Abstract
Preserving the ability to vividly recall emotionally rich experiences contributes to quality of life in older adulthood. While prior works suggest that moderate-intensity physical activity (MPA) may bolster memory, it is unclear whether this extends to emotionally salient memories consolidated during sleep. In the current study, older adults (mean age = 72.3 ± 5.8) completed an overnight polysomnography assessment with emotional memory tested before and after sleep and a self-report questionnaire assessing habitual PA. Results show that better negative emotional memory consolidation was associated with the frequency and duration of MPA. Statistically replacing 30 min of lower-intensity activity with MPA was associated with better negative emotional memory consolidation. MPA may enhance sleep-dependent consolidation of negative memories in older adults, with modest increases in MPA yielding significant consolidation benefits. Findings may guide interventions and inform public health recommendations by demonstrating that substituting even short durations of low-intensity activity for MPA could produce significant cognitive gains in older adulthood.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83336-0.
Subject terms: Sleep, Consolidation, Cognitive ageing
Introduction
As the global population continues to age1, identifying protective measures to preserve quality of life and stave off memory decline has become of critical importance. Excessive sedentary behavior2is highly prevalent among the elderly population, with some studies estimating that older adults are sedentary for 65–85% of the waking day3. Sedentary behaviors contribute to significant physical and cognitive health risks, including increased mortality4, impaired memory processing5, and affective dysfunction and disorders6. Aging is also associated with sleep changes, with more fragmented sleep and less time being spent in deeper sleep stages7. Sedentary behavior may partially contribute to these changes, as increased sedentary behavior is linked to sleep efficiency8. These behavioral shifts are particularly concerning given the critical role of sleep in the consolidation of memories9. Disruption in sleep due to aging can impair overnight memory consolidation, contributing to memory deficits7.
Frequent engagement in physical activity, on the other hand, seems to play a protective role against age-related memory decline10and may improve mood and affect11. Indeed, research from both animal models and human participants provide support for brain and cognitive benefits of physical activity (PA)12–16, particularly in the domains of episodic memory17and executive function18. In fact, PA improves subjective sleep quality19, sleep efficiency18, and bolsters neural oscillations involved in sleep-dependent memory consolidation20. However, not all memories are created equal, and physical activity21 and sleep may interactively contribute to the preferential consolidation and integrity of specific memory traces.
Research shows that memories containing emotional content are better remembered than non-emotional ones22. Sleep appears to selectively benefit the retention of emotional memories23–25, but there is age-related variability in this effect26. A recent study in younger to middle-aged adults suggests that positive memories may not always exhibit a benefit from sleep27and other work suggests that older adults may better remember the details of negative relative to positive stimuli28. Traditional emotional memory recognition tests, however, do not assess the quality of memory representations. This can be measured via mnemonic discrimination—a behavioral proxy for hippocampal pattern separation which facilitates the storage of similar experiences as unique, high fidelity memory traces29. While recognition memory for neutral events is generally preserved in aging30, older adults exhibit worse emotional (negative and positive) versus neutral memory recognition after a 24-hr consolidation period (which naturally encompasses sleep) and no consolidation benefit for emotional mnemonic discrimination31. It is possible that physical activity may bolster consolidation for emotional recognition memory and mnemonic discrimination in older adults, as work in young adults suggests that PA and fitness support mnemonic discrimination immediately following encoding32–36. Understanding these nuanced relationships among physical activity and sleep-related consolidation of emotional memory representations is important, as maintaining high fidelity, vivid representations of experiences embedded in rich emotional context may provide adaptive value and enhance quality of life in older adulthood37,38.
Given that moderate-vigorous PA is consistently tied to better cognitive and health outcomes39, an outstanding question is whether replacing sedentary behavior with moderate-intensity physical activity could yield significant improvements in sleep-related consolidation of emotional memories in older adults. Advances in statistical modelling enable the estimation of how health or behavior might change when substituting the time spent performing one type of activity with another40. This approach, called Isotemporal Substitution Modelling (ISM), aids in the identification of which activity substitutions are likely to facilitate better outcomes. A burgeoning literature using this theoretical modelling demonstrates that reallocating time spent in sedentary or non-exertive behavior to moderate-intensity physical activity (MPA) improves a wide range of health outcomes41,42. A recent study found that replacing sedentary time for light-intensity PA also improves episodic memory function in middle- and older-aged Latine adults43. However, this method has not yet been applied to sleep-related emotional memory consolidation in older adults, which is critical, as impairments in sleep-related memory processing contribute to age-related memory deficits7. It is unknown whether replacing time spent in lower-intensity activities with MPA supports the consolidation of memory for emotionally laden experiences in the elderly. Understanding these relationships is essential, as these findings can inform the development of targeted interventions and inform public health recommendations aimed at preserving memory in late life.
To address these knowledge gaps, the current study assessed memory performance on an emotional variant of the Mnemonic Discrimination Task (eMDT) prior to and following overnight sleep and self-reported habitual PA engagement over the four weeks prior to an in-lab polysomnography (PSG) study in a sample of cognitively unimpaired older adults. Two primary hypotheses were tested: (1) that more frequent and longer duration engagement in MPA would be specifically associated with better overnight retention of negative emotional memories when assessing both mnemonic discrimination and recognition memory, and that (2) replacing time spent in lower-intensity activities with MPA would yield a significant sleep-related negative memory retention benefit.
Methods
Study participant details
Forty cognitively unimpaired older adults between the ages of 60–85 years were recruited from the Biomarker Exploration in Aging, Cognition, and Neurodegeneration (BEACoN) cohort for an in-lab overnight polysomnography (PSG) assessment (µage = 72.3 ± 5.8, 26 Female, Mini Mental State Exam scores > 24, Table 1). Participants were free of neurological and psychiatric disorders and were not taking sleep or cognition-affecting medications such as sedative hypnotics, non-SSRI antidepressants, anxiolytics, or neuroleptics. Participants did not have any prior diagnosis of sleep disorders other than sleep-disordered breathing (SDB) prior to participating and those with a prior SDB diagnosis were not being treated with continuous positive airway pressure. The severity of SDB was evaluated using the apnea-hypopnea index (AHI) which was derived from the PSG assessment and included as a covariate in all statistical models. Participants had not traveled more than three time zones within the three weeks prior to participating. On the day of the sleep study, participants were instructed to not consume caffeine any time after 9:00 AM. All participants provided written informed consent according to procedures approved by the University of California, Irvine Institutional Review Board (UCI IRB). All protocols were approved by the UCI IRB and all methods were performed in accordance with the relevant guidelines and regulations of the IRB.
Table 1.
Sample characteristics.
| Sample Characteristics | N = 40 |
|---|---|
| Age, Mean [SD] | 72.3 [5.8] |
| Sex, No. Female (%) | 26 (65) |
| Years of Education, Mean [SD] | 16.7 [2.2] |
| BMI, Mean [SD] | 24.7 [5.6] |
| AHI, Mean [SD] | 13.4 [17.4] |
| Race, No. | |
| African American or Black | 1 |
| Asian | 10 |
| White | 2 |
| More than one race | 26 |
| Unknown/Not Reported | 1 |
| Ethnicity, No. | |
| Hispanic | 2 |
| Non-Hispanic | 38 |
| Sedentary Activity | |
| Frequency (bouts/wk), Mean [SD] | 18.7 [18.0] |
| Duration (hours/wk), Mean [SD] | 17.0 [5.9] |
| Non-Exertive Activity | |
| Frequency (bouts/wk), Mean [SD] | 7.8 [4.1] |
| Duration (hours/wk), Mean [SD] | 16.6 [9.8] |
| Light-Intensity Physical Activity | |
| Frequency (bouts/wk), Mean [SD] | 11.8 [5.0] |
| Duration (hours/wk), Mean [SD] | 9.8 [7.0] |
| Moderate-Intensity Physical Activity | |
| Frequency (bouts/wk), Mean [SD] | 8.8 [6.9] |
| Duration (hours/wk), Mean [SD] | 9.6 [9.1] |
Community Healthy Activities Model Program for Seniors (CHAMPS) Activities Questionnaire for Older Adults
The CHAMPS Activities Questionnaire for Older Adults is a 40-item self-report questionnaire assessing engagement in various age-appropriate physical and lifestyle activities of various intensities during a ‘typical week’ when considering the prior four weeks44. CHAMPS, as published, quantifies the frequency (number of times per week) and duration (total hours per week) of each reported activity to derive measures of frequency and duration of all exercise-related activities and moderate-intensity exercise-related activities. Given evidence that moderate-to-vigorous PA yields the greatest memory benefit17our analyses were initially a priori restricted to the frequency and duration of moderate-intensity exercise-related activities, as published. The frequency and duration of light-intensity PA were derived to explore the specificity of PA intensity effects. A strength of this questionnaire is the inclusion of other, non-exertive activities that reduce socially desirable responding by allowing older adults who are less physically active to report other meaningful activities44. Although not calculated in the original publication, the inclusion of these less physically active items allows for the quantification of frequency and duration of non-exertive and sedentary behavior. These variables were also derived and examined to determine the specificity of intensity-related findings. See Tables S1-S3 for questionnaire scoring and details on the categorization of items into PA intensities. For ISM, each activity type was categorized (MPA as published in CHAMPS), and total weekly durations of each activity type were converted to average daily minutes and then rescaled to 30 min intervals for substitution (Table S2 & S4). For greater detail on which activities were categorized as sedentary, non-exertive, light-intensity, and moderate-intensity, see Table S3.
Emotional mnemonic discrimination task
Participants performed the emotional version of the Mnemonic Discrimination Task (eMDT) prior to and following overnight sleep45. The eMDT is an established framework to measure emotionally-modulated hippocampal-dependent memory45,46. Participants performed an incidental encoding phase followed by a surprise memory test prior to sleep and a delayed memory test the following morning. During the incidental encoding phase, 180 emotionally salient images were presented on black background for 4 seconds. Participants were not informed that they would be subsequently tested on the stimuli presented during encoding. While viewing, participants rated each image on valence (i.e., positive, negative, or neutral) using three individually colored orby button switches (P.I. Engineering, Williamston, MI). A white fixation cross was displayed on a black background for 500ms between image presentations. Immediately following the encoding phase, participants underwent a surprise memory test (immediate test phase) wherein they were presented with another 120 emotionally salient images split evenly among images they had seen during encoding (targets, 30), entirely new images (foils, 30), and images similar to those they saw during encoding (lures, 60). Lures could be somewhat similar to targets (low similarity, 30) or highly similar to targets (high similarity, 30). Participants were informed prior to performing the test phases that some images would be identical to those in the incidental encoding phase, some would be new, and some would be similar to those in the encoding phase. Participants indicated via button press whether they had seen the image before (‘old’) or whether it was an entirely new image (‘new’). Although participants should classify both foils and lure images as ‘new’, it should be noted that the brain likely processes these two types of images differently; lures tax pattern separation processes reliant upon dentate gyrus/cornu ammonis 3 hippocampal subfields, whereas foil images tax neural circuits involved in recollection and familiarity, such as parahippocampal cortices47,48. Importantly, the inclusion of both of these image types in the task allowed us to test the relationships between physical activity and memory function under conditions of moderate-to-high (LDI) and no interference (d’), as outlined below. Following overnight PSG, participants completed another surprise memory test (delayed test phase), during which they viewed and responded to another 120 images split evenly among emotionally valent targets, foils, and lures as outlined above. No targets or corresponding lures presented in the immediate test phase were shown during the delayed test phase.
Recognition memory was calculated using d’, defined as z(‘old’|Targets) – z(‘old’|Foils). Mnemonic discrimination performance (behavioral proxy of hippocampal pattern separation) was calculated using the Lure Discrimination Index (LDI), defined as p(‘new’|lures) – p(‘new’|Targets).31Importantly, the calculation for LDI corrects for response bias, thereby assessing memory independent of overall response patterns26,29,45. LDI was calculated collapsing across similarity bins for each valence condition at both immediate and delayed testing timepoints; d’ was calculated for each valence at both timepoints. Overnight memory retention for mnemonic discrimination ability and recognition memory were measured via overnight change in LDI (LDIdelayed test-LDIimmediate test) and d’ (d’delayed test-d’immediate test), respectively. Both LDI and d’ were analyzed to assess memory processing under conditions of moderate-to-high levels of interference (as measured by LDI) or no interference (as indicated by d’).
Statistical analyses
All analyses were conducted in SPSS v27.0 (IBM SPSS Statistics, Inc., Chicago, IL) and figures were created in RStudio v2022.12.0 + 353. Assumptions of multiple linear regression were checked. Frequency of MPA and duration of light-intensity PA were logarithm transformed (i.e., log(1 + x)) and the duration of MPA was cube-root transformed to meet assumptions of normality.
Multiple regression analyses were used to examine relationships between self-reported MPA and overnight emotional memory retention (LDI and d’ for negative and positive stimuli) while adjusting for age, sex, and AHI as covariates. To show specificity of the relationships between MPA and the consolidation of negative emotional stimuli, exploratory analyses were conducted using d’ for neutral images. While prior work has shown that SDB severity impacts emotional memory consolidation and sleep neurophysiology49,50, AHI was not associated with overnight emotional recognition memory retention in the current sample (r = 0.060, p= 0.713). Despite the lack of a significant association between AHI and overnight emotional memory retention, AHI was included as a covariate due to the complexity of SDB effects on neurobiology and behavior50. Primary analyses focused on MPA frequency (total number of bouts per week) and duration (total number of hours per week), as prior works have demonstrated that MPA is most consistently tied to cognitive performance17,51,52. Follow-up multiple regression analyses were performed additionally adjusting for sleep duration (minutes). To examine potential effects of PA intensity, control analyses examined effects of light-intensity PA and non-exertive activity frequency and/or duration adjusting for covariates. Follow-up multiple regression analyses adjusting for the same covariates were performed to determine whether relationships between MPA and overnight emotional memory retention were driven by relationships with immediate or delayed test recognition memory (d’) performance. Similarly, follow-up multiple regression analyses were performed adjusting for sleep duration. False Discovery Rate (FDR) correction was used to adjust for multiple comparisons for models predicting overnight change in negative emotional recognition memory from MPA frequency (3 comparisons: MPA, LPA, non-exertive) and MPA duration (3 comparisons: MPA, LPA, non-exertive).
Isotemporal substitution model
Isotemporal substitution analyses evaluate the effect of reallocating a set duration of time from one activity type to another, while keeping total time constant40. ISM was performed to determine whether reallocating 30 min of sedentary behavior, non-exertive activity, or light-intensity PA to MPA would yield an overnight emotional memory consolidation benefit. A 30 min time allocation was selected based on previous literature40and public health relevance53. The ISM constructed for the current analyses can be expressed as:
Memory retention = b1(Sedentary behavior) + b2(Non−exertive activity) + b3(Light−intensity PA) + b4(MPA) + b5(Total time).
Where b1-b5 are the unstandardized regression coefficients. ISM is conducted by iteratively eliminating one activity component from the model and evaluating the remaining coefficients for each remaining activity type. The remaining coefficients represent the effect of substituting 30 min/day of the eliminated activity type for the remaining activities in the model, while holding total discretionary time constant. For example, by eliminating b1 (sedentary time) from the model, the remaining coefficients represent the effect of substituting 30 min of sedentary time for that respective activity (e.g., non-exertive activity, light-intensity PA, and MPA), while holding time spent in other activities and total time constant. Pearson’s correlations were performed to test for multicollinearity; importantly, average daily minutes spent in each activity type were not significantly correlated (See Table S5). Three separate ISMs were constructed separately to evaluate the effects of time reallocation on overnight negative, positive, and neutral memory retention, respectively. Given that the intensity of some activities could not be determined given the lack of specificity into some aspects of the questionnaire (e.g., type of musical instrument played), we re-constructed ISM re-categorizing some activities up an intensity category to test for consistency in our findings (by MCF & CEK). Namely, we recategorized item 17 from sedentary to light-intensity and item 13 from non-exertive to light-intensity. These models produced similar results (see Tables S6-S8).
Results
Moderate-intensity physical activity is associated with overnight consolidation of negative emotional memories
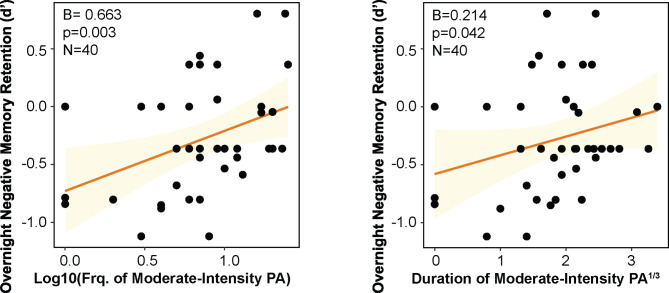
First, we examined whether MPA frequency was associated with overnight retention of emotional mnemonic discrimination. A multiple regression model adjusting for age, sex and AHI (hereafter referred to as covariates) revealed no statistically significant association between MPA and overnight retention of mnemonic discrimination for negative stimuli (B = 0.149, SE = 0.101, p = 0.149). Similarly, no relationships were found with overnight change in positive LDI (β=−0.034, SE = 0.092, p = 0.714) or neutral LDI (B = 0.025, SE = 0.095, p = 0.792). However, a regression model using overnight change in negative d’ to measure recognition memory performance revealed that greater frequency of MPA was associated with better overnight negative emotional recognition memory retention (B = 0.663, SE = 0.212, p = 0.003, FDR-adjusted p = 0.009; Fig. 1a). This result remained statistically significant when sleep duration was added into the model as an additional covariate (B = 0.666, SE = 0.221, p = 0.005). Importantly, this relationship was specific to negative emotional items, as MPA frequency was not associated with overnight change in positive recognition memory d’ (β=−0.304, SE = 0.363, p = 0.407) or neutral recognition memory d’ (B = 0.216, SE = 0.308, p = 0.487). This remained the case when sleep duration was added into the model (neutral recognition memory d’: B = 0.252, SE = 0.321, p = 0.438). The frequency of light-intensity PA (B = 0.002, SE = 0.018, p = 0.891, FDR-adjusted p = 0.891) and non-exertive activity (B=−0.020, SE = 0.022, p = 0.365, FDR-adjusted p = 0.548) was not associated with overnight change in negative emotional recognition memory. When CHAMPS items 13 and 17 were recategorized as light-intensity PA, the frequency of light intensity PA (B = 0.005, SE = 0.017, p = 0.748) and non-exertive activity (B=−0.019, SE = 0.022, p = 0.399) still exhibited no statistically significantly associations with overnight retention of negative recognition memory.
Fig. 1.
(A) More frequent and (B) longer duration moderate-intensity physical activity is associated with better overnight retention of negative emotional memories. Abbreviations: Frq—Frequency; PA—Physical activity.
Next, the relationship between MPA duration and overnight retention of emotional recognition memory was tested. Greater duration of MPA was also associated with better overnight negative recognition memory retention (d’), adjusting for covariates (B = 0.214, SE = 0.101, p = 0.042; Fig. 1b). However, this did not survive correction for multiple comparisons (FDR-adjusted p = 0.126). This relationship was trending when sleep duration was added as an additional model covariate (B = 0.210, SE = 0.106, p = 0.055). MPA duration was not related to positive recognition memory retention (d’), adjusting for covariates (β=−0.173, SE = 0.162, p = 0.292). Of note, duration of light-intensity PA (B = 0.006, SE = 0.012, p = 0.611, FDR-adjusted p = 0.873) and non-exertive activity (B=−0.001, SE = 0.009, p = 0.873, FDR-adjusted p = 0.873) was not associated with overnight negative recognition memory retention. These results remained when CHAMPS items 13 and 17 were recategorized as light-intensity PA (B = 0.009, SE = 0.012, p = 0.471) and non-exertive activity (B=−0.001, SE = 0.009, p = 0.939). These results suggest that frequency and duration of MPA, specifically, support overnight negative emotional recognition memory retention, but not mnemonic discrimination.
Follow-up multiple regression analyses were conducted to determine whether the relationship between MPA and overnight negative emotional recognition memory retention was driven by performance at immediate or delayed test. Neither the frequency (B=−0.242, SE = 0.193, p = 0.219) nor the duration (B=−0.029, SE = 0.088, p = 0.748) of MPA was associated with negative emotional recognition memory performance at the immediate testing timepoint, when adjusting for the same covariates. Results were similar when sleep duration was included as a covariate (MPA frequency: B=−0.225, SE = 0.202, p = 0.272; MPA duration: B=−0.016, SE = 0.092, p = 0.866). However, MPA frequency (B = 0.421, SE = 0.230, p = 0.076) and duration (B = 0.185, SE = 0.103, p = 0.082) exhibited trending relationships with performance at delayed test, further suggesting that MPA engagement supports the consolidation process in older adults. This finding was similar when sleep duration was included as an additional covariate in these models (MPA frequency: B = 0.441, SE = 0.240, p = 0.075; MPA duration: B = 0.195, SE = 0.108, p = 0.081).
To explore whether these relationships were due to MPA effects on sleep architecture, Pearson’s correlations were tested to examine if MPA frequency or duration was associated with measures of global sleep architecture. These models revealed no statistically significant associations between MPA frequency or duration with total sleep time, sleep efficiency (time spent asleep/time in bed), wake after sleep onset, percentage of time spent in non-rapid eye movement sleep (NREM) stage 1, stage 2, stage 3, or REM (Tables S9-S10).
Replacing 30 min of lower-intensity activity with moderate-intensity physical activity yields a negative emotional memory consolidation benefit
ISM was performed to determine whether theoretically replacing 30 min of lower-intensity activity (i.e., sedentary behavior, non-exertive activity, light-intensity PA) with MPA would be associated with better negative emotional memory consolidation. Reallocating 30 min of non-exertive activity to MPA was associated with better overnight negative emotional memory retention (B = 0.108, SE = 0.048, p = 0.030; Table 2, Model B). Reallocation of non-exertive activity to either sedentary time or light-intensity PA did not impact overnight negative emotional memory retention. Reallocation of 30 min of light-intensity PA to moderate-intensity activity showed a trending association with better overnight negative memory retention (B = 0.100, SE = 0.059, p = 0.099; Table 2, Model C). Importantly, the impact of time reallocation on overnight memory processing was specific to negative emotional events, as no relationships were observed with neutral (Table 3) or positive stimuli (Table 4). When CHAMPS items 13 and 17 were recategorized as light-intensity PA, the ISM analyses produced similar findings (Tables S6-S8). These findings provide further support for the specificity of MPA effects on sleep-related consolidation of negative memories and suggest that replacing even a short duration of lower-intensity activity for MPA enhances memory consolidation.
Table 2.
Isotemporal substitution model: The effect of reallocating 30 min of time spent engaged in sedentary behavior, non-exertive activities, light-intensity physical activity, and moderate-intensity physical activity on overnight negative emotional memory retention (N = 40).
| Isotemporal Substitution Model | Type of Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SED | Non-Exertive | LPA | MPA | |||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Model A: Substitution of SED | — | — | — | −0.082 | 0.051 | 0.117 | −0.073 | 0.060 | 0.235 | 0.027 | 0.063 | 0.675 |
| Model B: Substitution of Non-Exertive | 0.082 | 0.051 | 0.117 | — | — | — | 0.009 | 0.050 | 0.862 | 0.108 | 0.048 | 0.030* |
| Model C: Substitution of LPA | 0.073 | 0.060 | 0.235 | −0.009 | 0.050 | 0.862 | — | — | — | 0.100 | 0.059 | 0.099t |
| Model D: Substitution of MPA | −0.027 | 0.063 | 0.675 | −0.108 | 0.048 | 0.030* | −0.100 | 0.059 | 0.099t | — | — | — |
Data are unadjusted for covariates. Negative values indicate worse overnight negative emotional memory retention. tp < 0.10, *p < 0.05, **p < 0.01.
Table 3.
Isotemporal substitution model: The effect of reallocating 30 min of time spent engaged in sedentary behavior, non-exertive activities, light-intensity physical activity, and moderate-intensity physical activity on overnight neutral memory retention (N = 40).
| Isotemporal Substitution Model | Type of Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SED | Non-Exertive | LPA | MPA | |||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Model A: Substitution of SED | — | — | — | 0.051 | 0.073 | 0.490 | 0.087 | 0.087 | 0.323 | 0.022 | 0.091 | 0.808 |
| Model B: Substitution of Non-Exertive | −0.051 | 0.073 | 0.490 | — | — | — | 0.036 | 0.506 | 0.616 | −0.029 | 0.069 | 0.679 |
| Model C: Substitution of LPA | −0.087 | 0.087 | 0.323 | −0.036 | 0.072 | 0.616 | — | — | — | −0.065 | 0.085 | 0.449 |
| Model D: Substitution of MPA | −0.022 | 0.091 | 0.808 | 0.029 | 0.069 | 0.679 | 0.065 | 0.085 | 0.449 | — | — | — |
Data are unadjusted for covariates. Negative values indicate worse overnight neutral memory retention. tp < 0.10, *p < 0.05, **p < 0.01.
Abbreviations: SED—sedentary behavior; LPA—light-intensity physical activity; MPA—moderate intensity physical activity; B—unstandardized regression coefficient; SE—standard error; p—p-value.
Table 4.
Isotemporal substitution model: The effect of reallocating 30 min of time spent engaged in sedentary behavior, non-exertive activities, light-intensity physical activity, and moderate-intensity physical activity on overnight positive emotional memory retention (N = 40).
| Isotemporal Substitution Model | Type of Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SED | Non-Exertive | LPA | MPA | |||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Model A: Substitution of SED | — | — | — | 0.064 | 0.086 | 0.463 | −0.072 | 0.102 | 0.488 | −0.063 | 0.107 | 0.560 |
| Model B: Substitution of Non-Exertive | −0.064 | 0.086 | 0.463 | — | — | — | −0.135 | 0.084 | 0.115 | −0.127 | 0.081 | 0.126 |
| Model C: Substitution of LPA | 0.072 | 0.102 | 0.488 | 0.135 | 0.084 | 0.115 | — | — | — | 0.009 | 0.099 | 0.932 |
| Model D: Substitution of MPA | 0.063 | 0.107 | 0.560 | 0.127 | 0.081 | 0.126 | −0.009 | 0.099 | 0.932 | — | — | — |
Data are unadjusted for covariates. Negative values indicate worse overnight positive memory retention. tp < 0.10, *p < 0.05, **p < 0.01.
Abbreviations: SED—sedentary behavior; LPA—light-intensity physical activity; MPA—moderate intensity physical activity; B—unstandardized regression coefficient; SE—standard error; p—p-value.
Discussion
Results from the current study suggest that higher engagement in MPA (both frequency and duration) supports sleep-related negative emotional memory consolidation in older adults. Multiple regression analyses revealed that greater MPA was associated with better overnight negative emotional memory retention. ISM demonstrated that replacing 30 min of non-exertive activity with MPA yields an improvement in overnight negative emotional memory consolidation. Notably, each of these findings was specific to memories containing negative emotional content. These findings provide the first evidence that reallocating just 30 min of time spent in non-exertive activities to MPA yields a memory consolidation benefit in older adults, a population at risk for cognitive decline. Targeted interventions and policies aimed at promoting memory preservation in older adults should include the substitution of more sedentary behaviors with MPA, even for short durations.
Prior work in older adults using ISM has shown that substituting 30 min of lower-intensity activity (i.e., sedentary behavior, light-intensity physical activity) with MPA is associated with better mental health54, executive function43, self-regulation55, and episodic memory43. The current findings build on this literature, showing that substituting just 30 min of non-active time with MPA may result in significant improvements in sleep-dependent consolidation of negative emotional memories in older adults. Notably, MPA items of the CHAMPS questionnaire constitute aerobic PA, which has been consistently tied to better memory function. In fact, several studies even suggest that aerobic PA may bias offline processing towards retention of emotional stimuli via changes in stress hormone action, neurotransmitter release, increased levels of brain-derived neurotrophic factor, and changes in autonomic activity21,56,57. PA appears to have time-dependent or cumulative effects on memory processing depending on the frequency of engagement58. Acute exercise seems to yield the greatest memory boost when it is temporally coupled to learning, and recent work has demonstrated that a post-encoding aerobic exercise bout selectively promotes the consolidation of emotional experiences57. In contrast, the effects of long-term PA appear to be cumulative and less time-dependent, driving changes in the neurophysiology of the medial temporal lobe (MTL) memory system over time with repeated bouts facilitating maintenance of memory enhancement58,59. In line with this notion, our findings that MPA engagement (frequency and duration) is significantly associated with overnight negative emotional recognition memory consolidation and delayed retrieval—and not performance at immediate test—suggest that PA supports consolidation during sleep rather than simply enhancing encoding processes in older adulthood.
This study found that PA was specifically associated with negative recognition memory but not negative mnemonic discrimination ability. Furthermore, findings suggest that while MPA supports sleep-related memory consolidation, sedentary behavior and light-intensity activity do not necessarily actively impair this process, as evidenced by a lack of negative correlations between these measures. These findings underscore that engagement in MPA may bolster consolidation processes occurring during sleep in older adults. Our findings were specific to emotional images with negative but not positive valence, which may suggest that MPA augments the preferential consolidation of negative experiences during sleep. Indeed, prior work has shown that negative elements of an experience are preferentially consolidated during sleep24,27,60and that, irrespective of age, positive experiences do not broadly exhibit a sleep benefit27. However, it should be noted that positive and negative images in the eMDT are not matched on arousal, making it difficult to appropriately disentangle the effects of positive versus negative valence46. Future work should parse arousal and valence effects to determine which component modulates physical activity effects on sleep-related memory retention.
The finding that MPA was not associated with mnemonic discrimination performance contrasts research conducted in young adults, though these studies did not assess mnemonic discrimination performance over a period of sleep32–35. While a recent study in physically active older adults found that object recognition memory significantly improved following a moderate-intensity aerobic exercise bout, mnemonic discrimination did not61. However, findings in this domain are generally mixed62. One study in initially inactive older adults found improved mnemonic discrimination performance following a chronic high-intensity exercise paradigm—an effect related to improved cardiorespiratory fitness63—while other studies in physically fit older adults found no relationship with cardiorespiratory fitness or any intervention-related changes in performance64–66. On the other hand, another study found improved mnemonic discrimination after one bout of light-intensity aerobic exercise; however, the fitness level of participants prior to participation was not assessed67. It is important to note that participants in the current study were relatively physically active, with 80% of the study participants meeting guidelines for MPA (≥ 150 min per week)68. While several of these prior studies did not directly compare recognition memory and mnemonic discrimination63–66, our findings are consistent with those including samples of physically active older adults in that habitual PA was not associated with mnemonic discrimination performance.
The potential dissociation between mnemonic discrimination and recognition memory in our sample, and other works61, is interesting and may highlight the importance of considering the baseline fitness level of participants. Perhaps, in physically active older adults, PA-related memory improvements extend beyond the hippocampus (which facilitates mnemonic discrimination) to other MTL structures to drive improvements in other facets of memory processing that support recognition memory. One possibility is that MPA bolsters familiarity, which is a key component of recognition memory that is less reliant on the hippocampus and more closely tied to perirhinal and parahippocampal cortex function47,48. Indeed, MPA has been shown to strengthen functional connectivity in perirhinal and parahippocampal cortices17, and PA increases parahippocampal blood-oxygen-level-dependent signal activation, angiogenesis, and gray matter volumes21. Additionally, given that older adults often rely on familiarity-based strategies to compensate for memory deficits69, it is possible that engagement in MPA may bolster this adaptive function. Future studies should investigate whether acute manipulation of physical activity bolsters sleep-dependent consolidation of negative events.
This study is the first to demonstrate that MPA specifically supports sleep-related negative emotional memory retention. The use of the eMDT allowed us to investigate valence-specific differences, as well as differential effects on recognition memory and mnemonic discrimination. The use of the CHAMPS questionnaire enabled us to probe different aspects of PA across the human movement spectrum. The use of ISM in this context provided novel insights into the effects of reallocating sedentary time to PA, demonstrating that replacing just 30 min of low-intensity activity for MPA yields a memory consolidation benefit for in older adults. However, this study is not without limitations. PA was assessed using a self-report questionnaire; while allowing for the quantification of self-reported frequency and duration of engagement, this limited our ability to differentiate different activity types due to their inclusion in a single questionnaire item and limited our ability to evaluate the effects of physical fitness. Furthermore, as the questionnaire assessed typical activity engagement over the past four weeks, PA engagement on the day of the overnight sleep study visit was not assessed, thereby limiting the ability to differentiate acute versus chronic effects on memory performance. Additionally, total time spent in each activity was assumed to be equal across each day of the week for ISM. Future work should utilize device-based monitoring to assess daily sedentary behavior and PA engagement, as well as objective fitness assessments. Participants in the current study were relatively active, as evidenced by meeting PA guidelines for moderate intensity activity, and exhibited SDB symptoms, both of which may independently or interactively influence neurophysiology associated with sleep-dependent memory processing. For this reason, AHI was included as a covariate in our analyses. While the average AHI in our sample was representative of community dwelling older adults70, future studies should examine whether these relationships differ in older adult populations with lower SDB burden. While global measures of sleep architecture were not associated with MPA engagement, future studies utilizing larger samples should investigate whether MPA is associated with the expression of sleep oscillations implicated in the consolidation process, such as slow oscillations and sleep spindles9. Lastly, the cross-sectional design limits our ability to definitively infer causal relationships between PA and emotional memory consolidation. Future studies should use objective measures of fitness and PA (i.e., VO2 max, actigraphy) and/or exercise manipulations in a larger, more diverse sample free of SDB to determine whether reallocating 30 min of low intensity activity improves memory function. Despite these limitations, the findings from the current study have important public health implications and can inform interventions and recommendations to preserve memory function in later life.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Abbreviations
- SED
sedentary behavior
- LPA
light—intensity physical activity
- MPA
moderate intensity physical activity
- B
unstandardized regression coefficient
- SE
standard error
- p
p—value
- PA
physical activity
- eMDT
emotional Mnemonic Discrimination Task
- LDI
Lure Discrimination Index
- SDB
Sleep-disordered breathing
- AHI
Apnea-Hypopnea Index
- PSG
polysomnography
- ISM
Isotemporal substitution model
- FDR
False DiscoveryRate
- NREM
Non-rapid eye movement sleep
- MTL
Medial temporal lobe
- Frq
Frequency
Author contributions
Conceptualization, M.G.C-F., M.A.Y, B.A.M.; Data Acquisition, M.G.C-F, D.E.B, A.D., N.S., K.K.L., I.Y.C.; Methodology & Software, M.G.C-F., J.T.J., R.M.S., C.E.K.; Formal Analysis, M.G.C-F.; Writing – Original Draft, M.G.C-F; Writing – Reviewing and Editing, all authors; Funding Acquisition, M.G.C-F, D.E.B., R.M.B., M.A.Y., B.A.M.
Funding
This research was supported by grants NIA F31AG074703, NIA F31AG084308, NHLBI T32HL082610, NIA R21AG07955, NIA R01AG053555, NIA K01AG058353, and the American Academy of Sleep Medicine Strategic Research Award. We would like to thank all of the research participants who generously devoted their time to this study, as well as the laboratory technicians, research specialists, and undergraduate research assistants to aided in data collection.
Data availability
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Declarations
Competing interests
Dr. Mander has served as a consultant to Eisai and AstronauTx. Dr. Yassa has served as a consultant for Eisai, Pfizer, Cognito Therapeutics, Dart Neuroscience, Curasen Therapeutics, Myosin Therapeutics and BPT Pharma. He is also co-founder and scientific advisor for Augnition Labs and Enthorin Therapeutics. Dr. Benca has served as a consultant to Eisai, Idorsia, Merck, Sage, and Genentech. Dr. Chappel-Farley has served as a consultant to Apnimed. The other authors declare no conflicts of interest relevant to this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vincent, G. K., Velkoff, V. A. & Bureau, U. S. C. The Next Four Decades: The Older Population in the United States: 2010 to 2050 (U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau), 2010). [Google Scholar]
- 2.Tremblay, M. S. et al. Sedentary Behavior Research Network (SBRN) – Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Activity. 14, 75. 10.1186/s12966-017-0525-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey, J. A., Chastin, S. F. M. & Skelton, D. A. How Sedentary are Older People? A Systematic Review of the Amount of Sedentary Behavior. J. Aging Phys. Act.23, 471–487. 10.1123/japa.2014-0164 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Stamatakis, E. et al. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol.73, 2062–2072. 10.1016/j.jacc.2019.02.031 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Coelho, L. et al. The association between sedentary behavior and cognitive ability in older adults. Aging Clin. Exp. Res.32, 2339–2347. 10.1007/s40520-019-01460-8 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Luo, Y. et al. Symptoms of depression are related to sedentary behavior and sleep duration in elderly individuals: A cross-sectional study of 49,317 older Chinese adults. J. Affect. Disord.308, 407–412. 10.1016/j.jad.2022.04.102 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Mander, B. A., Winer, J. R. & Walker, M. P. Sleep. Hum. Aging Neuron94, 19–36. 10.1016/j.neuron.2017.02.004. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubelmann, C., Heinzer, R., Haba-Rubio, J., Vollenweider, P. & Marques-Vidal, P. Physical activity is associated with higher sleep efficiency in the general population: the CoLaus study. Sleep 41. (2018). 10.1093/sleep/zsy070 [DOI] [PubMed]
- 9.Klinzing, J. G., Niethard, N. & Born, J. Mechanisms of systems memory consolidation during sleep. Nature Neuroscience 22. (2019). 10.1038/s41593-019-0467-3 [DOI] [PubMed]
- 10.Yaffe, K., Barnes, D., Nevitt, M., Lui, L. Y. & Covinsky, K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch. Intern. Med.161, 1703–1708. 10.1001/archinte.161.14.1703 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Kompf, J. M. & Lachman, M. E. Daily Physical Activity: Associations With Memory and Affect. Am. J. Health Promot. 37, 602–613. 10.1177/08901171221139836 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neeper, S. A., Gómez-Pinilla, F., Choi, J. & Cotman, C. W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res.726, 49–56. 10.1016/0006-8993(96)00273-9 (1996). [PubMed] [Google Scholar]
- 13.Creer, D. J., Romberg, C., Saksida, L. M., Van Praag, H. & Bussey, T. J. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. U.S.A.107, 2367–2372. 10.1073/pnas.0911725107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature472, 466–470. 10.1038/nature09817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Praag, H., Christie, B. R., Sejnowski, T. J. & Gage, F. H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U.S.A.96, 13427–13431. 10.1073/pnas.96.23.13427 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voss, M. W., Vivar, C., Kramer, A. F. & van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci.17, 525–544. 10.1016/j.tics.2013.08.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voss, M. W. et al. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci.23, 318–333. 10.1016/j.tics.2019.01.006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilckens, K. A., Erickson, K. I. & Wheeler, M. E. Physical activity and cognition: A mediating role of efficient sleep. Behav. sleep. Med.16, 569–586. 10.1080/15402002.2016.1253013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappel-Farley, M. G. et al. Symptoms of obstructive sleep apnea are associated with less frequent exercise and worse subjective cognitive function across adulthood. Sleep45, 1–10. 10.1093/sleep/zsab240 (2022). [DOI] [PMC free article] [PubMed]
- 20.Mograss, M. et al. Exercising before a nap benefits memory better than napping or exercising alone. Sleep 1–9. 10.1093/sleep/zsaa062 (2020). [DOI] [PMC free article] [PubMed]
- 21.Loprinzi, P. D., Frith, E. & Edwards, M. K. Exercise and emotional memory: A systematic review. J. Cogn. Enhancement. 3, 94–103. 10.1007/s41465-018-0086-z (2019). [Google Scholar]
- 22.McGaugh, J. L. Making lasting memories: Remembering the significant. Proceedings of the National Academy of Sciences 110, 10402–10407. (2013). 10.1073/pnas.1301209110 [DOI] [PMC free article] [PubMed]
- 23.Wagner, U., Hallschmid, M., Rasch, B. & Born, J. Brief sleep after learning keeps emotional memories alive for years. Biol. Psychiatry. 60, 788–790. 10.1016/j.biopsych.2006.03.061 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Payne, J. D. et al. Sleep preferntially enhances memory for emotional components of scenes. Psychol. Sci.19, 781–788. 10.1111/j.1467-9280.2008.02157.x.Sleep (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne, J. D. & Kensinger, E. A. Sleep’s Role in the Consolidation of Emotional Episodic Memories. Curr. Dir. Psychol. Sci.19, 290–295. 10.1177/0963721410383978 (2010). [Google Scholar]
- 26.Leal, S. L., Noche, J. A., Murray, E. A. & Yassa, M. A. Age-related individual variability in memory performance is associated with amygdala-hippocampal circuit function and emotional pattern separation. Neurobiol. Aging. 49, 9–19. 10.1016/j.neurobiolaging.2016.08.018.Age-related (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denis, D., Sanders, K. E. G., Kensinger, E. A. & Payne, J. D. Sleep preferentially consolidatese negative aspects of human memory: Well-powered evidence from two online experiments. Proceedings of the National Academy of Sciences 119, e2202657119. (2022). 10.1073/pnas.2202657119 [DOI] [PMC free article] [PubMed]
- 28.Kensinger, E. A., Garoff-Eaton, R. J. & Schacter, D. L. Effects of emotion on memory specificity in younger and older adults. Journals Gerontol. Ser. B: Psychol. Sci. Social Sci.62, 208–215 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Yassa, M. A. & Stark, C. E. L. Pattern separation in the hippocampus. Trends Neurosci.34, 515–525. 10.1016/j.tins.2011.06.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yassa, M. A., Mattfeld, A. T., Stark, S. M. & Stark, C. E. L. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl. Acad. Sci. U.S.A.108, 8873–8878. 10.1073/pnas.1101567108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leal, S. L. & Yassa, M. A. Effects of aging on mnemonic discrimination of emotional information. Behav. Neurosci.128, 539–547. 10.1037/bne0000011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwabe, K. et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc. Natl. Acad. Sci.115, 10487–10492. 10.1073/pnas.1805668115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suwabe, K. et al. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus27, 229–234. 10.1016/j.physbeh.2017.03.040 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suwabe, K. et al. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: Evidence for memory flexibility in young adults. Sci. Rep.7, 1–10. 10.1038/s41598-017-04850-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein, E. E. & McNally, R. J. Examining the Effects of Exercise on Pattern Separation and the Moderating Effects of Mood Symptoms. Behav. Ther.50, 582–593. 10.1016/j.beth.2018.09.007 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Nauer, R. K., Schon, K. & Stern, C. E. Cardiorespiratory fitness and mnemonic discrimination across the adult lifespan. Learn. Memory. 27, 91–103. 10.1101/lm.049197.118.27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charles, S. T. & Carstensen, L. L. Social and emotional aging. Annu. Rev. Psychol.61, 383–409. 10.1146/annurev.psych.093008.100448 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowan, E. T., Schapiro, A. C., Dunsmoor, J. E. & Murty, V. P. Memory consolidation as an adaptive process. Psychon. Bull. Rev.28, 1796–1810. 10.3758/s13423-021-01978-x (2021). [DOI] [PubMed] [Google Scholar]
- 39.Sewell, K. R. et al. Relationships between physical activity, sleep, and cognitive function: A narrative review. Neurosci. Biobehav. Rev.130, 369–378. 10.1016/j.neubiorev.2021.09.003 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Mekary, R. A., Willett, W. C., Hu, F. B. & Ding, E. L. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am. J. Epidemiol.170, 519–527. 10.1093/aje/kwp163 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grgic, J. et al. Health outcomes associated with reallocations of time between sleep, sedentary behaviour, and physical activity: a systematic scoping review of isotemporal substitution studies. Int. J. Behav. Nutr. Phys. Act.15, 69. 10.1186/s12966-018-0691-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miatke, A. et al. The association between reallocations of time and health using compositional data analysis: a systematic scoping review with an interactive data exploration interface. Int. J. Behav. Nutr. Phys. Act.20, 127. 10.1186/s12966-023-01526-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canton, I. et al. Isotemporal Substitution of Sedentary Time With Physical Activity Among Middle-Aged and Older Latinos: Effects on Episodic Memory. Am. J. Health Promotion. 0, 08901171241233404. 10.1177/08901171241233404 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart, A. L. et al. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med. Sci. Sports. Exerc.33, 1126–1141. 10.1097/00005768-200107000-00010 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Leal, S. L., Tinghe, S. K., Jones, C. K. & Yassa, M. A. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus 24, 1146–1155. (2014). 10.1002/hipo.22298.Pattern [DOI] [PMC free article] [PubMed]
- 46.Leal, S. L., Tighe, S. K. & Yassa, M. A. Asymmetric effects of emotion on mnemonic interference. Neurobiol. Learn. Mem.111, 41–48. 10.1016/j.nlm.2014.02.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rugg, M. D. & Yonelinas, A. P. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn. Sci.7, 313–319 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Stark, S. M., Kirwan, C. B. & Stark, C. E. L. Mnemonic Similarity Task: A Tool for Assessing Hippocampal Integrity. Trends Cogn. Sci.23, 938–951. 10.1016/j.tics.2019.08.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bubu, O. M. et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med. Rev.50, 101250. 10.1016/j.smrv.2019.101250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahib, A. et al. Relationships between brain tissue damage, oxygen desaturation, and disease severity in obstructive sleep apnea evaluated by diffusion tensor imaging. Journal of Clinical Sleep Medicine 18, 2713–2721. https://doi.org/1.5664/jcsm.10192. (2022). [DOI] [PMC free article] [PubMed]
- 51.Best, J. R., Falck, R. S., Landry, G. J. & Liu-Ambrose, T. Analysis of dynamic, bidirectional associations in older adult physical activity and sleep quality. Journal of Sleep Research 28. (2019). 10.1111/jsr.12769 [DOI] [PubMed]
- 52.Kline, C. E. et al. Physical activity and sleep: An updated umbrella review of the 2018 Physical Activity Guidelines Advisory Committee report. Sleep Med. Rev.58, 101489. 10.1016/j.smrv.2021.101489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO Guidelines on Physical Activity and Sedentary Behaviour. (Geneva: World Health Organization). (2020).
- 54.Tully, M. A. et al. Sedentary behavior, physical activity, and mental health in older adults: An isotemporal substitution model. Scandinavian J. Med. Sports Sci.30, 1957–1965. 10.1111/sms.13762 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Fanning, J. et al. Replacing sedentary time with sleep, light, or moderate-to-vigorous physical activity: effects on self-regulation and executive functioning. J. Behav. Med.40, 332–342. 10.1007/s10865-016-9788-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keyan, D. & Bryant, R. A. The capacity for actue exercise to modulate emotional memories: A review of findings and mechanisms. Neurosci. Biobehav. Rev.107, 438–449. 10.1016/j.neubiorev.2019.09.033 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Jentsch, V. L. & Wolf, O. T. Acute physical exercise promotes the consolidation of emotional material. Neurobiology of Learning and Memory 127. (2020). 10.1016/j.nlm.2020.107252 [DOI] [PubMed]
- 58.Roig, M. et al. Time-dependent effects of cardiovascular exercise on memory. Exerc. Sport Sci. Reviews. 44, 81–88. 10.1249/JES.0000000000000078 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A.108, 3017–3022. 10.1073/pnas.1015950108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Payne, J. D., Chambers, A. M. & Kensinger, E. A. Sleep promotes lasting changes in selective memory for emotional scenes. Front. Integr. Nuerosci.6, 1–11. 10.3389/fnint.2012.00108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Callow, D. D., Pena, G. S., Stark, C. E. L. & Carson Smith, J. Effects of acute aerobic exercise on mnemonic discrimination performance in older adults. J. Int. Neuropsychol. Soc.29, 519–528. 10.1017/S1355617722000492 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callow, D. D., Kommula, Y., Stark, C. E. L. & Carson Smith, J. Acute cycling exercise and hippocampal subfield function and microstructure in healthy older adults. Hippocampus33, 1123–1138. 10.1002/hipo.23571 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovacevic, A., Fenesi, B. & Paolucci, E. The effects of aerobic exercise intensity on memory in older adults. Appl. Physiol. Nutr. Metab.45, 591–600. 10.1139/apnm-2019-0495 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Kern, K. L., Storer, T. W. & Schon, K. Cardiorespiratory fitness, hippocampal subfield volumes, and mnemonic discrimination task performance in aging. Hum. Brain. Mapp.42, 871–892. 10.1002/hbm.25259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basso, J. C. et al. Examining the effect of increased aerobic exercise in moderately fit adults on psychological state and cognitive function. Front. Hum. Neurosci.16, 833149 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pena, G. S., Callow, D. D., Evans, W. S., Prior, S. J. & Carson Smith, J. Associations between cardiorespiratory fitness, monocyte polarization, and exercise-related changes in mnemonic discrimination performance in older adults. Exp. Gerontol.169, 111973. 10.1016/j.exger.2022.111973 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Suwabe, K. et al. Improvement of mnemonic discimination with acute light exercise is mediated by pupil-linked arousal in older adults. Neurobiol. Aging. 10.1016/j.neurobiolaging.2023.09.006 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Physical Activity Guidelines for Americans. (U.S. Department of Health and Human Services, 2018).
- 69.Shing, Y. L. et al. Episodic memory across the lifespan: The contributions of associative and strategic components. Neurosci. Biobehavioral Reviews. 34, 1080–1091. 10.1016/j.neubiorev.2009.11.002 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Won, C. H. J. et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep43, zsz274. 10.1093/sleep/zsz274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during the current study is available from the corresponding author on reasonable request.