Abstract
The intraerythrocytic development of Plasmodium falciparum correlates with increasing levels of the polyamines putrescine, spermidine, and spermine in the infected red blood cells; and compartmental analyses revealed that the majority is associated with the parasite. Since depletion of cellular polyamines is a promising strategy for inhibition of parasite proliferation, new inhibitors of polyamine biosynthesis were tested for their antimalarial activities. The ornithine decarboxylase (ODC) inhibitor 3-aminooxy-1-aminopropane (APA) and its derivatives CGP 52622A and CGP 54169A as well as the S-adenosylmethionine decarboxlyase (AdoMetDC) inhibitors CGP 40215A and CGP 48664A potently affected the bifunctional P. falciparum ODC-AdoMetDC, with Ki values in the low nanomolar and low micromolar ranges, respectively. Furthermore, the agents were examined for their in vitro plasmodicidal activities in 48-h incubation assays. APA, CGP 52622A, CGP 54169A, and CGP 40215A were the most effective, with 50% inhibitory concentrations below 3 μM. While the effects of the ODC inhibitors were completely abolished by the addition of putrescine, growth inhibition by the AdoMetDC inhibitor CGP 40215A could not be antagonized by putrescine or spermidine. Moreover, CGP 40215A did not affect the cellular polyamine levels, indicating a mechanism of action against P. falciparum independent of polyamine synthesis. In contrast, the ODC inhibitors led to decreased cellular putrescine and spermidine levels in P. falciparum, supporting the fact that they exert their antimalarial activities by inhibition of the bifunctional ODC-AdoMetDC.
Malaria is the most prevailing parasitic disease worldwide, with an estimated 500 million people infected annually. The protist Plasmodium falciparum causes the most severe form of malaria and kills over 1 million people in the tropical regions of the world per year, mostly children under the age of 5 years. Treatment of the disease is being compromised by the spreading resistance to the commonly used antimalarial drugs. Therefore, the evaluation of new drug targets and the identification of compounds with plasmodicidal activities are of urgent need (http://www.who.int/health-topics/malaria.htm).
During erythrocytic schizogony, P. falciparum proliferates rapidly within host cells, leading to 12 to 18 new merozoites every 48 h. It has been shown for many organisms that growth and differentiation processes depend on adequate intracellular concentrations of the polyamines putrescine, spermidine, and spermine (23, 32). As a consequence, depletion of cellular polyamine levels has an antiproliferative effect on cells, including P. falciparum (23, 27, 32).
The polyamine synthesis pathway contains two regulatory steps, catalyzed by ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC). ODC converts the amino acid ornithine into putrescine. AdoMetDC generates decarboxylated S-adenosylmethionine (dcAdoMet), which is required as aminopropyl group donor by spermidine and spermine synthase to form spermidine and spermine, respectively (23, 32). Usually, ODC and AdoMetDC represent two separate proteins encoded by two individual genes. In P. falciparum, however, both enzymes are located on a single open reading frame, which encodes a bifunctional ODC-AdoMetDC protein (26). Although it has been shown that, despite this unusual organization, both domains act independently, P. falciparum ODC and AdoMetDC exhibit specific regulatory features that are distinct from those of the monofunctional host enzymes (21, 40). We propose that this distinct regulation of the key enzymes of the polyamine synthesis pathway in P. falciparum may offer possibilities for the design of new chemotherapies against malaria.
Classical ODC and AdoMetDC inhibitors, like α-difluoromethylornithine (DFMO), methylglyoxal bis(guanylhydrazone) (MGBG), and MDL 73811, have been used in previous attempts to interfere with the polyamine synthesis in P. falciparum and other Plasmodium species (3, 6, 7, 11, 12, 14, 41). Sporozoite formation in the insect vector as well as the development of liver stages was sensitive to DFMO, whereas the erythrocytic stages of Plasmodium were hardly affected by these inhibitors in vivo.
Meanwhile, a next generation of ODC and AdoMetDC inhibitors has been synthesized. The new inhibitors of ODC are related to 3-aminooxy-1-aminopropane (APA) (15, 17, 18, 24, 34), and those of AdoMetDC are derivatives of bis(guanylhydrazones) (29, 30, 35, 36) (Fig. 1). Several of these compounds were reported to be more potent in blocking the proliferation of tumor cells and parasites than their progenitors (8, 15, 17, 24, 25, 30, 36). In particular, the AdoMetDC inhibitor CGP 40215A is highly effective against trypanosomes in laboratory model infections (4).
FIG. 1.
Structural formulas of the ODC and AdoMetDC inhibitors.
In the study described in this paper we monitored the intracellular polyamine concentrations during the erythrocytic cycle of P. falciparum and determined the polyamine distribution within the parasite-host cell unit. Furthermore, the effects of various established ODC and AdoMetDC inhibitors as well as new ODC and AdoMetDC inhibitors on in vitro enzyme activities, parasite growth, and cellular polyamine concentrations were investigated. Our results show that some of the new ODC and AdoMetDC inhibitors are by far more potent antimalarials, at least in culture, than the classical agents.
(A part of this work was conducted in partial fulfillment of the requirement for a Ph.D. by R. Das Gupta and I. B. Müller from the University of Hamburg.)
MATERIALS AND METHODS
Culture of P. falciparum.
The P. falciparum 3D7 strain was maintained in continuous culture, according to Trager and Jensen (37). The parasites were grown in human red blood cells (RBCs blood group A positive), RPMI 1640 medium supplemented with 25 mM HEPES, 20 mM sodium bicarbonate, and 0.5% AlbuMAX (Invitrogen, Karlsruhe, Germany) or, alternatively, in 10% human plasma at 5% hematocrit. The flasks were gassed with 90% N2, 5% O2, and 5% CO2 and incubated at 37°C. The development of the cultures and the percentage of infected RBCs were determined by light microscopy of Giemsa-stained thin smears. Synchronization was carried out by incubation of the cells in 2 volumes of 0.3 M l-alanine, 10 mM HEPES, pH 7.4, for 5 min at 37°C (22). In order to isolate infected RBCs from noninfected erythrocytes and to separate different life stages of the parasite, a discontinuous Percoll-l-alanine gradient centrifugation was performed (16). The cell number was determined in a cell counter (Coulter Max M; Coulter Electronics, Krefeld, Germany). To perform compartmental analyses, parasites were separated from host RBCs by using the pore-forming toxin streptolysin O (SLO; kindly provided by S. Bhakti, University of Mainz) (5, 22). Trophozoite-infected RBCs (2 × 108) were diluted in Earle's balanced salt solution containing 1.43 μg SLO, and the mixture was incubated for 6 min at 20°C. The reaction mixture was centrifuged at 1,500 × g for 5 min at 4°C; and the supernatant, which contained the RBC fraction, as well as the isolated parasites in the pellet were examined to determine the polyamine contents.
Effects of ODC and AdoMetDC inhibitors on cell growth.
The ODC inhibitor 3-aminooxy-1-aminopropane was synthesized as described previously (19). The APA derivatives CGP 52622A and CGP 54169A as well as the AdoMetDC inhibitors CGP 40215A and CGP 48664A were kindly provided by Novartis Pharma AG (Basel, Switzerland). DFMO and MDL 73811 were a kind gift from Hoechst Marion Roussel (Cincinnati, OH). The activities of these ODC and AdoMetDC inhibitors against erythrocytic stages of P. falciparum were determined by a [3H]hypoxanthine incorporation assay (10). Dilutions of each drug (0.1 to 5 μM for APA, CGP 54169A, and CGP 52622A; 0.5 to 5 μM for CGP 40215A and MDL 73811; 2 to 20 μM for CGP 48644A; and 0.5 to 5 mM for DFMO) were added to 250-μl parasite cultures at 1.5% hematocrit with 1.5 to 2.0% parasitemia prepared in 96-well microtiter plates. To examine the effects of exogenous polyamines, the cultures were additionally supplemented with 500 μM putrescine or spermidine. After incubation for 24 h at 37°C, 0.1 μCi of [3H]hypoxanthine was added to each well. The plates were incubated for an additional 24 h under the same conditions and were subsequently harvested with a cell harvester system (Inotech, Dottikon, Switzerland). Infected RBCs were washed four times with distilled water before they were analyzed in a multidetector liquid scintillation counter (Wallac, Turku, Finland). The 50% inhibitory concentrations (IC50s) were calculated from sigmoidal inhibition curves by using GraphPad Prism 1.02 (GraphPad Software, San Diego, CA). To test the stage-specific effects of the polyamine synthesis inhibitors, the growth of highly synchronized ring-stage cultures at 0.8% parasitemia was monitored for 120 h. Inhibitors and polyamines were added as indicated. The development of P. falciparum was monitored by light microscopy of Giemsa-stained thin smears.
The ODC and AdoMetDC inhibitors were also tested against the murine B-cell lymphoma cell line A20/2J grown in 96-well tissue culture plates. The cells were incubated with increasing concentrations of the respective drugs for 48 h at 37°C. Cell viability was determined by a colorimetric assay with the tetrazolium salt WST-1 (Roche). After incubation for 3 h, formazan formation was quantified at 450 nm with a multiwell enzyme-linked immunosorbent assay reader (Multiskan MCC/340; Labsystems).
Polyamine analysis.
The analysis of the polyamine levels in the RBCs and the parasites was performed as described by Seiler and Knödgen (31). The samples were deproteinized by adding 0.2 M perchloric acid for 12 h at 4°C. A total of 49.3 nmol 1,6-diaminohexane was added to the homogenates as internal standard. The perchloric acid extracts were saturated with sodium carbonate and reacted with dansylchloride by addition of 3 volumes of dansylchloride in acetone (2 mg ml−1). The reactions took place at room temperature overnight in the dark. Subsequently, the dansyl derivatives were extracted with toluene. The organic phase was evaporated to dryness, and the residues were dissolved in methanol-acetic acid (95:5; vol/vol). A total of 50 μl of each of the dansyl derivative solutions was applied to a Spherisorb ODS II column (5 μm, 250 by 3 mm; Machery-Nagel, Düren, Germany) for reversed-phase high-pressure liquid chromatography (HPLC) analyses. The mobile phases consisted of methanol (solvent A) and water (solvent B). Separation was performed at a flow rate of 0.6 ml min−1 by application of the following gradient (% of solvent B): 0 min, 57.5%; 25 min, 67.5%; 37.5 min, 82.5%; 46.25 min, 100%; 50 min, 100%; 52.5 min 57.5%; 62.5 min, 57.5%. Dansylated polyamines were detected by a fluorescence spectrophotometer (excitation, 365 nm; emission, 485 nm; SFM 25; Kontron, Neufahrn, Germany).
Enzyme assays.
Recombinant expression and purification of the bifunctional P. falciparum ODC-AdoMetDC and its domains were carried out as described previously (21, 26, 40). ODC was assayed by measuring the formation of 14CO2 from l-[1-14C]ornithine at 37°C. The standard assay contained, in a final volume of 250 μl, 50 mM potassium phosphate buffer, pH 7.5, 1 mM dithiothreitol, 1 mM EDTA, 40 μM pyridoxal 5-phosphate (PLP), 0.02% Brij 35, 100 μM ornithine (0.27 μCi of l-[1-14C]ornithine), and 72 nM recombinant P. falciparum ODC-AdoMetDC or the P. falciparum ODC domain. The reaction time was 30 min. The inhibition constants for APA and its derivatives were determined under standard assay conditions with various concentrations of ornithine (10 to 200 μM) and by the addition of various concentrations of inhibitors (3 to 8 nM for APA, 5 to 10 nM for CGP 54169A, and 5 to 25 nM for CGP 52622A). The Ki values for the tightly binding inhibitors were calculated by using the Morrison equation (13).
AdoMetDC activity was assayed as described above, except that S-adenosyl-l-[methyl-14CO2]methionine was used instead of l-[1-14C]ornithine, with a standard assay mixture containing 50 mM potassium phosphate buffer, 1 mM dithiothreitol, 0.1 mM EDTA, 0.02% Brij 35, and 72 nM recombinant P. falciparum ODC-AdoMetDC or the P. falciparum AdoMetDC domain. The inhibition constants for CPG 40215A and CPG 48664A were determined under standard assay conditions with various concentrations of AdoMet (10 to 200 μM) and by the addition of various concentrations of inhibitors (0.5 to 3.0 μM for CGP 40215A and 2.5 to 10.0 μM for CGP 48664A). The corresponding Ki values were calculated from the Lineweaver-Burk plots by using GraphPad Prism 1.02. The Ki value for the irreversible inhibitor MDL 73811 was determined by preincubation of aliquots of the bifunctional recombinant P. falciparum ODC-AdoMetDC at 37°C with various concentrations of MDL 73811 ranging from 0.1 to 0.6 μM. At different time points, samples were withdrawn and assayed for residual enzyme activity. Kinetic analysis was carried out by the method of Kitz and Wilson (20).
RESULTS
Polyamine levels during the erythrocytic cycle of P. falciparum.
To monitor the polyamine content during the erythrocytic schizogony, P. falciparum-infected RBCs of a highly synchronized culture were separated from noninfected RBCs by use of a Percoll gradient at three different time points postinvasion (p.i.) representing rings (4 to 10 h p.i.), trophozoites (18 to 24 h p.i.), and schizonts (36 to 42 h p.i.). The level of parasitemia of the isolated cells was determined to be at least 95%. As shown in Table 1, a correlation was observed between the intracellular levels of putrescine, spermidine, and spermine and the developmental stage of the parasite. The total levels of all three polyamines increased from the ring to the schizont stage by 17-, 14-, and 22-fold, respectively. At all times spermidine represented the major polyamine, followed by putrescine and low levels of spermine.
TABLE 1.
Stage-specific polyamine content of P. falciparum-infected RBCsa
| Cell | Polyamine concn (nmol 1010 cells−1)
|
||
|---|---|---|---|
| Putrescine | Spermidine | Spermine | |
| Rings | 35.9 ± 24.2 | 93.4 ± 62.9 | 5.1 ± 3.3 |
| Trophozoites | 454.5 ± 195.8 | 1,079.0 ± 185.0 | 39.8 ± 17.2 |
| Schizonts | 624.0 ± 227.7 | 1,295.4 ± 398.9 | 112.5 ± 53.2 |
| Cocultured noninfected RBCs | 23.1 ± 10.3 | 43.5 ± 19.6 | 11.9 ± 8.0 |
Prior to polyamine determination, infected RBCs were separated from noninfected RBCs by discontinuous Percoll-l-alanine gradient centrifugation to yield parasitemias >95%. The results represent the means ± standard deviations of eight independent determinations.
To determine the polyamine distribution in the parasite-RBC unit, isolated trophozoite-infected RBCs were treated with SLO to separate both “compartments.” The following quantitative analyses revealed that the majority of the polyamines is associated with the parasite and that the RBC compartment contains markedly lower polyamine levels (Table 2). This is in accordance with the low polyamine levels found in cocultured noninfected RBCs (Table 1).
TABLE 2.
Compartmental analysis of polyamine distribution in P. falciparum-infected RBCs
| Compartment | Polyamine concn (nmol 1010 cells−1)a
|
||
|---|---|---|---|
| Putrescine | Spermidine | Spermine | |
| Parasite-host unit | 164.6 ± 69.8 | 865.9 ± 342.5 | 45.7 ± 21.5 |
| Parasite | 104.7 ± 54.1 | 686.8 ± 299.4 | 34.0 ± 19.3 |
| Host cell | 41.9 ± 9.6 | 140.4 ± 37.0 | 11.9 ± 6.8 |
The results are the means ± standard deviations of four independent determinations.
Effects of ODC and AdoMetDC inhibitors on cultured parasites.
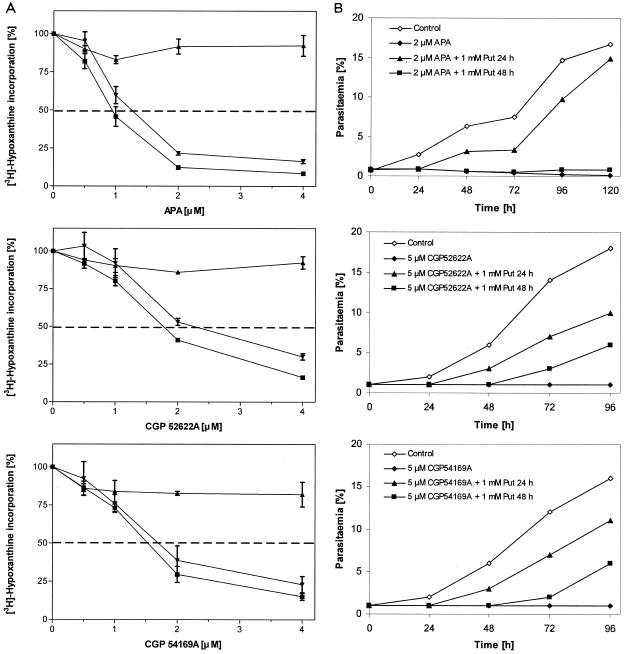
P. falciparum was cultured in the presence of increasing concentrations of various ODC and AdoMetDC inhibitors. All inhibitors tested affected the growth of P. falciparum, as determined by [3H]hypoxanthine incorporation. The impacts of the inhibitors on murine B-cell lymphoma cells were also examined. Table 3 summarizes the calculated IC50s, including data on a human bladder carcinoma cell line adopted from previous reports (30, 34, 35). Except for the AdoMetDC inhibitor CGP 48664A, the P. falciparum cultures exhibited higher sensitivities (4- to 40-fold) to the inhibitors than the mammalian cells (Table 3). The exogenous addition of 500 μM putrescine to the culture medium completely abrogated the growth arrest of P. falciparum caused by the ODC inhibitors (Fig. 2A), suggesting that their effects on parasite growth are attributable to the inhibition of putrescine synthesis. Spermidine did not antagonize the growth inhibition by ODC inhibitors (Fig. 2A) and only marginally altered the effects of the AdoMetDC inhibitors (data not shown). The ODC inhibitors APA, CGP 52622A, and CGP 54169A were found to be 500- to 1,300-fold more effective than the classical inhibitor DFMO. Among the AdoMetDC inhibitors, CGP 40215A exhibited the best IC50 (1.8 μM). Moreover, 2 μM APA, 5 μM CGP 52622A, and 5 μM CGP 54169A were found to arrest parasite development at the trophozoite stage (data not shown), which is in accordance with the findings from previous reports on the stage-specific effect of DFMO (1, 2, 41). The addition of 1 mM putrescine after 24 h or 48 h of drug treatment reversed the growth block, whereby APA-treated parasites only scarcely recovered when putrescine was added after 48 h (Fig. 2B).
TABLE 3.
Effects of ODC and AdoMetDC inhibitors on enzyme activities and the survival rate of cultured P. falciparum
| Inhibitor | Ki valuea | IC50 (μM) inb:
|
|
|---|---|---|---|
| P. falciparum culture | Mammalian cell culture | ||
| ODC inhibitors | |||
| APA | 2.7 ± 0.5 (n = 5) | 1.0 ± 0.3 (n = 6) | 12.8 ± 3.6/24.4c (n = 5)c |
| CGP 54169A | 7.9 ± 2.1d/5.9 ± 1.2 (n = 3/4) | 2.0 ± 0.3 (n = 4) | 40.8 ± 16.3 (n = 5) |
| CGP 52622A | 20.4 ± 8.1d (n = 3) | 2.7 ± 0.2 (n = 5) | 12.2 ± 3.2 (n = 5) |
| DFMO | 87,600 ± 14,300d (n = 3) | 1,250 ± 420 (n = 4) | NDe |
| AdoMetDC inhibitors | |||
| CGP 40215A | 0.8 ± 0.2f/1.3 (n = 3/2) | 1.8 ± 0.4 (n = 5) | 74.4 ± 3.8/>100g (n = 5) |
| CGP 48664A | 3.0 ± 0.9f (n = 3) | 8.8 ± 1.7 (n = 5) | 0.18 ± 0.07/0.6h (n = 5) |
| MDL 73811 | 1.6 ± 0.4 (n = 3) | 3.0 ± 0.4 (n = 4) | ND |
Ki values were determined with recombinant bifunctional P. falciparum ODC-AdoMetDC and/or (as indicated) with the respective separately expressed domains. The data are in nanomolar for the ODC inhibitors and micromolar for the AdoMetDC inhibitors.
IC50s were determined in cultured P. falciparum and murine B-cell lymphoma A20/J2 cells 48 h after the addition of the respective inhibitor.
Data for human T24 bladder carcinoma cells are adopted from reference 34.
Separately expressed ODC domain (21).
ND, not determined.
Separately expressed AdoMetDC domain.
Data for human T24 bladder carcinoma cells are adopted from reference 35.
Data for human T24 bladder carcinoma cells are adopted from reference 30.
FIG. 2.
Effects of exogenous polyamines on drug-treated P. falciparum cultures. (A) The P. falciparum culture medium containing various concentrations of APA, CGP 52622A, or CGP 54169A was supplemented with 500 μM putrescine (▴) or spermidine (▾). Control cells were cultured solely in the presence of the drug (▪). Proliferation was determined after 48 h incubation by the [3H]hypoxanthine incorporation assay described in Materials and Methods. (B) Starting with a synchronous culture (ring stages about 10 h postinvasion), the growth of P. falciparum was monitored over a period of 96 h or 120 h in the presence of 2 μM APA, 5 μM CGP 52622A, or 5 μM CGP 54169A. Putrescine (1 mM) was added after treatment for 24 h and 48 h, as indicated. Parasitemia levels were determined by use of Giemsa-stained thin smears.
Combination studies with APA-derived ODC inhibitors and the established AdoMetDC inhibitor MDL 73811 revealed an additive effect but no synergistic effect on P. falciparum growth (data not shown), which is in accordance with the previously obtained data for the combination of DFMO and MDL 73811 (41).
Effects of APA derivatives and bis(guanosylhydrazone) derivatives on P. falciparum ODC-AdoMetDC.
The inhibition constants for the ODC and AdoMetDC inhibitors were determined for the bifunctional ODC-AdoMetDC and/or its separately expressed domains (Table 3). We have previously demonstrated that the separately expressed domains exhibit similar kinetic constants as the bifunctional enzyme (21, 40). This was confirmed by the data presented herein. Regarding the AdoMetDC inhibitors, CGP 40215A was found to be the most effective compound, with Ki values for the bifunctional protein and the AdoMetDC domain of 1.3 μM and 0.8 μM, respectively. The Ki value of 3.0 μM for CGP 48664A was obtained with the separately expressed AdoMetDC domain. The classical irreversible AdoMetDC inhibitor MDL 73811 has a Ki value of 1.6 μM on the bifunctional enzyme. The half-life of inactivation at saturating MDL 73811 concentrations was 1.5 ± 0.6 min (n = 3). The kinetic analyses for the tightly binding ODC inhibitors APA and its derivatives revealed extremely low Ki values between 3 and 20 nM for the inhibition of the ODC domain and the bifunctional enzyme (Table 3).
Polyamine pattern of cultured P. falciparum after treatment with inhibitors of polyamine synthesis.
To determine whether the plasmodicidal effects of the most effective ODC and AdoMetDC inhibitors correspond to an alteration of the intracellular polyamine levels, P. falciparum cultures were supplemented with sublethal concentrations of the respective inhibitors for 24 h or 48 h. Subsequently, infected RBCs were separated from noninfected RBCs by use of a Percoll gradient, and the changes in the polyamine levels were analyzed by reversed-phase HPLC.
The ODC inhibitor APA drastically affects the putrescine levels in infected RBCs in a dose-dependent manner (Table 4). Treatment with 2 μM for 24 h almost completely depleted the infected RBCs of putrescine. The spermidine-to-putrescine ratio increased from 4:1 in the control cells up to 22:1 in the treated cells. As a consequence of the decreased putrescine levels, the spermidine concentrations declined by 80%, while the spermine levels were found to be elevated.
TABLE 4.
Effects of the ODC inhibitors APA, CGP 52622A, and CPG 54169A on the polyamine pattern of P. falciparum-infected RBCs
| Treatment (24 h) | Intracellular polyamine concn (nmol/1010 cells)a
|
||
|---|---|---|---|
| Putrescine | Spermidine | Spermine | |
| Control | 272.4 ± 37.1 | 1,007.8 ± 118.7 | 49.9 ± 5.5 |
| 1 μM APA | 52.6 ± 38.0 | 319.2 ± 180.1 | 69.4 ± 9.9 |
| 2 μM APA | 10.1 ± 4.8 | 222.7 ± 105.4 | 88.8 ± 6.6 |
| Control | 410.2 ± 87.0 | 1,550.5 ± 76.0 | 83.5 ± 3.3 |
| 1 μM CGP 54169A | 91.7 ± 28.5 | 589.2 ± 198.0 | 187.1 ± 35.6 |
| 5 μM CGP 54169A | 23.4 ± 13.3 | 287.5 ± 127.9 | 202.0 ± 59.3 |
| Control | 453.9 ± 100.4 | 888.9 ± 245.7 | 38.9 ± 23.5 |
| 1 μM CGP 52622A | 342.9 ± 99.4 | 641.0 ± 162.1 | 81.5 ± 67.2 |
| 5 μM CGP 52622A | 286.6 ± 85.6 | 489.1 ± 106.3 | 117.7 ± 100.8 |
The data are the means ± standard deviations of four to five independent determinations.
The addition of 5 μM of the ODC inhibitor CGP 52622A for 24 h also resulted in remarkable reductions of the putrescine and spermidine concentrations by approximately 50% (Table 4). Similar to APA treatment, the low but distinct spermine levels showed a threefold increase after the addition of 5 μM CGP 52622A. Incubation of P. falciparum cultures with 1 μM CGP 54169A for 24 h also resulted in a drastic decrease in the putrescine and spermidine concentrations. The amounts of both putrescine and spermidine were reduced to 22 and 38%, respectively (Table 4). As for APA and CGP 52622A, the spermine levels were elevated. Treatment with 5 μM CGP 54169A resulted in a 95% reduction of the putrescine level, and the spermidine level dropped by more than 80%.
The AdoMetDC inhibitor MDL 73811 exhibited a dose-dependent effect, resulting in drastically increased putrescine levels and decreased spermidine concentrations in treated cells. Incubation with 5 μM MDL 73811 for 24 h raised the putrescine level by threefold, and the spermidine level decreased by 67% (Table 5). Spermine levels were also lowered after 48 h of drug treatment. In contrast to MDL 73811, incubation with the AdoMetDC inhibitor CGP 40215A hardly affected the polyamine patterns of cultured P. falciparum-infected RBCs (Table 5).
TABLE 5.
Effects of the AdoMetDC inhibitors CGP 40215A and MDL 73811 on the polyamine pattern of P. falciparum-infected RBCs
| Treatment | Intracellular polyamine concn (nmol/1010 cells)a
|
||
|---|---|---|---|
| Putrescine | Spermidine | Spermine | |
| 24 h | |||
| Control | 172.9 ± 76.6 | 649.5 ± 129.2 | 24.6 ± 3.4 |
| 1 μM MDL 73811 | 367.3 ± 40.1 | 301.2 ± 22.3 | 29.4 ± 3.4 |
| 5 μM MDL 73811 | 515.0 ± 168.9 | 213.1 ± 25.6 | 33.4 ± 9.2 |
| Control | 334.1 ± 24.7 | 935.3 ± 93.2 | 64.7 ± 6.9 |
| 5 μM CGP 40215A | 262.2 ± 33.2 | 1,182.9 ± 179.6 | 84.5 ± 16.0 |
| 48 h | |||
| Control | 261.8 ± 40.6 | 743.9 ± 68.0 | 39.3 ± 15.6 |
| 1 μM MDL 73811 | 616.2 ± 124.3 | 594.8 ± 120.9 | 17.8 ± 4.4 |
| 5 μM MDL 73811 | 822.6 ± 151.9 | 335.0 ± 117.5 | 13.6 ± 5.0 |
| Control | 479.5 ± 128.1 | 1,045.6 ± 144.6 | 41.1 ± 8.4 |
| 2 μM CGP 40215A | 422.9 ± 124.9 | 1,246.5 ± 110.9 | 46.8 ± 13.0 |
| 4 μM CGP 40215A | 379.7 ± 200.2 | 1,169.3 ± 175.6 | 50.5 ± 14.2 |
The data are the means ± standard deviations of three to five independent determinations.
DISCUSSION
The intracellular polyamine concentrations of the asexual erythrocytic stages of P. falciparum increase during maturation from rings to schizonts, where the polyamine pool is most likely distributed between the arising merozoites. This finding correlates with the stage-specific expression pattern of the bifunctional P. falciparum ODC-AdoMetDC (26) and confirms the findings of previous studies, in which changes in the polyamine pattern and ODC and AdoMetDC activities were found to be associated in P. falciparum cultures (1, 39, 41). According to our analyses, the majority of the polyamines of the host-parasite cell unit is associated with the parasite. Generally, polyamines are important for cell growth and differentiation processes (23, 32). In accordance with that, the intracellular polyamine concentrations of P. falciparum increase with maturation and the metabolic activity of the developing parasite, whereas reduced polyamine levels are accompanied by decreased rates of DNA, protein, and RNA synthesis (3). Since inhibitors of polyamine synthesis block the development of the erythrocytic stages of P. falciparum at the trophozoite stage (1, 3, 39, 41; this study), polyamines were suggested to be involved in the control of the schizogony process in Plasmodium.
P. falciparum ODC activity is effectively inhibited by APA, CGP 52622A, and CGP 54169A, with Ki values in the low nanomolar range. Owing to the aminooxy group, these compounds exhibit carbonyl reagent activity and, thus, form an oxime with the PLP cofactor in the active site of the ODC. Since APA and its derivatives are structural analogs of putrescine, the inhibitory effect was shown to be very specific, whereas the activities of other PLP-dependent enzymes were far less affected (17). The ODC inhibitors block the erythrocytic development of P. falciparum, with in vitro IC50s below 3 μM. The plasmodicidal effect is most likely attributable to decreases in the intracellular concentrations of putrescine and spermidine and could be abolished by supplementing the medium with 500 μM putrescine but, interestingly, not when spermidine is added to the parasite cultures. For the classical irreversible ODC inhibitor DFMO, the Ki value of the P. falciparum ODC was determined to be 80 μM (21) and the estimated in vitro IC50 determined in the present study and previously (6) is 1.3 mM, which is up to 1,300-fold higher than the IC50s determined for the novel competitive, tightly binding ODC inhibitors. As was the case for APA and its CGP derivatives, the effect of DFMO was reversed by supplementation with 500 μM putrescine. However, in contrast to the data presented here, the addition of 0.25 mM spermidine also considerably restored the growth of DFMO-treated P. falciparum in culture (2). In addition, Bitonti et al. (6) reported that the effect of DFMO is antagonized by the concurrent addition of spermidine and spermine, while spermine had no effect in another study of DFMO (2). Putrescine was reported to penetrate P. falciparum-infected RBCs much better than noninfected RBCs, while the uptake of spermidine was merely slightly enhanced, and no change of the low spermine uptake rate was determined (2). Therefore, it should be taken into consideration that uptake of putrescine by infected RBCs might weaken the efficacies of ODC inhibitors. To date, only a Plasmodium knowlesi-induced putrescine transporter has been characterized (33).
The ODC inhibitors APA, CGP 52622A, and CGP 54169A lead to enhanced spermine concentrations in P. falciparum-infected RBCs. This was also reported for DFMO-treated P. falciparum-infected RBCs (6). Since the genome of P. falciparum seems to lack a spermine synthase gene (as determined from a BLAST search of the P. falciparum genome database), it is remarkable that parasitized RBCs accumulate spermine when the intracellular putrescine concentration decreases due to ODC inhibition. This effect implies that the parasites have means to synthesize the polyamine, and indeed, it has been shown that P. falciparum spermidine synthase also catalyzes at a low rate the formation of spermine from spermidine and dcAdoMet (12a). For mammalian cells treated with ODC inhibitors, spermine levels were reported to remain nearly unchanged (23, 32).
The bicyclic analogue of MGBG, CGP 40215A, is a potent inhibitor of P. falciparum AdoMetDC activity, with a Ki value of about 1 μM. Furthermore, the compound has a plasmodical effect in vitro, with an estimated IC50 of 3 μM. CGP 40215A is a very promising drug candidate for the treatment of protozoan infections. The compound was found to have an in vitro trypanocidal activity as well as antileishmanial activity in the lower micromolar range (8, 25). Moreover, CGP 40215A cures acute laboratory model infections caused by Trypanosoma brucei subspecies and Trypanosoma congolense. When it is given in combination with DFMO, it acts synergistically against model central nervous system infections caused by T. brucei brucei. The trypanosomal AdoMetDC was suggested to be a main target, because reduced spermidine concentrations and enhanced putrescine concentrations were determined in treated Trypanosoma cells (4). In contrast, sublethal doses of CGP 40215A do not affect the polyamine concentrations in P. falciparum even after treatment for 48 h, suggesting a mechanism of action that does not depend on polyamine depletion. In this regard, it is noteworthy that the structure of CGP 40215A is similar to those of the diamidine Berenil (diminazene) and pentamidine (Fig. 1). For both compounds the mechanism of action is unknown; however, nucleic acids and their metabolism are suggested to be targeted (38).
In contrast to CGP 40215A, the potent irreversible AdoMetDC inhibitor MDL 73811 exhibits a dose-dependent effect on the cellular polyamine levels of P. falciparum-infected RBCs, which confirms the findings of previous studies by Wright et al. (41). The effect of the AdoMetDC inhibitor was only slightly abolished by supplementation with spermidine, which might be indicative of the inefficient uptake of the triamine. These data are in sharp contrast to those from previous studies, in which the addition of 250 μM spermidine as well as of 250 μM spermine abrogated the effect of MDL 73811 (41). Byers et al. (9) reported on the effect of the AdoMetDC inhibitor MDL 73811 on T. brucei brucei and found an enormous accumulation of AdoMet levels within 1 h of treatment, while polyamine levels were not affected within this time span. In that study, parasite viability was discussed to be impaired by elevated methylation reactions. In contrast, exposure of mammalian cells to MDL 73811 for 6 h resulted only in a 1.5- to 2-fold increase in AdoMet levels.
The AdoMetDC inhibitor CGP 48664A (also known as SAM486A), which potently blocks mammalian cell growth (30) and which has already been tested in phase I and phase II trials for its activities against solid tumors and relapsed or refractory non-Hodgkin's lymphoma (28, 32), turned out to be less effective against P. falciparum.
Inhibition of polyamine synthesis is a promising approach to the identification of antiprotozoan drugs with chemotherapeutic potential. Previous studies have shown that depletion of polyamines interferes with the development of P. falciparum and other Plasmodium species. However, the classical inhibitor of polyamine synthesis, DFMO, has only limited efficacy against the erythrocytic stages of Plasmodium in vivo (6). Moreover, although the AdoMetDC inhibitor MDL 73811 is very potent against cultured P. falciparum, it was found to be ineffective against Plasmodium berghei-infected mice (41). This is probably attributable to a rapid clearance of the inhibitor with a plasma half-life of about 10 min. The ODC and AdoMetDC inhibitors examined in this study are very potent plasmodicidal agents in culture, although it appears that CGP 40215A acts on targets distinct from those involved in the parasite's polyamine biosynthesis. The inhibitory effects of the new ODC inhibitors APA, CGP 52622A, and CGP 54169A on the enzyme activity of the bifunctional ODC-AdoMetDC correlate well with their effects on parasite growth and the depletion of putrescine and spermidine levels in treated parasites. These inhibitors are notably more potent than the classical compound DFMO against P. falciparum in vitro, and it is certainly worthwhile to analyze their antimalarial activities in animal model systems.
Acknowledgments
We thank Novartis Pharma AG and Aventis Pharmaceuticals Inc. (Bridgewater, N.J.) for kindly providing inhibitors. We are grateful to Thomas Jacobs and Volker Heuβler for providing mammalian cell lines and cultivation facilities.
This work was supported by Deutsche Forschungsgemeinschaft grant WA 395/10. S.M. is a Wellcome Trust Senior Fellow in Basic Biomedical Science.
REFERENCES
- 1.Assaraf, Y. G., J. Golenser, D. T. Spira, and U. Bachrach. 1984. Polyamine levels and the activity of their biosynthetic enzymes in human erythrocytes infected with the malarial parasite, Plasmodium falciparum. Biochem. J. 222:815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaraf, Y. G., J. Golenser, D. T. Spira, G. Messer, and U. Bachrach. 1987. Cytostatic effect of dl-alpha-difluoromethylornithine against Plasmodium falciparum and its reversal by diamines and spermidine. Parasitol. Res. 73:313-318. [DOI] [PubMed] [Google Scholar]
- 3.Assaraf, Y. G., L. Abu-Elheiga, D. T. Spira, H. Desser, and U. Bachrach. 1987. Effect of polyamine depletion on macromolecular synthesis of the malarial parasite, Plasmodium falciparum, cultured in human erythrocytes. Biochem. J. 242:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchi, C. J., R. Brun, S. L. Croft, K. Alicea, and Y. Buhler. 1996. In vivo trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob. Agents Chemother. 40:1448-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi, S., J. Tranum-Jensen, and A. Sziegoleit. 1985. Mechanism of membrane damage by streptolysin O. Infect. Immun. 47:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitonti, A. J., P. P. McCann, and A. Sjoerdsma. 1987. Plasmodium falciparum and Plasmodium berghei: effects of ornithine decarboxylase inhibitors on erythrocytic schizogony. Exp. Parasitol. 64:237-243. [DOI] [PubMed] [Google Scholar]
- 7.Bitonti, A. J., J. A. Dumont, T. L. Bush, M. L. Edwards, D. M. Stemerick, P. P. McCann, and A. Sjoerdsma. 1989. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc. Natl. Acad. Sci. USA 86:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brun, R., Y. Buhler, U. Sandmeier, R. Kaminsky, C. J. Bacchi, D. Rattendi, S. Lane, S. L. Croft, D. Snowdon, V. Yardley, G. Caravatti, J. Frei, J. Stanek, and H. Mett. 1996. In vitro trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob. Agents Chemother. 40:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byers, T. L., T. L. Bush, P. P. McCann, and A. J. Bitonti. 1991. Antitrypanosomal effects of polyamine biosynthesis inhibitors correlate with increases in Trypanosoma brucei brucei S-adenosyl-l-methionine. Biochem. J. 274:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins, R. E., C. J. Canfield, D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillet, J. M., G. Bone, and F. Herman. 1982. Inhibitory action of alpha-difluoromethylornithine on rodent malaria (Plasmodium berghei). Trans. R. Soc. Trop. Med. Hyg. 76:776-777. [DOI] [PubMed] [Google Scholar]
- 12.Gillet, J. M., J. Charlier, G. Bone, and P. L. Mulamba. 1983. Plasmodium berghei: inhibition of the sporogonous cycle by alpha-difluoromethylornithine. Exp. Parasitol. 56:190-193. [DOI] [PubMed] [Google Scholar]
- 12a.Haider, N., M. L. Eschbach, S. de Souza Dias, T. W. Gilberger, R. D. Walter, and K. Lüersen. The spermidine synthase of the malaria parasite Plasmodium falciparum: molecular and biochemical characterisation of the polyamine synthesis enzyme. Mol. Biochem. Parasitol., in press. [DOI] [PubMed]
- 13.Henderson, P. J. F. 1972. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem. J. 127:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollingdale, M. R., P. P. McCann, and A. Sjoerdsma. 1985. Plasmodium berghei: inhibitors of ornithine decarboxylase block exoerythrocytic schizogony. Exp. Parasitol. 60:111-117. [DOI] [PubMed] [Google Scholar]
- 15.Hyvonen, T., L. Alakuijala, L. Andersson, A. R. Khomutov, R. M. Khomutov, and T. O. Eloranta. 1988. 1-Aminooxy-3-aminopropane reversibly prevents the proliferation of cultured baby hamster kidney cells by interfering with polyamine synthesis. J. Biol. Chem. 263:11138-11144. [PubMed] [Google Scholar]
- 16.Kanaani, J., and H. Ginsburg. 1989. Metabolic interconnection between the human malarial parasite Plasmodium falciparum and its host erythrocyte. Regulation of ATP levels by means of an adenylate translocator and adenylate kinase. J. Biol. Chem. 264:3194-3199. [PubMed] [Google Scholar]
- 17.Khomutov, R. M., G. F. Denisova, A. R. Khomutov, K. M. Belostotskaya, R. B. Schlosman, and E. Yu. Artamonova. 1985. Aminooxypropylamine as an effective inhibitor of ornithine decarboxylase in vitro and in vivo. Bioorgan. Khim. 11:1574-1576. [PubMed] [Google Scholar]
- 18.Khomutov, R. M., T. Hyvonen, E. Karvonen, L. Kauppinen, T. Paalanen, L. Paulin, T. Eloranta, R. L. Pajula, L. C. Andersson, and H. Pösö. 1985. 1-Aminooxy-3-aminopropane, a new and potent inhibitor of polyamine biosynthesis that inhibits ornithine decarboxylase, adenosylmethionine decarboxylase and spermidine synthase. Biochem. Biophys. Res. Commun. 130:596-602. [DOI] [PubMed] [Google Scholar]
- 19.Khomutov, A. R., J. J. Vepsalainen, A. S. Shvetsov, T. Hyvonen, T. A. Keinanen, V. N. Pustobaev, T. O. Eloranta, and R. M. Khomutov. 1996. Synthesis of hydroxylamine analogues of polyamines. Tetrahedron 52:13751-13766. [Google Scholar]
- 20.Kitz, R., and I. Wilson. 1962. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J. Biol. Chem. 237:3245-3249. [PubMed] [Google Scholar]
- 21.Krause, T., K. Lüersen, C. Wrenger, T. W. Gilberger, S. Müller, and R. D. Walter. 2000. The ornithine decarboxylase domain of the bifunctional ornithine decarboxylase/S-adenosylmethionine decarboxylase of Plasmodium falciparum: recombinant expression and catalytic properties of two different constructs. Biochem. J. 352:287-292. [PMC free article] [PubMed] [Google Scholar]
- 22.Lüersen, K., R. D. Walter, and S. Müller. 2000. Plasmodium falciparum-infected red blood cells depend on a functional glutathione de novo synthesis attributable to an enhanced loss of glutathione. Biochem. J. 346:545-552. [PMC free article] [PubMed] [Google Scholar]
- 23.Marton, L. J., and A. E. Pegg. 1995. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 35:55-91. [DOI] [PubMed] [Google Scholar]
- 24.Mett, H., J. Stanek, J. A. Lopez-Ballester, J. Janne, L. Alhonen, R. Sinervirta, J. Frei, and U. Regenass. 1993. Pharmacological properties of the ornithine decarboxylase inhibitor 3-aminooxy-1-propanamine and several structural analogues. Cancer Chemother. Pharmacol. 32:39-45. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay, R., P. Kapoor, and R. Madhubala. 1996. Antileishmanial effect of a potent S-adenosylmethionine decarboxylase inhibitor: CGP 40215A. Pharmacol. Res. 33:67-70. [DOI] [PubMed] [Google Scholar]
- 26.Müller, S., A. Da'dara, K. Lüersen, C. Wrenger, R. Das Gupta, R. Madhubala, and R. D. Walter. 2000. In the human malaria parasite Plasmodium falciparum, polyamines are synthesized by a bifunctional ornithine decarboxylase, S-adenosylmethionine decarboxylase. J. Biol. Chem. 275:8097-8102. [DOI] [PubMed] [Google Scholar]
- 27.Müller, S., G. H. Coombs, and R. D. Walter. 2001. Targeting polyamines of parasitic protozoa in chemotherapy. Trends Parasitol. 17:242-249. [DOI] [PubMed] [Google Scholar]
- 28.Pless, M., K. Belhadj, H. D. Menssen, W. Kern, B. Coiffier, J. Wolf, R. Herrmann, E. Thiel, D. Bootle, I. Sklenar, C. Muller, L. Choi, C. Porter, and R. Capdeville. 2004. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin's lymphoma: results from a phase II multicenter study. Clin. Cancer Res. 10:1299-1305. [DOI] [PubMed] [Google Scholar]
- 29.Regenass, U., G. Caravatti, H. Mett, J. Stanek, P. Schneider, M. Müller, A. Matter, P. Vertino, and C. W. Porter. 1992. New S-adenosylmethionine decarboxylase inhibitors with potent antitumor activity. Cancer Res. 52:4712-4718. [PubMed] [Google Scholar]
- 30.Regenass, U., H. Mett, J. Stanek, M. Mueller, D. Kramer, and C. W. Porter. 1994. CGP 48664, a new S-adenosylmethionine decarboxylase inhibitor with broad spectrum antiproliferative and antitumor activity. Cancer Res. 54:3210-3217. [PubMed] [Google Scholar]
- 31.Seiler, N., and B. Knödgen. 1978. Determination of di- and polyamines by high-performance liquid chromatographic separation of their 5-dimethylaminonaphthalene-1-sulfonyl derivatives. J. Chromatogr. 145:29-39. [DOI] [PubMed] [Google Scholar]
- 32.Seiler, N. 2003. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr. Drug Targets 4:537-564. [DOI] [PubMed] [Google Scholar]
- 33.Singh, S., S. K. Puri, S. K. Singh, R. Srivastava, R. C. Gupta, and V. C. Pandey. 1997. Characterization of simian malarial parasite (Plasmodium knowlesi)-induced putrescine transport in rhesus monkey erythrocytes. J. Biol. Chem. 272:13506-13511. [DOI] [PubMed] [Google Scholar]
- 34.Stanek, J., J. Frei, H. Mett, P. Schneider, and U. Regenass. 1992. 2-Substituted 3-(aminooxy)propanamines as inhibitors of ornithine decarboxylase: synthesis and biological activity. J. Med. Chem. 35:1339-1344. [DOI] [PubMed] [Google Scholar]
- 35.Stanek, J., G. Caravatti, H. G. Capraro, P. Furet, H. Mett, P. Schneider, and U. Regenass. 1993. S-Adenosylmethionine decarboxylase inhibitors: new aryl and heteroaryl analogues of methylglyoxal bis(guanylhydrazone). J. Med. Chem. 36:46-54. [DOI] [PubMed] [Google Scholar]
- 36.Stanek, J., G. Caravatti, J. Frei, P. Furet, H. Mett, P. Schneider, and U. Regenass. 1993. 4-Amidinoindan-1-one 2′-amidinohydrazone: a new potent and selective inhibitor of S-adenosylmethionine decarboxylase. J. Med. Chem. 36:2168-2171. [DOI] [PubMed] [Google Scholar]
- 37.Trager, W., and J. D. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 38.Wang, C. C. 1995. Molecular mechanisms and therapeutic approaches to the treatment of African trypanosomiasis. Annu. Rev. Pharmacol. Toxicol. 35:93-127. [DOI] [PubMed] [Google Scholar]
- 39.Whaun, J. M., and N. D. Brown. 1985. Ornithine decarboxylase inhibition and the malaria-infected red cell: a model for polyamine metabolism and growth. J. Pharmacol. Exp. Ther. 233:507-511. [PubMed] [Google Scholar]
- 40.Wrenger, C., K. Lüersen, T. Krause, S. Müller, and R. D. Walter. 2001. The Plasmodium falciparum bifunctional ornithine decarboxylase, S-adenosyl-l-methionine decarboxylase, enables a well balanced polyamine synthesis without domain-domain interaction. J. Biol. Chem. 276:29651-29656. [DOI] [PubMed] [Google Scholar]
- 41.Wright, P. S., T. L. Byers, D. E. Cross-Doersen, P. P. McCann, and A. J. Bitonti. 1991. Irreversible inhibition of S-adenosylmethionine decarboxylase in Plasmodium falciparum-infected erythrocytes: growth inhibition in vitro. Biochem. Pharmacol. 41:1713-1718. [DOI] [PubMed] [Google Scholar]