Abstract
Prediction of the relative efficacies of different fluoroquinolones is often based on the ratios of the clinically achievable area under the concentration-time curve (AUC) to the MIC, usually with incorporation of the MIC50 or the MIC90 and with the assumption of antibiotic-independent patterns of the AUC/MIC-response relationships. To ascertain whether this assumption is correct, the pharmacodynamics of seven pharmacokinetically different quinolones against two clinical isolates of Staphylococcus aureus were studied by using an in vitro model. Two differentially susceptible clinical isolates of S. aureus were exposed to two 12-h doses of ciprofloxacin (CIP) and one dose of gatifloxacin (GAT), gemifloxacin (GEM), grepafloxacin (GRX), levofloxacin (LVX), moxifloxacin (MXF), and trovafloxacin (TVA) over similar AUC/MIC ranges from 58 to 932 h. A specific bacterial strain-independent AUC/MIC relationship with the antimicrobial effect (IE) was associated with each quinolone. Based on the IE-log AUC/MIC relationships, breakpoints (BPs) that are equivalent to a CIP AUC/MIC ratio of 125 h were predicted for GRX, MXF, and TVA (75 to 78 h), GAT and GEM (95 to 103 h) and LVX (115 h). With GRX and LVX, the predicted BPs were close to those established in clinical settings (no clinical data on other quinolones are available in the literature). To determine if the predicted AUC/MIC BPs are achievable at clinical doses, i.e., at the therapeutic AUCs (AUCthers), the AUCther/MIC50 ratios were studied. These ratios exceeded the BPs for GAT, GEM, GRX, MXF, TVA, and LVX (750 mg) but not for CIP and LVX (500 mg). AUC/MIC ratios above the BPs can be considered of therapeutic potential for the quinolones. The highest ratios of AUCther/MIC50 to BP were achieved with TVA, MXF, and GEM (2.5 to 3.0); intermediate ratios (1.5 to 1.6) were achieved with GAT and GRX; and minimal ratios (0.3 to 1.2) were achieved with CIP and LVX.
Prediction of comparative efficacies among fluoroquinolones is often based on the ratios of the clinically achievable area under the concentration-time curve (AUC) to the MIC, usually with incorporation of the MIC50 or MIC90 (31). In fact, these antimicrobial effect predictors rather than the effect itself are often used in these comparisons. Such a replacement might seem appropriate, although it would be correct only if it were assumed that the AUC/MIC-response relationships are antibiotic independent. However, our in vitro pharmacodynamic studies that simulate quinolone pharmacokinetics (13, 18, 19, 43) did not support this assumption: a specific AUC/MIC-response relationship was shown to be inherent in each individual quinolone.
The present study was designed to compare the pharmacodynamics of seven pharmacokinetically different fluoroquinolones against Staphylococcus aureus, to delineate AUC/MIC-response relationships, and to predict the respective AUC/MIC breakpoints (BPs) relative to clinically achievable AUC/MIC ratios.
MATERIALS AND METHODS
Antimicrobial agents.
Ciprofloxacin (CIP), gatifloxacin (GAT), gemifloxacin (GEM), grepafloxacin (GRX), levofloxacin (LVX), moxifloxacin (MXF), and trovafloxacin (TVA) were kindly provided by Bayer Corporation (West Haven, CT), Bristol-Myers Squibb Pharmaceutical (New Brunswick, NJ), SmithKline Beecham Pharmaceutical (Collegeville, PA), Glaxo-Wellcome (Research Triangle Park, NC) Ortho-McNeil Pharmaceutical (Raritan, NJ), Bayer Corporation, and Pfizer Inc. (Groton, CT), respectively.
Bacterial strains.
Two clinical isolates of methicillin-resistant Staphylococcus aureus, S. aureus 944 and 916, were used in this study. Susceptibility testing was performed in triplicate by broth microdilution techniques at 24 h postexposure with the organism grown in Ca2+ (20 to 25 mg/liter)- and Mg2+ (10 to 12.5 mg/liter)-supplemented Mueller-Hinton broth (BBL, Becton Dickinson and Company, Sparks, MD) at an inoculum size of 106 CFU/ml. To determine precise values, the MICs of the quinolones were determined in parallel by using doubling dilutions with starting concentrations of 3, 4, and 5 mg/liter, as described previously (16). The MICs of the quinolones are presented in Table 1, and the respective weighted mean geometric values of the reported MIC50s are presented in Table 2.
TABLE 1.
MICs of the fluoroquinolones for S. aureus
| S. aureus strain | Quinolone MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| CIP | GAT | GEM | GRX | LVX | MXF | TVA | |
| 944 | 0.30 | 0.15 | 0.01 | 0.06 | 0.25 | 0.18 | 0.15 |
| 916 | 1.25 | 1.25 | 0.04 | 0.30 | 0.60 | 0.38 | 0.60 |
TABLE 2.
Quinolone doses, MIC50s, clinically achievable AUCs, and AUC/MIC ratios
| Quinolone | Dose (mg) | AUCther (μg · h/ml) | MIC50 (μg/ml)a | AUCther/MIC50 (h) | Free fraction (%)b | AUCther,f (μg · h/ml) | AUCther,f/MIC50 (h) |
|---|---|---|---|---|---|---|---|
| CIP | 500 | 22 | 0.62 | 35 | 74 | 16 | 26 |
| GAT | 400 | 32.9 | 0.23 | 143 | 80 | 26 | 114 |
| GEM | 320 | 12.4 | 0.04 | 310 | 30 | 4 | 93 |
| GRX | 600 | 19 | 0.15 | 127 | 57 | 11 | 72 |
| LVX | 500 | 45.6 | 0.7 | 65 | 70 | 32 | 46 |
| LVX | 750 | 82.6 | 0.7 | 118 | 70 | 58 | 83 |
| MXF | 400 | 32.9 | 0.16 | 206 | 60 | 20 | 123 |
| TVA | 200 | 19 | 0.1 | 190 | 28 | 5 | 53 |
Weighted geometric mean MIC50s from reported data on CIP (1, 3, 8, 21, 27, 29, 37, 42, 44; F. Daschner, U. Frank, and K. Huber, Abstr. 6th Int. Symp. New Quinolones, abstr. S12.27, 1998; D. Felmingham, M. J. Robbins, I. Mathias, K. Ingley, H. Bhogal, and R. N. Gruneberg, Abstr. 20th Int. Congr. Chemother., abstr. 3276, 1997; F. H. Kayser, P. Santanam, and E. Huf, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-197, 1998; E. Loza, M. I. Morosini, F. Almaraz, M. C. Negri, F. Baquero, and Spanish Collaborative Group Microbiology Service, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-206, 1998; B. Minassian, G. Warr, B. Kolek, B. Ryan, J. Fung-Tomc, and D. Bonner, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-181, 1998; K. Paek, M.-Y. Kim, and Y. S. Choo, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-090, 1998; F. J. Schmitz, J. Verhoef, A. C. Fluit, H. P. Heinz, U. Hadding, and M. E. Jones, Abstr. 8th Int. Congr. Infect. Dis., abstr. 13.007, 1998; C. Torres-Viera, C. Wennersten, R. C. Moellering, Jr., and G. Eliopoulos, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-193, 1998; C. von Eiff and G. Peters, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-212a, 1998; B. Wiedemann, Abstr. 8th Int. Congr. Infect. Dis., abstr. 13.035, 1998) GAT (3, 26, 27; B. Minassian, G. Warr, B. Kolek, B. Ryan, J. Fung-Tomc, and D. Bonner, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-181, 1998; C. Torres-Viera, C. Wennersten, R. C. Moellering, Jr., and G. Eliopoulos, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-193, 1998), GEM (K. Paek, M.-Y. Kim, and Y. S. Choo, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-090, 1998; K. Paek, M.-Y. Kim, and Y. S. Choo, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-092, 1998), GRX (1, 21, 25, 29, 42, 44; F. Daschner, F., U. Frank, and K. Huber, Abstr. 6th Int. Symp. New Quinolones, abstr. S12.27, 1998; K. Paek, M.-Y. Kim, and Y. S. Choo, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-090, 1998; B. Wiedemann, Abstr. 8th Int. Congr. Infect. Dis., abstr. 13.035, 1998), LVX (3, 25, 35, 37; D. Felmingham, M. J. Robbins, I. Mathias, K. Ingley, H. Bhogal, and R. N. Gruneberg, Abstr. 20th Int. Congr. Chemother., abstr. 3276, 1997; F. Kayser, P. Santanam, and E. Huf, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-197, 1998; C. Torres-Viera, C. Wennersten, R. C. Moellering, Jr., and G. Eliopoulos, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-193, 1998), MXF (3, 21, 25, 28, 35, 37; J. Blondeau, R. Laskowski, and D. Vaughan, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F155, 1997; J. Dubois and C. St-Pierre, Abstr. 6th Int. Symp. New Quinolones, abstr. S10.03, 1998; Kayser, F. H., P. Santanam, and E. Huf, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-197, 1998; E. Loza, M. I. Morosini, F. Almaraz, M. C. Negri, F. Baquero, and Spanish Collaborative Group Microbiology Service, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-206, 1998; F. Schmitz, J. Verhoef, A. C. Fluit, H. P. Heinz, U. Hadding, and M. E. Jones, Abstr. 8th Int. Congr. Infect. Dis., abstr. 13.007, 1998; L. Verbist and J. Verhaegen, Abstr. 8th Int. Congr. Infect. Dis., abstr. 13.015, 1998; C. von Eiff and G. Peters, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-212a, 1998; B. Wiedemann, Abstr. 8th Int. Congr. Infect. Dis., abstr. 13.035, 1998), and TVA (3, 8, 21, 25-28, 33; C. Torres-Viera, C. Wennersten, R. C. Moellering, Jr., and G. Eliopoulos, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-193, 1998; C. von Eiff and G. Peters, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-212a, 1998).
Free fractions of CIP (5, 9, 45, 50), GAT (30), GEM (48), GRX (7, 11, 50), LVX (20, 50), MXF (36, 39, 40, 47), and TVA (6, 41, 50) in plasma.
In vitro dynamic model and simulated pharmacokinetic profiles.
A previously described dynamic model (17) was used in the study. The operation procedure, the reliability of the simulations of the quinolone pharmacokinetic profiles, and the high degree of reproducibility of the time-kill curves provided by the model have been reported elsewhere (14).
A series of monoexponential profiles that mimic two consecutive 12-h doses of CIP and single-dose administrations of GAT, GEM, GRX, LVX, MXF, and TVA were simulated. The simulated half-lives (4, 7, 7.4, 11.6, 6.8, 12.1, and 10 h, respectively) represent the weighted medians of the values reported for humans: 3.2 to 5.0 h (4, 24), 6.0 to 8.4 h (30), 5.9 to 8.8 h (2), 10.1 to 12.7 h (10), 6.0 to 7.4 h (20), 9.3 to 14.0 h (38), and 7.2 to 9.9 h (41, 46), respectively. The simulated AUC/MIC ratios of CIP and LVX varied from 116 to 932 h; and those of GAT, GEM, GRX, MXF, and TVA varied from 58 to 466 h.
Quantitation of the time-kill curves.
In each experiment multiple sampling of bacterium-containing medium from the central compartment was performed throughout the observation period. The duration of the experiments was defined in each case as the time until antibiotic-exposed bacteria reached the maximum numbers observed in the absence of antibiotic (≥109 CFU/ml). The procedure used to quantitate the viable counts has been reported elsewhere (14). The limit of accurate detection was 2 × 102 CFU/ml.
Quantitation of the antimicrobial effect and the relationships to its predictors.
Based on the time-kill data, the intensity of the antimicrobial effect (IE; which is the area between control growth and time-kill curves) (12, 17) was determined from time zero to the time that the effect could no longer be detected, i.e., the time after the last fluoroquinolone dose at which the number of antibiotic-exposed bacteria reached 109 CFU/ml.
Correlation and regression analyses of the relationships between IE and log AUC/MIC were performed at a level of significance of P equal to 0.05.
The IE-log AUC/MIC relationships were used to predict the effects of each individual quinolone on a hypothetical strain of S. aureus with MICs equal to the respective MIC50s (Table 2) at therapeutic AUCs (AUCthers), i.e., the AUCs that correspond to two 500-mg doses of CIP, a 400-mg dose of GAT, a 320-mg dose of GEM, a 400-mg dose of GRX, a 500-mg dose of LVX, a 400-mg dose of MXF, and a 200-mg dose of TVA. In addition, the effect of LVX at the AUC that corresponds to that achieved with its 750-mg dose was predicted. The necessary AUCthers were calculated by using linear dose relationships (GEM [2], LVX [20], and MXF [38]) or curvilinear dose relationships (CIP [4], GAT [30], GRX [10], and TVA [41, 46]) of the AUC. To consider the different levels of protein binding of CIP, GAT, GEM, GRX, LVX, MXF, and TVA, the AUCthers were corrected by factors of 0.74, 0.80, 0.30, 0.57, 0.70, 0.60, and 0.28, respectively (the reported free fractions of the quinolones in plasma are presented in Table 2). Then, the unbound AUCthers (AUCther,fs) were related to the respective IEs.
RESULTS
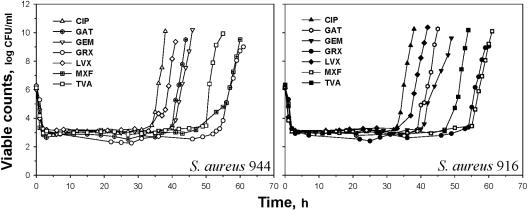
The time-kill curves for S. aureus 944 and 916 exposed to seven fluoroquinolones at one of the simulated AUC/MIC ratios are shown in Fig. 1. With each quinolone, bacterial regrowth followed the rapid killing of the bacteria during the first 3 to 4 h, which led to almost identical minimal numbers of surviving organisms. Despite similar initial killing rates, the times to regrowth were quinolone specific. For example, treatment with CIP resulted in the earliest regrowth of S. aureus 944, and this was 20 h shorter than that with treatment with GRX, with which the regrowth was the latest. Based on the time to regrowth, the quinolones may be arranged as follows: CIP < LVX < GAT ≤ GEM < TVA < MXF ≤ GRX. Similar patterns of quinolone pharmacodynamics were established at other simulated AUC/MICs with S. aureus 944 and 916 (data not shown).
FIG. 1.
Time-kill curves of quinolone-exposed S. aureus 944 and S. aureus 916 at comparable AUC/MIC ratios (466 h).
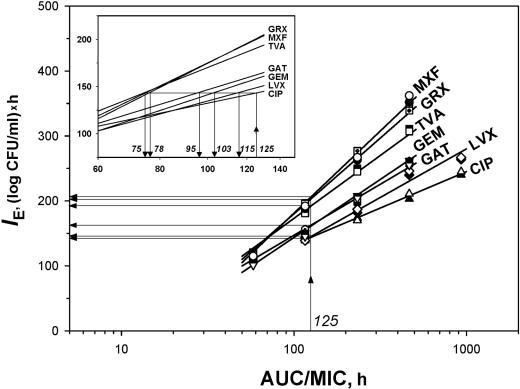
With each quinolone, the antistaphylococcal effect expressed by IE correlated well with the log AUC/MIC in a strain-independent (r2 ≥ 0.98) but quinolone-specific fashion (Fig. 2). The IE-log AUC/MIC relationships were of different slopes: minimal with CIP and maximal with MXF and GRX. This resulted in distinct differences among the IEs produced by a given AUC/MIC ratio of the different quinolones. For example, at an AUC/MIC of 125 h, the antistaphylococcal effects of GRX and MXF were 1.4 times greater than that of CIP.
FIG. 2.
AUC/MIC-dependent antistaphylococcal effects of seven quinolones. The equivalent AUC/MIC BPs are indicated by the italicized numbers in the inset. The symbols are the same as those in Fig. 1.
On the other hand, the IE-versus-log AUC/MIC plots allow prediction of the AUC/MIC ratios for new quinolones that may be equivalent to the clinically proven AUC/MIC BPs for an older quinolone. By using a 125-h AUC/MIC ratio of CIP as a reference, the BPs predicted for six other quinolones were estimated to be 75 h (GRX and MXF), 78 h (TVA), 95 h (GAT), 103 h (GEM), and 115 h (LVX) (Fig. 2, inset). Based on these predictions, 1.5-fold smaller AUC/MIC ratios of GRX, MXF, and TVA should provide the antimicrobial effect that is considered acceptable for CIP.
DISCUSSION
This study further demonstrates the bacterial strain-independent but quinolone-specific patterns of AUC/MIC relationships of the antistaphylococcal effect as expressed by the IE parameter. Earlier, similar relationships were reported with GAT-exposed Streptococcus pneumoniae (49), CIP- and GAT-exposed Escherichia coli and Klebsiella pneumoniae (43), as well as CIP- and TVA-exposed Pseudomonas aeruginosa (15). Based on the IE-log AUC/MIC relationships that were established with seven pharmacokinetically different quinolones against S. aureus, equiefficient BPs of the AUC/MIC ratio were predicted. To achieve the same antistaphylococcal effect provided by a clinically proven 125-h BP of the CIP AUC/MIC (23), the respective AUC/MIC ratios of six other quinolones were shown to be lower: 75 to 78 h (GRX, MXF, and TVA), 95 to 103 h (GAT and GEM), and 115 h (LVX).
Are these predictions clinically relevant? To answer this question, the BPs predicted in vitro should be compared with proven BPs that have been reported from clinical studies. Unfortunately, such BPs have been established for only two novel quinolones. With GRX, the AUC/MIC BP was estimated to be 75 h (22); and with LVX, the respective peak concentration-to-MIC ratio (Cmax/MIC) was estimated to be 12.2 (32), which corresponds to an AUC/MIC of 110 h (34). Based on the IE-log AUC/MIC relationships established in the present study, the AUC/MIC BP for grepafloxacin (78 h) is very close to the 75-h value established in the clinical setting and the AUC/MIC BP (115 h) predicted for LVX is close to the 110-h value established in a clinical setting. There are only two examples of the in vitro-in vivo correlations. Further evidence is needed to confirm the clinical relevance of AUC/MIC breakpoints predicted in in vitro studies by the use of dynamic models.
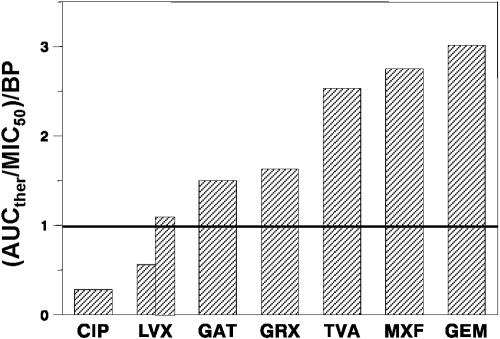
Are the predicted AUC/MIC BPs attainable in patients treated with the recommended quinolone doses? More specifically, can these doses provide AUC/MICs above the BPs? By taking the therapeutic value of AUC (AUCther) related to the MIC50 (Table 2) as the clinically achievable AUC/MIC ratio, the respective AUCther/MIC50 ratios exceed the BPs for GAT, GEM, GRX, MXF, TVA, and LVX (750 mg) but not CIP and LVX (500 mg). The ratios of AUCther/MIC50 to BP can be considered an index of the quinolone therapeutic potential. As seen in Fig. 3, the highest ratios of AUCther/MIC50 to BP are achieved with TVA, MXF, and GEM (2.5 to 3.0), intermediate values are achieved with GAT and GRX (1.5 to 1.6), and minimal values are achieved with CIP (0.3) and LVX (0.6 and 1.2 for 500- and 750-mg doses, respectively). This analysis predicts the greater therapeutic potentials of TVA, MXF, and GEM than GAT, GRX, and LVX (750 mg) but the lack of such potentials for LVX (500 mg) or CIP.
FIG. 3.
Index of quinolone therapeutic potentials expressed as the clinically achievable ratio of AUCther/MIC50 related to the predicted AUC/MIC BPs. For LVX, the left bar reflects the 500-mg dose and the right bar reflects the 750-mg dose.
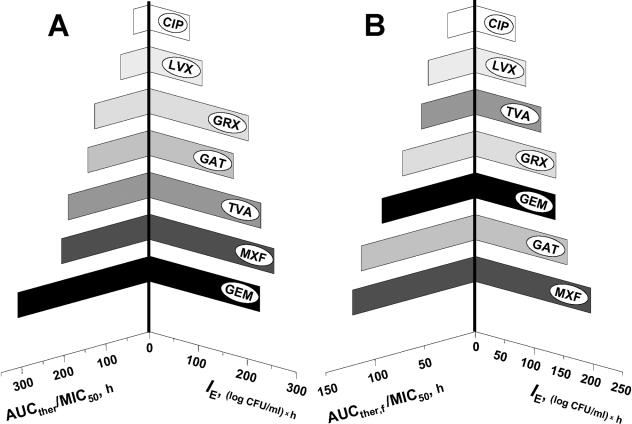
As pointed out in the introduction, the ratios of the AUCther or the therapeutic value of Cmax to MIC50 or MIC90 rather than determination of the antimicrobial effect itself are often considered as a basis for the direct comparison of antibiotics: the greater that AUCther/MIC50(90) or Cmax/MIC50(90) is, the better. Our study gives further evidence that different quinolones may produce different effects at a given AUC/MIC ratio. Quinolone-specific AUC/MIC-antimicrobial effect relationships are illustrated by comparing the AUCther/MIC50s with their respective IEs (Fig. 4A). Despite the similar AUCther/MIC50s achieved with GRX and GAT, the predicted IE of GRX appeared to be greater than that of GAT. Moreover, despite the higher AUCther/MIC50 ratio for GEM, its effect was less pronounced than that of MXF at a lower AUCther/MIC50 ratio. Therefore, plotting of the AUCther/MIC50 ratios versus IEs results in a tree with asymmetric branches, and AUCther/MIC50 correlates rather loosely with IE (r2 = 0.85). This analysis demonstrates the lack of correspondence between the sequence of quinolones arranged by their AUCther/MIC50 ratios and the sequence of quinolones arranged by their antimicrobial effects. So, neither our in vitro study nor others' clinical studies (different BPs reported for CIP [23] and GRX [22]) support the replacement of the effect by its predictor in comparisons among the quinolones.
FIG. 4.
AUCther/MIC50 (A) and AUCther,f/MIC50 (B) compared with the respective IEs.
It is interesting that a tree of the quinolones arranged by the AUCther/MIC50 ratios that consider only free concentrations (AUCther,f/MIC50) (Table 2) is almost symmetric (Fig. 4B). Furthermore, there is a stronger correlation between AUCther,f/MIC50 and IE (r2 0.94). In this light, AUCther,f/MIC50 (based on the free drug AUC) might be a better interquinolone predictor of the clinically achievable antimicrobial effect than AUCther/MIC50 (based on total drug AUC).
Acknowledgments
This study was supported in part by grants from the Bayer Corporation; Bristol-Myers Squibb; Glaxo-Wellcome; Roerig, a division of Pfizer Pharmaceuticals; and SmithKline-Beecham Pharmaceuticals.
REFERENCES
- 1.Adam, B. 1998. Comparative in vitro activity of grepafloxacin against fresh bacterial isolates from paediatric patients. Antiinfect. Drug Chemother. 16:70. [Google Scholar]
- 2.Allen, A., E. Bygate, S. Oliver, M. Johnson, C. Ward, A. J. Cheon, Y. S. Choo, and I. C. Kim. 2000. Pharmacokinetics and tolerability of gemifloxacin (SB-265805) after administration of single oral doses to healthy volunteers. Antimicrob. Agents Chemother. 44:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind, A. 1997. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J. Antimicrob. Chemother. 40:639-651. [DOI] [PubMed] [Google Scholar]
- 4.Bergan, T., and S. B. Thorsteinsson. 1986. Pharmacokinetics and bioavailability of ciprofloxacin, p. 111-121, Proceedings of the First International Ciprofloxacin Workshop, 1985, vol. 34. Elsevier Science Publishers B.V. (Excerpta Medica), Amsterdam, The Netherlands. [Google Scholar]
- 5.Bergan, T., S. B. Thorsteinsson, R. Solberg, L. Bjornskau, I. M. Kolstad, and S. Johnsen. 1987. Pharmacokinetics of ciprofloxacin: intravenous and increasing oral doses. Am. J. Med. 82:97-102. [PubMed] [Google Scholar]
- 6.Child, J., J. Andrews, F. Boswell, N. Brenwald, and R. Wise. 1995. The in-vitro activity of CP 99,219, a new naphthyridone antimicrobial agent: a comparison with fluoroquinolone agents. J. Antimicrob. Chemother. 35:869-876. [DOI] [PubMed] [Google Scholar]
- 7.Cook, P. J., J. M. Andrews, R. Wise, D. Honeybourne, and H. Moudgil. 1995. Concentrations of OPC-17116, a new fluoroquinolone antibacterial, in serum and lung compartments. J. Antimicrob. Chemother. 35:317-326. [DOI] [PubMed] [Google Scholar]
- 8.Coque, T. M., K. V. Singh, and B. E. Murray. 1996. Comparative in-vitro activity of the new fluoroquinolone trovafloxacin (CP-99,219) against gram-positive cocci. J. Antimicrob. Chemother. 37:1011-1016. [DOI] [PubMed] [Google Scholar]
- 9.Davis, R., A. Markham, and J. A. Balfour. 1996. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs 51:1019-1074. [DOI] [PubMed] [Google Scholar]
- 10.Efthymiopoulos, C. 1997. Pharmacokinetics of grepafloxacin. J. Antimicrob. Chemother. 40(Suppl. A):35-43. [DOI] [PubMed] [Google Scholar]
- 11.Efthymiopoulos, C., S. L. Bramer, and A. Maroli. 1997. Pharmacokinetics of grepafloxacin after oral administration of single and repeat doses in healthy young males. Clin. Pharmacokinet. 33:1-8. [DOI] [PubMed] [Google Scholar]
- 12.Firsov, A. A., V. M. Chernykh, and S. M. Navashin. 1990. Quantitative analysis of antimicrobial effect kinetics in an in vitro dynamic model. Antimicrob. Agents Chemother. 34:1312-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firsov, A. A., I. Y. Lubenko, S. N. Vostrov, O. V. Kononenko, S. H. Zinner, and Y. A. Portnoy. 2000. Comparative pharmacodynamics of moxifloxacin and levofloxacin in an in vitro dynamic model: prediction of the equivalent AUC/MIC breakpoints and equiefficient doses. J. Antimicrob. Chemother. 46:725-732. [DOI] [PubMed] [Google Scholar]
- 14.Firsov, A. A., A. A. Shevchenko, S. N. Vostrov, and S. H. Zinner. 1998. Inter- and intraquinolone predictors of antimicrobial effect in an in vitro dynamc model: new insight into a widely used concept. Antimicrob. Agents Chemother. 42:659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firsov, A. A., R. G. Vasilov, S. N. Vostrov, O. V. Kononenko, I. Y. Lubenko, and S. H. Zinner. 1999. Prediction of the antimicrobial effects of trovafloxacin and ciprofloxacin on staphylococci using an in-vitro dynamic model. J. Antimicrob. Chemother. 43:483-490. [DOI] [PubMed] [Google Scholar]
- 16.Firsov, A. A., S. N. Vostrov, I. Y. Lubenko, S. H. Zinner, and Y. A. Portnoy. 2004. Concentration-dependent changes in the susceptibility and killing of Staphylococcus aureus in an in vitro dynamic model that simulates normal and impaired gatifloxacin elimination. Int J. Antimicrob. Agents 23:60-66. [DOI] [PubMed] [Google Scholar]
- 17.Firsov, A. A., S. N. Vostrov, A. A. Shevchenko, and G. Cornaglia. 1997. Parameters of bacterial killing and regrowth kinetics and antimicrobial effect examined in terms of area under the concentration-time curve relationships: action of ciprofloxacin against Escherichia coli in an in vitro dynamic model. Antimicrob. Agents Chemother. 41:1281-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firsov, A. A., S. N. Vostrov, A. A. Shevchenko, Y. A. Portnoy, and S. H. Zinner. 1998. A new approach to in vitro comparisons of antibiotics in dynamic models: equivalent area under the curve/MIC breakpoints and equiefficient doses of trovafloxacin and ciprofloxacin against bacteria of similar susceptibilities. Antimicrob. Agents Chemother. 42:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firsov, A. A., S. H. Zinner, I. Y. Lubenko, and S. N. Vostrov. 2000. Gemifloxacin and ciprofloxacin pharmacodynamics in an in-vitro dynamic model: prediction of the equivalent AUC/MIC breakpoints and doses. Int. J. Antimicrob. Agents 16:407-414. [DOI] [PubMed] [Google Scholar]
- 20.Fish, D. N., and A. T. Chow. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32:101-119. [DOI] [PubMed] [Google Scholar]
- 21.Focht, J. 1998. In vitro activity of BAY 12-8039 compared with other fluoroquinolones against bacterial strains from upper and lower respiratory tract infections in general practice. Antiinfect. Drug Chemother. 16:76. [Google Scholar]
- 22.Forrest, A., S. Chodosh, M. A. Amantea, D. A. Collins, and J. J. Schentag. 1997. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 40(Suppl. A):45-57. [DOI] [PubMed] [Google Scholar]
- 23.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffken, G., H. Lode, C. Prinzing, K. Borner, and P. Koeppe. 1985. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob. Agents Chemother. 27:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, M. E., M. R. Visser, M. Klootwijk, P. Heisig, J. Verhoef, and F. J. Schmitz. 1999. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linozelid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 43:421-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, R. N., M. L. Beach, M. A. Pfaller, and G. V. Doern. 1998. Antimicrobial activity of gatifloxacin tested against 1676 strains of ciprofloxacin-resistant gram-positive cocci isolated from patient infections in North and South America. Diagn. Microbiol. Infect. Dis. 32:247-252. [DOI] [PubMed] [Google Scholar]
- 27.Jones, R. N., M. A. Croco, M. A. Pfaller, M. L. Beach, and K. C. Kugler. 1999. Antimicrobial activity evaluations of gatifloxacin, a new fluoroquinolone: contemporary pathogen results from a global antimicrobial resistance surveillance program (SENTRY, 1997). Clin. Microbiol. Infect. 5:540-546. [DOI] [PubMed] [Google Scholar]
- 28.Malathum, K., K. V. Singh, and B. E. Murray. 1999. In vitro activity of moxifloxacin, a new 8-methoxyquinolone, against gram-positive bacteria. Diagn. Microbiol. Infect. Dis. 35:127-133. [DOI] [PubMed] [Google Scholar]
- 29.Marco, F., R. N. Jones, D. J. Hoban, A. C. Pignatari, N. Yamane, and R. Frei. 1994. In-vitro activity of OPC-17116 against more than 6000 consecutive clinical isolates: a multicentre international study. J. Antimicrob. Chemother. 33:647-654. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima, M., T. Uematsu, K. Kosuge, H. Kusajima, T. Ooie, Y. Masuda, R. Ishida, and H. Uchida. 1995. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob. Agents Chemother. 39:2635-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nightingale, C. H. 1993. Pharmacokinetic considerations in quinolone therapy. Pharmacotherapy 13:34S-38S. [PubMed] [Google Scholar]
- 32.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 33.Rodloff, A. C., and A. F. Schmalreck. 1998. In vitro susceptibility of the fluoroquinolones trovafloxacin, ciprofloxacin, ofloxacin, sparflloxacin and 21 other antibiotics against consecutively obtained clinical isolates (1996-1997) from the university hospitals of Leipzig. Antiinfect. Drug Chemother. 16:77. [Google Scholar]
- 34.Schentag, J. J., A. K. Meagher, and A. Forrest. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 1. In vitro and animal models. Ann. Pharmacother. 37:1287-1298. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz, F. J., B. Hofmann, B. Hansen, S. Scheuring, M. Luckefahr, M. Klootwijk, J. Verhoef, A. Fluit, H. P. Heinz, K. Kohrer, and M. E. Jones. 1998. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 41:481-484. [DOI] [PubMed] [Google Scholar]
- 36.Siefert, H. M., A. Domdey-Bette, K. Henninger, F. Hucke, C. Kohlsdorfer, and H. H. Stass. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43(Suppl. B):69-76. [DOI] [PubMed] [Google Scholar]
- 37.Souli, M., C. B. Wennersten, and G. M. Eliopoulos. 1998. In vitro activity of BAY 12-8039, a new fluoroquinolone, against species representative of respiratory tract pathogens. Int. J. Antimicrob. Agents 10:23-30. [DOI] [PubMed] [Google Scholar]
- 38.Stass, H., A. Dalhoff, D. Kubitza, and U. Schuhly. 1998. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 42:2060-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stass, H., and D. Kubitza. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 43(Suppl. B):83-90. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan, J. T., M. Woodruff, J. Lettieri, V. Agarwal, G. J. Krol, P. T. Leese, S. Watson, and A. H. Heller. 1999. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 43:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng, R., S. C. Harris, D. E. Nix, J. J. Schentag, G. Foulds, and T. E. Liston. 1995. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J. Antimicrob. Chemother. 36:385-394. [DOI] [PubMed] [Google Scholar]
- 42.Verbist, L., and J. Verhaegen. 1998. In vitro activity of a new fluoroquinolone, grepafloxacin, compared with other antibacterials against gram-positive bacteria. Antiinfect. Drug Chemother. 16:73. [Google Scholar]
- 43.Vostrov, S. N., O. V. Kononenko, I. Y. Lubenko, S. H. Zinner, and A. A. Firsov. 2000. Comparative pharmacodynamics of gatifloxacin and ciprofloxacin in an in vitro dynamic model: prediction of equiefficient doses and the breakpoints of the area under the curve/MIC ratio. Antimicrob. Agents Chemother. 44:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallrauch, C., and I. Braveny. 1998. In vitro activity of grepafloxacin against H. influenzae, M. catarrhalis, S. pneumoniae, S. pyogenes and S. aureus. Antiinfect. Drug Chemother. 16:71. [Google Scholar]
- 45.Wise, R., J. M. Andrews, and L. J. Edvards. 1983. In vitro activity of Bay-08967, a new quinolone derivative, compared with those of other antimicrobial agents. Antimicrob. Agents Chemother. 23:559-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise, R., D. Mortiboy, J. Child, and J. M. Andrews. 1996. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219). Antimicrob. Agents Chemother. 40:47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodcock, J. M., J. M. Andrews, F. J. Boswell, N. P. Brenwald, and R. Wise. 1997. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob. Agents Chemother. 41:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhanel, G. G., K. Ennis, L. Vercaigne, A. Walkty, A. S. Gin, J. Embil, H. Smith, and D. J. Hoban. 2002. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs 62:13-59. [DOI] [PubMed] [Google Scholar]
- 49.Zinner, S. H., A. A. Firsov, D. Gilbert, K. Simmons, and I. Y. Lubenko. 2001. The pharmacodynamics of gatifloxacin and ciprofloxacin for pneumococci in an in vitro dynamic model: prediction of equiefficient doses. J. Antimicrob. Chemother. 48:821-826. [DOI] [PubMed] [Google Scholar]
- 50.Zlotos, G., A. Bucker, U. Holzgrabe, M. Kinzig-Schippers, and F. Sorgel. 1998. Protein binding of gyrase inhibitors in clinical practice: trovafloxacin, sparfloxacin, rufloxacin and older compounds. Antiinfect. Drug Chemother. 16:64. [Google Scholar]