Abstract
The transferrin binding proteins (TbpA and TbpB) comprise the gonococcal transferrin receptor and are considered potential antigens for inclusion in a vaccine against Neisseria gonorrhoeae. Intranasal (IN) immunization has shown promise in development of immunity against sexually transmitted disease pathogens, in part due to the induction of antigen-specific genital tract immunoglobulin A (IgA) and IgG. Conjugation of antigens to the highly immunogenic cholera toxin B subunit (Ctb) enhances antibody responses in the serum and mucosal secretions following IN vaccination. In the current study, we characterized the anti-Tbp immune responses following immunization of mice IN with recombinant transferrin binding proteins (rTbpA and rTbpB) conjugated to rCtb. We found that both rTbpA-Ctb and rTbpB-Ctb conjugates administered IN induced antibody responses in the serum and genital tract. IN immunization resulted in both IgA and IgG in the genital tract; however, subcutaneous immunization mainly generated IgG. Surprisingly, rTbpA alone was immunogenic and induced serum and mucosal antibody responses similar to those elicited against the rTbpA-Ctb conjugate. Overall, rTbpB was much more immunogenic than rTbpA, generating serum IgG levels that were greater than those elicited against rTbpA. Bactericidal assays conducted with sera collected from mice immunized IN with TbpA and/or TbpB indicated that both antigens generated antibodies with bactericidal activity. Anti-TbpA antibodies were cross-bactericidal against heterologous gonococcal strains, whereas TbpB-specific antibodies were less cross-reactive. By contrast, antibodies elicited via subcutaneous immunization were not cross-bactericidal against heterologous strains, indicating that IN vaccination could be the preferred route for elicitation of biologically functional antibodies.
A 1995 World Health Organization report estimated that there were 62.2 million cases of the sexually transmitted infection gonorrhea worldwide (19). This number is considered to be an underestimate of the actual incidence, due in part to inadequate reporting by physicians and clinics, as well as to the prevalence of asymptomatic carriage (8, 40). One study found that asymptomatic carriage in women can be as high as 55% (17). Uncomplicated gonorrhea manifests as urethritis in men and as endocervitis and/or urethritis in women. Serious downstream sequelae can afflict those individuals with asymptomatic infection, since the infection can spread to the upper genital tract. Ascension can result in epididimytis, salpingitis, ectopic pregnancy, sterility, and disseminated gonococcal infection. Antibiotics are the treatment of choice for gonorrhea, but the increasing emergence of drug-resistant strains has made treatment more difficult and expensive (25). Furthermore, it has been shown that coinfection with Neisseria gonorrhoeae and human immunodeficiency virus (HIV) can increase the risk of transmission of HIV (10). These findings have made the need for an effective vaccine more imperative. To date, attempts to develop a gonococcal vaccine have been disappointing. Human trials using partially lysed gonococci, purified pilin, or purified porin all failed to confer protection upon natural exposure (4, 20, 45). These vaccine formulations, although immunogenic, failed to protect, likely due in part to the intrinsic ability of the gonococcus to undergo high-frequency phase and antigenic variation of surface structures (28).
The gonococcal transferrin binding proteins, TbpA and TbpB, have generated particular interest as vaccine antigens because they are ubiquitously expressed among clinical isolates, they exhibit low strain-to-strain variability, and they are not subject to high-frequency antigenic or phase variation (11, 12, 29). Furthermore, their importance in gonococcal virulence has been established in a human male challenge model of infection (14). Subjects inoculated with a mutant strain of N. gonorrhoeae that lacked the transferrin receptor showed no signs or symptoms of urethritis, in contrast to subjects inoculated with the parental strain (14). In spite of their expression in vivo, we demonstrated that antibody responses to the transferrin binding proteins resulting from natural infections were weak in the serum and nonexistent in vaginal washes and seminal fluid (34). We postulate that the induction and sustained production of an appropriate antibody response to one or both Tbps in the genital tract could prevent colonization.
One of the shortcomings of parenteral immunization is its relatively poor ability to induce genital-tract-specific immunoglobulin A (IgA) antibodies (5, 30). IgA is considered important in protecting the genital tract from infection, as its presence is correlated with a protective role against chlamydia and HIV (6, 7). Intranasal (IN) immunization, on the other hand, has been more promising in terms of eliciting genital-tract antigen-specific IgA and IgG in mice (18, 21, 47), primates (42), and humans (3, 38). In addition, the genital-tract antibodies generated as a function of IN immunization have been demonstrated to be long lasting in mice (37, 47)
Cholera toxin B (Ctb), the nonenzymatic, nontoxic component of cholera toxin, has been studied extensively for its ability to augment antibody responses to coadministered and physically conjugated antigens following intranasal application (21, 23, 24, 48, 49). In this study, we evaluated the immunogenicity of IN administered recombinant TbpA and TbpB, alone and in combination with recombinant Ctb. We demonstrate that IN immunization can elicit serum and vaginal antigen-specific antibody responses. Furthermore, this route of immunization was superior to subcutaneous immunization in the induction of specific genital tract IgA. IN immunization generated antibodies with greater serum bactericidal activity than did subcutaneous immunization. Importantly, this bactericidal activity was detected against both homologous and heterologous gonococcal strains.
(A preliminary account of these results was presented previously [G. A. Price, M. W. Russell, and C. N. Cornelissen, 14th Int. Pathog. Neisseria Conf., abstr. 188, 2004].)
MATERIALS AND METHODS
Construction of expression plasmids.
The tbpA expression plasmid, pUNCH412, was described previously (13). The tbpB expression plasmid, pVCU711, was constructed by PCR amplification using a proofreading Taq polymerase (Platinum Pfx; Invitrogen) of a previously described tbpB expression plasmid, pVCU705 (34). The forward primer, oVCU240 (GGATCCTGTCTGGGCGGAGGCGGCAGTTTCG), contained a BamHI site (shown in boldface) and amplified the FA19 tbpB gene from the sequence that encodes amino acid 2 of the mature protein. The reverse primer, oVCU241 (CCCGGGTTATTTCACAAGCTTTTGGCGTTTCG), contained a SmaI site (shown in boldface) and the stop codon of the FA19 tbpB gene. The PCR product was ligated into the pQE-80L expression vector (QIAGEN). The resultant plasmid, pVCU711, encoded a recombinant TbpB in which the N-terminal six-histidine tag was fused to amino acid 2 of the mature protein. The resulting protein lacked the amino-terminal cysteine residue and was expressed under the control of the T5 promoter. The ctb expression plasmid, pVCU710, was constructed by PCR amplification of the plasmid pCTΔA1 (21). The forward primer, oVCU238 (TGGCCACACCTCAAAATATTACTGATTTGTGTG) contained an MscI site (shown in boldface) and amplified the mature ctb gene product. The reverse primer, oVCU239 (CTCGAGTTAATTTGCCATACTAATTGCGGCAATCG), contained an XhoI site and amplified the 3′ end of the ctb gene, including the stop codon. The PCR product was ligated into the pET-22b(+) (Novagen) expression vector. The resultant plasmid, pVCU710, contained the mature ctb gene product fused with the Escherichia coli pelB leader sequence immediately upstream. Gene expression was under the control of the T7 promoter. The expression hosts for pVCU710 and pVCU711 were the E. coli strains BL21(DE3) (Novagen) and TOP10 (Invitrogen), respectively.
Recombinant protein expression and purification.
Recombinant proteins were expressed in 1-liter cultures of Luria-Bertani broth containing 1% glucose and 500 μg/ml of carbenicillin for recombinant TbpA (rTbpA) expression or 200 μg/ml of ampicillin for rTbpB and rCtb expression. When the cultures reached an optical density at 600 nm of 0.4 to 0.6, they were induced with IPTG (isopropyl-β-d-thiogalactopyranoside). For rTbpA, prior to induction, cultures were centrifuged for 15 min at 6,000 × g to pellet the bacteria. The pellets were then resuspended in fresh medium as described above with 0.5 mM IPTG and allowed to express overnight at 27°C (∼16 h). For rTbpB and rCtb expression, 0.5 mM IPTG was added, and the cultures were allowed to express for 3 h at 30°C. After induction, the cells were pelleted as described above and stored at −80°C.
For rTbpA and rTbpB purification, the pellets were thawed on ice and resuspended in Tris buffer (100 mM Tris [pH 8.0] and 0.5 M NaCl). After the cells were completely resuspended, Elugent (Calbiochem) was added to a final concentration of 2%. Protease inhibitors (Sigma), lysozyme, and DNase were added, and the mixture was allowed to incubate overnight at 4°C. Solubilized preparations were centrifuged at 18,000 × g for 30 min to remove insoluble material. TbpA was purified using a transferrin affinity column (26). The rTbpA-transferrin column was washed with 20 bed volumes of 50 mM potassium phosphate (pH 8.0)-0.5 M NaCl-0.05% lauryl maltoside (n-dodecyl-β-d-maltopyranoside; Anatrace, Maumee, OH) and eluted with the above-mentioned buffer at pH 2.0. The eluted proteins were immediately neutralized by the addition of 1 M potassium phosphate, pH 8.0, and 0.05% lauryl maltoside. rTbpB was purified as described previously (34). Ctb pellets were resuspended in 50 mM potassium phosphate buffer, pH 6.8, and 100 μg/ml lysozyme and placed at 30°C for 15 min. Following the 15-min incubation, the cell pellets were subjected to sonication on ice for 30 bursts repeated three times. Following centrifugation, the supernatants were subjected to precipitation by ammonium sulfate, where Ctb precipitated at 60 to 80% saturation. The resulting precipitate was collected by centrifugation and dissolved in 20 mM potassium phosphate buffer, pH 6.8. The dissolved precipitate was dialyzed three times against a 1,000-fold excess of potassium phosphate buffer. The dialyzed preparation was centrifuged to remove precipitated material and then passed through a 0.45-μm-pore-size syringe filter. Ctb was then purified by anion-exchange chromatography using an Econo-Pac High S Cartridge (Bio-Rad) and gel filtration using a Superdex 200 column (Amersham). Following purification, TbpB and Ctb were dialyzed four times against a 1,000-fold excess of phosphate-buffered saline (PBS), and TbpA was dialyzed against PBS plus 0.05% lauryl maltoside.
Tbp-Ctb conjugate preparation.
TbpA (1 mg in 1 ml PBS plus 0.05% lauryl maltoside), TbpB (2 mg in 1 ml PBS), and Ctb (2 mg in 1 ml PBS) were treated with 5 μl of a 20 mM stock solution of SPDP [N-succinimidyl 3-(2-pyridyldithio) proprionate; Pierce] in dimethyl sulfoxide for 1 h at room temperature. Each protein was dialyzed against the corresponding initial buffers to remove free SPDP. To 1 ml of derivatized TbpA or TbpB, 0.5 ml of acetate buffer (100 mM sodium acetate, 100 mM NaCl, and 0.05% lauryl maltoside for TbpA only) containing 12 mg of dithiothreitol was added, and the mixture was incubated for 30 min at room temperature. The reduced proteins were passed through a desalting column (Pierce), and protein concentrations were determined by bicinchoninic acid assay (Pierce). Equimolar amounts of derivatized Ctb were added to the reduced proteins and allowed to incubate overnight at 4°C. For TbpA conjugation, the derivatized Ctb was diluted to half by the addition of PBS plus 0.1% lauryl maltoside in order to keep the detergent concentration at 0.05%. Conjugated proteins were separated from unconjugated proteins by size exclusion chromatography using a Superdex 200 column (Amersham).
GM1 ganglioside ELISA.
Purified conjugates were analyzed for the presence of Ctb and TbpA or TbpB using the GM1 ganglioside enzyme-linked immunosorbent assay (ELISA). ELISA plates (Nunc) were coated with 0.05 ml GM1 ganglioside (Sigma) diluted at 2 μg/ml in methanol. Following evaporation of the methanol, the plates were blocked with 0.2 ml of PBS plus 1% skim milk for 1 h at 37°C. The test samples were diluted at 1/100 in PBS or PBS plus 0.05% lauryl maltoside for the TbpA conjugate and applied to each well in 0.1-ml volumes. The plate was then incubated at 30°C for 1 h. The plates were washed three times with PBS to remove unbound material, and bound conjugates were probed for 1 h at room temperature with 0.05 ml of either anti-TbpA, anti-TbpB, or anti-CT (Sigma) rabbit serum diluted in PBS plus 1% skim milk. The plates were again washed as described above and probed with 0.05 ml of alkaline phosphatase-conjugated goat anti-rabbit IgG (Bio-Rad) for 1 h at room temperature. The plates were washed again and developed with 0.05 ml of p-nitrophenylphosphate substrate (Sigma) diluted in carbonate buffer (0.05 M sodium carbonate, 1 mM MgCl2, pH 9.8). After sufficient color developed, the optical density of each well was measured at 405 nm and compared to those of blank and control wells.
Immunizations and sample collection.
Female BALB/c mice, 7 to 8 weeks old, were purchased from Harlan-Sprague-Dawley (Indianapolis, IN). The mice were housed in microisolator cages and were under the care and supervision of the Division of Animal Resources. The protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. At the start of the experiment, the mice were approximately 10 weeks old. Groups of five mice were immunized either intranasally or subcutaneously with Tbp-Ctb conjugate(s) or Tbps with or without Ctb as an adjuvant (Table 1 shows immunization details). All groups were immunized three times at 10-day intervals. Sera and vaginal secretions were collected on days 0, 17, 28, 35, and 65. Sera were obtained from tail vein blood samples and stored at −20°C. Vaginal secretions were obtained by pipetting 0.05 ml of PBS in and out of the vaginal vault three times. This procedure was repeated twice, and the vaginal washes were pooled. The protease inhibitor phenylmethylsulfonyl fluoride (Sigma) was added to each wash sample at a concentration of 1 mM following collection. The vaginal washes were kept at −80°C until use.
TABLE 1.
Immunization groups
| Group (immunization route) | Immunogen | Amt administereda (μg) |
|---|---|---|
| A − Ctb (IN) | Ctb − TbpA conjugate | 20 |
| B − Ctb (IN) | Ctb − TbpB conjugate | 20 |
| A − Ctb + B − Ctb (IN) | Ctb − TbpA + Ctb − TbpB conjugates | 20 + 20 |
| A + Ctb (IN) | Ctb + TbpA admixed | 10 + 10 |
| B + Ctb (IN) | Ctb + TbpB admixed | 10 + 10 |
| A + B + Ctb (IN) | Ctb + TbpA + TbpB admixed | 10 + 10 + 10 |
| A only (IN) | TbpA | 10 |
| B only (IN) | TbpB | 10 |
| Control | PBS only | 0 |
| S.c.A + B + Ctb (s.c.b) | Ctb + TbpA + TbpB admixed | 10 + 10 + 10 |
Groups of mice (n = 5) were immunized three times at 10-day intervals.
One group was immunized subcutaneously (s.c.) with an admixture of TbpA, TbpB, and Ctb.
ELISAs.
Serum and vaginal washes were assayed for total and specific antibodies as described previously (34). For antibodies specific to Ctb, plates were first coated with 0.1 ml of GM1 ganglioside as described above. All capture antibodies and alkaline phosphatase-conjugated goat anti-mouse isotype-specific antibodies were purchased from Southern Biotechnology Associates (Birmingham, AL). The standard curve was generated using a mouse reference serum (Bethyl Laboratories).
Serum bactericidal assays.
Mouse sera were pooled by group and heat inactivated at 56°C for 30 min. Gonococcal strains were plated from freezer stocks directly onto plates containing GC medium base (Difco) plus Kellogg’s supplement I (25a) and 5 μM desferal to induce iron stress. For strains FA19 and FA1090, plates were allowed to incubate at 37°C and 5% CO2 for approximately 24 h, at which time they were passed again as described above. Following the second passage, the plates were allowed to incubate for 16 to 18 h. Isolated colonies were picked from the plate and suspended in prewarmed 37°C Gey's balanced salt solution (Sigma) containing 0.1% gelatin and 5 μM desferal (GBSS+G+D). The optical density of the inoculum at 600 nm was monitored until it reached 0.20 (0.23 for strain MS11), and then it was serially diluted to 10−5 in prewarmed GBSS+G+D. Immediately following dilution, 80 μl of the diluted cell suspension was added to a prewarmed 96-well microtiter plate containing 10 μl of the appropriate serum samples diluted in GBSS+G+D. The plate was incubated at 37°C and 5% CO2 for 15 min, and then 10 μl of normal human pooled serum (Quidel Corp.) was added and the plate was again incubated as described above for 45 min. After incubation, viable gonococci were detected by plating them onto plates containing GC medium base plus Kellogg's supplement I and 12.5 μM ferric nitrate. The plates were incubated for approximately 24 h as described above, after which colonies were enumerated. The bactericidal titer was determined as the lowest dilution that gave ≥50% killing compared to control sera at the same dilution. Strain MS11 was plated only once directly from the freezer stock and allowed to grow for 16 to 18 h. Because of its moderate sensitivity to human serum, bactericidal activity against strain MS11 was tested in 5% human serum with incubation for 15 min.
Statistics.
Analysis of variance for multiple group comparisons was performed using the Tukey-Kramer multiple-comparison test or the Kruskal-Wallis multiple-comparison Z value test where appropriate. A P value of < 0.05 was considered significant. These comparisons were performed on logarithmically transformed data. Group data are therefore presented as geometric means ×/÷ standard deviation, after back transformation of the logarithmic means ± standard deviation.
RESULTS
Serum antibody responses against TbpA and TbpB.
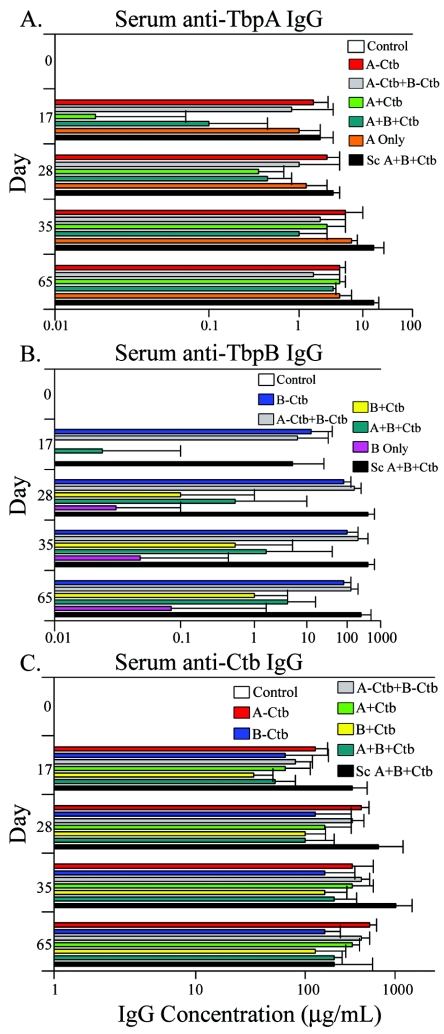
The serum antibody responses were measured over time using a quantitative ELISA, with which we measured antibody levels following each immunization. Sera were collected at days 0, 17, 28, 35, and 65. All day zero sera were assayed and found to be negative for antibodies specific to all antigens tested. The antibody responses to TbpA and TbpB following vaccination were strikingly different and were dependent on antigen preparation and route of immunization. For TbpA, the highest antibody responses were seen in the subcutaneously immunized group, in which antibodies to TbpA peaked on day 35 and remained high through day 65 (Fig. 1A). The groups receiving TbpA conjugated to Ctb and TbpA alone generated the next-highest responses through day 28. Interestingly, the presence of Ctb in admixtures with TbpA appeared to delay the immune response against TbpA. However, by day 65, TbpA levels were similar for all IN immunized groups (Fig. 1A).
FIG. 1.
Serum IgG levels specific for TbpA, TbpB, and Ctb. (A) Serum IgG levels specific for TbpA detected at days 17, 28, 35, and 65. (B) Serum IgG levels specific for TbpB detected at the same time points. (C) Serum IgG levels specific for Ctb detected at the same time points. Results are expressed as the geometric mean of antibody titers ×/÷ standard deviation. For all immunization groups, n = 5.
Unlike TbpA, conjugation of TbpB to Ctb significantly enhanced antibody titers compared to the groups where Ctb was admixed with TbpB (all comparisons, P < 0.05, days 17 to 65) (Fig. 1B). Another important difference between TbpA and TbpB was that TbpB was poorly immunogenic when administered by itself (Fig. 1B) whereas TbpA alone was as immunogenic as the conjugated form (Fig. 1A). IN immunization with TbpB conjugate or with TbpB and TbpA conjugates together did not result in antibody levels that were significantly different from those elicited in the subcutaneously immunized group (Fig. 1B). In terms of antibody response, the groups immunized with both Tbps did not differ significantly from the group immunized with only one antigen on any day tested (Fig. 1A and B). Thus, although each antigen individually elicited distinct antibody responses, the presence of a coadministered antigen did not adversely affect antibody levels generated by IN vaccination. Antibody responses to Ctb were robust in all groups tested (Fig. 1C). Not surprisingly, the subcutaneously immunized group elicited the highest Ctb antibody titers, except on day 65 (Fig. 1C).
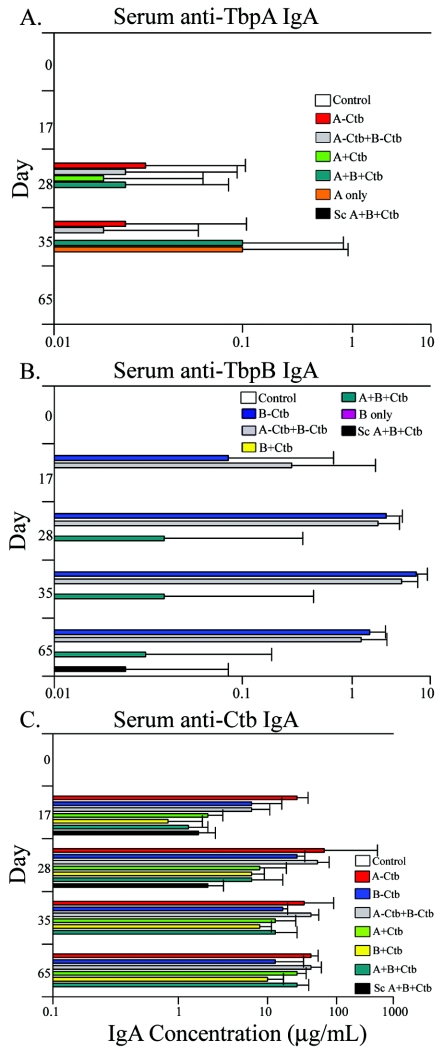
We were also able to detect serum IgA antibody responses specific for TbpA, TbpB, and Ctb (Fig. 2). Serum IgA levels against TbpA were transient and not measurable until day 28 and were completely undetectable in all groups by day 65 (Fig. 2A). The low IgA levels detected against TbpA were probably reflective of the antigen's lower overall immunogenicity, as shown by lower serum IgG titers against TbpA than against TbpB (Fig. 1A and B). Serum IgA responses to TbpB were much higher than those measured against TbpA, with the highest detected serum IgA antibody responses found in the groups immunized with the TbpB-Ctb conjugates (Fig. 2B). The levels measured in the conjugate groups were not significantly different from one another but were different from the only other groups with measurable serum IgA against TbpB, namely, the group immunized with TbpA plus TbpB plus Ctb and the subcutaneously immunized group (P < 0.05; days 28 to 65) (Fig. 2B). The subcutaneously immunized animals had the highest serum IgG antibody titers against TbpB; however, the serum IgA titers elicited by this route were detectable only on day 65 (Fig. 2B). Serum IgA titers to Ctb (Fig. 2C) initially were highest in the animals immunized with the TbpA-Ctb conjugate, followed by the other two conjugate groups. By day 65, all IN immunized groups had similar levels of Ctb-specific IgA antibody. Interestingly, on days 17 and 28, we were able to measure serum IgA to Ctb in the subcutaneously immunized group, but by day 35, Ctb-specific serum IgA was undetectable and remained so on day 65 (Fig. 2C).
FIG. 2.
Serum IgA levels specific for TbpB and Ctb. (A) Serum IgA levels specific for TbpA detected at days 17, 28, 35, and 65. (B) Serum IgA levels specific for TbpB detected at the same time points. (C) Serum IgA levels specific for Ctb detected at the same time points. Results are expressed as the geometric mean of antibody titers ×/÷ standard deviation. For all immunization groups, n = 5.
Vaginal antibody responses to TbpA and TbpB.
The relative immunogenicities of TbpA and TbpB were also reflected in the detectable antibody levels measured in the vaginal washes. Vaginal-wash antibodies to TbpA detected on day 28 (7 days after the final immunization) (Table 2) were not as robust as those detected against TbpB (Table 3). For TbpA-specific IgA (Table 2), on day 28 the highest response measured was generated by the group of animals immunized with the TbpA-Ctb conjugate: TbpA-specific IgA represented 1% of the total IgA antibody detected. This level, however, was only significantly different from the group immunized with both Tbp conjugates (P < 0.05) among the groups in which we were able to measure TbpA-specific IgA. Furthermore, only in the IN immunized groups were we able to detect TbpA-specific IgA. Interestingly, TbpA-specific IgA responses declined on day 35 in all groups with measurable IgA; however, these levels had returned to similar or slightly higher levels by day 65 (Table 2). Although the group receiving both TbpA and TbpB conjugates had increased antibody levels by day 65, they were still significantly lower than those of the groups immunized with TbpA-Ctb and with both Tbps admixed with Ctb (P < 0.05) (Table 2). TbpA-specific IgG levels were undetectable on day 28. We were unable to measure vaginal IgG until days 35 and 65 (Table 2). For the most part, vaginal IgG antibody levels specific for TbpA were lower and more sporadic than vaginal IgG measured against TbpB (Table 3).
TABLE 2.
Vaginal antibody levels specific for TbpA detected at days 28, 35, and 65a
| Immunization group | Day 28b
|
Day 35
|
Day 65
|
|||
|---|---|---|---|---|---|---|
| IgA | IgG | IgA | IgG | IgA | IgG | |
| TbpA − Ctb | 1.0 ×/÷ 1.8 | 0 | 0.2 ×/÷ 1.7 | 0 | 0.9 ×/÷ 1.4 | 0.6 ×/÷ 1.7f |
| TbpA − Ctb + TbpB − Ctb | <0.1c | 0 | <0.1d | 0 | 0.3 ×/÷ 2.3 | 0 |
| TbpA + Ctb | 0 | 0 | 0 | 0 | 0 | 0.5 ×/÷ 1.3e |
| TbpA + TbpB + Ctb | 0.4 ×/÷ 6.3e | 0 | 0.2 ×/÷ 3.8 | 0.6 ×/÷ 10.6d | 1.5 ×/÷ 3.1 | 0.3 ×/÷ 2.7f |
| TbpA | 0.5 ± 4.1e | 0 | 0.2 ± 2.0 | 2.8 ×/÷ 3.8 | 0.6 ×/÷ 10.8e | 0.2 ×/÷ 4.4g |
| S.c.h TbpA + TbpB + Ctb | 0 | 0 | 0 | 0 | 0 | 1.2 ×/÷ 1.3 |
Data are expressed as the geometric mean of the percentage of total corresponding antibody isotype concentrations ×/÷ standard deviation.
Day 28 is 7 days after final immunization.
Only one mouse had detectable TbpA-specific antibodies.
Only two mice had detectable TbpA-specific antibodies.
Only three mice had detectable TbpA-specific antibodies.
n = 4; one mouse removed due to very low total IgG.
n = 3; two mice removed due to very low total IgG.
S.c., subcutaneous.
TABLE 3.
Vaginal antibody levels specific for TbpB detected at days 28, 35, and 65a
| Immunization group | Day 28b
|
Day 35
|
Day 65
|
|||
|---|---|---|---|---|---|---|
| IgA | IgG | IgA | IgG | IgA | IgG | |
| TbpB − Ctb | 12.2 ×/÷ 2.9 | 32.5 ×/÷ 1.4f | 6.4 ×/÷ 2.7 | 15.9 ×/÷ 1.5f | 3.5 ×/÷ 1.7 | 2.0 ×/÷ 3.8d |
| TbpA − Ctb + TbpB − Ctb | 8.2 ×/÷ 3.6 | 20.2 ×/÷ 5.5 | 2.6 ×/÷ 2.9 | 20.4 ×/÷ 1.5 | 1.9 ×/÷ 2.9 | 9.7 ×/÷ 4.0e |
| TbpB + Ctb | 0 | 0 | 0 | 0 | 0 | 0 |
| TbpA + TbpB + Ctb | <0.2c | 0 | <0.1d | 1.8 ×/÷ 2.9e | <0.1d | 1.6 ×/÷ 2.7c |
| TbpB | <0.2c | 0 | 0 | 0.5 ×/÷ 1.3c,f | 0 | 0 |
| S.c.g TbpA + TbpB + Ctb | <0.1d | 20.5 ×/÷ 1.8 | <0.1d | 12.9 ×/÷ 1.5 | <0.1d | 15.8 ×/÷ 1.5 |
Data are expressed as the geometric mean of the percentage of total corresponding antibody isotype concentrations ± standard deviation.
Day 28 is 7 days after final immunization.
Only one mouse had detectable TbpB-specific antibodies.
Only two mice had detectable TbpB-specific antibodies.
Only three mice had detectable TbpA-specific antibodies.
n = 4; one mouse removed due to very low total IgG.
S.c., subcutaneous.
In contrast to antibody levels measured against TbpA, TbpB-specific IgA and IgG levels were robust as early as day 28 (Table 3). Vaginal IgA levels specific for TbpB were highest in groups immunized with the Ctb conjugates and were statistically different from those of the other IN immunized and subcutaneous groups (P < 0.05 day 28), and they remained significantly different through day 65 (P < 0.05). The day 28 TbpB-specific IgG responses were also robust, with the highest levels measured in the IN immunization groups immunized with the Ctb conjugates and in the subcutaneously immunized group. In the IN groups immunized with admixed Ctb, we were unable to detect TbpB-specific IgG on day 28; however, levels increased on subsequent days (Table 3), consistent with serum IgG increases (Fig. 1B). Though TbpB-specific levels of IgA and IgG were initially robust in the groups immunized with the TbpB-Ctb conjugate, they were in decline by day 65 (Table 3).
Vaginal antibody responses to Ctb were also robust and were generally higher than those responses measured against TbpA or TbpB (data not shown). This is presumably reflective of this antigen's higher immunogenicity and is consistent with the higher serum IgG levels shown in Fig. 1. The groups immunized with the Ctb conjugates, as opposed to the admixtures, generally induced the highest Ctb-specific antibody responses. Interestingly, the subcutaneously immunized group had high levels of Ctb-specific IgA, whereas subcutaneous immunization with the Tbps resulted in IgA levels that were almost zero (Tables 2 and 3 and data not shown).
Serum bactericidal activity.
In order to determine whether serum antibodies had bactericidal activity, we performed in vitro serum bactericidal assays using pooled mouse serum from day 35 with human serum as a complement source. The data demonstrate that those animals immunized with both Tbp-Ctb conjugates had the greatest bactericidal activity against both homologous and heterologous strains tested (Table 4). The sera from the group immunized with the TbpA-Ctb conjugate was more effective at killing the homologous strain (FA19) and one heterologous strain (MS11) than were the TbpB-Ctb sera. This outcome is interesting, considering the significantly lower serum antibody titers generated against TbpA in comparison to those generated against TbpB (Fig. 1A and B). The subcutaneously immunized group had the highest TbpA and TbpB antibody titers on day 35 (Fig. 1A and B); however, these sera were the least bactericidal against the homologous strain of all the groups tested. Furthermore, sera from this group were the only ones that failed to show bactericidal activity against any of the heterologous strains tested.
TABLE 4.
Serum bactericidal activities of sera collected at day 35
| Immunization group | Serum bactericidal titera for strain:
|
||
|---|---|---|---|
| FA19 | FA1090 | MS11 | |
| TbpA − Ctb + TbpB − Ctb | 800 (84 ± 5.7) | 200 (64 ± 1.4) | 400 (60 ± 0.7) |
| TbpA − Ctb | 400 (83 ± 4.2) | 25 (<50)b | 200 (64 ± 1.4) |
| TbpB − Ctb | 200 (64 ± 1.4) | 25 (<50)b | 50 (56 ± 4.9) |
| S.c.d TbpA + TbpB + Ctb | 100 (70 ± 1.4) | 25 (<50)b | 25 (78)b |
| TbpA only | 400 (71 ± 9.9) | NDc | ND |
Data are represented as the lowest reciprocal dilution that gave >50% killing. The average percent killing determined from duplicate assays ± standard deviation is shown in parentheses.
Assays conducted at 1/25 dilution were performed only once, and lower dilutions were not tested.
ND, not determined.
S.c., subcutaueous.
IgG subclass analysis.
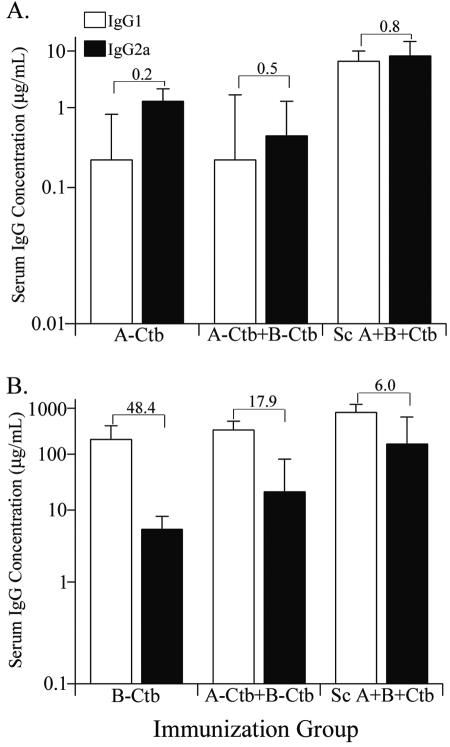
We performed IgG subclass analysis on selected serum samples in an effort to gain insight into why some serum pools performed better than others in bactericidal assays. It had been shown previously that mouse IgG2a is the most efficient IgG subclass in activating complement, while IgG1 is poor and may be inhibitory (16, 27, 44). We found that animals immunized with the TbpA-Ctb conjugate had higher IgG2a antibody responses, and hence a lower IgG1/IgG2a ratio (Fig. 3A). Those animals immunized with the TbpB-Ctb conjugate produced significantly more IgG1 than IgG2a antibodies and had a very high IgG1/IgG2a ratio (Fig. 3B). Interestingly, in the animals immunized simultaneously with both Ctb conjugates, the presence of TbpA and TbpB in the same antigen preparation influenced the IgG1-to-IgG2a ratio of antibodies elicited against the individual antigens. The presence of TbpA increased the level of IgG2a compared to TbpB, whereas the presence of TbpB resulted in increased production of IgG1 and decreased levels of IgG2a against TbpA. Contrary to expectations, the subcutaneously immunized animals had low IgG1/IgG2a ratios against both TbpA and TbpB; however, as demonstrated above, sera from this group performed the most poorly in terms of bactericidal activity.
FIG. 3.
IgG1 and IgG2a subtype analysis. (A) IgG1 and IgG2a antibody levels specific for TbpA detected in sera collected at day 35. (B) IgG1 and IgG2a antibody levels specific for TbpB detected in sera collected at day 35. The bars represent the geometric mean ×/÷ standard deviation. The values above the bars represent the IgG1/IgG2a ratios of the corresponding immunization groups, indicated below each graph. For all groups, n = 5.
DISCUSSION
Previous studies have shown IN immunization to be an effective means for the induction of serum and mucosal antigen-specific antibodies (21, 23, 24, 48, 49). The prolonged induction of genital tract antigen-specific antibodies following IN vaccination has highlighted this route of immunization as an attractive potential method for preventing sexually transmitted infections (37, 47). We explored this possibility by immunizing mice IN with recombinant transferrin binding protein A and/or B in conjunction with the mucosal adjuvant cholera toxin B. We demonstrated that IN immunization with these antigens is an effective means of eliciting specific serum and vaginal anti-Tbp antibodies. However, each Tbp antigen behaved differently in regard to overall immunogenicity.
TbpB was the more immunogenic of the two proteins. Large differences in immunogenicity between the antigens were apparent regardless of the route of immunization. IN immunization elicited the highest anti-TbpB titers when TbpB was conjugated to Ctb. Admixing TbpB with Ctb improved the immunogenicity of TbpB over control groups; however, in general, differences between admixed groups and those in which TbpB was conjugated to Ctb were statistically significant. TbpB was poorly immunogenic if administered alone in the absence of the Ctb adjuvant. By contrast, maximal TbpA-specific serum antibody responses following IN immunization were not dependent on the presence of Ctb. Mice immunized IN with TbpA alone elicited serum antibody titers similar to those generated by the group immunized with the TbpA-Ctb conjugate. This may have been the result of the inclusion of the nonionic detergent lauryl maltoside in the TbpA antigen preparations. Lauryl maltoside has been shown to act as an absorption enhancer in the nasal cavity (1, 32). This may have allowed better absorption of TbpA, as high-molecular-weight proteins are usually poorly absorbed in the nasal cavity without enhancers (36). On the other hand, the relatively poor immunogenicity of TbpB administered alone was likely due to the solubility of the TbpB used in this study. Native TbpB is a lipoprotein and is anchored to the bacterial outer membrane via a lipid tail, and it contains no predicted transmembrane segments. To simplify recombinant protein expression and purification, we expressed TbpB in E. coli without the amino-terminal cysteine, where lipidation normally occurs. Because of its overall hydrophilicity, it is likely that overexpressed, lipid-free TbpB would have been excluded from detergent micelles (22). The enhanced immunomodulatory effects with TbpB conjugated to Ctb, therefore, are likely due in part to binding of Ctb to GM1 ganglioside on nasal mucosa cells, which is thought to enhance antigen uptake and presentation to the immune system.
Interestingly, Ctb admixed with TbpA delayed the generation of antibodies against TbpA, as shown by the statistically significant differences in antibody titers measured on days 17 and 28. However, this effect was abrogated by day 65, at which time there were no significant differences in the levels of TbpA-specific antibody titers against TbpA among any of the IN immunized groups. The concurrent IN immunization with both TbpA and TbpB did not have a negative effect on levels of antibodies to either antigen compared to groups in which each antigen was administered alone; however, the IgG subclass distribution was influenced by the presence of either antigen. These alterations in IgG subclass distribution, however, did not appear to be deleterious, as the bactericidal activity of pooled sera from the group immunized with both TbpA and TbpB was superior to those of sera from animals immunized with a single antigen. This demonstrates that the two antigens can be administered simultaneously without negatively influencing antibody levels or serum bactericidal activity.
Similar to the situation with specific antibody levels in the serum, vaginal antibody responses to TbpB were generally much higher than those elicited against TbpA. The robust genital tract TbpB-specific antibody responses measured were also dependent on conjugation to Ctb, whereas this was not the case with TbpA. Immunization with TbpA elicited mostly IgA, while measurable IgG responses were low and sporadic. The low levels of TbpA-specific vaginal IgG were not surprising, as it is thought that most vaginal IgG originates from serum transudation (41). Although TbpA IgA levels were low, they remained mostly steady through day 65, except for a transient decrease on day 35. It is possible that this decrease could have resulted from the mouse estrus cycle, as levels of genital tract immunoglobulins fluctuate during the cycle (37). The levels of TbpB-specific IgA and IgG, though initially robust, decreased significantly during the course of the study. This decline in antibody levels over time is not uncommon. Wu et al. followed the genital tract antibody levels in IN immunized mice for a 1-year period (47). They demonstrated that by 4 months postimmunization, antibody levels had decreased extensively from their initial analysis but appeared to level out throughout the course of 1 year (47). The aim of the current study was not to characterize the duration of anti-Tbp immune responses, but future studies will address the longevity of the antibody response following IN immunization and whether these immune responses are protective.
We performed serum bactericidal assays as a correlate for the induction of protective antibody responses. We detected serum bactericidal activity against the homologous gonococcal strain (FA19) and two heterologous strains (FA1090 and MS11) using human serum as a complement source. We found that all IN immunization groups yielded sera with greater bactericidal activity than the subcutaneously immunized group. The group immunized IN with both TbpA and TbpB gave the highest serum bactericidal titers and was the only pool of sera that contained bactericidal antibodies reactive against all three strains tested. Surprisingly, the group immunized IN with TbpA elicited the second-highest bactericidal titers, in spite of the fact that serum IgG levels were approximately 20-fold lower than TbpB titers at that time point. This suggests that TbpA may be the more ideal target in the development of a vaccine. Studies have shown that TbpA is the more conserved of the two proteins (11, 12), which may be why TbpA elicited antibodies with more cross-bactericidal activity. Furthermore, in a meningococcal-vaccine study, mice immunized with TbpA or TbpA and TbpB were completely protected following lethal challenge, but the group immunized with TbpB only was not (46).
The obvious discrepancies between antibody titers and serum bactericidal activities suggested that qualitative rather than quantitative differences existed among antibody preparations, which prompted us to perform IgG subclass analysis. We found that those animals immunized IN with the TbpA-Ctb conjugate elicited higher levels of IgG2a than did those animals immunized with the TbpB-Ctb conjugate. In mice, the IgG2a isotype is the most efficient activator of complement, while the IgG1 isotype is the poorest complement activator (16, 27, 44). Thus, the lower IgG1/IgG2a ratio detected could in part explain the enhanced bactericidal activity observed with the TbpA antiserum. The results of the IgG subclass analysis do not, however, explain why the subcutaneously immunized group, immunized with both Tbps, differed so dramatically from its IN immunized counterpart in terms of bactericidal activity. The IgG1/IgG2a ratios against TbpA for both IN and subcutaneously immunized groups were similar (0.5 and 0.8) (Fig. 3). By contrast, the IgG1/IgG2a ratio against TbpB in the subcutaneously immunized group was nearly three times lower than that of the IN group (6.0 and 17.9) (Fig. 3). In spite of this, the bactericidal activity of the subcutaneously immunized group was comparatively poor. These results suggest that antigens delivered by IN immunization may better retain a native conformation than those delivered by subcutaneous immunization. Bactericidal activity is associated with high-avidity antibodies, elicitation of which correlates with the ability to keep protein antigens in native conformation (9). Furthermore, vaccine studies using meningococcal PorA demonstrated that PorA is immunogenic when administered via subcutaneous immunization in conjunction with a variety of adjuvants; however, only mice immunized with PorA contained in outer membrane vesicles or liposomes generated antibodies with bactericidal activity (2, 9). This suggests that antigens delivered subcutaneously may be subject to misfolding or possibly proteolysis unless they are protected in a membrane. By contrast, the current study indicates that protein degradation or misfolding, resulting in nonnative presentation, may not be as problematic if the antigens are delivered intranasally.
Whether bactericidal activity is an important mediator of immunity in the genital tract is a matter of speculation. Though complement lytic activity has been demonstrated in human cervical mucus (35), complement levels are highly variable among individuals and are influenced by hormonal cycles (43). Furthermore, IgA levels are high in the female genital tract, and IgA has been shown to be inhibitory to IgG complement activation (39). Therefore, bactericidal activity in the female genital tract may not be an important mediator of protection. Mucosal IgA has been shown to be important in the protection of the mucosal surfaces from invading bacteria, viruses, and toxins (39). Furthermore, studies have shown enhanced protective abilities of polymeric IgA compared to IgG in passive protective studies in mice (39). The precise roles of the different antibody isotypes in protection of the genital tract remain to be elucidated. However, studies performed with mice have shown that protection against Chlamydia trachomatis genital infection is attributable to IgA. Cui et al. showed that, following immunization and subsequent Chlamydia challenge, clearance of cervical chlamydial antigen correlated with increases in cervical IgA, but not in IgG (15). Furthermore, Pal et al. showed that a monoclonal IgA antibody against the chlamydial major outer membrane protein could confer passive protection in mice (31). Finally, in humans, levels of IgA in vaginal secretions and the amount of C. trachomatis isolated from the cervix are inversely correlated (7). In a recent study, IN immunization of mice with gonococcal outer membrane preparations resulted in strong serum bactericidal activity, and it decreased the gonococcal vaginal colonization of estradiol-treated mice (33). This study also showed that antigen-specific IgA titers were 8- to 16-fold higher than IgG titers in the mice with reduced vaginal colonization (33). These studies suggest that IgA may be more important than IgG for protection against sexually transmitted bacterial infections and highlight the importance of inducing genital IgA following vaccination.
In conclusion, we have demonstrated induction of both serum and vaginal antibodies following IN immunization with TbpA, TbpB, or both. TbpA was poorly immunogenic in comparison to TbpB in both serum and vaginal antibody responses; however, the bactericidal activities of TbpA-specific sera were much greater. This, combined with the greater degree of TbpA sequence conservation among gonococcal strains, suggests that TbpA may be the more efficacious vaccine target. Future studies will be aimed at enhancement of TbpA immunogenicity in an effort to elicit higher serum and vaginal antibody titers and at determining whether Tbp-specific antibodies in the genital tract can confer protection.
Acknowledgments
We gratefully acknowledge Heather Strange for excellent technical assistance and Chris Elkins and Heather Masri for advice on bactericidal assay methodology.
This work was supported by NIH grant AI47141 to C.N.C. and NIH grants AI46561 and DE06746 to M.W.R. G.A.P was supported by a Training in Molecular Pathogenesis grant (T32 AI07617) from the NIH.
Editor: J. T. Barbieri
REFERENCES
- 1.Ahsan, F., J. J. Arnold, E. Meezan, and D. J. Pillion. 2001. Mutual inhibition of the insulin absorption-enhancing properties of dodecylmaltoside and dimethyl-beta-cyclodextrin following nasal administration. Pharm. Res. 18:608-614. [DOI] [PubMed] [Google Scholar]
- 2.Arigita, C., G. F. Kersten, T. Hazendonk, W. E. Hennink, D. J. Crommelin, and W. Jiskoot. 2003. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 21:950-960. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist, C., E. L. Johansson, T. Lagergard, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boslego, J. W., E. C. Tramont, R. C. Chung, D. G. McChesney, J. Ciak, J. C. Sadoff, M. V. Piziak, J. D. Brown, C. C. Brinton, Jr., S. W. Wood, et al. 1991. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9:154-162. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet, J. P., L. Belec, R. Pires, and J. Pillot. 1994. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect. Immun. 62:3957-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broliden, K., J. Hinkula, C. Devito, P. Kiama, J. Kimani, D. Trabbatoni, J. J. Bwayo, M. Clerici, F. Plummer, and R. Kaul. 2001. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol. Lett. 79:29-36. [DOI] [PubMed] [Google Scholar]
- 7.Brunham, R. C., C. C. Kuo, L. Cles, and K. K. Holmes. 1983. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect. Immun. 39:1491-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2001. Sexually transmitted disease surveillance, 2001. Centers for Disease Control and Prevention, Atlanta, Ga.
- 9.Christodoulides, M., J. L. Brooks, E. Rattue, and J. E. Heckels. 1998. Immunization with recombinant class 1 outer-membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144:3027-3037. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, J. J. Eron, Jr., et al. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868-1873. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen, C. N., J. E. Anderson, I. C. Boulton, and P. F. Sparling. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect. Immun. 68:4725-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect. Immun. 65:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen, C. N., G. D. Biswas, and P. F. Sparling. 1993. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J. Bacteriol. 175:2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 15.Cui, Z. D., D. Tristram, L. J. LaScolea, T. Kwiatkowski, Jr., S. Kopti, and P. L. Ogra. 1991. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect. Immun. 59:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ey, P. L., G. J. Russell-Jones, and C. R. Jenkin. 1980. Isotypes of mouse IgG-I. Evidence for ‘non-complement-fixing’ IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol. Immunol. 17:699-710. [DOI] [PubMed] [Google Scholar]
- 17.Farley, T. A., D. A. Cohen, and W. Elkins. 2003. Asymptomatic sexually transmitted diseases: the case for screening. Prev. Med. 36:502-509. [DOI] [PubMed] [Google Scholar]
- 18.Gallichan, W. S., and K. L. Rosenthal. 1995. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine 13:1589-1595. [DOI] [PubMed] [Google Scholar]
- 19.Gerbase, A. C., J. T. Rowley, and T. E. Mertens. 1998. Global epidemiology of sexually transmitted diseases. Lancet 351:2-4. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg, L., B. B. Diena, F. A. Ashton, R. Wallace, C. P. Kenny, R. Znamirowski, H. Ferrari, and J. Atkinson. 1974. Gonococcal vaccine studies in Inuvik. Can. J. Public Health 65:29-33. [PubMed] [Google Scholar]
- 21.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 22.Helenius, A., and K. Simons. 1975. Solubilization of membranes by detergents. Biochim. Biophys. Acta 415:29-79. [DOI] [PubMed] [Google Scholar]
- 23.Isaka, M., Y. Yasuda, S. Kozuka, Y. Miura, T. Taniguchi, K. Matano, N. Goto, and K. Tochikubo. 1998. Systemic and mucosal immune responses of mice to aluminium-adsorbed or aluminium-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine 16:1620-1626. [DOI] [PubMed] [Google Scholar]
- 24.Isaka, M., Y. Yasuda, M. Mizokami, S. Kozuka, T. Taniguchi, K. Matano, J. Maeyama, K. Mizuno, K. Morokuma, K. Ohkuma, N. Goto, and K. Tochikubo. 2001. Mucosal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine 19:1460-1466. [DOI] [PubMed] [Google Scholar]
- 25.Ison, C. A., J. A. Dillon, and J. W. Tapsall. 1998. The epidemiology of global antibiotic resistance among Neisseria gonorrhoeae and Haemophilus ducreyi. Lancet 351:8-11. [DOI] [PubMed] [Google Scholar]
- 25a.Kellogg, D. S., Jr., W. L. Peacock, W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed]
- 26.Kenney, C. D., and C. N. Cornelissen. 2002. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J. Bacteriol. 184:6138-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koolwijk, P., J. H. Boot, R. Griep, and B. J. Bast. 1991. Binding of the human complement subcomponent C1q to hybrid mouse monoclonal antibodies. Mol. Immunol. 28:567-576. [DOI] [PubMed] [Google Scholar]
- 28.Meyer, T. F., J. Pohlner, and J. P. van Putten. 1994. Biology of the pathogenic Neisseriae. Curr. Top. Microbiol. Immunol. 192:283-317. [DOI] [PubMed] [Google Scholar]
- 29.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nardelli-Haefliger, D., R. Roden, C. Balmelli, A. Potts, J. Schiller, and P. De Grandi. 1999. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J. Virol. 73:9609-9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15:575-582. [DOI] [PubMed] [Google Scholar]
- 32.Pillion, D. J., S. Hosmer, and E. Meezan. 1998. Dodecylmaltoside-mediated nasal and ocular absorption of lyspro-insulin: independence of surfactant action from multimer dissociation. Pharm. Res. 15:1637-1639. [DOI] [PubMed] [Google Scholar]
- 33.Plante, M., A. Jerse, J. Hamel, F. Couture, C. R. Rioux, B. R. Brodeur, and D. Martin. 2000. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J. Infect. Dis. 182:848-855. [DOI] [PubMed] [Google Scholar]
- 34.Price, G. A., M. M. Hobbs, and C. N. Cornelissen. 2004. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect. Immun. 72:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price, R. J., and B. Boettcher. 1979. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil. Steril. 32:61-66. [DOI] [PubMed] [Google Scholar]
- 36.Quraishi, M. S., N. S. Jones, and J. D. Mason. 1997. The nasal delivery of drugs. Clin. Otolaryngol. 22:289-301. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal, K. L., and W. S. Gallichan. 1997. Challenges for vaccination against sexually-transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin. Immunol. 9:303-314. [DOI] [PubMed] [Google Scholar]
- 38.Rudin, A., G. C. Riise, and J. Holmgren. 1999. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 67:2884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell, M. W., and M. Kilian. 2005. Biological activities of IgA, p. 267-289. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, W. Strober, and J. R. McGhee (ed.), Mucosal immunology, 3rd ed., Elsevier/Academic Press, Amsterdam, The Netherlands.
- 40.Russell, M. W., P. F. Sparling, R. P. Morrison, S. Cauci, P. L. Fidel, D. Martin, E. W. Hook, and J. Mestecky. 2004. Mucosal immunology of sexually transmitted diseases, p. 1693-1720. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, W. Strober, and J. R. McGhee (ed.), Mucosal immunology, 3rd ed. Elsevier/Academic Press, Amsterdam, The Netherlands.
- 41.Russell, M. W., and J. Mestecky. 2002. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 4:667-677. [DOI] [PubMed] [Google Scholar]
- 42.Russell, M. W., Z. Moldoveanu, P. L. White, G. J. Sibert, J. Mestecky, and S. M. Michalek. 1996. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect. Immun. 64:1272-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumacher, G. F. 1988. Immunology of spermatozoa and cervical mucus. Hum. Reprod. 3:289-300. [DOI] [PubMed] [Google Scholar]
- 44.Seino, J., P. Eveleigh, S. Warnaar, L. J. van Haarlem, L. A. van Es, and M. R. Daha. 1993. Activation of human complement by mouse and mouse/human chimeric monoclonal antibodies. Clin. Exp. Immunol. 94:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tramont, E. C. 1989. Gonococcal vaccines. Clin. Microbiol. Rev. 2(Suppl.):S74-S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, H. Y., S. Abdu, D. Stinson, and M. W. Russell. 2000. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect. Immun. 68:5539-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, H. Y., and M. W. Russell. 1998. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16:286-292. [DOI] [PubMed] [Google Scholar]
- 49.Wu, H. Y., and M. W. Russell. 1993. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect. Immun. 61:314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]