Abstract
Helicobacter pylori colonizes the mucus layer of the human stomach and duodenum, causes chronic gastritis, gastric ulcer, and is a risk factor for gastric adenocarcinoma. There is a 20% failure rate in antibiotic therapy, which is increasingly due to antibiotic resistance and necessitates the search for alternative antimicrobial methods. We have discovered that H. pylori when cultured in liquid medium, accumulates significant quantities of coproporphyrin and protoporphyrin IX, both in the cells and secreted into the medium. These photoactive porphyrins lead to cell death (up to 5 logs) by photodynamic action upon illumination with low doses of visible light, with blue/violet light being most efficient. The degree of killing increases with the age of the culture and is greater than that found with Propionibacterium acnes (another bacterium known to be photosensitive due to porphyrin accumulation). Both virulent and drug-resistant strains are killed. The data suggest that phototherapy might be used to treat H. pylori infection in the human stomach.
Helicobacter pylori is a gram-negative microaerophilic bacterium which selectively colonizes the mucus layer of the human stomach and duodenum (7). As more than 50% of the world population is infected and in some countries infection rates approach 90%, H. pylori can be termed the world's commonest infectious agent (19). H. pylori infection has been shown to be strongly associated with the presence of inflammation and chronic gastritis, and once acquired, H. pylori persists, usually for life, unless eradicated by antimicrobial therapy (15). It is now known that H. pylori is a major cause of peptic ulcer disease, and in 1994, the International Agency for Cancer Research declared that H. pylori was a carcinogen of humans and was implicated in the development of gastric cancer (11).
The most common antibacterial treatment regimens include bismuth, metronidazole, tetracycline, and a proton pump inhibitor, or clarithromycin in combination with a proton pump inhibitor and amoxicillin (10). Combinations such as the above lead to eradication rates of about 80% but at the expense of side effects and possible poor patient compliance. Increasing development of antibiotic resistance among H. pylori isolates and the existence of nonresponsive patients suggest that alternative strategies for H. pylori eradication be sought (22).
Photodynamic therapy uses the combination of nontoxic dyes (frequently porphyrins or their derivatives) and harmless visible light to produce cytotoxicity via generation of reactive oxygen species (25). Photodynamic therapy has been clinically approved for various malignant, premalignant, and ophthalmologic conditions (6). It has long been known that many microorganisms including gram-negative and gram-positive bacteria, mycoplasma, fungi, and viruses can be killed by photodynamic therapy, and recently photodynamic therapy has been investigated as a treatment for infectious disease (12). The gram-positive bacterium that causes acne, Propionibacterium acnes, is killed by both blue and red light in the absence of any added photosensitizers or dyes (2, 18); recently the Food and Drug Administration approved a high-intensity narrow-band blue light therapy for the treatment of acne vulgaris (8).
In the present report we demonstrate that all tested strains of H. pylori, including virulent and multiply drug-resistant strains, are killed by exposure to otherwise harmless levels of visible light. We have identified the porphyrins responsible for this photosensitivity and the optimum wavelength of light. We suggest that delivery of light into the human stomach can be used as an effective therapy for H. pylori infection.
(Presented in part at the 30th Annual Meeting of the American Society for Photobiology, Quebec City, Canada, July 2002; Digestive Disease Week 2003, Orlando, Fla., May 2003; and 10th Congress of the European Society for Photobiology, Vienna, Austria, September 2003.)
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The following H. pylori strains were used: ATCC 43504 and 49503, are laboratory-adapted strains. ATCC 700824, also known as J99, is a cagA vacA virulent strain whose genome has been sequenced. Clinical isolate (CI) 1 (previously known as Leung) was provided by David Cave (St. Elizabeth's Medical Center, Brighton, MA), while CI2 (42-year-old female with gastritis) and CI3 (75-year-old female with gastric ulcer) are from patient biopsies taken at Abbott-Northwestern Hospital, Minneapolis, MN. CI4 is a clinical isolate provided by D. Y. Graham (VA Medical Center, Houston, TX) from a patient with gastritis who had failed antibiotic therapy. It exhibited MICs for clarithromycin >32 μg/ml and for metronidazole >128 μg/ml.
Bacteria were routinely grown in liquid medium consisting of Brucella broth supplemented with 10% fetal bovine serum and an antibiotic mixture of 10 μg/ml vancomycin, 5 μg/ml trimethoprim, 6 μg/ml nalidixic acid, and 5 μg/ml amphotericin B. The solid medium consisted of the former ingredients with 1.5% agar. One ml of frozen stock (medium described above plus 20% glycerol) was added to 10 ml medium in a 25-ml Erlenmeyer flask and incubated in a microaerophilic atmosphere (GasPak jar with Campypak generators, Fisher Scientific, Hampton, NH) and rotation at 200 rpm at 37°C. After 24 h the optical density (OD) had generally risen to 0.5, after 48 h to 1, after 72 h to 2, and after 96 h to ≥3. P. acnes (ATCC 6919) was grown in reduced thioglycolate broth (Difco 243210) in anaerobic jars for 48 h when the OD was >3 and on agar plates prepared from the same medium. Escherichia coli (ATCC 53868) was grown aerobically in brain heart infusion broth and on agar plates prepared from the same medium.
In vitro light delivery to H. pylori.
After incubation, 3 ml of bacterial suspension was removed and spun down in a centrifuge (2,000 × g), and the pellet was resuspended in 3 ml of sterile phosphate-buffered saline. Broad-band white light illumination was carried out with a Spot Light Source with adjustable power output (model L2859-07, Hamamatsu Photonics KK, Bridgewater, NJ) fitted with a 400-nm long-pass filter and adjusted to give a 2-cm-diameter spot with an irradiance of 100 mW/cm2 measured with a power meter (model K1700, International Light Inc, Newburyport, MA). Fifty-nm interference band pass filters (Omega Optical Inc, Brattleboro, VT) were used in conjunction with the Spot Light Source to deliver light confined to the following wavelength ranges: 375 to 425, 425 to 475, 475 to 525, 525 to 575, 575 to 625, and 625 to 675 nm with spot sizes 2 cm in diameter and irradiances of 100 mW/cm2 as measured with the power meter.
A diode laser capable of emitting 400 mW total power at 405 ± 5 nm was obtained from Nichia Chemical Industries Corp. (Mountville, PA). It consisted of 20 individual GaN laser diodes coupled into 20 individual fibers and brought together to form a 650-μm coupling diameter, numerical aperture of 0.20, at the laser output port (SMA Connector). It provided a 2-cm-diameter spot with an irradiance of 100 mW/cm2 to illuminate H. pylori. In some experiments E. coli was illuminated in the same manner as a control nonphotosensitive organism. Two hundred μl of bacterial suspension was added to each well of a hanging drop slide (Fisher Scientific) that was placed on a black background to avoid reflectance of light. The temperature of the suspension did not rise during the illumination.
Survival fraction determination.
After each predefined fluence of light had been delivered to one well, a 20-μl aliquot of suspension was removed and 10 μl of this was subjected to 5 serial tenfold dilutions in PBS. The original 10 μl and 10 μl of each dilution were streaked horizontally on square agar plates according to the method of Jett et al. (16). The maximum time between illumination and plating was 10 minutes. These plates were incubated at 37°C in stationary microaerophilic jars for 4 days (for H. pylori) or anaerobic jars for 2 days (P. acnes) until countable colonies appeared. Surviving CFU were counted and recorded for analysis. Survival fractions were determined relative to unilluminated bacterial suspensions that had been exposed to laboratory air in the same wells for the same time as the illumination.
Fluorescence spectroscopy.
The culture supernatant (2 ml) from suspensions of H. pylori that would subsequently be illuminated was added to 1 ml of a mixture of 0.1 M NaOH/1% sodium dodecyl sulfate (SDS) and allowed to stand in the dark for 1 day. Fluorescence was measured on a fluorimeter (Fluoromax 3, SPEX Industries, Edison, NJ), with excitation at 405 nm and emission scanned from 580 to 720 nm. Peak heights were correlated with the reciprocal of the survival fraction after 10 J/cm2 405-nm light had been delivered.
Identification of porphyrins.
H. pylori culture medium supernatant (4 ml) was added to 1 ml of 0.1 M NaOH and 1 ml of chloroform. The aqueous layer was extracted with a mixture of ethyl acetate and glacial acetic acid (10 ml, 8:1) and the organic layer washed with 1 ml of 0.1 M sodium bicarbonate and then deionized water and evaporated. The residue was dissolved in 200-μl capillary electrophoresis (CE) buffer (10 mM 2-[N-cyclohexylamino]ethanesulfonic acid and 75 mM sodium dodecyl sulfate at pH 10) for CE-laser-induced fluorescence (CE-LIF) analysis. The weighed dry pellet of H. pylori or P. acnes (ca. 20 mg) was added to 4 ml of 0.1 M NaOH and digested in the dark at room temperature for 48 h. The mixture was extracted as described for the supernatant.
Coproporphyrin I dichloride (CP, FW 727.6) and protoporphyrin IX (PPIX, FW 562.7) were from Sigma. PPIX was dissolved in CE buffer to give 1.2 × 10−4 M solution and CP in 0.1 N NaOH to give a 1.6 × 10−3 M solution. Calibration curves were constructed with dilutions from 2.5 × 10−8 to 64 × 10−8 M. CE-LIF analysis used a Beckman P/ACE MDQ system (Beckman Coulter, Fullerton, CA, USA) and a fused silica capillary (60 cm by 150 μm internal diameter) with a detection window at 50 cm from the inlet and a 488-nm argon laser for excitation. Separation voltage was +30 kV in CE buffer. The recorded peak areas were used in plotting the standard curves and determining sample concentrations of CP and PPIX.
RESULTS
H. pylori is killed upon illumination.
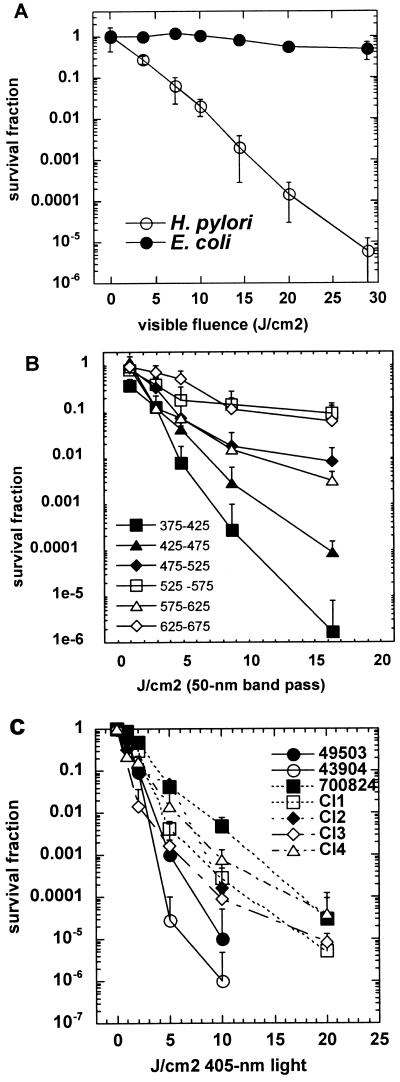
Initial experiments showed that H. pylori was killed by illumination with low levels of visible light. As shown in Fig. 1A, a suspension obtained from a 3-day-old culture of ATCC 49503 illuminated with broad-band white light (>400 nm delivered at an irradiance of 100 mW/cm2) gave a linear semilogarithmic plot of survival fraction versus fluence. Thirty J/cm2 gave greater than 5 logs of killing (>99.999%). The time for 30 J/cm2 to be delivered at 100 mW/cm2 is 5 min, and control suspensions of this microaerophilic organism exposed to the laboratory air in the dark did not suffer any significant loss of viability. Indeed in later work we exposed H. pylori suspensions to room air for up to 1 hour without loss of viability (data not shown). As a control for a nonphotosensitive bacterium, we used E. coli and illuminated it under the same conditions as H. pylori. There was no loss of viability of E. coli after 30 J/cm2 of white light had been delivered, suggesting that H. pylori is at least 100,000 times more photosensitive than E. coli.
FIG. 1.
A) Fluence-dependent killing of H. pylori ATCC 49503 after exposure to broad-band white light, >400 nm. E. coli is unharmed after similar illumination. Values are means of three independent experiments and bars are standard errors. B) Fluence-dependent killing of H. pylori ATCC 49503 after exposure to light produced by 50-nm band-pass filters. Values are means of three independent experiments and bars are standard errors. C) Comparison of photosensitivity of seven strains of H. pylori illuminated with light from a 405-nm diode laser. Values are means of three independent experiments and bars are standard errors.
Violet/blue light is most effective.
To determine which wavelengths of the visible range between 400 and 700 nm were most effective in killing H. pylori we used six 50-nm band pass filters centered on wavelengths of 400, 450, 500, 550, 600, and 650 nm to isolate specific colors of light. As shown in Fig. 1B the range between 375 and 425 nm (blue/violet light) was clearly the most effective followed by the range of 425 to 475 nm (blue), with the other four ranges producing only a slight killing effect.
All tested strains are photosensitive.
We therefore obtained a diode laser that delivered 405 nm light (±2 nm) at a total power of 160 mW out of the fiber. This was used to illuminated bacterial suspensions and to test generality of this observation using more strains of H. pylori as shown in Fig. 1C. All tested strains were killed at least 99.9% by 20 J/cm2 of 405-nm light. However there were differences between strains. It appears that the laboratory-adapted strains (ATCC 49503 and 43504) are more sensitive (1 to 2 logs) than the more recent clinical isolates. The fact that strain J99 that expresses virulence factors and a doubly antibiotic resistant strain (CI4) were both killed suggests that phototherapy might be applied to treat antibiotic-resistant disease.
Photosensitivity depends on culture age and is greater in H. pylori than P. acnes.
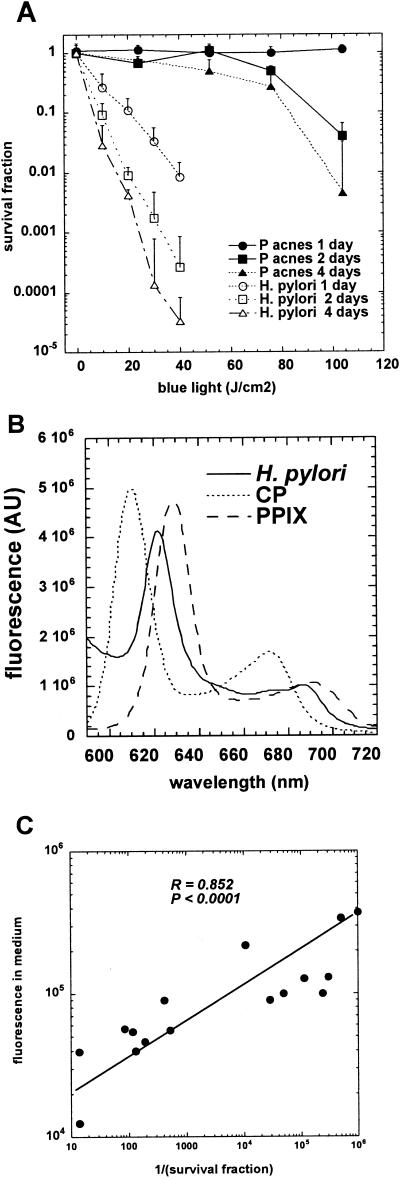
We observed that the extent of killing of H. pylori bacterial suspensions observed by delivering defined fluences depended on the age of the liquid culture providing the suspension. We also wished to compare the relative photosensitivity of the previously best-known porphyrin accumulating bacterium, P. acnes. Figure 2A compares the killing curves of 1-, 2-, and 4-day-old cultures of H. pylori and P. acnes. As can be seen there is a very large difference between the photosensitivity of the two bacteria. P. acnes requires two to three times the light to produce killing 100 to 1,000 times less than that seen with H. pylori. The degree of photosensitivity of H. pylori increases with the age of the culture with a 4 day old culture being most sensitive. Cultures significantly older than 4 days run the risk of becoming coccoid and nonculturable (5, 27). There is some variability (1 to 2 logs) in the light-induced killing of H. pylori cultures even of the same strain and chronological age. This is due to the variation in the amounts of porphyrins (1- to 2-fold) produced by these cultures. The factors that influence the quantitative differences in porphyrin accumulation in H. pylori cultures of the same strain and chronological age are at present unknown.
FIG. 2.
A) Comparison of photoinactivation using 405-nm laser light of H. pylori ATCC 49503 and P. acnes grown in liquid medium for various lengths of time. Values are means of three independent experiments and bars are standard errors. B) Fluorescence spectroscopy of 4-day-old culture medium from ATCC 49503 compared to CP and PPIX standards. C) Correlation between height of fluorescence emission from culture supernatants and cytotoxicity expressed as reciprocal of surviving fraction after 10 J/cm2 of 405-nm light.
H. pylori produces porphyrin fluorescence that correlates with phototoxicity.
The data suggested that the reason H. pylori was killed by visible light with a peak in the action spectrum about 400 nm was due to the bacteria accumulating a metal-free porphyrin that produces reactive oxygen species upon illumination. This hypothesis was tested by carrying out fluorescence emission spectroscopy on the 4-day-old H. pylori pellet that had been dissolved in NaOH/SDS. We excited the solution at 405 nm and scanned the emission between 580 and 720 nm.
Figure 2B shows that the supernatant contains an emission peak centered at 622 nm almost midway between the emission peaks of CP (610 nm) and PPIX (632 nm). We correlated the measures of the fluorescence intensity in the culture supernatants with the amount of killing observed in many experiments that gave variable degrees of light-mediated killing and were carried out with different strains of H. pylori and particularly with cultures of different ages. The reciprocal of the survival fraction after 10 J/cm2 blue light was taken as the measure of cytotoxicity. There was an excellent positive correlation over several orders of magnitude between the porphyrin fluorescence in the medium and the cytotoxicity with an R-value of 0.878 (P < 0.001) as shown in Fig. 2C. The variation in porphyrin content and photosensitivity was largely due to using cultures that had been grown for various lengths of time (1, 2, 3, and 4 days).
H. pylori produces both CP and PPIX.
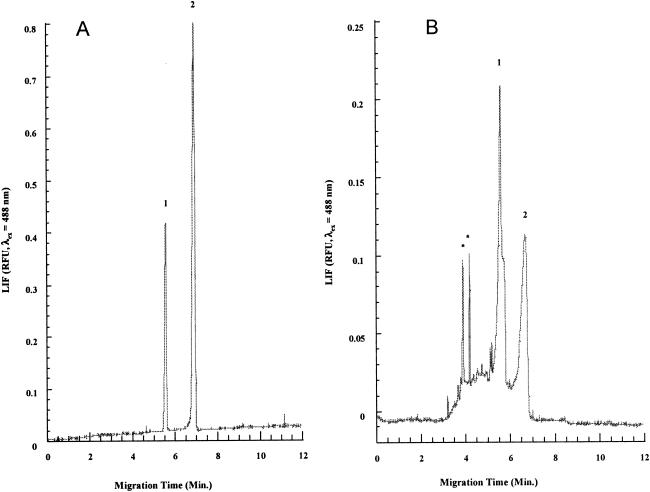
To further identify and confirm the existence of free porphyrins in the four-day old culture supernatant and bacterial pellets, we carried out analysis by solvent extraction and CE-LIF. Representative electropherograms of the culture supernatant from ATCC 49503 and a mixture prepared from authentic CP and PPIX are shown in Fig. 3. We were able to prepare a calibration curve from various concentrations of CP and PPIX standards that allowed us to quantitatively compare the concentrations of the porphyrins in supernatants from both the H. pylori strains and from P. acnes.
FIG. 3.
A) Capillary electrophoresis of standard mixture of CP (peak 1) and PPIX (peak 2). B) Capillary electrophoresis of extract from culture supernatant of 4-day-old H. pylori ATCC 49503. Peaks marked * may be artifacts or very low concentrations of porphyrins with more than four carboxyl groups.
We were also able to measure concentrations of porphyrins extracted from bacterial cell pellets but due to the sensitivity of the laser-induced fluorescence detector we needed to grow enough bacteria to make a pellet weighing about 15 mg dry weight (from approximately 20 ml of 4-day-old suspension). As the pellet isolated from P. acnes was much larger than that isolated from H. pylori we determined the dry weight after freeze drying before dissolving the pellet in NaOH/SDS. The results are presented in Table 1. The concentrations of both CP and PPIX measured in the supernatants from the five different H. pylori strains and from P. acnes were roughly comparable, ranging from 10 to 20 nM for CP and from 12 to 42 nM for PPIX. In all cases the PPIX concentration was larger than that of CP (between 1.2 and 2.7 times higher).
TABLE 1.
Strains useda
| Strain | Coproporphyrin (M) | Protoporphyrin (M) |
|---|---|---|
| H. pylori ATCC 43904 | 1.57 × 10−8 | 4.27 × 10−8 |
| H. pylori ATCC 700824 | 1.97 × 10−8 | 3.82 × 10−8 |
| H. pylori ATCC 49503 | 1.53 × 10−8 | 3.44 × 10−8 |
| H. pylori CI3 | 1.59 × 10−8 | 2.7 × 10−8 |
| H. pylori CI1 | 1.02 × 10−8 | 1.23 × 10−8 |
| P. acnes supernatant | 1.37 × 10−8 | 3.07 × 10−8 |
| P. acnes pellet (16.5 mg) | 0.68 × 10−8 (1.08 ppm wt/wt) | 1.12 × 10−8 (1.53 ppm wt/wt) |
| H. pylori pellet (15.2 mg) | 0.56 × 10−8 (0.96 ppm wt/wt) | 0.88 × 10−8 (1.3 ppm wt/wt) |
Concentrations of CP and PPIX in H. pylori and P. acne supernatants and pellets were determined by solvent extraction, CE-LIF separation, and comparison with calibration curves.
DISCUSSION
We have shown for the first time that H. pylori, when grown in liquid medium, naturally accumulates sufficient photoactive porphyrins to allow it to be efficiently killed after illumination with low fluences of blue light. This finding suggests that a novel form of phototherapy could be applied in the human stomach to eradicate the infection. There have been scattered reports of different species of bacteria exhibiting photosensitivity due to the accumulation of porphyrins. Many of these concern the gram-positive anaerobe P. acnes that is the cause of the human skin disease acne. P. acnes has been reported to be killed by both blue and red light (2, 18), and recently the Food and Drug Administration approved a high-intensity narrow-band blue light therapy for the treatment of acne vulgaris (8). However, our data comparing the photosensitivity of H. pylori and P. acnes demonstrate that, although they accumulate comparable amounts of porphyrins, the former is several thousandfold more photosensitive.
Our finding of the relative resistance of P. acnes to blue light killing is in agreement with a report from Ashkenazi et al. (2), who needed to use repetitive illumination of P. acnes in order to achieve satisfactory levels of killing. They achieved less than 2 logs of killing after 75 J/cm2 blue light, but this increased to 4 logs of killing after two similar illuminations 24 h apart. The explanation for the observation that P. acnes and H. pylori make similar amounts of porphyrins but have very different responses to illumination is at present unclear. There are clearly important differences between H. pylori and P. acnes, such as Gram stain status (H. pylori is gram-negative while P. acnes is gram-positive) and H. pylori is microaerophilic while P. acnes is anaerobic, but there are so few examples of natural porphyrin-producing bacteria that the determinants of relative susceptibilities to photoinactivation remain unknown.
It has long been known that some bacteria accumulate porphyrins under some circumstances, and consequently exhibit a red fluorescence under UVA or blue light illumination and are susceptible to killing upon illumination (18). This was reported to be common among anaerobic species (4) and red-fluorescent pus was reported to be present in infections (3). The phenomenon has been noted in a group of anaerobic species that are mainly oral pathogens and were previously known as black-pigmented Bacteroides species but have now been reclassified as Porphyromonas and Prevotella species (17). These bacteria depend largely on external heme (either hemoglobin or hemopexin) to satisfy their demand for iron (20) and to a greater or lesser extent accumulate a black pigment intracellularly that consists of an iron-containing heme aggregate (e.g., hematin) together with various amounts of iron-free PPIX (28, 29). If they accumulate PPIX they will be photosensitive (13), however, if they mainly have hematin they will not be photosensitive, as iron-containing tetrapyrroles do not carry out the appropriate photochemistry. Henry et al. showed that these species were more sensitive when grown on media containing hemin as an iron source compared to hemoglobin (14).
A second group of porphyrin-accumulating bacteria comprises species such as P. acnes and now H. pylori. Here the porphyrins consist of mixtures of PPIX with CP and sometimes uroporphyrin (26). The vast majority of bacterial species use the heme biosynthetic pathway by which (in a similar fashion to mammalian cells) porphyrins are produced from the precursor 5-aminolevulanic acid by a well-conserved set of enzymes. The finding that both H. pylori and P. acnes accumulate a tetracarboxylic porphyrin (CP) as well as PPIX shows that the porphyrins must arise from endogenous heme biosynthesis rather than accumulation of the porphyrins from exogenous heme, as there is no known biochemical mechanism for producing a tetracarboxylic porphyrin from a dicarboxylic porphyrin present in heme. Normally the heme biosynthetic cycle is subject to tight feedback control to avoid producing photoactive free porphyrins, and it is presently unclear if there is any evolutionary advantage to H. pylori accumulating these compounds.
The finding that H. pylori porphyrin levels and susceptibility to photokilling increases with increasing age of the culture is also in agreement with a report of a similar finding concerning P. acnes (23). It is possible that the porphyrin accumulation is controlled by quorum-sensing mechanisms whereby bacteria secrete small molecules termed autoinducers that are detected by neighboring cells and induce expression of a set of genes involving survival protection and increased virulence (24, 31). H. pylori, while not possessing autoinducer system one involving acylhomoserine lactones, does possess the luxS gene that codes for the biosynthesis of autoinducer AI2 (9).
By analogy with P. acnes (1), the mechanism of killing of H. pylori is likely to involve photodynamic action whereby the porphyrins absorb light and produce, via the excited singlet state, a long-lived triplet state that can interact with molecular oxygen to produce the cytotoxic species singlet oxygen. This mechanism implies that the killing will be oxygen dependent, and consideration must be given to whether there will be sufficient oxygen in the stomach mucosa to allow complete killing. There is known to be enough oxygen present in the gastric mucosa for phagocytic cells to generate reactive oxygen species in a respiratory burst. In addition it is presently uncertain whether light penetration into the lining of the human stomach will be a barrier to photoeradication. The gastric glands and crypts will be situated deep within the mucosa, and tissue is an effective absorber and scatterer of blue light. The surface area of the stomach is approximately 800 cm2 and if illumination is to be carried out in a reasonable time during endoscopy it may be necessary to deliver optical powers as high as 50 to 100 W into the stomach.
It has been proposed to treat H. pylori by administering oral 5-aminolevulanic acid that acts as a metabolic precursor of PPIX followed by illumination of the stomach lining by blue light (30). However, it is well known that 5-aminolevulanic acid can produce PPIX in human mucosal linings (21) and the use of this drug may increase the chances of damaging the stomach lining.
In conclusion, we have shown that all tested strains of H. pylori naturally accumulate a mixture of PPIX and CP that can sensitize the bacteria to killing by visible light, particularly blue light. This finding suggests that a novel phototherapy approach may be applied in the human stomach to eliminate H. pylori infection.
Acknowledgments
This work was supported by grants from LumeRx Inc, Seedling Enterprises LLC, and Public Health Service grant R01AI050879 to M.R.H. from the National Institute for Allergy and Infectious Disease.
We thank Touqir Zahra and Aamir Ahmad for technical assistance and Philip Levin and Robert Arcangeli for helpful discussions. We are grateful to David Cave, David Y. Graham, and Anne Kane for generous gifts of strains.
The authors have commercial associations that might pose a conflict of interest. Michael R. Hamblin is a consultant to LumeRx and Seedling and received research support from LumeRx and Seedling. Robert A. Ganz is a stockholder in LumeRx and is a member of their scientific advisory board and an employee of Sterilite. Sterilite received research support from LumeRx and Seedling. M. Joshua Tolkoff is a stockholder in LumeRx and Seedling and is an employee of Seedling.
REFERENCES
- 1.Arakane, K., A. Ryu, C. Hayashi, T. Masunaga, K. Shinmoto, S. Mashiko, T. Nagano, and M. Hirobe. 1996. Singlet oxygen (1 delta g) generation from coproporphyrin in Propionibacterium acnes on irradiation. Biochem. Biophys. Res. Commun. 223:578-582. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, H., Z. Malik, Y. Harth, and Y. Nitzan. 2003. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 35:17-24. [DOI] [PubMed] [Google Scholar]
- 3.Brazier, J. S. 1990. Analysis of the porphyrin content of fluorescent pus by absorption spectrophotometry and high performance liquid chromatography. J. Med. Microbiol. 33:29-34. [DOI] [PubMed] [Google Scholar]
- 4.Brazier, J. S. 1986. A note on ultra-violet red fluorescence of anaerobic bacteria in vitro. J. Appl. Bacteriol. 60:121-126. [DOI] [PubMed] [Google Scholar]
- 5.Cellini, L., I. Robuffo, E. Di Campli, S. Di Bartolomeo, T. Taraborelli, and B. Dainelli. 1998. Recovery of Helicobacter pylori ATCC43504 from a viable but not culturable state: regrowth or resuscitation? APMIS 106:571-579. [PubMed] [Google Scholar]
- 6.Dougherty, T. J. 2002. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 20:3-7. [DOI] [PubMed] [Google Scholar]
- 7.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elman, M., M. Slatkine, and Y. Harth. 2003. The effective treatment of acne vulgaris by a high-intensity, narrow band 405-420 nm light source. J. Cosmet. Laser Ther. 5:111-117. [PubMed] [Google Scholar]
- 9.Forsyth, M. H., and T. L. Cover. 2000. Intercellular communication in Helicobacter pylori: luxS is essential for the production of an extracellular signaling molecule. Infect. Immun. 68:3193-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gene, E., X. Calvet, R. Azagra, and J. P. Gisbert. 2003. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Aliment. Pharmacol. Ther. 17:1137-1143. [DOI] [PubMed] [Google Scholar]
- 11.Graham, D. Y., M. F. Go, and R. M. Genta. 1995. Helicobacter pylori, duodenal ulcer, gastric cancer: tunnel vision or blinders? Ann. Med. 27:589-594. [DOI] [PubMed] [Google Scholar]
- 12.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry, C. A., B. Dyer, M. Wagner, M. Judy, and J. L. Matthews. 1996. Phototoxicity of argon laser irradiation on biofilms of Porphyromonas and Prevotella species. J. Photochem. Photobiol. B 34:123-128. [DOI] [PubMed] [Google Scholar]
- 14.Henry, C. A., M. Judy, B. Dyer, M. Wagner, and J. L. Matthews. 1995. Sensitivity of Porphyromonas and Prevotella species in liquid media to argon laser. Photochem. Photobiol. 61:410-413. [DOI] [PubMed] [Google Scholar]
- 15.Hunt, R. H. 1996. The role of Helicobacter pylori in pathogenesis: the spectrum of clinical outcomes. Scand. J. Gastroenterol. Suppl. 220:3-9. [PubMed] [Google Scholar]
- 16.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 17.Jousimies-Somer, H., and P. Summanen. 2002. Recent taxonomic changes and terminology update of clinically significant anaerobic gram-negative bacteria (excluding spirochetes). Clin. Infect. Dis. 35:S17-21. [DOI] [PubMed] [Google Scholar]
- 18.Konig, K., M. Teschke, B. Sigusch, E. Glockmann, S. Eick, and W. Pfister. 2000. Red light kills bacteria via photodynamic action. Cell. Mol. Biol. (Noisy-le-Grand) 46:1297-1303. [PubMed] [Google Scholar]
- 19.Lacy, B. E., and J. Rosemore. Helicobacter pylori: ulcers and more: the beginning of an era. J. Nutr. 131:2789S-2793S, 2001. [DOI] [PubMed]
- 20.Leung, K. P., P. S. Subramaniam, M. Okamoto, H. Fukushima, and C. H. Lai. 1998. The binding and utilization of hemoglobin by Prevotella intermedia. FEMS Microbiol. Lett. 162:227-233. [DOI] [PubMed] [Google Scholar]
- 21.Loh, C. S., D. Vernon, A. J. MacRobert, J. Bedwell, S. G. Bown, and S. B. Brown. 1993. Endogenous porphyrin distribution induced by 5-aminolaevulinic acid in the tissue layers of the gastrointestinal tract. J. Photochem. Photobiol. B 20:47-54. [DOI] [PubMed] [Google Scholar]
- 22.Megraud, F., and H. Lamouliatte. 2003. Review article: the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 17:1333-1343. [DOI] [PubMed] [Google Scholar]
- 23.Melo, T. B., and G. Reisaeter. 1986. Photodestruction of endogenous porphyrins in relation to cellular inactivation of Propionibacterium acnes. Z. Naturforsch. 41:867-872. [PubMed] [Google Scholar]
- 24.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 25.Ochsner, M. 1997. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B 39:1-18. [DOI] [PubMed] [Google Scholar]
- 26.Romiti, R., M. Schaller, K. Jacob, and G. Plewig. 2000. High-performance liquid chromatography analysis of porphyrins in Propionibacterium acnes. Arch. Dermatol. Res. 292:320-322. [DOI] [PubMed] [Google Scholar]
- 27.Saito, N., K. Konishi, F. Sato, M. Kato, H. Takeda, T. Sugiyama, and M. Asaka. 2003. Plural transformation-processes from spiral to coccoid Helicobacter pylori and its viability. J. Infect. 46:49-55. [DOI] [PubMed] [Google Scholar]
- 28.Shah, H. N., R. Bonnett, B. Mateen, and R. A. Williams. 1979. The porphyrin pigmentation of subspecies of Bacteroides melaninogenicus. Biochem. J. 180:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah, H. N., and S. E. Gharbia. 1993. Biochemical and chemical analyses of black-pigmented gram-negative anaerobes. FEMS Immunol. Med. Microbiol. 6:89-96. [DOI] [PubMed] [Google Scholar]
- 30.Wilder-Smith, C. H., P. Wilder-Smith, P. Grosjean, H. van den Bergh, A. Woodtli, P. Monnier, G. Dorta, F. Meister, and G. Wagnieres. 2002. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: preliminary human studies. Lasers Surg. Med. 31:18-22. [DOI] [PubMed] [Google Scholar]
- 31.Winzer, K., and P. Williams. 2001. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291:131-143. [DOI] [PubMed] [Google Scholar]