Abstract
Aspartic proteases play key roles in the biology of malaria parasites and human immunodeficiency virus type 1 (HIV-1). We tested the activity of seven HIV-1 protease inhibitors against cultured Plasmodium falciparum. All compounds inhibited the development of parasites at pharmacologically relevant concentrations. The most potent compound, lopinavir, was active against parasites (50% inhibitory concentration [IC50], 0.9 to 2.1 μM) at concentrations well below those achieved by ritonavir-boosted lopinavir therapy. Lopinavir also inhibited the P. falciparum aspartic protease plasmepsin II at a similar concentration (IC50, 2.7 μM). These findings suggest that use of HIV-1 protease inhibitors may offer clinically relevant antimalarial activity.
The human immunodeficiency virus type 1 (HIV-1) pandemic has emerged in many regions of the developing world already suffering from the burden of malaria (5). In developed countries, HIV-1 protease inhibitors have dramatically improved the outcome of HIV disease. The target of these inhibitors is the HIV-1 protease, a member of the aspartic protease family (6). Plasmodium falciparum, the most virulent human malaria parasite, expresses a number of aspartic proteases, known as plasmepsins (2). Recent studies suggest that three HIV-1 protease inhibitors, saquinavir, ritonavir, and indinavir, inhibit the growth of Plasmodium falciparum parasites in vitro at clinically relevant concentrations (13). In addition, evidence suggests that HIV-1 protease inhibitors may protect against malaria through the inhibition of CD36-mediated cytoadherence of P. falciparum-infected erythrocytes (9). We hypothesized that HIV-1 aspartic protease inhibitors exert antimalarial activity by acting against plasmepsins. To test this hypothesis, we investigated the effects of seven available HIV-1 protease inhibitors on the in vitro development of cultured malaria parasites and on the P. falciparum aspartic protease plasmepsin II.
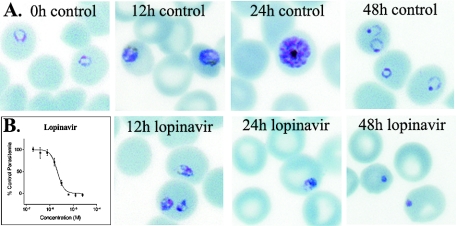
To evaluate the antiparasitic effects of HIV-1 protease inhibitors, we incubated cultured parasites with multiple concentrations of seven inhibitors. P. falciparum parasites were cultured with human erythrocytes (2% hematocrit) in RPMI medium and 10% human serum (11). Four laboratory strains of P. falciparum (acquired from the Malaria Research and Reference Reagent Center) with a wide range of sensitivities to standard antimalarial drugs were studied (12). Parasites were synchronized by serial treatments with 5% d-sorbitol (11). Microwell cultures of synchronized parasites were incubated with HIV-1 protease inhibitors (from 1,000× stocks in dimethyl sulfoxide [DMSO]; final concentrations ranged from 100 μM to 25 nM) for 48 h beginning at the ring stage. The effects of inhibitors upon P. falciparum morphology were assessed by light microscopy of Giemsa-stained smears. After 12 h of incubation, beginning at the late ring stage, synchronized parasites treated with concentrations of lopinavir achievable with standard dosing (10 μM) exhibited markedly altered morphology (Fig. 1A). Parasite abnormalities were more marked after 24 h, and after 48 h, when control cultures contained normal rings, treated cultures contained only very abnormal pyknotic parasites. The morphological changes caused by the protease inhibitors were rather nonspecific, but similar to those caused by the generic aspartic protease inhibitor pepstatin (1, 10).
FIG. 1.
Effects of HIV-protease inhibitors on cultured parasites. A. Parasite morphology. Synchronized ring stage parasites were incubated with 10 μM lopinavir. Treated and control (with equivalent concentrations of DMSO) parasites were evaluated at the indicated time points on Giemsa-stained smears. B. Parasite development. Synchronized parasites were incubated with multiple concentrations of lopinavir, beginning at the ring stage. After 48 h, ring parasitemias were determined by flow cytometry analysis of YOYO-1-stained parasites, as previously described (11). Results represent two independent experiments, each performed in duplicate using the HB3 strain of P. falciparum. Error bars represent standard deviations.
To quantify antimalarial activity, new ring stage parasites were counted after incubating parasites with protease inhibitors for one 48-hour life cycle, beginning at the ring stage. Ring parasitemias were assessed by fluorescence-activated cell sorter analysis and compared with those of control cultures incubated with the same concentration of DMSO, as previously described (11, 12). Fifty percent inhibitory concentrations (IC50s) were calculated by nonlinear regression with the Prism 3.0 program (GraphPad Software). All tested HIV-1 protease inhibitors demonstrated antimalarial activity at low micromolar concentrations (Table 1). Results were similar for all four tested P. falciparum strains. Calulated IC50s were higher than those previously reported for the HIV-1 protease inhibitors saquinavir, ritonavir, and indinavir (13), probably due to differences in assay methods, but nonetheless all tested compounds exerted antimalarial activity at concentrations near those achievable in the bloodstream with standard dosing. Importantly, combination regimens that take advantage of the boosting of levels of other protease inhibitors by the strong cytochrome P450 inhibitor ritonavir are increasingly advocated for standard antiretroviral therapy (8). In this regard, it is of interest that the most potent antimalarial protease inhibitor was lopinavir, which demonstrated an IC50 nearly 10-fold below the trough blood concentration achieved with standard dosing of a lopinavir/ritonavir combination (Fig. 1B). Ritonavir also demonstrated potent antimalarial activity at levels achievable with high dosages (600 mg twice daily [b.i.d.]), and at lower dosage (100 mg b.i.d.) it boosted the levels of several coadministered protease inhibitors to concentrations at which antimalarial activity was seen (Table 1). Caution should be exercised, however, as ritonavir's potent inhibition of cytochrome P450 may lead to complex drug interactions in coinfected patients (4).
TABLE 1.
Activity of HIV-1 protease inhibitors against cultured P. falciparumh
| Drug |
P. falciparum IC50 (μM) for:
|
Serum concn (μM) with:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Standard dosing
|
Ritonavir coadministration
|
|||||||
| HB3 | D6 | Dd2 | W2 | Cmax | Cmin | Cmax | Cmin | |
| Saquinavira | 5.6 ± 0.4 | 4.8 ± 1.2 | 4.3 ± 1.2 | 1.1 ± 0.5 | 3.7 | 0.3 | 5.5 | 0.6 |
| Ritonavirb | 4.7 ± 0.2 | 7.9 ± 1.9 | 6.9 ± 2.5 | 1.2 ± 0.5 | 15.5 | 5.1 | NA | NA |
| Indinavirc | 5.8 ± 0.9 | 15.6 ± 2.6 | 31.2 ± 9.4 | 4.1 ± 2.4 | 10.3 | 0.3 | 17.2 | 0.4 |
| Nelfinavird | 15.2 ± 0.1 | 23.0 ± 3.5 | 19.1 ± 5.5 | 6.5 ± 2.3 | 6.0 | 3.3 | NA | NA |
| Amprenavire | 51.9 ± 22.4 | 25.0 ± 8.3 | 17.4 ± 12.2 | 33.3 ± 6.6 | 15.2 | 0.6 | 14.1 | 3.8 |
| Lopinavirf | 1.4 ± 0.2 | 2.0 ± 0.4 | 2.1 ± 0.2 | 0.9 ± 0.2 | NA | NA | 15.6 | 8.8 |
| Atazanavirg | 6.8 ± 0.3 | 11.6 ± 2.5 | 7.1 ± 1.3 | 2.5 ± 1.0 | 3.3 | 0.2 | 8.7 | 1.7 |
Based on saquinavir 1,200 mg three times daily (as free base) in HIV-infected individuals and coadministered saquinavir soft gel capsule 1,000 mg/ritonavir 100 mg b.i.d. in HIV-infected individuals (Roche prescribing information) (14).
Based on ritonavir 600 mg b.i.d. in healthy and HIV-infected individuals (Abbott prescribing information).
Based on indinavir sulfate 800 mg every 8 h and coadministered indinavir 800 mg/ritonavir 100 mg b.i.d. in healthy individuals with low-fat meal (3).
Based on nelfinavir mesylate 1,250 mg b.i.d. in HIV-infected individuals; Cmin was determined prior to morning dosage (Agouron prescribing information).
Based on amprenavir 1,200 mg b.i.d. in healthy individuals and coadministered amprenavir 600 mg/ritonavir 100 mg b.i.d. in HIV-infected individuals (GlaxoSmithKline prescribing information) (7).
Based on lopinavir 400 mg/ritonavir 100 mg b.i.d. in HIV-infected individuals (Abbott prescribing information).
Based on atazanavir sulfate 400 mg once a day (q.d.) in HIV-infected subjects and coadministered atazanavir 300 mg/ritonavir 100 mg q.d. (Bristol-Myers Squibb prescribing information); serum concentrations are given as geometric means of atazanavir, as free base.
IC50 data are means ± standard deviations from four experiments. Serum concentrations are from published information. Cmax and Cmin are the mean maximum and minimum serum levels achieved under standard dosing intervals, respectively. Ritonavir-boosted serum concentrations are those achieved with coadministration with ritonavir, as indicated in the other footnotes. NA, not applicable, as these formulations are not used clinically.
The predicted antimalarial mechanism of action of HIV-1 protease inhibitors is the inhibition of plasmepsins. The P. falciparum genome predicts the existence of 10 plasmepsins. The best characterized is plasmepsin II, an acidic food vacuole enzyme that appears to play a role in the initial hydrolysis of hemoglobin by intraerythrocytic malaria parasites (2). To determine if HIV-1 protease inhibitors also inhibit the P. falciparum protease, the effects of lopinavir and ritonavir on the hydrolysis of a hemoglobin-based peptide substrate by recombinant plasmepsin II were assessed. Plasmepsin II was expressed, purified, and studied as described previously, with the exception that proplasmepsin II was preactivated for 60 min at 37°C, pH 5.2, and inhibitors were preincubated with enzyme for 30 min prior to addition of 0.5 μM substrate (2). Hydrolysis was recorded as the increase in fluorescence over 10 minutes using a Molecular Devices FlexStation II fluorometer. Both tested protease inhibitors inhibited plasmepsin II at concentrations (IC50, 2.7 μM for lopinavir and 3.1 μM for ritonavir) near those that were inhibitory for cultured malaria parasites. However, it remains unclear if the protease inhibitors achieve adequate intracellular concentrations to inhibit plasmepsin II, and additional studies will be needed to fully characterize their specific enzymatic targets.
Antiretroviral therapy is increasingly available to HIV-infected individuals in malaria-endemic regions. Currently, nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens are preferred because of cost, simplicity of dosing, modest storage requirements, and availability of coformulated preparations (15). However, protease inhibitors are advocated in some regions where the HIV type is insensitive to NNRTIs and for the treatment of viruses that are resistant to other classes of drugs. The use of protease inhibitors will likely increase as NNRTI resistance rises and as protease inhibitor regimens are simplified (15).
Our results support the intriguing possibility that HIV-infected individuals receiving protease inhibitor therapy may also benefit from an antimalarial effect due to inhibition of plasmepsins. In vitro, lopinavir demonstrated potent activity against P. falciparum at concentrations well below those achievable with standard dosing of a ritonavir-boosted lopinavir regimen. Due to concerns regarding cost, toxicity, and potential selection of resistant viruses, it is unlikely that currently available HIV-1 protease inhibitors will gain roles as standard treatments for malaria. Nonetheless, it seems likely that, for select protease inhibitors, the concentrations achieved during chronic antiretroviral therapy will offer some protection against malaria. If standard regimens for HIV-1 offer chemoprophylaxis against malaria, particularly in children, in whom the burden of malaria is greatest, the clinical consequences of this effect will be great. However, it is unclear if in vitro results showing antimalarial activity of HIV-1 protease inhibitors predict clinical efficacy. Therefore, clinical trials to test the hypothesis that HIV-1 protease inhibitors confer protection against malaria are urgently needed.
Acknowledgments
We thank members of the Rosenthal laboratory (Puran Sijwali, Kailash Pandey, Julie Lehman, and Anthony Lau) and Jun Liu of the Goldberg laboratory for their expert technical assistance.
Financial support was provided by the National Institutes of Health. Protease inhibitors were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: lopinavir, ritonavir, saquinavir (as free base), atazanavir, indinavir, nelfinavir, and amprenavir. P. falciparum strains were obtained from the Malaria Research and Reference Reagent Center (Manassas, Virginia).
REFERENCES
- 1.Bailly, E., R. Jambou, J. Savel, and G. Jaureguiberry. 1992. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and pepstatin A (aspartyl protease inhibitor). J. Protozool. 39:593-599. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, R., J. Liu, W. Beatty, L. Pelosof, M. Klemba, and D. E. Goldberg. 2002. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. USA 99:990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boffito, M., S. Bonora, R. Raiteri, H. E. Reynolds, P. G. Hoggard, A. Sinicco, D. J. Back, and G. Di Perri. 2002. Pharmacokinetic evaluation of indinavir and indinavir/ritonavir-containing antiretroviral regimens in a clinical setting. Ther. Drug Monit. 24:574-576. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, C. L., R. P. van Heeswijk, K. Gallicano, and D. W. Cameron. 2003. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin. Infect. Dis. 36:1585-1592. [DOI] [PubMed] [Google Scholar]
- 5.Corbett, E. L., R. W. Steketee, F. O. ter Kuile, A. S. Latif, A. Kamali, and R. J. Hayes. 2002. HIV-1/AIDS and the control of other infectious diseases in Africa. Lancet 359:2177-2187. [DOI] [PubMed] [Google Scholar]
- 6.Dash, C., A. Kulkarni, B. Dunn, and M. Rao. 2003. Aspartic peptidase inhibitors: implications in drug development. Crit. Rev. Biochem. Mol. Biol. 38:89-119. [DOI] [PubMed] [Google Scholar]
- 7.Goujard, C., I. Vincent, J. L. Meynard, N. Choudet, D. Bollens, C. Rousseau, D. Demarles, C. Gillotin, R. Bidault, and A. M. Taburet. 2003. Steady-state pharmacokinetics of amprenavir coadministered with ritonavir in human immunodeficiency virus type 1-infected patients. Antimicrob. Agents Chemother. 47:118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King, J. R., R. Yogev, G. Aldrovandi, E. Chadwick, and E. P. Acosta. 2004. Pharmacokinetics of antiretrovirals administered to HIV-infected children via gastrostomy tube. HIV Clin. Trials 5:288-293. [DOI] [PubMed] [Google Scholar]
- 9.Nathoo, S., L. Serghides, and K. C. Kain. 2003. Effect of HIV-1 antiretroviral drugs on cytoadherence and phagocytic clearance of Plasmodium falciparum-parasitised erythrocytes. Lancet 362:1039-1041. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal, P. J. 1995. Plasmodium falciparum: effects of proteinase inhibitors on globin hydrolysis by cultured malaria parasites. Exp. Parasitol. 80:272-281. [DOI] [PubMed] [Google Scholar]
- 11.Sijwali, P. S., and P. J. Rosenthal. 2004. Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 101:4384-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh, A., and P. J. Rosenthal. 2001. Comparison of efficacies of cysteine protease inhibitors against five strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 45:949-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner-Adams, T. S., J. S. McCarthy, D. L. Gardiner, P. M. Hilton, and K. T. Andrews. 2004. Antiretrovirals as antimalarial agents. J. Infect. Dis. 190:1998-2000. [DOI] [PubMed] [Google Scholar]
- 14.Veldkamp, A. I., R. P. van Heeswijk, J. W. Mulder, P. L. Meenhorst, G. Schreij, S. van der Geest, J. M. Lange, J. H. Beijnen, and R. M. Hoetelmans. 2001. Steady-state pharmacokinetics of twice-daily dosing of saquinavir plus ritonavir in HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 27:344-349. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 30 November 2003, posting date. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. 2003 revision. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/3by5/publications/documents/arv_guidelines/en/.