Figure 1.
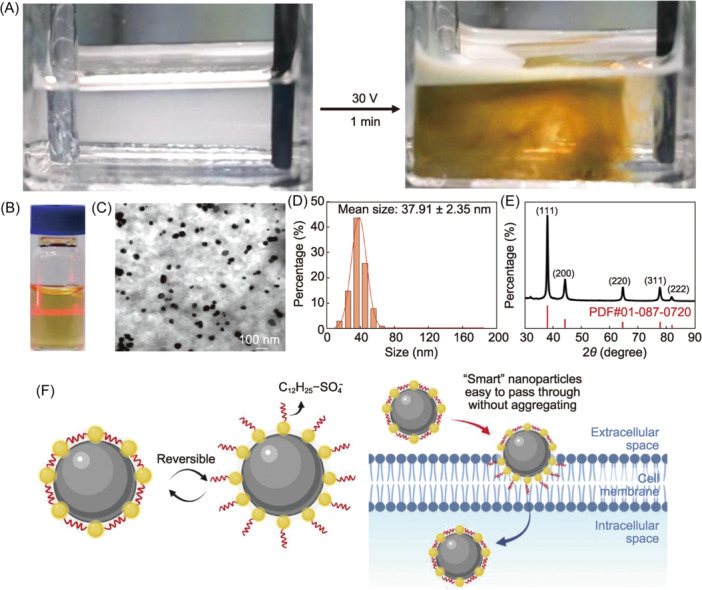
Sustained electrochemical preparation of silver nanoparticles (AgNPs) in electrolyte solution. (A) Production of AgNPs. AgNPs were obtained by electrodeposition in an electrolyte solution of silver nitrate (AgNO3) and sodium dodecyl sulfate (SDS). The AgNPs prepared here were continuously emitted from the electrode interface into the electrolyte solution, resulting in a yellow colloidal solution that formed within 1 min. (B) AgNPs showing the Tyndall effect when dispersed in water and indicating the homogeneity of the synthetic colloidal solution system. (C) Transmission electron microscope analysis of the AgNPs. (D) Particle size distribution of the AgNPs. (E) X‐ray diffraction analysis of the AgNPs. (F) Proposed mechanism for adapting ligands. The reversible orientation change of the surface ligands allowed them to adapt to surrounding liquids and easily pass through the bacterial membrane.
