involved in various biological processes, including the renewal and survival of embryonic, hematopoietic, and intestinal stem cells, neuronal development, and cancer proliferation through its regulation of mRNA transportation, our findings warrant further investigation into the role of necdin in these processes.
Supplementary Information
Acknowledgements
This project is funded by the National Natural Science Foundation of China (32371218, 81770780, 31972913, 82101960, 31970504); Key Research and Development Programs from Hunan Province (2021DK2001); Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” (2018B030335001); Strategic Priority Research Program of Central South University (ZLXD2017004); China Postdoctoral Science Foundation (2021T140746); grant 2023ZZTS0575 of the Postgraduate Scientific Research Innovation Project of Central South University (to YH); and grant CX20220318 of the Postgraduate Scientific Research Innovation Project of Hunan Province (to XRL).
Author contributions
RBL and XL designed the methodology and conducted the investigation. RBL and XL completed formal raw data analyses and curated the raw data. YH and YCZ gave assistance to supplementary experiments. YH, YCZ, XYT, ZSG, and JDL provided insights or reagents. All authors analyzed the processed data. XL wrote the original draft, which all other authors reviewed and edited. RBL and JDL supervised the project.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data is located at controlled access data storage at Central South University.
Declarations
Ethics approval
All procedures regarding the care and use of animals were approved by the ethics committee of Center for Medical Genetics, School of Life Sciences, Central South University of China. All methods were performed in accordance with approved guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klein, M. O. et al. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol.39, 31–59. 10.1007/s10571-018-0632-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damier, P., Hirsch, E. C., Agid, Y. & Graybiel, A. M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 122, 1437–1448 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Salamone, J. D. & Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 76, 470–485. 10.1016/j.neuron.2012.10.021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise, R. A. Drug-activation of brain reward pathways. Drug Alcohol Depend.51, 13–22 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Hu, Z., Cooper, M., Crockett, D. P. & Zhou, R. Differentiation of the midbrain dopaminergic pathways during mouse development. J. Comp. Neurol.476, 301–311. 10.1002/cne.20230 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Wolters, E. C. Psychiatric complications in Parkinson’s disease. J. NeuraL Transm Suppl.60, 291–302. 10.1007/978-3-7091-6301-6_20 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Tamura, T. et al. Niemann. FMIP, a novel fms-interacting protein, affects granulocyte/macrophage differentiation. Oncogene18, 6488–6495 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Doan, D. H. & Tran, A. K. a. T. T. THOC5, a member of the mRNA export complex a novel link between mRNA export machinery and signal transduction pathways in cell proliferation and differentiation. Cell. Commun Signal.12, (2014). [DOI] [PMC free article] [PubMed]
- 9.Sträßer, K. M. et al. Robin. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 417, 304–308 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Maeder, C. I. et al. The THO Complex Coordinates Transcripts for Synapse Development and Dopamine Neuron Survival. Cell174, 1436-1449 e1420. 10.1016/j.cell.2018.07.046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, R., Dong, Y. & Li, J. D. Necdin regulates BMAL1 stability and circadian clock through SGT1-HSP90 chaperone machinery. Nucleic Acids Res.48, 7944–7957. 10.1093/nar/gkaa601 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran, D. D. et al. THOC5 controls 3’end-processing of immediate early genes via interaction with polyadenylation specific factor 100 (CPSF100). Nucleic Acids Res.42, 12249–12260. 10.1093/nar/gku911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki, I. & Asanuma, M. Neuron-Astrocyte Interactions in Parkinson’s Disease. Cells. 910.3390/cells9122623 (2020). [DOI] [PMC free article] [PubMed]
- 14.Francois, S. et al. Necdin enhances myoblasts survival by facilitating the degradation of the mediator of apoptosis CCAR1/CARP1. PLoS One. 7, e43335. 10.1371/journal.pone.0043335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gur, I., Fujiwara, K., Hasegawa, K. & Yoshikawa, K. Necdin promotes ubiquitin-dependent degradation of PIAS1 SUMO E3 ligase. PLoS One. 9, e99503. 10.1371/journal.pone.0099503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon, H. E. et al. Negative regulation of hypoxia inducible factor-1alpha by necdin. FEBS Lett.579, 3797–3801. 10.1016/j.febslet.2005.05.072 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa, K. et al. Promotion of mitochondrial biogenesis by necdin protects neurons against mitochondrial insults. Nat. Commun.7, 10943. 10.1038/ncomms10943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijesuriya, T. M. et al. The Prader-Willi syndrome proteins MAGEL2 and necdin regulate leptin receptor cell surface abundance through ubiquitination pathways. Hum. Mol. Genet.26, 4215–4230. 10.1093/hmg/ddx311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa, K. et al. Necdin controls Foxo1 acetylation in hypothalamic arcuate neurons to modulate the thyroid axis. J. Neurosci.32, 5562–5572. 10.1523/JNEUROSCI.0142-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa, K. & Yoshikawa, K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J. Neurosci.28, 8772–8784. 10.1523/JNEUROSCI.3052-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu, N. L., Wang, J. & Tsukamoto, H. The necdin-wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells. J. Biol. Chem.285, 30463–30471. 10.1074/jbc.M110.156703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniguchi, N. et al. The postmitotic growth suppressor necdin interacts with a calcium-binding protein (NEFA) in neuronal cytoplasm. J. Biol. Chem.275, 31674–31681. 10.1074/jbc.M005103200 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Mancini, A., Koch, A., Whetton, A. D. & Tamura, T. The M-CSF receptor substrate and interacting protein FMIP is governed in its subcellular localization by protein kinase C-mediated phosphorylation, and thereby potentiates M-CSF-mediated differentiation. Oncogene. 23, 6581–6589. 10.1038/sj.onc.1207841 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Kubota, Y., Osawa, M., Jakt, L. M., Yoshikawa, K. & Nishikawa, S. Necdin restricts proliferation of hematopoietic stem cells during hematopoietic regeneration. Blood. 114, 4383–4392. 10.1182/blood-2009-07-230292 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Takazaki, R., Nishimura, I. & Yoshikawa, K. Necdin is required for terminal differentiation and survival of primary dorsal root ganglion neurons. Exp. Cell. Res.277, 220–232. 10.1006/excr.2002.5558 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Lee, S. et al. Essential role for the Prader-Willi syndrome protein necdin in axonal outgrowth. Hum. Mol. Genet.14, 627–637. 10.1093/hmg/ddi059 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data is located at controlled access data storage at Central South University.