Abstract
Adherence of Candida albicans to buccal epithelial cells via its fimbrial subunit requires the minimal disaccharide sequence β-GalNAc(1-4)-β-galactosidase in host cell receptors asialo-GM1 or asialo-GM2. This and other disaccharides and some of its synthetic derivatives have been shown to inhibit purified fimbrial or pathogen binding in vitro. This study evaluates the in vivo efficacy of the propyl derivative of this disaccharide, octyl O-(2-acetamido-2-deoxy-β-d-galactopyranosyl)-(1-4)-2-O-propyl-β-d-galactopyranoside, or Fimbrigal-P, incorporated into a mucoadhesive polymer formulation in a rat oral candidiasis model. Colony counts of microcurette samples from the oral cavity and tongue homogenates were used to estimate the effectiveness of four treatment modalities to reduce oral fungal burden. All treatment modalities (preventative, premixing with the Candida inoculant, drinking water, and treatment) significantly reduced fungal burden compared to untreated control animals by day 9; however, the preventative and premixing approaches provided a faster rate of fungal clearance. The low toxicity and immunogenicity of this synthetic carbohydrate and its stability in saliva, as demonstrated by high-performance liquid chromatography, make it a promising candidate for the prevention and treatment of microbial infections in which the pathogen relies on the β-GalNAc(1-4)-β-galactosidase disaccharide to establish adherence.
As conventional antibiotics become less effective due to increasing resistance, alternative means of preventing and eradicating infections must be developed. Because the ability to adhere to host cells is directly related to the potential infectivity of a microorganism (2), one promising approach may involve the prevention of microbial adhesion to host cells by blocking the attachment of microbial appendages known as pili or fimbriae to their cellular receptors (14, 27). These structures are a feature of many gram-negative bacteria such as Pseudomonas aeruginosa, Helicobacter pylori, and Escherichia coli, as well as fungi such as Candida albicans (3, 4, 10, 19).
C. albicans is a dimorphic yeast capable of causing opportunistic infections ranging from topical to systemic, particularly in immunocompromised individuals. C. albicans contains a fimbrial subunit, a major component of the fungal fimbria, which is responsible for adherence to the host cell surface. The adherence of C. albicans to host cells in the oral cavity appears to be a two-step process. After the initial fimbria-mediated binding, the hyphae invade the mucosal surface and initiate an inflammatory process (13, 22). Recently, several genes (HWP1, ALS1, ALA1, and INT1) were identified that encode proteins with adherent properties (7-9, 12, 21). The adherence may involve lectin, protein-protein, or hydrophobic interactions (5).
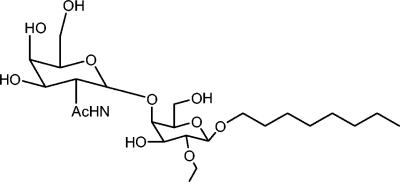
A Candida fimbrial adhesin (MP66) has been identified and partially characterized to be a hydrophobic mannoprotein with a mature molecular mass of 66 kDa, of which 85% is carbohydrate and 15% is protein (24). This molecule mediates fungal adhesion to human buccal epithelial cells (BEC) via an interaction with asialo-GM1 and asialo-GM2 glycolipids on the host cell surface (25). Binding to these host cell receptors occurs via the carbohydrate moiety of the glycolipids, as synthetic disaccharides have been shown to competitively inhibit in vitro BEC binding by C. albicans (25). The minimum domain required for binding to BEC asialo-GM1 and asialo-GM2 is the disaccharide βGalNAc (1-4)-βGal [octyl O-(2-acetamido-2-deoxy-β-d-galactopyranosyl)-(1-4) β-d-galactopyranoside](18). The receptor-binding domain of the C. albicans adhesin is conserved with that of P. aeruginosa, and synthetic derivatives of this disaccharide can also competitively inhibit the binding of this bacterium to immobilized asialo-GM1 in vitro (15, 24, 25). The propyl derivative of the disaccharide appears to be particularly effective, with a pathogen-binding affinity 10-fold higher than that of the native receptor analogue (17). In this study, we present results evaluating the therapeutic efficacy of Fimbrigal-P (Fig. 1) in an in vivo rat oral candidiasis model.
FIG. 1.
Octyl O-(2-acetamido-2-deoxy-β-d-galactopyranosyl)-(1-4)-2-O-propyl-β-d-galactopyranoside (Fimbrigal-P) is one of a family of compounds based on the β-GalNAc(1-4)-β-galactosidase disaccharide structure.
MATERIALS AND METHODS
Formulation of Fimbrigal-P for buccal administration.
Fimbrigal-P was provided by R. T. Irvin, University of Alberta. Fimbrigal-P was formulated in Carbopol EX 214 (BF Goodrich) gel. The aqueous gel was used at 0.8% (wt/wt) polymer concentration without neutralization. The gel was combined with a 50-mg/ml solution of Fimbrigal-P in distilled water to achieve a final Fimbrigal-P concentration of 25 mg/ml and a gel concentration of 0.4% (wt/wt).
Stability studies of Fimbrigal-P.
Various molecular weight mucoadhesive polyacrylic acid polymers (Carbopol 934P, 940P, 981, 1342, 1382, and EX 214) were evaluated for physical stability. The pH of formulations was adjusted to neutrality with a small amount of NaOH for all polymer dispersions except EX 214, which forms a gel without neutralization. Changes in flow and viscosity characteristics of these gels were evaluated in the presence of artificial saliva (24 mM Na2HPO4, 25 mM K2HPO4, 150 mM KHCO3, 100 mM NaCl, and 1.5 mM MgCl2). Viscosity was measured by using a Brookfield cone and plate viscometer, model RV (Brookfield Engineering Labs, Inc.) with spindle CP51 at 25°C.
A Beckman high-performance liquid chromatograph employing a System Gold V810 data system/controller equipped with a Beckman System Gold programmable solvent module 126 and a Sedex Model 55 evaporative light-scattering detector was used to analyze the Fimbrigal-P samples. The column was Lichrospher Diol, 250 by 4 mm (Astec). The mobile phase consisted of methanol/water, 70%/30% (vol/vol). The flow rate was 1 ml/min, and the eluting components were monitored using an evaporative light-scattering detector set at 50°C, at a pressure of 2.2 bar and gain of 6.
To determine whether Fimbrigal-P is enzymatically degraded by salivary enzymes, 1 mg Fimbrigal-P was dissolved in 200 μl of fresh natural saliva (collected from one individual), incubated in a 37°C water bath for 24 h, and subjected to high-performance liquid chromatography analysis.
Oral candidiasis in a rat model.
An immunosuppressed candidiasis model described previously (6) adapted for the oral cavity was used. Sprague-Dawley rats (each, 250 to 300 g; Charles River, Montreal, Canada) were initially supplemented with 0.1% (wt/vol) tetracycline HCl for 1 week and then with 0.01% (wt/vol) tetracycline until the end of the experiment. On day 1 of the experiment, animals were given 150 mg of cyclophosphamide (Cytoxan; Bristol Laboratories of Canada)/kg of body weight intraperitoneally (i.p.) 14 to 24 h before infection. This animal model provides an approximately 2-week acute colonization/infection model with sustained high Candida counts suitable for screening various topical therapeutic agents or treatment protocols.
Animals were inoculated by oral administration of 2 × 107 blastospores of C. albicans (ATCC 90028) in 200 μl of normal saline on 3 consecutive days. Groups of 15 animals each were treated with Fimbrigal-P in one of four different treatment modalities, as summarized in Table 1. Group 1 (control) animals were treated with cyclophosphamide only and inoculated with Candida. Rats in group 2 (preventative group) were given a dose of Fimbrigal-P (6.25 mg/250 μl of 0.4% Carbopol EX 214 gel/dose) 1 h prior to each Candida inoculation and once each 12-h interval for a total of six doses, into the oral cavity. Fimbrigal-P was made continuously available in drinking water (0.5 mg/ml) to group 3 (drinking water group) animals. The medicated water was provided to the animals beginning 2 h before the first inoculation until the day of sacrifice. Group 4 (premixing group) animals were inoculated with a suspension of Candida (2 × 107 blastospores) which were mixed with 6.25 mg Fimbrigal-P in 200 μl normal saline (100 μl of 50-mg/mlFimbrigal-P mixed with 100 μl of normal saline containing 2 × 107 blastospores). The mixture was applied to the oral cavity immediately after mixing. Animals in group 5 (treatment group) received Fimbrigal-P (6.25 mg/250 μl in 0.4% Carbopol EX 214 gel/dose) starting 1 h after the final Candida inoculation, twice a day for 3 days (for a total of six doses) into the oral cavity.
TABLE 1.
Fimbrigal-P treatment modalities for rat oral candidiasisa
| Group (n) and treatment | Day
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Group 1: control group (15) | |||||||||
| Cyclophosphamide 150 mg/kg i.p. | x | ||||||||
| Candida inoculation | x | x | x | ||||||
| MC: 15, 10, 5; 5 rats for HP and TH | x | x | x | ||||||
| Group 2: preventative group (15) | |||||||||
| Cyclophosphamide 150 mg/kg i.p. | x | ||||||||
| Candida inoculation | x | x | x | ||||||
| Fimbrigal-P dose: 6.25 mg/250 μl twice a day | x | x | x | ||||||
| MC: 15, 10, 5; 5 rats for HP and TH | x | x | x | ||||||
| Group 3: drinking water group (15) | |||||||||
| Cyclophosphamide 150 mg/kg i.p. | x | ||||||||
| Candida inoculation | x | x | x | ||||||
| Fimbrigal-P dose: 0.5 mg/ml | x | x | x | x | x | x | x | x | |
| MC: 15, 10, 5; 5 rats for HP and TH | x | x | x | ||||||
| Group 4: premixing group (15) | |||||||||
| Cyclophosphamide 150 mg/kg i.p. | x | ||||||||
| Candida inoculation: C. albicans 2 × 107 blastospores + 6.25 mg Fimbrigal-P in 200 μl normal saline | x | x | x | ||||||
| MC: 15, 10, 5; 5 rats for HP and TH | x | x | x | ||||||
| Group 5: treatment group (15) | |||||||||
| Cyclophosphamide 150 mg/kg i.p. | x | ||||||||
| Candida inoculation | x | x | x | ||||||
| Fimbrigal-P dose: 6.25 mg/250 μl orally twice a day | x | x | x | ||||||
| MC: 15, 10, 5; 5 rats for HP and TH | x | x | x | ||||||
Candida inoculation for all groups: C. albicans 90028 (ATCC), 2 × 107 blastospores/200 μl of normal saline orally. For groups 2 and 5, Fimbrigal-P (25 mg/ml in 0.4% of Carbopol Ex214) was administered at a dose of 250 μl/animal orally, using tuberculin syringes. For group 3, Fimbrigal-P was supplied in drinking water (0.5 mg/ml) from day 2 to the end of the experiment. For group 4, 100 μl Fimbrigal-P (50 mg/ml) was mixed with 2 × 107 blastospores/100 μl of normal saline and then immediately administered orally to animals. In all test groups except group 3, the animals were supplemented orally with 0.1% tetracycline HCl for 1 week and then with 0.01% tetracycline until the end of the experiment. MC, microcurette sampling for fungal burden; TH, tongue homogenate fungal burden; HP, histopathology.
Animals were tested on day 6 (15 animals), day 8 (10 animals), and day 9 (5 animals) for the presence of Candida by microcurette sampling from the oral cavity. After the sampling, five animals were terminated for tongue homogenate and histopathology studies. To accomplish this, animals were anesthetized using methoxyflurane and placed on a surgical pad in a lumbar supine position. The mouth was kept open with a retractor, and the oral cavity was examined with an otoscope. Five individual regional samples were then taken from each animal by striking the end of a 1.5-mm-diameter microcurette five times on the surface of each of five specific regions of the oral cavity, including the surface of the tongue, right buccal mucosa, left buccal mucosa, margin of gingiva, and palate. Individual regional samples were dispersed in 100 μl of sterile normal saline, and the regional samples were then pooled into a single sample for each animal. Samples were stored at 4°C for a maximum of 24 h before further processing. Samples were vortexed and diluted prior to inoculation onto inhibitory mold agar at 30°C in triplicate. The amount of C. albicans present in each sample was determined by colony counting.
On day 9 following the last microcurette sampling, the five remaining animals from each group were sacrificed to quantitate the amount of C. albicans present in the tongues. Animals were anesthetized with methoxyflurane and placed on a surgical pad in a supine position. A blood sample (3.5 ml) was taken from each animal by cardiac puncture. Using scissors, the tongues were removed and divided into two equal parts. One half was fixed in 10% formalin for histopathological examination, and the other half was immersed in 3 ml sterile normal saline for homogenization. Tongues were weighed, minced, and then homogenized with a Cyclon Virtishear homogenizer. Homogenate samples were diluted in saline, and aliquots were inoculated on inhibitory mold agar plates at 30°C for 24 to 48 h. The amount of C. albicans in each sample was determined quantitatively by colony counting. Values for microcurette samples are reported as the number of CFU per milliliter or CFU per gram of tissue (fungal burden) for each treatment group.
Dose-response study.
The effect of a concentration range of Fimbrigal-P (0.2 mg/ml to 25 mg/ml) on oral fungal burden was evaluated using the treatment modality (group 5 for 25 mg/ml). Additional groups (five animals per group) were used for the concentrations of 0.2, 1, and 5 mg/ml and for tongue homogenate analysis at these concentrations to analyze tissues sampled on day 9 only. Microcurette and tongue homogenate samples were analyzed as described in the previous section. In a separate experiment, the effect of Carbopol EX 214 vehicle on baseline fungal counts was also evaluated.
Statistical evaluation.
The various treatment groups and doses applied were compared by analysis of variance and the multiple-comparison Tukey test.
Histopathology.
One half of the excised tongue was fixed in 10% formalin and embedded into paraffin blocks. Sections were cut and stained with hematoxylin and eosin and periodic acid-Schiff reagent. The number of sections taken for each case: 3-μm-thick sections cut at three levels with each level including 10 sections, for total of 30 sections examined per case. Tissue sections were evaluated for the presence of fungal elements and general pathological features.
RESULTS
Preparation of a mucoadhesive dosage form of Fimbrigal-P.
Fimbrigal-P was formulated in a polymeric mucoadhesive dosage form to prolong the presence of the drug in the oral cavity. Among the various mucoadhesive polyacrylic acid polymers, Carbopol EX 214 was found to be the most suitable, since it forms a gel without neutralization and is compatible with electrolytes. The final formulation of 25 mg Fimbrigal-P/ml in 0.4% Carbopol EX 214 was made by adding 0.4% (wt/vol in water) Carbopol EX 214 to an equal volume of Fimbrigal-P (50 mg/ml in water). The resulting mixture formed a clear gel with a plastic flow behavior (plastic viscosity, 4,500 cP; yield stress, 200 D/cm2; results not shown) and pH of 7.8, which was stable during storage at 4°C.
Stability of Fimbrigal-P in saliva.
An evaluation of the susceptibility of Fimbrigal-P to degradation by salivary enzymes indicates that Fimbrigal-P is stable in human saliva (used as a possible substitute for rat saliva, since suitable quantities could be obtained) for 24 h at 37°C. Fimbrigal-P eluted as a single peak with a retention time of 3.55 min in chromatograms of both saliva-incubated Fimbrigal-P and a control solution of Fimbrigal-P in water (chromatograms not shown). These results are consistent with the ability of salivary α-amylase (also present in rat saliva) (23) to hydrolyze α(1-4) but not β(1-4) glycosidic bonds, such as those found between the two galactose molecules in Fimbrigal-P.
Effect of Fimbrigal-P on fungal burden in the oral cavity.
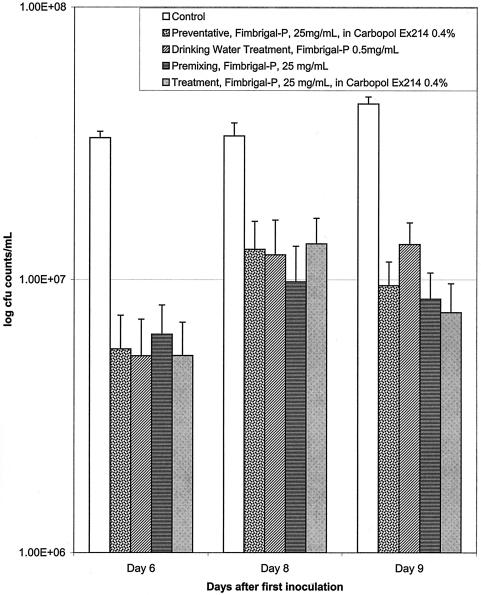
Several different treatment modalities were employed to evaluate the antifungal effect of Fimbrigal-P on oral candidiasis and to elucidate its possible mechanism of action. Evaluation of oral fungal burden by microcurette sampling showed significant clearance of fungus by all the treatment modalities applied (Fig. 2). Significant differences were observed between the control group and all treatment groups on days 6, 8, and 9 (P < 0.05), but there was no apparent difference between individual treatment modalities by the microcurette sampling method. On average, fungal burden decreased by 70% as a result of Fimbrigal-P application (25 mg/ml) for all groups.
FIG. 2.
Effect of Fimbrigal-P on fungal burden in the oral cavity evaluated by microcurette sampling of the surface in the oral cavity after different treatment modalities. All treatment groups were different from control on days 6, 8, and 9 (P < 0.05). There was no statistical difference between the different treatment groups.
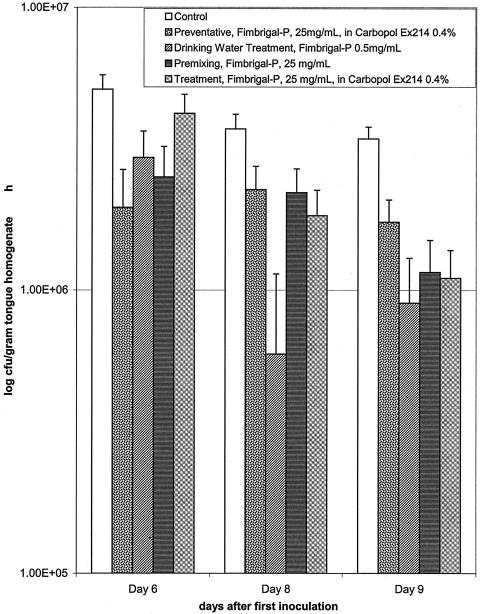
Differences between treatment modalities became apparent, however, when the fungal burden was evaluated in tongue homogenates (Fig. 3). On Day 6, significant difference was observed between the control group and the preventative group (group 2) (P < 0.05). However, the control group did not differ significantly from the drinking water group (group 3) (P < 0.2), the premixing group (group 4) (P < 0.1), or the treatment group (group 5) at this time. By day 8, fungal burden had significantly decreased by about 70 to 80% in the drinking water group (P < 0.05). Although there was no statistical difference between the other treatment modalities at this time point (P < 0.3), there appeared to be a slower rate of fungal elimination by these treatments. By day 9, however, fungal burden had decreased by 60 to 70%, and all treatment groups were significantly different from the control (P < 0.05). Two response patterns are apparent with the different treatments. The preventative and the premixing approaches provided an initial rapid response and continued slower rate of fungal elimination, whereas the delivery of the Fimbrigal-P in the drinking water and as a treatment after the infection showed a slower initial rate of fungal elimination with an exponential pattern, but essentially reducing fungal burden to the same level as the former two delivery approaches by day 9.
FIG. 3.
Effect of Fimbrigal-P on fungal burden in the oral cavity evaluated by analysis of tongue homogenates after different treatment modalities. Statistical evaluation indicated significant differences on days 6 and 8, preventative group versus control (P < 0.05); day 8, drinking water group versus control (P < 0.05); and day 9, all treatment groups versus control (P < 0.05).
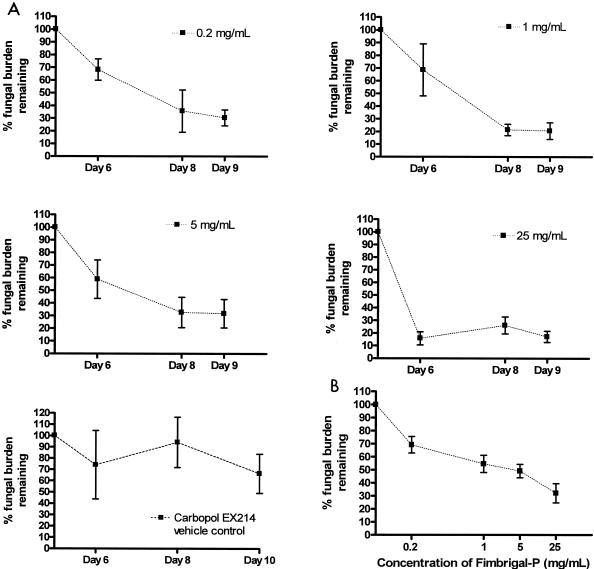
The time curves for the different concentrations of Fimbrigal-P with the treatment modality indicated a gradual response at the lower concentrations and a rapid response at 25 mg/ml (0.2 versus 25 mg/ml on day 6; P < 0.05) (Fig. 4A). At the lower concentrations of Fimbrigal-P (0.2, 1, and 5 mg/ml) fungal burden decreased by 30 to 40% and at 25 mg/ml by 80 to 90%. At later stages, i.e., days 8 and 9, even the lower concentrations of Fimbrigal-P appeared as effective as the higher concentrations. Control experiments indicated that the placebo Carbopol EX 214 gel may have had a slight effect on fungal counts by microcurette sampling; at the early stages of treatment (day 6) and at low Fimbrigal-P concentration, the gel may mask the effect of the active compound due to the variability of the assay (Fig. 4A). However, after day 8 at all concentrations tested, the effect was attributable to Fimbrigal-P.
FIG. 4.
Time curves (A) for the efficacy of Fimbrigal-P at four different concentrations measured by microcurette sampling from the oral cavity. Statistical evaluation indicated a significant difference between 0.2 and 25 mg/ml concentrations on day 6 (P < 0.5). A concentration-response curve (B) obtained with the treatment modality on tongue fungal burden, i.e., administration of Fimbrigal-P after the establishment of infection. The graph shows the percent fungal burden remaining on the tongue (tongue homogenate samples) versus concentration of Fimbrigal-P.
Evaluation of the fungal burden in the tongue homogenates clearly showed a dose-dependent decrease in C. albicans (Fig. 4B). The decrease in fungal burden was approximately 30% at 0.2 mg/ml Fimbrigal-P and 45%, 55%, and 70% at 1, 5, and 25 mg/ml Fimbrigal-P, respectively. The concentration-response curve appears to indicate the possibility of more than one binding site for Fimbrigal-P (Fig. 4B).
Histopathology.
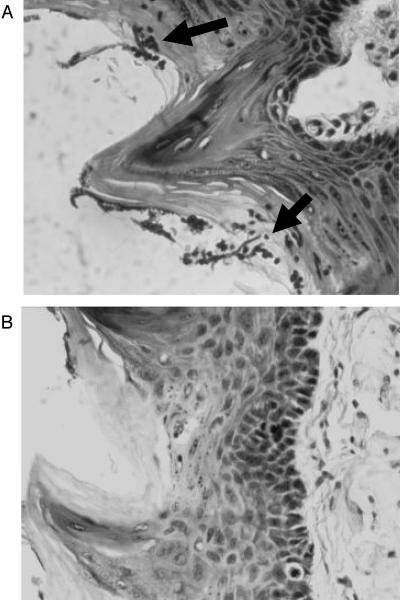
Examination of sections taken from the tongues of the untreated rat control group demonstrated heavy mucosal fungal hyphae and blastospores with associated acute inflammation (Fig. 5A). Similar tongue sections taken from treated rats (any of the four treatment modalities) showed significantly fewer fungal elements and a minor degree of inflammatory reaction. Figure 5B shows a representative micrograph from the treatment group.
FIG. 5.
Qualitative histopathology of rat tongue sections stained with hematoxylin and eosin. Cross-sections of a representative excised rat tongues from the control (untreated) group (A) showing the presence of fungal hyphae and blastospores (arrows) as an indication of fungal colonization and a tongue section from the treatment group (B), indicating clearance of fungal elements. Tongue samples for both groups were taken on day 9.
DISCUSSION
The purpose of this investigation was to evaluate the in vivo efficacy of a novel carbohydrate-based anti-infective agent. Many pathogens use carbohydrate-binding proteins (adhesins or lectins) as receptors to establish adherence to host cells, and the infectivity of these pathogens depends on their adhesive ability. Among the host defenses present at mucosal surfaces and in secretions are oligosaccharides, which act as decoys for adhesions (27). These carbohydrates inhibit microbial attachment to host cells by occupying adhesin-binding sites. In theory, the therapeutic administration of oligosaccharides might achieve the same antiadhesive effect.
The minimum host epithelial cell carbohydrate structure required for binding by C. albicans is the disaccharide β-GalNAc(1-4)-β-galactosidase found in surface glycolipids such as asialo-GM1 (gangliotetraosylceramide) and asialo-GM2 (gangliotriosylceramide) (24-26). A number of pulmonary pathogens, including P. aeruginosa, Haemophilus influenzae, and Staphylococcus aureus, are able to utilize this disaccharide structure as a receptor, facilitating their attachment to host cells (14). This disaccharide and various derivatives thereof have been shown to inhibit fungal adherence to cultured epithelial cells in vitro (15, 18, 26). The greater affinity of the propyl derivative of this disaccharide for the adhesin in vitro led to interest in its potential as an anti-infective agent (17).
In this study, Fimbrigal-P was shown to be stable in human saliva, making it a suitable compound for buccal administration. The effect of Fimbrigal-P on rat oral fungal burden was evaluated in two ways. Direct microcurette sampling of various regions of the oral cavity indicated similar responses between the different Fimbrigal-P treatment modalities. Overall, the fungal burden was substantially reduced for all modalities by 70 to 85% compared to untreated controls, starting on day 6 after infection, and remained at about 70% reduction on day 9. This is significant, as the administration of Fimbrigal-P did not continue beyond day 6 in the treatment group (group 5) and day 4 in the preventative group (group 4). The similarly positive responses to the different delivery methods can be explained by the common mechanism of Fimbrigal-P binding to the yeast, regardless of the delivery system or method. In the preventative group, the oral cavity was covered with a mucoadhesive dosage form of Fimbrigal-P, providing a reservoir of this compound for binding with the yeast. Similarly, in the premixing approach, the yeast cells were preincubated with the Fimbrigal-P solution; therefore, the bound yeast cells were less likely to bind the mucosal surfaces. In the treatment modality, the twice-daily administration of Fimbrigal-P following the inoculation provided a continuous supply of the compound from the mucoadhesive dosage form for binding to the yeast. Probably, a similar effect was achieved by using the administration with the drinking water. The overall dose of Fimbrigal-P received by the animals was also similar in all treatment modalities.
Evaluation of tongue homogenates demonstrated a more distinct pattern between treatment modalities. Initially, the largest decrease in fungal burden was observed in the animals given the preventative and the premixing treatment modality prior to C. albicans exposure (Fig. 3). This is intriguing, in light of previously published data suggesting that soluble oligosaccharides might be able to prevent initial adherence as well as eliminate established infections (11). By day 8, a significant lowering of fungal burden was also observed in animals given access to Fimbrigal-P in drinking water; by day 9, all animals exhibited a low fungal burden compared to untreated controls, regardless of treatment modality.
The differences in results between the two assessment methods, i.e., the microcurette sampling and tongue homogenate analysis, could indicate the differences in surfaces sampled within the oral cavity and the differences in the interaction of the mucoadhesive formulation with mucosal versus tongue surfaces within the oral cavity.
While the efficacy studies indicated that administration of Fimbrigal-P led to a significant reduction in fungal burden in the oral cavity and tongue, complete elimination of C. albicans was not achieved. There are several potential explanations for this. First, the compound may have been cleared from the oral cavity too rapidly to provide sufficient active agent to bind to yeast cells. If so, this might be overcome by the use of improved bioadhesive or slow-release formulations. Second, C. albicans-mediated adhesion included receptors other than the carbohydrate types utilized here. Third, a number of studies have reported increased efficacy using multivalent rather than monovalent soluble oligosaccharides, which may more accurately mimic microbial attachment in vivo (1, 20). It is possible, therefore, that a multivalent form of Fimbrigal-P may exhibit improved antiadhesive properties. It can be argued, however, that significant therapeutic benefit might be achieved without complete blockade by Fimbrigal-P, as a simple reduction in adhesion may allow normal mucosal mechanisms to successfully complete microbial clearance (27).
The ability of these soluble carbohydrates to prevent fungal adherence lends them clinical potential as a new class of antimicrobial agents. To date, oligosaccharide receptor analogues have been investigated as antiadhesive compounds in a number of infections. Sialylated oligosaccharides, for example, have been shown to inhibit the attachment of Streptococcus pneumoniae to respiratory epithelium both in vitro and in vivo (1, 11). Another receptor analogue, 3′ sialyllactose, can block Heicobacter pylori adherence to intestinal epithelial cells in vitro (20) and has been shown to successfully clear H. pylori infection from some rhesus monkeys in vivo (16). The clinical potential of therapeutic carbohydrates may extend beyond their antimicrobial applications, for example, xenotransplantation (20), where soluble carbohydrate therapy may complement other approaches to eliminating graft reactions.
There are numerous potential advantages to the clinical use of these types of compounds. Their toxicity is low, and they can be used in a prophylactic manner. In addition, the risk of resistance or tolerance developing to these compounds is low. Finally, these agents are nonimmunogenic and stable in solution, and they can be administered by a variety of routes, including orally, topically, and intravenously. However, administration of soluble carbohydrate compounds such as Fimbrigal-P may require a suitable delivery system to increase the residence time in the oral cavity. Potential vehicles would include mucoadhesive Carbopol polymers (e.g., the one used in this study, cross-linked acrylic acid derivatives that are pharmaceutical grade, biocompatible hydrogels with good safety profiles) or potentially others that could provide an extended-release kinetic profile in maintaining appropriate antiadhesin levels within the oral cavity.
Acknowledgments
We thank W. Y. Wong, R. T. Irvin, O. Hindsgaul, and R. Hodges, Department of Medical Microbiology and Infectious Diseases, University of Alberta, Protein Engineering Network Centers of Excellence (PENCE) Edmonton, Alberta, Canada, for providing the compound Fimbrigal-P.
REFERENCES
- 1.Barthelson, R., A. Mobasseri, D. Zopf, and P. Simon. 1998. Adherence of Streptococcus pneumoniae to respiratory epithelial cells is inhibited by sialylated oligosaccharides. Infect. Immun. 66:1439-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 3.Calderone, R. 1993. Recognition between Candida albicans and host cells. Trends Microbiol. 1:55-58. [DOI] [PubMed] [Google Scholar]
- 4.Calderone, R. A. 1993. Molecular interactions at the interface of Candida albicans and host cells. Arch. Med. Res. 24:275-279. [PubMed] [Google Scholar]
- 5.Cannon, R. D., and W. L. Chaffin. 1999. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 10:359-383. [DOI] [PubMed] [Google Scholar]
- 6.Foldvari, M., J. Radhi, G. Yang, Z. He, R. Rennie, and L. Wearley. 2000. Experimentally induced vaginal candidiasis model in the immunocomprised rat. Mycoses 43:393-401. [PubMed] [Google Scholar]
- 7.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y.-C. Chen, S. W. French, J. Cutler, S. G. Filler, and J. E. Edwards, Jr. 2002. Candida albicans Als1p: an adhesion that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44:61-72. [DOI] [PubMed] [Google Scholar]
- 8.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 9.Gaur, N. K., and S. A. Klotz. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hostetter, M. K. 1994. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin. Microbiol. Rev. 7:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idanpaan-Heikkila, I., P. M. Simon, D. Zopf, T. Vullo, P. Cahill, K. Sokol, and E. Tuomanen. 1997. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J. Infect. Dis. 176:704-712. [DOI] [PubMed] [Google Scholar]
- 12.Kamai, Y., M. Kubota, Y. Kamai, T. Hosokawa, T. Fukuoka, and S. G. Filler. 2002. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect. Immun. 70:5256-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klotz, S. 1994. Plasma and extracellular matrix proteins mediate in the fate of Candida albicans in the human host. Med. Hypotheses 42:328-334. [DOI] [PubMed] [Google Scholar]
- 14.Krivan, H. C., D. D. Roberts, and V. Ginsburg. 1988. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc1-4Gal found in some glycolipids. Proc. Natl. Acad. Sci. USA 85:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, K. K., L. Yu, D. L. Macdonald, W. Paranchych, R. S. Hodges, and R. T. Irvin. 1996. Anti-adhesin antibodies that recognize a receptor-binding motif (adhesintope) inhibit pilus/fimbrial-mediated adherence of Pseudomonas aeruginosa and Candida albicans to asialo-GM1 receptors and human buccal epithelial cell surface receptors. Can. J. Microbiol. 42:479-486. [DOI] [PubMed] [Google Scholar]
- 16.Mysore, J. V., T. Wigginton, P. M. Simon, and D. Zopf. 1999. Treatment of a Helicobacter pylori infection in rhesus monkeys using a novel antiadhesin compound. Gastroenterology 117:1316-1325. [DOI] [PubMed] [Google Scholar]
- 17.Schweizer, F., H. Jiao, O. Hindsgaul, W. Y. Wong, and R. T. Irvin. 1998. Interaction between the pili of Pseudomonas aeruginosa PAK and its carbohydrate receptor-D-GalNAc(164)-D-Gal analogs. Can. J. Microbiol. 44:307-311. [PubMed] [Google Scholar]
- 18.Sheth, H. B., K. K. Lee, W. Y. Wong, G. Srivastava, O. Hindsgaul, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1994. The pili of Pseudomonas aeruginosa strains PAK and PAO bind specifically to the carbohydrate sequence β GalNAc(1-4)β Gal found in glycosphingolipids asialo-GM1 and asialo-GM2. Mol. Microbiol. 11:715-723. [DOI] [PubMed] [Google Scholar]
- 19.Simon, P. M., P. L. Goode, A. Mobasseri, and D. Zopf. 1997. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect. Immun. 65:750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon, P. M., F. A. Neethling, S. Taniguchi, P. L. Goode, D. Zopf, W. W. Hancock, and D. K. C. Cooper. 1998. Intravenous infusion of Gal1-3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation 65:346-353. [DOI] [PubMed] [Google Scholar]
- 21.Staab, J. F., S. D. Bradway, P. L. Fidel, and P. Sundstorm. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535-1538. [DOI] [PubMed] [Google Scholar]
- 22.Vitkov, L., W. D. Krautgartner, M. Hannig, R. Weitgasser,and W. Stoiber. 2002. Candida attachment to oral epithelium. Oral Microbiol. Immunol. 17:60-64. [DOI] [PubMed] [Google Scholar]
- 23.Yamahara, H., and V. H. L. Lee. 1993. Drug metabolism in the oral cavity. Adv. Drug Deliv. Rev. 12:25-40. [Google Scholar]
- 24.Yu, L., K. K. Lee, K. Ens, P. C. Doig, M. R. Carpenter, W. Staddon, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1994. Partial characterization of a Candida albicans fimbrial adhesin. Infect. Immun. 62:2834-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, L., K. K. Lee, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1994. Adherence of Pseudomonas aeruginosa and Candida albicans to glycosphingolipid (asialo-GM1) receptors is achieved by a conserved receptor-binding domain present on their adhesins. Infect. Immun. 62:5213-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu, L., K. K. Lee, H. B. Sheth, P. Lane-Bell, G. Srivastava, O. Hindsgaul, W. Paranchych, R. S. Hodges, and R. T. Irvin. 1994. Fimbria-mediated adherence of Candida albicans to glycosphingolipid receptors on human buccal epithelial cells. Infect. Immun. 62:2843-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zopf, D., and S. Roth. 1996. Oligosaccharide anti-infective agents. Lancet 347:1017-1021. [DOI] [PubMed] [Google Scholar]