Abstract
The ability to maximize bactericidal activity while minimizing toxicity is a therapeutic goal in the treatment of infective endocarditis. We evaluated the impact of administering short-course regimens of gentamicin in combination with daptomycin or vancomycin against one methicillin-susceptible (MSSA 1199) and one methicillin-resistant (MRSA 494) Staphylococcus aureus isolate using an in vitro pharmacodynamic model with simulated endocardial vegetations over 96 h. Human therapeutic dosing regimens for daptomycin (6 and 8 mg/kg of body weight), vancomycin, and gentamicin were simulated. Short-course combination regimens involving gentamicin were administered either as a single 5-mg/kg dose or three 1-mg/kg doses for only the first 24 h and compared to the regimens administered for the full 96-h duration. For all experiments, physiologic conditions of albumin, calcium, and pH were simulated. Both regimens of daptomycin achieved 99.9% kill by 32 h and maintained bactericidal activity against both isolates, which was significantly different from vancomycin, which displayed bacteriostatic activity (P < 0.05). The effects of all short-course regimens of gentamicin were equal to those of the full-duration regimens in combination with daptomycin. Adding three doses of gentamicin (1 mg/kg) to daptomycin resulted in enhancement and bactericidal activity at 24 h against both MRSA and MSSA. The addition of a single dose of gentamicin (5 mg/kg) enhanced or improved the activity of daptomycin and resulted in early bactericidal activity at 4 h against both isolates. The addition of three doses of gentamicin (1 mg/kg) did not improve the activity of vancomycin. However, the addition of a single 5-mg/kg dose of gentamicin to vancomycin resulted in early enhancement at 4 h and 99.9% kill at 32 h for MRSA. These results suggest that a single high dose of gentamicin in combination with daptomycin or vancomycin may be of utility to maximize synergistic and bactericidal activity and minimize toxicity. Further investigation is warranted.
Infective endocarditis (IE) continues to be a challenging therapeutic problem associated with a high degree of morbidity and mortality (5, 28, 32, 45). Staphylococcus aureus has emerged as the most common cause of IE, with mortality rates as high as 47% (32). Multidrug resistance, the difficulty in employing antimicrobial agents which can overcome a high bacterial density, and the need to utilize bactericidal antimicrobial agents in S. aureus endocarditis limit therapeutic options (7, 13, 15, 23, 33, 38). In order to optimize therapy of IE, it would be ideal to utilize a dosing strategy and antimicrobial that maximizes bactericidal activity while minimizing toxicity. Employing synergistic antimicrobial combinations, shorter courses of therapy, and optimizing pharmacodynamic principles of new and existing antimicrobials is one potential strategy to achieve these goals.
Bactericidal activity is essential in treating IE to achieve rapid sterilization of the cardiac vegetation (15, 32, 33, 41). While vancomycin is currently the drug of choice in treating methicillin-resistant S. aureus (MRSA) endocarditis, many investigations have demonstrated that it is slowly bactericidal or bacteriostatic (24, 29, 43). Slow clinical responses to vancomycin have been reported, with bacteremia persisting for up to 7 days in patients with MRSA endocarditis (29). In addition, decreased bactericidal activity of vancomycin against MRSA may predict a higher probability of clinical failure in the treatment of MRSA bacteremia (41). Furthermore, the increasing prevalence of S. aureus isolates displaying reduced vancomycin susceptibility worldwide and subsequently resulting in treatment failure, especially in IE, is a cause for serious concern (8, 9, 18, 47). Daptomycin is a novel lipopeptide that possesses rapid concentration-dependent, bactericidal activity against multidrug-resistant gram-positive organisms, including those with reduced susceptibility to vancomycin (2, 4, 10, 46). Daptomycin at a dose of 6 mg/kg of body weight every day is currently undergoing clinical evaluation for the treatment of endocarditis and bacteremia due to S. aureus. Limited data exist on the most optimal dose and whether combination therapy is warranted in treating IE.
The optimal dosing regimen for gentamicin necessary for synergistic killing and bactericidal activity in IE remains of critical importance (28, 32). Current guidelines for the treatment of S. aureus endocarditis recommend the use of a 4- to 6-week course of vancomycin or an anti-staphylococcal beta-lactam with the optional addition of traditional gentamicin therapy (thrice daily) (14, 45). Combination regimens involving aminoglycosides have demonstrated a more rapid clinical response and a reduced duration of bacteremia (25, 28). However, when aminoglycosides are combined with vancomycin, there is an increased incidence of nephrotoxicity (37). Limited data exist on the use of high-dose once-daily aminoglycosides in serious gram-positive bacterial infections, including IE.
In an attempt to further minimize nephrotoxicity and maximize bactericidal activity, employing shorter courses of aminoglycosides is another potential strategy. Two-week combination regimens involving traditional gentamicin therapy have been successful in the treatment of right-sided endocarditis caused by S. aureus and viridans group streptococci (12, 17, 42). Furthermore, the administration of even shorter combination regimens of gentamicin at the beginning of therapy may be advantageous but has not been studied in IE. Administering a short-course regimen of gentamicin in combination with a highly active antimicrobial in the initial stages or beginning of therapy has the potential to eradicate a large number of susceptible organisms within a high inoculum and rapidly achieve bactericidal activity in sequestered infections. In a search for optimal antimicrobial dosing regimens in endocarditis, we investigated the bactericidal effect of administering a single high dose or three doses of gentamicin, only for the first 24 h, in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations (SEVs).
(A portion of this work was presented at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., 30 October to 2 November 2004.)
MATERIALS AND METHODS
Bacterial strains.
Clinical isolates evaluated were MSSA 1199 (methicillin-susceptible Staphylococcus aureus) and MRSA 494 (methicillin-resistant S. aureus). Both isolates were isolated from patients with bacterial endocarditis and were provided by Glenn W. Kaatz, John D. Dingell VA Medical Center, Detroit, MI.
Antibiotics.
Daptomycin (lot CM2:282A) analytical powder was provided by its manufacturer, Cubist Pharmaceuticals, Inc., Lexington, Massachusetts. Vancomycin and gentamicin were commercially purchased (Sigma Chemical Company, St. Louis, Missouri). Stock solutions of each antibiotic were freshly prepared at the beginning of each week and kept frozen daily at −4°C.
Media.
Mueller-Hinton broth (Difco, Detroit, Michigan) supplemented with 25 μg/ml calcium and 12.5 μg/ml magnesium (SMHB) was used for in vitro pharmacodynamic models and susceptibility testing involving vancomycin and gentamicin. For simulated regimens and susceptibility testing involving daptomycin, Mueller-Hinton broth supplemented with calcium, titrated to physiologic levels (1.1 to 1.3 mmol/liter) and 12.5 μg/ml magnesium was used due to daptomycin's dependency on calcium (20, 26). Colony counts were determined using tryptic soy agar (TSA) (Difco, Detroit, Michigan) plates.
Protein binding.
Human albumin 25% USP (American Red Cross, Detroit, MI) (NDC 52769-451-05) was incorporated into the SMHB to achieve a concentration of 4 g/dl for all simulated regimens, including daptomycin, vancomycin, gentamicin, and growth control, and for all susceptibility testing. All albumin-supplemented broth media for daptomycin experiments were further titrated with calcium as necessary to maintain physiological calcium levels and account for decreases in calcium concentrations in the presence of albumin resultant from binding to albumin (20). Samples from SMHB were monitored for total and ionized calcium, pH, and albumin at the beginning of each experiment and every day during all experiments by Detroit Medical Center Laboratories.
Susceptibility testing.
The MICs and minimum bactericidal concentrations (MBCs) of all antimicrobials were determined by broth microdilution in SMHB according to the Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) guidelines. The MICs for all antimicrobials were also determined in the presence of 4 g/dl of human albumin (MIC(albumin)) (American Red Cross, Detroit, MI) and at higher inoculum (5 × 109 CFU/ml) to simulate bacterial densities in the high-inoculum models as previously described (27). Five-microliter samples from clear wells were plated onto TSA plates for the determination of MBCs. All samples were incubated at 35°C for 24 h prior to interpretation of results.
SEVs.
Organism stocks were prepared by inoculating 5-ml test tubes of SMHB with colonies harvested from fresh overnight growth on TSA. Test tubes were incubated for 24 h on a rotator at 37°C. The inoculated test tubes were centrifuged for 15 min at 3,500 rpm, followed by removal of the supernatant. The remaining organism pellet was collected and resuspended to achieve a concentration of approximately 1010 CFU/ml. SEVs were prepared by mixing 0.1 ml of the organism suspension (final inoculum, 9 log10 CFU/g), 0.5 ml of human cryoprecipitate antihemolytic factor from volunteer donors (American Red Cross, Detroit, MI), and 0.025 ml of platelet suspension (platelets mixed with normal saline; 250,000 to 500,000 platelets per clot in 1.5-ml siliconized Eppendorf tubes). Bovine thrombin (0.05 ml; 5,000 units/ml) was added to each tube, after insertion of a sterile monofilament line into the mixture. The resultant SEVs were then removed from the Eppendorf tubes with a sterile 21-gauge needle and introduced into the model (31).
In vitro pharmacodynamic infection model.
An in vitro infection model consisting of a 250-ml one-compartment glass apparatus with ports in which the SEVs were suspended was utilized for all simulations (31). The apparatus was prefilled with media, and antibiotics were administered as boluses over a 96-h period into the central compartment via an injection port. The SEVs and supplemental models were placed in a 37°C water bath throughout the procedure, and a magnetic stir bar was placed in the media for thorough mixing of the drug in the model. Fresh media were continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Illinois) set to simulate the half-lives of the antibiotics. For combination regimen experiments, the elimination rate was set for the drug with the shortest half-life, and the drug with the longer half-life was supplemented (6). All model experiments were performed in triplicate to quintuplicate to ensure reproducibility. In addition, simulations in the absence of antibiotics were performed over 96 h to assure adequate growth of the organisms in the model.
Simulated short-course and full-duration regimens.
The following regimens were simulated and administered over the full 96-h duration: daptomycin, 6 mg/kg (D6) and 8 mg/kg (D8) every 24 h (q24h) (peak, 98.6 and 133.0 μg/ml, respectively; average half-life, 8 h); and vancomycin, 1 g every 12 h (q12h) (peak, 40 μg/ml; average half-life, 6 h). For gentamicin, two dosing regimens were simulated in combination: (i) full-duration regimens, administered for the entire 96 h: gentamicin, 1 mg/kg (G1) every 8 h (q8h) (peak, 4 μg/ml; average half-life, 3 h); gentamicin, 5 mg/kg (G5) q24h (peak, 15 μg/ml; average half-life, 3 h); (ii) short-course regimens, administered for only the first 24 h: one 5-mg/kg dose of gentamicin (G5x1) or three 1-mg/kg doses q8h (G1x3).
Pharmacodynamic analysis.
Two simulated endocardial vegetations were removed from each model at each time point (total of six) at 0, 4, 8, 24, 32, 48, 72, 80, and 96 h. The SEVs were then homogenized, diluted, plated onto TSA, and incubated at 35°C for 24 h. Colonies were enumerated after 24 h of incubation, and the number of CFU per gram was calculated. This method results in a lower limit of detection of 2.0 log10 CFU/ml. Antimicrobial carryover was taken into account by serial dilutions (10 to 10,000) of plated samples in conjunction with vacuum filtration where samples were washed through a 0.22-micron filter with normal saline. These filters were plated onto TSA and incubated at 35°C for 24 h to confirm colony counts. The total reductions in log10 CFU/gram over 96 h were determined by plotting time-kill curves based on the number of remaining organisms over the 96 h. Bactericidal activity (99.9% kill) was defined as a ≥3-log10 CFU/ml reduction in colony count from the initial inoculum. Bacteriostatic activity was defined as a <3-log10 CFU/ml reduction in colony count from the initial inoculum. The time to achieve a 99.9% bacterial load reduction (T99) was determined by linear regression if r2 was ≥0.95 or by visual inspection. The effects of the antimicrobial combinations were interpreted as follows. Enhancement of activity was defined as an increase in kill of ≥2-log10 CFU/ml by a combination of antimicrobials versus the most active single agent of that combination at 4, 24, 48, 72, and 96 h. Improvement was defined as a 1- to 2-log10 CFU/ml increase in kill in comparison to the most active single agent, while combinations that resulted in ≥1-log10 bacterial growth in comparison to the least-active single agent were considered antagonism. The terms “improvement” and “enhancement” were used because our simulations used therapeutically obtained serum concentration, which did not permit the mathematical modeling necessary to consider the standard terms “additivity” and “synergy” (3).
Pharmacokinetic analysis.
Samples for pharmacokinetic analysis were obtained through the injection port at 0.5, 1, 2, 4, 8, 24, 32, 48, 72, and 96 h for verification of target antibiotic concentrations. In addition, all simulated endocardial vegetations were assayed for antimicrobial concentrations after homogenization and were compared to model concentrations at the same time points over time for 24 h. All samples were stored at −70°C until analysis. Vancomycin and gentamicin concentrations were determined by fluorescence polarization immunoassay (Abbott Diagnostics TDx). The vancomycin and gentamicin assay has a limit of detection of 2.0 mg/liter for vancomycin and 0.27 mg/liter for gentamicin with an interday percent coefficient of variation (CV%) of ≤1% and 3%, respectively. We have previously determined that vancomycin and gentamicin concentrations determined by fluorescence polarization immunoassay in SMHB were comparable to concentrations of the respective antimicrobial determined in serum (22).
Concentrations of daptomycin were determined by microbioassay utilizing Micrococcus luteus ATCC 9341. Blank 0.25-in. disks were spotted with 20 μl of the standards or samples. Each standard was tested in triplicate by placing the disk on Mueller-Hinton agar plates which were preswabbed with a 0.5 McFarland suspension of the test organism. Plates were incubated for 18 to 24 h at 37°C. The diameters of inhibition for samples and standards were measured to the nearest 0.1 mm with a vernier caliper. Daptomycin concentrations in samples were calculated by using the data from the curves derived from the drug standards. The standard curves of the zone sizes versus the natural logarithm of the drug concentration were linear between 1 and 150 μg/ml when the standards were prepared in SMHB (r = 0.98; intraday CV% of ≤8% and an interday CV% of ≤10%). Concentrations of 150, 50, 10, 5, and 1 μg/ml were used as standards for daptomycin. The daptomycin bioassay has a lower limit of detection of 1 μg/ml for control standards. The half-lives, area under the curve (AUC), and peak concentrations of the antibiotics were determined by the trapezoidal method utilizing PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Statistical analysis.
Differences between regimens in log10 CFU/gram per milliliter at 4, 24, 48, 72, and 96 h and T99 were determined using analysis of variance with Tukey's test for multiple comparisons. For all experiments, a P value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software (release 12.0; SPSS, Inc., Chicago, Illinois).
Resistance.
Development of resistance was evaluated at multiple time points throughout the simulation at 24, 48, 72, and 96 h. Samples (100 μl) from each time point were plated on TSA plates containing four- and eightfold the MIC of the respective antibiotic to assess the development of resistance and examined for growth after 48 h of incubation at 35°C. Colonies which exhibited growth on antibiotic-containing TSA plates were subsequently examined by Etest.
RESULTS
Susceptibility testing and medium parameters.
All susceptibility results for daptomycin, gentamicin, and vancomycin against the MRSA and MSSA isolates are reported in Table 1. Overall, MICs for both isolates increased fourfold for daptomycin and zero- to twofold for vancomycin in the presence of albumin for both isolates. The calcium and albumin concentrations and pH were confirmed for each batch of broth before the start of each experiment (mean ± standard deviation): total calcium, 9.7 ± 0.8 mg/dl; ionized calcium, 1.2 ± 0.2 mmol/liter; albumin, 4.1 ± 0.3 g/dl; and pH, 7.1 ± 0.2.
TABLE 1.
Susceptibility results for three antimicrobial agents in the presence and absence of albumin against S. aureus
| Antimicrobial | MIC (μg/ml) fora:
|
|
|---|---|---|
| MSSA 1199 | MRSA 494 | |
| Daptomycin | 0.25 (8.0) | 0.25 (8.0) |
| Daptomycin with albuminb | 1.0 (16.0) | 1.0 (16.0) |
| Vancomycin | 1.0 (4.0) | 0.5 (2.0) |
| Vancomycin with albumin | 1.0 (8.0) | 1.0 (4.0) |
| Gentamicin | 0.5 (0.5) | 0.5 (1.0) |
| Gentamicin with albumin | 0.5 (1.0) | 0.5 (2.0) |
The standard inoculum was 5 × 105 CFU/g, and the high inoculum was 5 × 109 CFU/g. Data for the high inoculum are presented parenthetically.
Albumin added to broth to achieve a final concentration of 4 g/dl.
Pharmacokinetics.
Pharmacokinetic parameters (mean ± standard deviation) for the tested agents are shown in Table 2. Observed pharmacokinetic parameters were within 10% of targeted values. The area under the concentration-time curve from 0 to 24 h (AUC0-24) for D6 and D8 regimens were 1,059.5 ± 85.5 μg/ml/h and 1,415.5 ± 106.6 μg/ml/h, respectively. The maximum concentration of drug in serum (Cmax) was 101.7 ± 7.2 and 140.2 ± 7.1 μg/ml for the D6 and D8 regimens, respectively. For vancomycin, the AUC0-24 was 579.4 ± 19.65 μg/ml/h. For regimens in which gentamicin was given q8h and q24h, the values for Cmax were 4.0 ± 0.5 and 15.9 ± 1.4 μg/ml, respectively. The maximal observed concentrations in serum compared to those in the SEVs in SMHB in the first 24 h for daptomycin, gentamicin, and vancomycin ranged from 25 to 76%.
TABLE 2.
Pharmacokinetic parameters obtained with pharmacodynamic infection models
| Parameter | Value (mean ± SD)a
|
||||
|---|---|---|---|---|---|
| V | G1 | G5 | D6 | D8 | |
| Half-life (h) | 6.5 ± 0.3 | 3.2 ± 0.6 | 3.1 ± 0.4 | 8.2 ± 0.7 | 8.7 ± 0.8 |
| Cmax (μg/ml) | 41.8 ± 1.4 | 4.0 ± 0.5 | 15.9 ± 1.4 | 101.7 ± 7.2 | 140.2 ± 7.1 |
| Cmin (μg/ml)b | 11.6 ± 0.3 | 0.7 ± 0.4 | 0.1 ± 0.02 | 13.8 ± 1.2 | 18.2 ± 0.6 |
| SEV Cmax | 25.9 ± 0.7 | 1.0 ± 0.4 | 5.39 ± 0.71 | 76.9 ± 4.7 | 95.1 ± 7.8 |
| AUC0-24 (μg/ml · h−1) | 579.4 ± 19.6 | 45.8 ± 3.4 | 69.7 ± 4.14 | 1,059.5 ± 85.5 | 1,415.5 ± 106.6 |
| AUC/MIC(albumin) MSSAc | 579.4 | 91.6 | 139.4 | 1,059.5 | 1,415.5 |
| AUC/MIC(albumin) MRSAd | 579.4 | 91.6 | 139.4 | 1,059.5 | 1,415.5 |
| Cmax/MIC(albumin) MSSA | 25.9 | 8.0 | 31.8 | 101.7 | 140.2 |
| Cmax/MIC(albumin) MRSA | 25.9 | 8.0 | 31.8 | 101.7 | 140.2 |
V, vancomycin q12h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; D6, daptomycin in 6-mg/kg doses q24h; D8, daptomycin in 8-mg/kg doses q24h.
Cmin, minimum concentration of drug in serum.
MSSA 1199.
MRSA 494.
Pharmacodynamics.
Quantitative changes in log10 CFU/gram over 96 h with each antimicrobial regimen exposure are listed in Table 3 and are graphically displayed in Fig. 1 to 8. For all model simulations, calcium and albumin concentrations and pH were monitored throughout all experiments (mean ± standard deviation): total calcium, 9.9 ± 1.4 mg/dl; ionized calcium, 1.3 ± 0.3 mmol/liter; albumin, 3.9 ± 0.3 g/dl; pH, 7.2 ± 0.4. The temperature was maintained at 37°C throughout the 96 h.
TABLE 3.
Changes in bacterial densities at multiple time points observed over 96 hours in the in vitro pharmacodynamic model
| Isolate and drug treatment | Time to 99.9% kill (h) | Changes in bacterial density (CFU/g) at:
|
||||
|---|---|---|---|---|---|---|
| 4 h | 24 h | 48 h | 72 h | 96 h | ||
| MRSA 494 | ||||||
| Daptomycin 6 mg/kg alone | 32 | −1.49 | −2.31 | −6.85 | −7.06 | −6.64 |
| In combination with: | ||||||
| Gentamicin q8h | 24 | −1.49 | −6.56a | −7.14 | −7.36 | −7.36 |
| Gentamicin q8h (3 doses) | 24 | −1.64 | −6.41a | −7.17 | −7.17 | −7.17 |
| Gentamicin q24h | 4 | −3.78a | −6.64a | −7.12 | −7.28 | −7.28 |
| Gentamicin q24h (1 dose) | 4 | −4.49a | −6.67a | −7.47 | −7.47 | −7.47 |
| Daptomycin 8 mg/kg alone | 24 | −2.16 | −5.85 | −7.39 | −7.39 | −7.39 |
| In combination with: | ||||||
| Gentamicin q8h | 24 | −2.39 | −7.13b | −7.42 | −7.42 | −7.42 |
| Gentamicin q8h (3 doses) | 24 | −2.42 | −6.74b | −6.83 | −7.06 | −7.06 |
| Gentamicin q24h | 4 | −6.21a | −7.31b | −7.31 | −7.31 | −7.31 |
| Gentamicin q24h (1 dose) | 4 | −6.46a | −6.92b | −7.19 | −7.19 | −7.19 |
| Vancomycin alone | NAc | −0.02 | −0.49 | −0.51 | −2.36 | −2.89 |
| In combination with: | ||||||
| Gentamicin q8h | 72 | −0.54 | −0.77 | −2.23a | −3.87b | −4.95a |
| Gentamicin q8h (3 doses) | NA | −0.40 | −0.65 | −0.86 | −2.59 | −2.91 |
| Gentamicin q24h | 24 | −1.73 | −3.07a | −4.82a | −6.86a | −7.16a |
| Gentamicin q24h (1 dose) | 32 | −1.89b | −2.86a | −3.43a | −4.64a | −5.79a |
| MSSA 1199 | ||||||
| Daptomycin 6 mg/kg alone | 24 | −2.13 | −3.17 | −6.13 | −6.42 | −6.82 |
| In combination with: | ||||||
| Gentamicin q8h | 24 | −1.87 | −5.86a | −7.38b | −7.38 | −7.38 |
| Gentamicin q8h (3 doses) | 24 | −1.89 | −5.99a | −7.38b | −7.38 | −7.08 |
| Gentamicin q24h | 4 | −3.53b | −6.87a | −7.32b | −7.32 | −7.32 |
| Gentamicin q24h (1 dose) | 4 | −3.17b | −6.49a | −7.33b | −6.88 | −6.80 |
| Daptomycin 8 mg/kg alone | 8 | −2.99 | −5.63 | −6.63 | −7.36 | −7.10 |
| In combination with: | ||||||
| Gentamicin q8h | 4 | −3.23 | −7.06 | −7.36 | 7.36 | 7.36 |
| Gentamicin q8h (3 doses) | 4 | −3.74 | −6.60 | −7.46 | 7.46 | 7.16 |
| Gentamicin q24h | 4 | −4.75b | −7.16b | −7.28 | 7.28 | 7.28 |
| Gentamicin q24h (1 dose) | 4 | −4.29b | −6.73b | −7.31 | 7.31 | 7.31 |
| Vancomycin alone | NA | −0.13 | −0.17 | −0.29 | 0.52 | 0.87 |
| In combination with: | ||||||
| Gentamicin q8h | NA | −0.38 | −1.37b | −1.31b | 2.40b | 2.73b |
| Gentamicin q8h (3 doses) | NA | −0.30 | −1.42b | −1.27 | 1.52 | 1.28 |
| Gentamicin q24h | 72 | −1.27 | −1.32b | −1.81b | 3.17a | 4.34a |
| Gentamicin q24h (1 dose) | 80 | −1.11 | −1.50b | −1.31 | 2.20b | 3.20a |
Enhancement in kill observed with combination.
Improvement in kill observed with combination.
NA, not applicable.
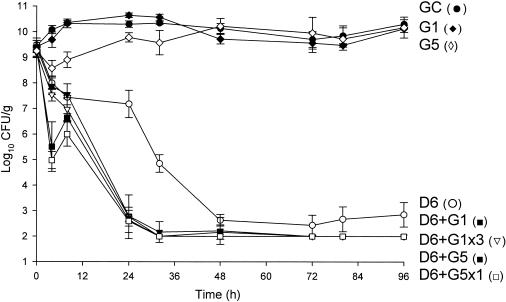
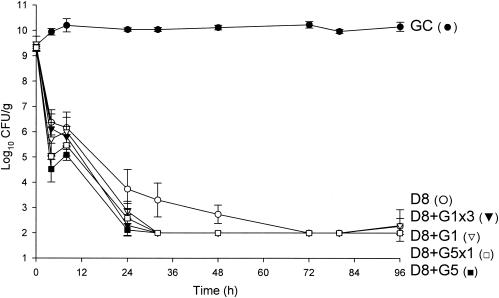
FIG. 1.
Activities of daptomycin (6 mg/kg/day) alone and in combination with gentamicin versus MRSA 494. D6, daptomycin in 6-mg/kg doses given q24h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; G5x1, gentamicin in one 5-mg/kg dose; G1x3, gentamicin (three 1-mg/kg doses); GC, growth control.
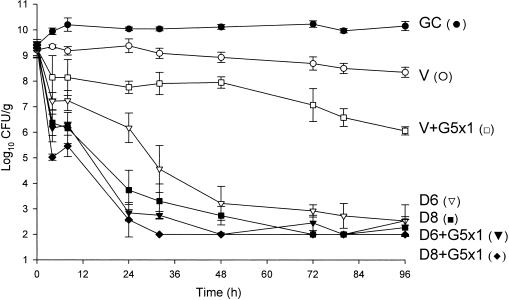
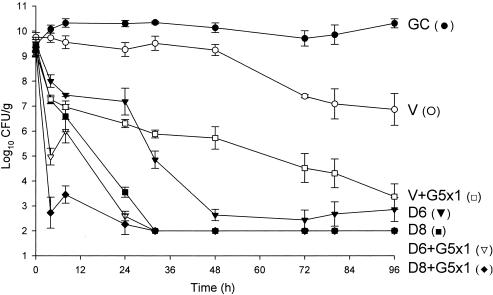
FIG. 8.
Comparison of activities of daptomycin (in 6-mg/kg and 8-mg/kg doses) and vancomycin alone and in combination with a single dose of gentamicin versus MSSA 1199. D6, daptomycin in 6-mg/kg doses given q24h; D8, daptomycin in 8-mg/kg doses q24h; V, vancomycin q12h; G5x1, gentamicin in one 5-mg/kg dose; GC, growth control.
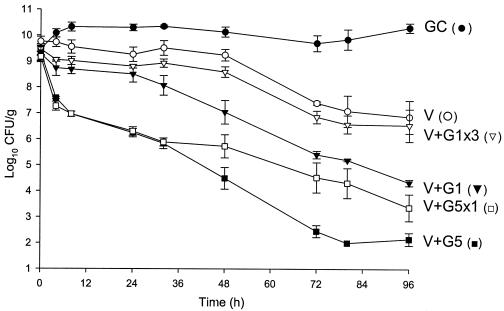
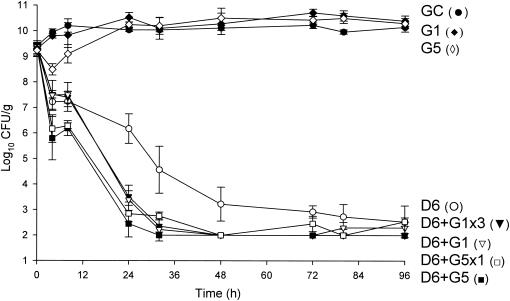
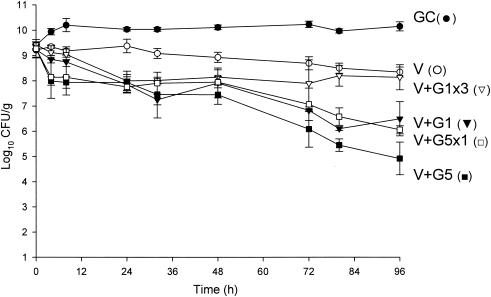
Against the MSSA and MRSA isolates, D6 and D8 alone achieved 99.9% kill by 32 h and 24 h, respectively, and maintained bactericidal activity for the duration of 96 h (P < 0.001). T99 was achieved as early as 8 h for D8 against the MSSA isolate. The achieved AUC/MIC(albumin) for D6 and D8 for both isolates were 1,059.5 and 1,415.5 μg · h/ml, respectively. Vancomycin alone did not achieve bactericidal activity at any time point throughout the 96-h study period against both isolates with an AUC/MIC(albumin) of 579.4 μg · h/ml for both isolates. Against MSSA, a <1-log10 CFU/ml kill was achieved at 96 h and was not significantly different from growth control. For MRSA, a >2-log10 CFU/ml decrease in bacterial density was noted at 72 h and 96 h. For both isolates, the rapid and sustained bactericidal activity achieved by D6 and D8 was significantly different from the delayed, bacteriostatic activity demonstrated by vancomycin (P < 0.001).
All short-course regimens of gentamicin were statistically similar to the full-duration regimens in combination with daptomycin. The achieved Cmax/MIC(albumin) for G1 and G5 were 8.0 and 31.8 μg/ml, respectively. The addition of G1x3 to D6 resulted in enhancement and bactericidal activity at 24 h against both isolates. Adding G1x3 to D8 resulted in improvement against MRSA 494 and 99.9% kill for both isolates at 24 h. The addition of G5x1 to D6 or D8 achieved enhancement or improvement and bactericidal activity as early at 4 h against MRSA and MSSA. All regimens of gentamicin in combination with both regimens of daptomycin achieved bactericidal activity by 24 h and maintained 99.9% kill until the 96-h endpoint. Enhancement or improvement for the combination of gentamicin and daptomycin was noted only from 0 to 24 h, as daptomycin alone demonstrated potent and sustained bactericidal activity from 32 to 96 h.
For vancomycin, the addition of G1x3 did not improve activity and was not significantly different from vancomycin alone for both isolates. However, the addition of G5x1 resulted in enhancement at 4 h and 99.9% kill at 32 h for MRSA 494, which was sustained until 96 h. For MSSA 1199, this combination resulted in bactericidal activity by 96 h and improvement at 24 h. At 96 h, vancomycin and G5x1 achieved enhancement in activity against both MRSA and MSSA isolates. Overall, at 96 h, in combination with vancomycin, the effect of adding G5x1 was similar to the effect of adding G1 for the full duration and significantly better than the effect of adding G1x3 (P < 0.001).
Detection of resistance.
Resistance was detected in the gentamicin q8h and q24h monotherapy model simulation. There was growth on 4× MIC gentamicin plates and a four- to sixfold increase in MIC there, as detected by Etest, at 48, 72, and 96 h for both regimens against both isolates. No resistance was noted at any time point in the daptomycin or vancomycin models with monotherapy or combination regimens.
DISCUSSION
Employing novel dosing regimens and antimicrobials are advantageous to combat the therapeutic problems associated with S. aureus IE. The administration of short courses of combination regimens involving aminoglycosides has previously demonstrated benefit and efficacy in treating IE. Korzeniowski and Sande found that the administration of nafcillin for 6 weeks and gentamicin for 2 weeks resulted in a more rapid mean clinical response and a reduced duration of bacteremia in patients with right-sided endocarditis (25). In addition, other investigators have shown that the outcomes of patients receiving 2-week combination aminoglycoside therapy were similar to the outcomes of patients receiving 4- to 6-week regimens (12, 17, 42). There have been no human studies investigating the effect of administering aminoglycosides in combination for less than 24 h against S. aureus IE. We attempted to investigate whether short-course, 24-h regimens of gentamicin in combination with vancomycin or daptomycin can improve bactericidal and synergistic activity by comparing four different dosing regimens of gentamicin (high dose once daily, single high dose, low dose thrice daily, and low dose thrice daily for only 24 h).
Agents that possess bactericidal activity are necessary to combat S. aureus endocarditis (15, 32, 33). In the presence of protein and high inocula, the administration of a single high dose of gentamicin at the beginning of therapy in combination with both regimens of daptomycin (6 mg/kg or 8 mg/kg) resulted in early enhancement and rapid bactericidal activity against both isolates. By 24 h, all regimens involving daptomycin and short-course gentamicin resulted in a >6-log10 CFU/ml reduction, which was maintained for the duration of the study. When a single high dose of gentamicin was added to either regimen of daptomycin, time to 99.9% kill is dramatically decreased by six- to eightfold. For vancomycin, while enhancement and 99.9% kill was not achieved early, a single high dose of gentamicin in combination resulted in enhancement and bactericidal activity at the final 96-h endpoint. In addition, a single high dose of gentamicin resulted in improvement with vancomycin and 99.9% kill by 32 h. While the clinical implications of the time to 99.9% kill and the magnitude of bacterial reduction remain controversial, the utility of strategies which exhibit a pronounced bactericidal effect which may overcome therapeutic problems associated with resistance, penetration barriers, and high inoculum cannot be underestimated (15, 28, 33, 40).
While the exact mechanism of early synergistic activities resultant from a single high dose of gentamicin of therapy in combination with daptomycin or vancomycin is unknown, these beneficial effects are of interest. The pronounced bacterial reduction which occurs within the first 24 h of combination therapy may eradicate susceptible organisms from a heterogeneous population at high inocula, which may potentially provide an explanation of the mechanism of synergy. The current MRSA strain utilized was not a heterogeneous vancomycin-intermediate S. aureus isolate (data not shown) but did have subpopulations which grew on agar plates containing 1 and 2 μg/ml of vancomycin. In addition, we have previously demonstrated that there are significant differences in the killing profiles of nafcillin, vancomycin, and to some extent daptomycin if the starting inocula is high (9 log10 CFU/ml) versus low (6 log10 CFU/ml) (27). At low inocula, nafcillin, vancomycin, and daptomycin alone result in rapid bactericidal activity. In contrast, against high inocula, the activities of vancomycin and nafcillin were severely hampered against a large bacterial density, while daptomycin was modestly affected. Therefore, the addition of a single high dose of gentamicin at the start of therapy may help to overcome the inoculum effect associated with vancomycin, as bacterial densities approach 6 log10 CFU/ml within 24 h against MRSA. For daptomycin, the addition of a single high dose of gentamicin may provide additional, early and rapid concentration-dependent killing, which may assist in further overcoming the high bacterial density in IE. It is interesting that while the activities of bacteria treated with low or high doses of gentamicin alone resembled growth curves at 96 h, at the 4-h time point, gentamicin at 5 mg/kg achieved a >1-log10 CFU/g reduction in bacterial densities compared to the 1 mg/kg regimen for both isolates. In addition, time-kill experiments performed in our laboratory over 4 h illustrated the concentration-dependent nature of gentamicin with significantly greater kill when concentrations are sequentially increased from 1/8 to 4× MIC against MRSA 494 (data not shown).
In human clinical trials of S. aureus IE, the benefit of combination therapy with aminoglycosides has not been definitively established (28). Clinical investigations have examined the potential for synergy employing a combination of gentamicin and vancomycin and report mixed results (1, 12, 16, 21, 25, 28, 35, 44). In the present investigation, high-dose regimens involving gentamicin combined with vancomycin resulted in enhancement, while high-dose gentamicin regimens combined with daptomycin did not result in enhancement at the 4-day endpoint. However, the addition of gentamicin significantly enhanced the activities of both regimens of daptomycin and vancomycin within the first 24 h of combined therapy.
Significant controversy exists regarding the effect of protein binding on antimicrobial activity. To simulate in vivo conditions, we incorporated human albumin at physiologic concentrations into SMHB for all susceptibility testing and pharmacodynamic model experiments. In addition, for all experiments involving daptomycin and albumin, we titrated ionized calcium concentrations after albumin incorporation further to physiologic levels to account for the loss of calcium after binding to albumin (11). Hanberger et al. noted a decrease of nearly 25% from 1.1 to 0.86 mM of ionized calcium of broth (50 mg/liter) in the presence of 4 g/dl albumin, which subsequently increased the daptomycin MIC by twofold (20). Two- to fourfold dilution differences in daptomycin MIC90 were observed when broth calcium concentrations were 25, 50, and 75 mg/dl (34). To ensure reproducibility, we repeated MIC tests eight times and also verified these results by having an independent laboratory (Jones Group/JMI Laboratories, North Liberty, IA) perform MIC testing in the presence and absence of albumin for these isolates under controlled calcium conditions. Overall, MICs in the presence of albumin for daptomycin increased fourfold in our laboratories and four- to eightfold when performed by the Jones Group/JMI Laboratories for both isolates. For vancomycin MICs in the presence of albumin, we found zero to twofold increases, which was consistent with the findings of the Jones Group/JMI Laboratories. These findings are also consistent with previous studies which have also found similar increases in the presence of albumin in broth or serum under controlled conditions of calcium (19, 20, 30). Furthermore, Craig et al. noted that the extent of increase of MICs of daptomycin in albumin or serum were less than expected from its protein binding levels, 48.2 to 81.1% as calculated by increased MICs (W. A. Craig, S. Kiem, and D. R. Andes, Program Abstr. 42nd Infect. Dis. Conf. Am., abstr. 302, p. 92, 2004).
Daptomycin at a dose of 6 mg/kg is currently being investigated for the treatment of infective endocarditis. By comparing the activities of daptomycin given in doses of 6 mg/kg and 8 mg/kg, we found that all regimens of daptomycin with or without gentamicin were not significantly different at the 96-h endpoint in our study. However, in the first 32 h, daptomycin in 8-mg/kg doses resulted in significantly greater kill than 6 mg/kg against both isolates (P < 0.001). However, when daptomycin in 6-mg/kg doses was combined with all regimens of gentamicin, killing profiles were similar to that of daptomycin alone (in 8-mg/kg doses) at 96 h.
The AUC/MIC ratio is the pharmacodynamic outcome parameter which is the best predictor of efficacy for daptomycin. Attaining an AUC/MIC ratio of greater than 516.5 has been correlated with the effective dose necessary to achieve 80% maximal kill. In our study, in the presence of albumin, both regimens of 6 and 8 mg/kg achieved AUC/MIC(albumin) ratios of 1,059.5 and 1,415.5, respectively. In addition, the peak/MIC ratio has also been associated with in vivo efficacy (39). The achieved Cmax/MIC and AUC/MIC ratios for both regimens of daptomycin were also consistent with previous findings.
It is well established that increasing exposure of aminoglycosides in combination with vancomycin increase the risk of nephrotoxicity. Combining vancomycin and gentamicin may increase the risk of nephrotoxocity as high as three to four times above baseline (36). Traditionally, in the treatment of gram-negative bacterial infections, the evolution of gentamicin dosing regimens to the high-dose-once-daily regimen has attempted to circumvent this problem by demonstrating a predictably lower probability of causing nephrotoxicity compared to traditional regimens. Therefore, it would be ideal to decrease aminoglycoside exposure yet achieve similar activity. We have demonstrated that vancomycin in combination with a single high dose of gentamicin achieved a killing profile similar to that of thrice-daily gentamicin administered for the full duration. In addition, in combination with daptomycin, similar activity was achieved with administration of a high dose of gentamicin as either a single dose or for the full duration. Decreasing the total exposure of gentamicin from an AUC0-96 of 183.2 or 278.8 μg · h/ml when using full-duration thrice-daily or high-dose once-daily dosing, respectively, to 69.7 μg · h/ml by using a single high dose, may be advantageous, as the total AUC exposure has been shown to be a predictor of nephrotoxicity (36). Dosing gentamicin in this fashion may have the potential to decrease nephrotoxicity by avoiding prolonged combination regimens involving gentamicin yet potentially achieve similar activity.
The present investigation had potential limitations. We acknowledge the use of one MSSA isolate and one MRSA isolate; however, we found similar results when utilizing identical regimens for daptomycin (6 mg/kg) and vancomycin and short-course gentamicin against two community-acquired MRSA strains using this model over 72 h (B. T. Tsuji and M. J. Rybak, 8th Int. Symp. Cardio. Infect. Dis. 2005). In addition, while we were able to determine maximal concentrations of antimicrobials in the SEV within 24 h, we did not fully characterize the pharmacokinetic profile within the second compartment. Finally, while the current investigation was performed over 4 days, we cannot conclude with certainty that our results will hold true with treatment durations longer than 96 h, as patients with IE may receive up to 6 weeks of therapy. The most ideal regimen and duration which can be applied to humans remains unknown. There have been no studies in humans with S. aureus IE which demonstrate that early bactericidal activity improves reduction in metastatic complications or mitigation of valvular damage. Therefore, further in vivo studies must first be performed in order to be applied to clinical practice.
In summary, we have shown that short-course gentamicin in combination with both regimens of daptomycin result in rapid bactericidal activity and early enhancement against a high inoculum of S. aureus. Short-course gentamicin and vancomycin also resulted in enhancement and 99.9% kill, while vancomycin alone did not result in bactericidal activity. Further investigation in animal models of IE and humans is warranted.
FIG. 2.
Activities of daptomycin (8 mg/kg/day) alone and in combination with gentamicin versus MRSA 494. D8, daptomycin in 8-mg/kg doses q24h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; G5x1, gentamicin in one 5-mg/kg dose; G1x3, gentamicin in three 1-mg/kg doses; GC, growth control.
FIG. 3.
Activities of vancomycin alone and in combination with gentamicin versus MRSA 494. V, vancomycin q12h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; G5x1, gentamicin in one 5-mg/kg dose; G1x3, gentamicin in three 1-mg/kg doses; GC, growth control.
FIG. 4.
Activities of daptomycin (6 mg/kg/day) alone and in combination with gentamicin versus MSSA 1199. D6, daptomycin in 6-mg/kg doses given q24h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; G5x1, gentamicin in one 5-mg/kg dose; G1x3, gentamicin in three 1-mg/kg doses; GC, growth control.
FIG. 5.
Activities of daptomycin (8 mg/kg/day) alone and in combination with gentamicin versus MSSA 1199. D8, daptomycin in 8-mg/kg doses q24h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; G5x1, gentamicin in one 5-mg/kg dose; G1x3, gentamicin in three 1-mg/kg doses; GC, growth control.
FIG. 6.
Activities of vancomycin alone and in combination with gentamicin versus MSSA 1199. V, vancomycin q12h; G1, gentamicin in 1-mg/kg doses q8h; G5, gentamicin in 5-mg/kg doses q24h; G5x1, gentamicin in one 5-mg/kg dose; G1x3, gentamicin in three 1-mg/kg doses; GC, growth control.
FIG. 7.
Comparison of activities of daptomycin (in 6-mg/kg and 8-mg/kg doses) and vancomycin alone and in combination with a single dose of gentamicin versus MRSA 494. D6, daptomycin in 6-mg/kg doses given q24h; D8, daptomycin in 8-mg/kg doses q24h; V, vancomycin q12h; G5x1, gentamicin in one 5-mg/kg dose; GC, growth control.
Acknowledgments
We thank Ronald Jones and Paul Rhomberg (Jones Group/JMI Laboratories, North Liberty, IA) for performing additional MIC testing, Edward Capellari (DMC Laboratories, Detroit, MI) for chemistry analysis, and Chrissy Cheung and Doina Plesoianu for technical assistance.
This work was supported by a grant from Cubist Pharmaceuticals, Lexington, MA.
REFERENCES
- 1.Abrams, B., A. Sklaver, T. Hoffman, and R. Greenman. 1979. Single or combination therapy of staphylococcal endocarditis in intravenous drug abusers. Ann. Intern. Med. 90:789-791. [DOI] [PubMed] [Google Scholar]
- 2.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1919-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer, A. S., A. F. Bolger, K. A. Taubert, W. Wilson, J. Steckelberg, A. W. Karchmer, M. Levison, H. F. Chambers, A. S. Dajani, M. H. Gewitz, J. W. Newburger, M. A. Gerber, S. T. Shulman, T. J. Pallasch, T. W. Gage, and P. Ferrieri. 1998. Diagnosis and management of infective endocarditis and its complications. Circulation 98:2936-2948. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 7.Caron, F., M. D. Kitzis, L. Gutmann, A. C. Cremieux, B. Maziere, J. M. Vallois, A. Saleh-Mghir, J. F. Lemeland, and C. Carbon. 1992. Daptomycin or teicoplanin in combination with gentamicin for treatment of experimental endocarditis due to a highly glycopeptide-resistant isolate of Enterococcus faecium. Antimicrob. Agents Chemother. 36:2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention.1997. Reduced susceptibility of Staphylococcus aureus to vancomycin-Japan, 1996. Morb. Mortal. Wkly. Rep. 46:624-626. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention.1997. Staphylococcus aureus with reduced susceptibility to vancomycin-United States, 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 10.Cha, R., W. J. Brown, and M. J. Rybak. 2003. Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 47:3960-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha, R., and M. J. Rybak. 2004. Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 54:259-262. [DOI] [PubMed] [Google Scholar]
- 12.Chambers, H. F., R. T. Miller, and M. D. Newman. 1988. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann. Intern. Med. 109:619-624. [DOI] [PubMed] [Google Scholar]
- 13.Cremieux, A. C., B. Maziere, J. M. Vallois, M. Ottaviani, A. Azancot, H. Raffoul, A. Bouvet, J. J. Pocidalo, and C. Carbon. 1989. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J. Infect. Dis. 159:938-944. [DOI] [PubMed] [Google Scholar]
- 14.Das, M., A. D. Badley, F. R. Cockerill, J. M. Steckelberg, and W. R. Wilson. 1997. Infective endocarditis caused by HACEK microorganisms. Annu. Rev. Med. 48:25-33. [DOI] [PubMed] [Google Scholar]
- 15.Finberg, R. W., R. C. Moellering, F. P. Tally, W. A. Craig, G. A. Pankey, E. P. Dellinger, M. A. West, M. Joshi, P. K. Linden, K. V. Rolston, J. C. Rotschafer, and M. J. Rybak. 2004. The importance of bactericidal drugs: future directions in infectious disease. Clin. Infect. Dis. 39:1314-1320. [DOI] [PubMed] [Google Scholar]
- 16.Fortun, J., E. Navas, J. Martinez-Beltran, J. Perez-Molina, P. Martin-Davila, A. Guerrero, and S. Moreno. 2001. Short-course therapy for right-side endocarditis due to Staphylococcus aureus in drug abusers: cloxacillin versus glycopeptides in combination with gentamicin. Clin. Infect. Dis. 33:120-125. [DOI] [PubMed] [Google Scholar]
- 17.Francioli, P., W. Ruch, and D. Stamboulian. 1995. Treatment of streptococcal endocarditis with a single daily dose of ceftriaxone and netilmicin for 14 days: a prospective multicenter study. Clin. Infect. Dis. 21:1406-1410. [DOI] [PubMed] [Google Scholar]
- 18.Fridkin, S. K., J. Hageman, L. K. McDougal, J. Mohammed, W. R. Jarvis, T. M. Perl, and F. C. Tenover. 2003. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997-2001. Clin. Infect. Dis. 36:429-439. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49:467-470. [DOI] [PubMed] [Google Scholar]
- 20.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldman, A. W., T. V. Hartert, S. C. Ray, E. G. Daoud, T. E. Kowalski, V. J. Pompili, S. D. Sisson, W. C. Tidmore, K. A. vom Eigen, S. N. Goodman, P. S. Lietman, B. G. Petty, and C. Flexner. 1996. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am. J. Med. 101:68-76. [DOI] [PubMed] [Google Scholar]
- 22.Huang, V., and M. J. Rybak. 2005. Pharmacodynamics of cefepime alone and in combination with various antimicrobials against methicillin-resistant Staphylococcus aureus in an in vitro pharmacodynamic infection model. Antimicrob. Agents Chemother. 49:302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaatz, G. W., S. L. Barriere, D. R. Schaberg, and R. Fekety. 1987. The emergence of resistance to ciprofloxacin during treatment of experimental Staphylococcus aureus endocarditis. J. Antimicrob. Chemother. 20:753-758. [DOI] [PubMed] [Google Scholar]
- 24.Karchmer, A. W. 1991. Staphylococcus aureus and vancomycin: the sequel. Ann. Intern. Med. 115:739-741. [DOI] [PubMed] [Google Scholar]
- 25.Korzeniowski, O., and M. A. Sande. 1982. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann. Intern. Med. 97:496-503. [DOI] [PubMed] [Google Scholar]
- 26.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le, T., and A. S. Bayer. 2003. Combination antibiotic therapy for infective endocarditis. Clin. Infect. Dis. 36:615-621. [DOI] [PubMed] [Google Scholar]
- 29.Levine, D. P., B. S. Fromm, and B. R. Reddy. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674-680. [DOI] [PubMed] [Google Scholar]
- 30.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath, B. J., S. L. Kang, G. W. Kaatz, and M. J. Rybak. 1994. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob. Agents Chemother. 38:2034-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mylonakis, E., and S. B. Calderwood. 2001. Infective endocarditis in adults. N. Engl. J. Med. 345:1318-1330. [DOI] [PubMed] [Google Scholar]
- 33.Pankey, G. A., and L. D. Sabath. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 38:864-870. [DOI] [PubMed] [Google Scholar]
- 34.Petersen, P. J., P. A. Bradford, W. J. Weiss, T. M. Murphy, P. E. Sum, and S. J. Projan. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribera, E., J. Gomez-Jimenez, E. Cortes, O. del Valle, A. Planes, T. Gonzalez-Alujas, B. Almirante, I. Ocana, and A. Pahissa. 1996. Effectiveness of cloxacillin with and without gentamicin in short-term therapy for right-sided Staphylococcus aureus endocarditis. A randomized, controlled trial. Ann. Intern. Med. 125:969-974. [DOI] [PubMed] [Google Scholar]
- 36.Rybak, M. J., B. J. Abate, S. L. Kang, M. J. Ruffing, S. A. Lerner, and G. L. Drusano. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 43:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybak, M. J., L. M. Albrecht, S. C. Boike, and P. H. Chandrasekar. 1990. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J. Antimicrob. Chemother. 25:679-687. [DOI] [PubMed] [Google Scholar]
- 38.Rybak, M. J., E. M. Bailey, K. C. Lamp, and G. W. Kaatz. 1992. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob. Agents Chemother. 36:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sexton, D. J., M. J. Tenenbaum, W. R. Wilson, J. M. Steckelberg, A. D. Tice, D. Gilbert, W. Dismukes, R. H. Drew, D. T. Durack, and the Endocarditis Treatment Consortium Group. 1998. Ceftriaxone once daily for four weeks compared with ceftriaxone plus gentamicin once daily for two weeks for treatment of endocarditis due to penicillin-susceptible streptococci. Clin. Infect. Dis. 27:1470-1474. [DOI] [PubMed] [Google Scholar]
- 43.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanakunakorn, C., and I. M. Baird. 1977. Prognostic factors in Staphylococcus aureus endocarditis and results of therapy with a penicillin and gentamicin. Am. J. Med. Sci. 273:133-139. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, W. R., A. W. Karchmer, A. S. Dajani, K. A. Taubert, A. Bayer, D. Kaye, A. L. Bisno, P. Ferrieri, S. T. Shulman, D. T. Durack, and the American Heart Association. 1995. Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci, and HACEK microorganisms. JAMA 274:1706-1713. [PubMed] [Google Scholar]
- 46.Wise, R., J. M. Andrews, and J. P. Ashby. 2001. Activity of daptomycin against Gram-positive pathogens: a comparison with other agents and the determination of a tentative breakpoint. J. Antimicrob. Chemother. 48:563-567. [DOI] [PubMed] [Google Scholar]
- 47.Woods, C. W., A. C. Cheng, V. G. Fowler, Jr., M. Moorefield, J. Frederick, G. Sakoulas, V. G. Meka, F. C. Tenover, P. Zwadyk, and K. H. Wilson. 2004. Endocarditis caused by Staphylococcus aureus with reduced susceptibility to vancomycin. Clin. Infect. Dis. 38:1188-1191. [DOI] [PubMed] [Google Scholar]