Abstract
We have sequenced the penicillin-binding domains of the complete repertoire of penicillin-binding proteins and MurM from 22 clinical isolates of Streptococcus pneumoniae that span a wide range of β-lactam resistance levels. Evidence of mosaicism was found in the genes encoding PBP 1a, PBP 2b, PBP 2x, MurM, and, possibly, PBP 2a. Five isolates were found to have identical PBP and MurM sequences, even though the MICs for penicillin G ranged from 0.25 to 2.0 mg/liter. When the sequences encoding PBP 1a, PBP 2b, and PBP 2x from one of these isolates were used to transform laboratory strain R6, the resulting strain had a resistance level higher than that of the less resistant isolates carrying that PBP set but lower than that of the most resistant isolates carrying that PBP set. This result demonstrates that if the R6 strain is arbitrarily defined as the standard genotype, some wild genetic backgrounds can either increase or decrease the PBP-based resistance phenotype.
The mounting rates of resistance of Streptococcus pneumoniae to antibiotics is an important concern, as this human pathogen is responsible for serious diseases, such as otitis, pneumonia, meningitis, and bacteremia. In clinical isolates, resistance to β-lactams is mostly due to the expression of modified penicillin-binding proteins (PBPs), the targets of the β-lactams, with reduced affinities for the antibiotics (3). The low-affinity PBPs are the products of mosaic genes that result from multiple events of homologous recombination with genes from other strains or closely related species (3). The presence of a functional murM gene (also called fibA), which is involved in the synthesis of an alternative physiological substrate for the PBPs, is required for the expression of β-lactam resistance (9, 31). Mosaic murM genes can dramatically increase the level of resistance due to low-affinity PBPs (9, 28).
S. pneumoniae has five high-molecular-weight PBPs, three of which (PBP 2x, PBP 2b, and PBP 1a) are definitely involved in β-lactam resistance (23), and one low-molecular-weight PBP. A laboratory experiment showed that the five high-molecular-weight PBPs were modified upon transfer of high-level resistance from Streptococcus mitis to S. pneumoniae (13). Other works have suggested a role for PBP 2a (26, 27) and the low-molecular-weight PBP, PBP 3 (17), in β-lactam resistance. These findings raised the possibility that previously undetected variability in all the pbp genes may account for the wide range of levels of resistance to β-lactams.
On the basis of the premise that knowledge of the sequence of MurM and all the PBPs might be sufficient to predict the level of β-lactam resistance, we sequenced murM and the region coding the penicillin-binding domain of the six PBPs from 22 isolates of S. pneumoniae. Five isolates were found to have very different MICs for β-lactams, even though they had the same six penicillin-binding domains and MurM. This demonstrates that other genes modulate significantly the resistance provided by the low-affinity PBPs. By transferring to the susceptible R6 strain the three mosaic pbp1a, pbp2b, and pbp2x sequences, which are identical in the five isolates that differ in β-lactam resistance, we generated a strain with a resistance level inferior to that of the most resistant originating isolate but higher than that of the less resistant isolate.
MATERIALS AND METHODS
Bacterial strains.
The 22 S. pneumoniae isolates for this study were isolated at the University Hospital in Grenoble, France, between 1997 and 2000. They were chosen from a collection of more than 500 isolates, based on the hospital record of their antibiotic susceptibilities measured at the time of their isolation. The isolates were chosen to exhibit a series of penicillin MICs as complete as possible between highly susceptible and highly resistant. Two isolates were included because of their unusually high levels of resistance to amoxicillin. Clones were reisolated on Columbia agar (Becton Dickinson) plates with 5% sheep blood incubated at 37°C in an atmosphere supplemented with 5% CO2. Liquid cultures were in glucose buffered broth (Diagnostic Pasteur) at 37°C.
Antibiotic susceptibility and typing.
Susceptibilities were determined with Etest strips (AB Biodisk) on Mueller-Hinton agar plates supplemented with 5% horse blood. Serogroups were determined by a latex agglutination test (kindly provided by bioMérieux). Multilocus sequence typing (MLST) was performed by the standard procedure (7), and the results were deposited in the S. pneumoniae MLST database (http://spneumoniae.mlst.net/).
Gene amplification and sequencing.
Genomic DNA was prepared with a High Pure PCR Template preparation kit from Roche. The region coding the transpeptidase domain of each PBP was amplified by PCR with the pairs of primers given in Table 1 by using the VENT polymerase (New England Biolabs). The PCR products were sequenced either directly by using the amplification primers or following insertion into pCRscript (Stratagene). Sequencing was performed by GenomeExpress (Grenoble, France).
TABLE 1.
Oligonucleotides used for PCR amplification of pbp and murM gene fragments
| Gene fragment | Primera | Sequence (5′→3′) |
|---|---|---|
| pbp1a-pbdb | 5′-PBP1a1 | CCCTATTCACATCCAGAAGC |
| 5′-PBP1a2 | CATCTCTGCTGAACAGTATG | |
| 3′-PBP1a1 | CTGTGAAGTTGAACTATCTGATG | |
| 3′-PBP1a2 | CTGATGATGAGCTTGAACTTTC | |
| pbp1a-ecdc | PBP1aUP | GGGGATCCAGCAAGGCTCCTAGCCTATCCG |
| PBP1aDN | CCGCTCGAGCGGTTATGGTTGTGCTGGTTGAGG | |
| pbp1b-pbd | 5′-PBP1b1 | GTTGACTGTTCCTCAAGCAGC |
| 5′-PBP1b2 | CTTACTCTCCTTATGAAAATACTGG | |
| 3′-PBP1b1 | GAGCTGGATGGAGTTGGTAG | |
| pbp2a-pbd | 5′-PBP2a1 | GATTGAATAGTTTCTCGAACCAC |
| 5′-PBP2a2 | CGCACAAGCTAGGCTTGC | |
| 3′-PBP2a1 | GGTTGCAGCAGGATATATTG | |
| 3′-PBP2a2 | GATAAAAACCAAGAAACCGAAGC | |
| pbp2b-pbd | 5′-PBP2b1 | ACCTTACAAGGAAACGCTCGG |
| 5′-PBP2b2 | GAAATCCATCTGGATAAATATGG | |
| 3′-PBP2b1 | CGTCTTAATCCCGATACCTGG | |
| 3′-PBP2b2 | CATCCCAATCGTATAAAAGGCC | |
| pbp2b-ecd | PBP2bUP | CGCGGATCCCAGGTTTTGAACAAGGATTTTTACGAAAAAAAGCTA |
| PBP2bDN | CCGCTCGAGAGCATAATTTCCTTTCTAATTCATTGGATGGTATTTTTG | |
| pbp2x-pbd | 5′-PBP2x1 | GGGACAGACGGCATTATTACCTATG |
| 3′-PBP2x1 | GGTGAAATATCCTTGATGCTAGGC | |
| pbp2x-ecd | PBP2xUP | CGGGATCCGGGACAGGCACTCGC |
| PBP2xDN | TCCCCCGGGTTAGTCTCCTAAAGTTAATTTAAT | |
| pbp3 | 5′-PBP3 | ATGAAAAAAATATTTTTAACTTTG |
| 3′-PBP3 | AGTTGGATAAAATTTGATTTTATAC | |
| murM | 5′-MurM | CTTTCTATGTTTTTTTCTTAATGTTTTACGG |
| 3′-MurM | ATGTACCGTTATCAAATTGGC |
Depending on the isolates, alternative pairs of primers were sometimes used to obtain proper amplification.
PCR fragments used for sequencing the penicillin-binding domain (pbd).
PCR fragments used for cloning the complete extracellular domain (ecd).
Transformation.
The gene fragments encoding the extracellular domains of PBP 1a (residues 37 to 719), PBP 2b (residues 35 to 683), and PBP 2x (residues 49 to 750) were PCR amplified from isolate 5031 with the primers given in Table 1 and cloned as BamHI-XhoI or BamHI-SmaI (PBP 2x) digests into pGex-4T1.
The nonencapsulated S. pneumoniae R6 strain was used as the recipient for genetic triple transformation. Bacteria were grown in C medium supplemented with 0.18% albumin (8%, boiled) to the onset of the exponential phase. Aliquots were stored in 20% glycerol at −80°C until they were used. About 50 ng of each plasmid DNA was added to 100 μl of competent cells (thawed on ice and diluted 10-fold in C medium with 0.18% albumin). After incubation for 30 min at 30°C and 120 min at 37°C, the cells were plated on Columbia blood agar enriched with 6% horse blood containing concentrations of cefotaxime ranging from 0 to 0.3 μg · ml−1.
Immunoblotting.
Pneumococci were harvested at an optical density at 600 nm of 0.4. The cells were lysed by resuspension in a 1/100 volume of CelLytic BII buffer (Sigma) supplemented with lysozyme (1 kU/ml; Sigma) and RQ1 DNase (80 U/ml; Promega) on ice for an hour. The total protein content was determined by the bicinchoninic acid assay (Uptima), with bovine serum albumin used as a standard. Samples containing 10 μg proteins were analyzed by gel electrophoresis and immunoblotting with rabbit antisera against PBP 1a, PBP 2b, and PBP 2x (19). The immunoblots were revealed with an enhanced chemiluminescence kit (Amersham).
Nucleotide sequence accession numbers.
The EMBL accession numbers for the new sequences are AJ698085 to AJ698090 and AJ698959 for PBP 2x, AJ842012 to AJ842018 for PBP 2b, AJ842019 to AJ842021 for PBP 1b, AJ842755 to AJ842758 for PBP 2a, AJ842759 and AJ842760 for PBP 3, and AJ842957 to AJ842961 for MurM.
RESULTS
The sequences of the penicillin-binding domains of the six PBPs and MurM of the 22 S. pneumoniae isolates of the small collection that we chose were determined, with a few exceptions. The sequences of PBP 1a, PBP 2b, and PBP 2x exhibited mosaicism in all the isolates with reduced susceptibilities to β-lactams. The MurM sequence showed mosaicism in two isolates. For PBP 1a, PBP 2b, PBP 2x, and MurM, the amino acid sequences were aligned and clustered with the CLUSTAL W program. Sequences that differed from each other by at most 10 residues were grouped together. These groups of similar sequences are designated by capital letters and are further divided into subgroups of sequences that were identical or that differed by a single substitution. These subgroups are designated by Arabic numerals, with primes used to indicate various single substitutions (Table 2). No evidence of mosaicism was found for PBP 1b and PBP 3, although minor differences were found in a few sequences. PBP 2a sequences had only a few substitutions, but one sequence showed a pattern consistent with a possible mosaicism. The sequences of PBP 1b, PBP 2a, and PBP 3 that differed by even a single amino acid were assigned to different subgroups (Table 2).
TABLE 2.
Characteristics of the strains and their PBP and MurM sequence typesa
| Strain | Yr | Source | Serogroup | MIC (μg/ml)
|
Sequence typeb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PenG | AMX | CTX | CRO | 1a | 1b | 2a | 2b | 2x | 3 | MurM | ||||
| 5204 | 1999 | Sputum | 14 | 6.0 | 6.0 | 12.0 | 3.0 | C3 | A5 | A3 | D1 | C3 | A1 | A1 |
| 5268 | 2000 | Blood | 14 | 3.0 | 4.0 | 4.0 | 2.0 | C3 | A5 | A3 | D1′ | C3 | A1 | A1 |
| 4843 | 1997 | Trachea | 23 | 2.0 | 1.5 | 1.0 | 1.0 | C1 | A3 | A6 | C1 | C1 | A1 | A1 |
| 4790 | 1996 | Blood | 23 | 1.5 | 1.5 | 1.0 | 1.0 | C1 | A3 | A6 | C1 | C1 | A1 | A1 |
| 5062 | 1999 | Trachea | 14 | 1.5 | 3.0 | 2.0 | 1.0 | C3 | A5 | A3 | ND | ND | A1 | A1 |
| 5245 | 2000 | Sputum | 6 | 1.5 | 3.0 | 0.75 | 0.75 | C2 | A1 | A4 | E1 | C2 | A1 | C1 |
| 5180 | 1999 | Sputum | 15 | 1.5 | 1.0 | 2.0 | 1.0 | C1 | A3 | A6 | C1 | C1′ | A1 | A1 |
| 4883 | 1997 | Blood | 9 | 1.0 | 1.5 | 0.75 | 0.75 | C1 | A3 | A3 | C1 | C1 | A1 | B1 |
| 5047 | 1999 | Nose swab | 9 | 0.75 | 0.50 | 2.0 | 0.50 | C1 | A4 | A3 | C1 | C1 | A1 | A1 |
| 5104 | 1999 | Sputum | 23 | 0.50 | 0.50 | 0.75 | 0.50 | C1 | A3 | A6 | C1 | C1 | A1 | A1 |
| 4935 | 1998 | Blood | 3 | 0.50 | 0.25 | 0.38 | 0.25 | C1 | A3 | A6 | C1 | C1 | A1 | A1 |
| 5024 | 1999 | Bronchus | 15 | 0.25 | 0.094 | 0.38 | 0.38 | A1 | A2 | ND | F1 | A5 | A1 | A1 |
| 5031 | 1999 | Bronchus | 23 | 0.25 | 0.19 | 0.19 | 0.125 | C1 | A3 | A6 | C1 | C1 | A1 | A1 |
| 5259 | 2000 | Trachea | 15 | 0.19 | 0.023 | 0.094 | 0.064 | B1 | A3 | A5 | B1 | B1 | A2 | A2 |
| 4816 | 1997 | CSF | 15 | 0.094 | <0.016 | 0.064 | 0.032 | B1 | A3 | A5 | B1 | B1 | A2 | A2 |
| 5023 | 1999 | Blood | 3 | 0.064 | 0.032 | 0.032 | 0.047 | A1′′ | A1 | A1 | A3 | A3 | A1 | A1 |
| 4942 | 1998 | Blood | 6 | 0.047 | 0.064 | 0.032 | 0.047 | A1 | A3 | A7 | B2 | A4 | A1 | A1 |
| 5084 | 1999 | Eye swab | ND | 0.023 | <0.016 | <0.016 | <0.016 | A1 | ND | A8 | A1′ | A1′ | A1 | A1′ |
| 5074 | 1999 | Blood | 23 | 0.016 | <0.016 | <0.016 | <0.016 | A1′′ | A3 | A2 | A1 | A1 | A1 | A1 |
| 4910 | 1998 | Pleural liquid | 19 | <0.016 | <0.016 | <0.016 | 0.016 | A1′′ | A1 | A7 | A1′′ | A1 | A1 | A1 |
| 5075 | 1999 | Blood | 6 | <0.016 | <0.016 | <0.016 | <0.016 | C1 | A3 | A9 | A1 | A1 | A1 | A2 |
| 5223 | 2000 | Bronchus | 6 | <0.016 | <0.016 | <0.016 | <0.016 | A1′ | A1 | A4 | A2 | A2 | A1 | A1 |
| R6 | <0.016 | <0.016 | <0.016 | <0.016 | A1 | A1 | A1 | A1 | A1 | A1 | A1 | |||
| R65031 | 1.0 | 0.75 | 0.75 | 0.5 | C1 | A3 | A6 | C1 | C1 | A1 | A1 | |||
Data for isolates with identical sequences are highlighted in boldface. Abbreviations: CSF, cerebrospinal fluid; ND, not determined; PenG, penicillin G; AMX, amoxicillin; CTX, cefotaxime; CRO, ceftriaxome.
For PBP1a, PBP 2b, PBP 2x, and MurM, sequences that differed from each other by at most 10 residues are grouped under a capital letter, sequences that differed by at most a single residue are grouped under a single number, and primes denote single substitutions. For PBP 1b, PBP 2a, and PBP 3, each sequence that differs at a single position is designated by a number.
The sequences are briefly described below, and Table 3 provides a list of the Uniprot database accession numbers, which will allow access to either the new sequences or sequences that are identical to those encountered in this study.
TABLE 3.
Uniprot accession numbers of the sequences determined in this studya
| PBP 1a
|
PBP 1b
|
PBP 2a
|
PBP 2b
|
PBP 2x
|
PBP 3
|
MurM
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Accession no. | Group | Accession no. | Group | Accession no. | Group | Accession no. | Group | Accession no. | Group | Accession no. | Group | Accession no. |
| A1 | Q5ZFP6 | A1 | Q7CRA4 | A1 | Q8DNB6 | A1 | P10524 | A1 | P59676 | A1 | Q75Y43 | A1 | Q5ZFT4 |
| A1′ | Q75YN0 | A2 | Q5ZGA4 | A2 | Q5ZG20 | A1′ | Q5ZGA8 | A1′ | Q6ZXK2 | A2 | Q5ZG15 | A1′ | Q5ZFT3 |
| A1′′ | Q04707 | A3 | Q75YK0 | A3 | Q97NL3 | A1′′ | Q54532 | A2 | Q6ZXJ9 | A2 | Q5ZFT0 | ||
| B1 | Q9RET8 | A4 | Q5ZGA3 | A4 | Q75YF1 | A2 | Q5ZGB0 | A3 | Q8G8S1 | B1 | Q5ZFT2 | ||
| C1 | Q54946 | A5 | Q5ZGA2 | A5 | Q75YE4 | A3 | Q8G819 | A4 | Q6ZXK1 | C1 | Q5ZFT1 | ||
| C2 | Q9RET6 | A6 | Q5ZG19 | B1 | Q9RES3 | A5 | Q6ZXK0 | ||||||
| C3 | Q9RET4 | A7 | Q75YD8 | B2 | Q5ZGA9 | B1 | Q9R315 | ||||||
| A8 | Q5ZG17 | C1 | Q9RES0 | C1 | Q9RES9 | ||||||||
| A9 | Q5ZG18 | D1 | Q5ZGA5 | C1′ | Q6ZXK4 | ||||||||
| D1′ | Q5ZGA6 | C2 | Q6ZXK3 | ||||||||||
| E1 | Q5ZGA7 | C3 | Q83XA7 | ||||||||||
| F1 | Q5ZGB1 | ||||||||||||
New sequences are in boldface type. For sequences that were already present in the database, the identity is complete for the fragments that we sequenced, i.e., PBP 1a (residues 264 to 654), PBP 1b (residues 310 to 752), PBP 2a (residues 301 to 431), PBP 2b (residues 313 to 680), PBP 2x (residues 266 to 616), PBP 3 (residues 25 to 297), and MurM (residues 21 to 386).
PBP 1a.
Seven different sequences of the penicillin-binding domain of PBP 1a (residues 264 to 654) were found in our study, only one of which was new. All the sequences had the E388D substitution with respect to the R6 PBP 1a sequence. In fact, R6 PBP 1a is the only publicly available sequence with a glutamate in position 388.
Seven isolates, including six of the seven most susceptible isolates and one isolate with an intermediate level of resistance, had type A1 sequences that were nearly identical to that of PBP 1a from R6. A second group consisted of type B1 sequences from isolates 5259 and 4816, which had 33 substitutions compared to the R6 sequence. The strains with B-type PBP 1a sequences exhibited an intermediate level of resistance. A group of four adjacent substitutions in positions 574 to 577 (TSQF to NTGY) characterized the sequences of group C, which originated from isolates with intermediate to high levels of resistance. These substitutions are known to be major determinants of β-lactam resistance (29). Sequences from subgroups C2 and C3 differed from C1 sequences by nine additional common substitutions scattered between positions 495 and 570. Finally, the three sequences that formed subgroup C3 lacked seven mutations in the C terminus of the penicillin-binding domain that were present in the single C2 sequence. The two most resistant isolates from our collection had C3 sequences.
A surprising finding was the presence of a mosaic PBP 1a of type C1 in a susceptible isolate (isolate 5075). As a mosaic PBP 1a does not confer resistance in the absence of low-affinity PBP 2x or PBP 2b, it is difficult to imagine that it was acquired on its own in the absence of a selective advantage. A more likely scenario is that an ancestor of isolate 5075 was resistant and had low-affinity PBP 2x and PBP 2b but that it reverted to a susceptible phenotype by acquiring again high-affinity PBP 2x and PBP 2b. If this hypothesis is correct, it implies a fitness cost of the low-affinity PBPs in the absence of selective pressure.
PBP 2b.
Twelve different sequences of the penicillin-binding domain of PBP 2b (residues 313 to 680) were found in our collection of 22 isolates, including seven new ones. PBP 2b showed the greatest complexity of mosaicism. Consequently, the PBP 2b sequences fell into six different groups, according to our classification scheme.
Six of the seven most susceptible isolates had group A PBP 2b sequences, identical or nearly identical (subgroup A1) or very similar (subgrous A2 and A3) to that of the PBP 2b sequence from the R6 reference strain. The singly represented type A3 sequence had six substitutions, five of which were also found in other sequence groups, including T446A (see below). These mutations may be related to the low resistance level of isolate 5023, which harbors sequence type A3. Group B sequences were found in three isolates with reduced susceptibilities or low levels of resistance. Sequences B1 and B2 differed from the R6 sequence by 15 and 17 substitutions, respectively, that were well spread over the entire penicillin-binding domain. Eight isolates with intermediate and high levels of resistance contained the PBP 2b C1 sequence, which was characterized by 13 substitutions, including 11 in the second quarter of the sequenced domain (residues 412 to 489). The two isolates with the highest levels of resistance had PBP 2b D1 sequences, which had 42 and 43 substitutions, respectively, including 8 that are common to sequence type C1 and 11 that are also found in the type B1 sequence. Finally, sequence types E1 and F1 most resembled sequence type D1 but were sufficiently different to define their own groups. Sequence type E1 had 29 substitutions, 25 of which were also found in sequence type D1. Sequence type F1 had 38 mutations, 15 of which were common to sequence type D1.
Substitution T446A was found in all sequences except A1 and A2, and its effect on the affinity of PBP 2b for β-lactams has been well characterized (24). Substitution E476G was also present in all sequences except A1 and A2. The same sequences also have a threonine-to-serine or -alanine mutation in position 489. These substitutions in positions 476 and 489 are therefore potentially important determinants of β-lactam resistance.
PBP 2x.
Eleven different sequences of the PBP 2x penicillin-binding domain (residues 266 to 616) were found among the 22 sequences determined. Six of these sequences were not present in public databases. Three groups could be identified.
Group A contained sequences that were very similar to that from the reference susceptible strain R6, including three that were identical (subgroup A1). The other group A sequences had 9 or 10 substitutions in comparison with the R6 sequence. As expected, all sequences in group A originated from the most susceptible isolates, with the exception of isolate 5024. Note that sequence types A3 (isolate 5023) and A4 (isolate 4942) contained the T338A mutation, although they differed from the R6 sequence by only 9 and 10 scattered substitutions, respectively. The mutation at position 338, next to the active-site Ser337, is a major determinant of resistance (21); and isolates 5023 and 4942 exhibited slightly reduced susceptibilities. The threonine in position 338 is replaced by a proline in sequence type A5 from isolate 5024, which had an intermediate level of resistance. This T338P substitution has already been reported in four PBP 2x sequences that have a limited number of other mutations in the penicillin-binding domain, like sequence type A5. A single sequence found in two isolates with an intermediate level of resistance (isolates 5259 and 4816) constituted group B1. This penicillin-binding domain sequence differed from that of R6 at 23 positions. Sequence type B1 lacked the T338A substitution but was characterized by the Q552E mutation, which typifies a second family of sequences. Based on the crystal structure of PBP 2x from isolate 5259, it has been proposed that the mechanism of affinity reduction for β-lactams in PBP 2x sequences that have the Q552E substitution is distinct from that of sequences with a mutation in position 338 (20, 25). The T338A substitution is found in the three sequences of group C, which differed from the R6 PBP 2x penicillin-binding domain by 39 to 41 mutations. Group C PBP 2x was found in isolates with intermediate to high levels of β-lactam resistance. Eight isolates harbored the type C1 sequence, including isolate 4790. PBP 2x from isolate 4790 has been characterized enzymatically in detail, and the crystal structure of the very similar PBP 2x from strain Sp328 has been determined (5). The Sp328 PBP 2x sequence differed from the 4790 PBP 2x sequence at only two positions in the penicillin-binding domain. Sequence type C2 differed from type C1 by the absence of the A346S and A347S substitutions, which are often associated with the T338A substitution. Sequence type C3 was present in isolates 5204 and 5268, which exhibited the greatest resistance in our collection. The C3 penicillin-binding domain is characterized by the M339F and M400T substitutions. The role of the active-site M339F mutation in decreasing the reactivity of PBP 2x for β-lactams has been extensively studied enzymatically and structurally (2).
PBP 1b.
Five different sequences of PBP 1b (residues 310 to 752) were represented in our collection of isolates, including three new ones. All sequences were very similar to that from strain R6, including sequence A1, which was identical to that from strain R6 and which was found in four isolates. Isolate 5024 has a unique PBP 1b sequence with the two substitutions R526S and M527L. Sequence type A3, found in 11 isolates, was characterized by the N531K substitution. Sequence type A4 is like sequence type A3 but with an additional A575V substitution. Sequence type A5 is like sequence type A3 but with an additional G436C substitution and originates from the most resistant isolates, isolates 5204, 5268, and 5062. Only the latter mutation appears to be correlated with the resistance of the isolates that carry it. However, this correlation may simply result from the clonal origins of isolates 5204, 5268, and 5062.
PBP 2a.
Nine different sequences of the PBP 2a penicillin-binding domain (residues 301 to 431) were found in our collection of clinical isolates, including four new sequences. The sequence from only one isolate was identical to that of PBP 2a from the susceptible laboratory strain R6 (sequence type A1); the other isolates contained up to five mutations (sequence type A9). Five of the 8 most resistant isolates of our collection had the G425S mutation (sequence type A3), and 6 of the 13 most resistant isolates had the D440N substitution (sequence type A4). PBP 2a sequence types A3 and A4 were thus found in 11 of the 13 isolates with the highest level of β-lactam resistance. At this stage, it is not possible to determine, without experimental testing, whether these mutations are neutral and simply reflect the lineage of the resistant isolates or if they were selected and contribute to the resistance phenotype.
A possible mosaicism was detected. Sequence type A8 from susceptible isolate 5084 had four amino acid substitutions compared to the R6 sequence. The G425S and S461A substitutions were also found in sequence type A3, whereas T354I, S461A, V586I, and P656S were also found in sequence type A7. Further examination of the nucleotide sequences of groups A8, A3, and A7 revealed that the first half of the sequence encoding the penicillin-binding domain is identical between groups A8 and A3, whereas the second half is identical between groups A8 and A7. Table 4 shows the positions that are not identical between the three sequences. Although there were too few differences from which a definitive conclusion could be drawn, the differences are consistent with an event of homologous recombination that occurred between nucleotides 1489 and 1755. This is somewhat surprising, as sequence type A8 was found in a susceptible isolate.
TABLE 4.
Differences between three PBP 2a sequences suggestive of mosaicism
| PBP 2a type | Location | Sequence difference for the following amino acid position, mutated nucleotide(s)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| 354, 1061 | 372, 1116 | 425, 1273 | 496, 1488 | 586, 1756/1758 | 598, 1794 | 656, 1966 | ||
| A7 | Amino acid | I | A | G | V | I | F | S |
| Codon | ATA | GCT | GGC | GTT | ATT | TTT | TCA | |
| A8 | Amino acid | T | A | S | V | I | F | S |
| Codon | ACA | GCC | AGC | GTC | ATT | TTT | TCA | |
| A3 | Amino acid | T | A | S | V | V | F | P |
| Codon | ACA | GCC | AGC | GTC | GTC | TTC | CCA | |
Boldface indicates the sequence difference.
PBP 3.
Most isolates from our collection had a PBP 3 penicillin-binding domain (residues 25 to 297) that was identical to that of strain R6 (sequence type A1). The single point mutation G215R defines sequence type A2, which was found in two isolates and which is new.
MurM.
As some mosaic alleles of murM are known to increase resistance to β-lactams, we also sequenced the region that encodes MurM residues 21 to 386 (of 406 residues) from all isolates in our collection. The sequences were classified by use of the same scheme used to classify PBP 1a, PBP 2b, and PBP 2x. The group A sequences did not exhibit mosaicism and were all very similar to MurM from reference strain R6, differing at least at two positions (sequence type A1) and at most by five substitutions (sequence type A2; isolates 5075 and 4816). Only isolates 4883 and 5245 had mosaic MurMs (sequence types B1 and C1, respectively), with 18 and 50 substitutions over the sequenced region, respectively, compared to the R6 reference sequence. Six substitutions were common to the B1 and C1 MurM sequence types. The publicly available sequence most similar to that of sequence type C1 was from strain 149193, although they differed at 24 positions (25). The sequence most similar to that of sequence type B1 was from strain TX7, with 14 differences (7). It is noteworthy that isolates 4883 and 5245 are not extremely resistant, despite their mosaic MurM sequences.
Global analysis.
Regarding the distribution of the sequences among the isolates of our collection, it appears that the various alleles are not randomly associated. Table 2 shows that the resistant isolates have one of the following combinations of alleles for PBP 1a, PBP 2b, and PBP 2x, respectively: C3-D1-C3, C1-C1-C1, C2-E1-C2, A1-F1-A5, or B1-B1-B1. Note that a given allele for one of the three mosaic PBPs was never found to be associated with more than one allele of the two other mosaic PBPs. These associations certainly reflect the history of the emergence and the spread of resistant strains. However, some functional relationships related to possible protein-protein interactions cannot be excluded.
When the resistance levels and the sequences are considered, the first observation that can be made is that isolates 5031, 4935, 5104, 4790, and 4843 have identical penicillin-binding domains (the C1-C1-C1 combination) and MurM proteins, although they display significantly different levels of β-lactam resistance. MLST analysis revealed that they have the same sequence type as the reference global clone Spain 23F-1 (strain ATCC 700669). The MICs for penicillin G in these five isolates range from 0.25 to 2.0 μg/ml and from 0.19 to 2.0 μg/ml for cefotaxime. Isolate 5180 also had PBPs identical to those of clone Spain 23F-1, with the exception of a L600S substitution in PBP 2x.
Isolates 5259 and 4816 also had identical penicillin-binding domains and MurM proteins, but the 5259 isolate exhibited slightly greater resistance. All the PBPs of the two most resistant isolates, isolates 5204 and 5268, had nearly identical sequences. Although the PBP 2b sequences of these two isolates belonged to the same subgroup, they nevertheless differed at a single position. Isolate 5204 had an additional K371Q mutation in PBP 2b. We cannot exclude the possibility that the higher level of resistance of isolate 5204 results from this minor difference.
Transfer of resistance to R6.
It is generally reported that the transfer of the mosaic genes for PBP 1a, PBP 2b, and PBP 2x from a resistant strain to susceptible strain R6 confers the resistance of the originating strain to the recipient strain (1, 22, 28). More recently, the additional transfer of mosaic murM alleles was found to be required to express in R6 the full resistance of the original clinical isolate (9, 28). The finding that five isolates with identical penicillin-binding domains and MurM proteins exhibit very different levels of β-lactam resistance calls these tenets into question. To clarify the matter, we decided to measure the level of resistance provided to the R6 recipient strain by transfer of the three mosaic genes for PBP 1a, PBP 2b, and PBP 2x that are present in our five isolates with different resistance levels.
We chose the least resistant isolate, isolate 5031, as the donating strain. Complete genes encoding the three mosaic PBPs were cloned and transformed simultaneously into R6 cells. Resistant clones were selected on cefotaxime. The levels of resistance of three independent transformed clones were measured and were found to exceed that of originating isolate 5031 for all antibiotics tested. The three clones had the same MICs, which were about threefold higher than that of the originating isolate. The data in Table 2 show that the level of resistance of the transformed R65031 strains is intermediate between those of isolate 5031 and isolate 4843, the least and most resistant isolates, respectively, of our five isolates with identical penicillin-binding domains.
Insertion of the mosaic pbp genes was checked in the three independent clones by sequencing following PCR amplification of the fragment encoding the penicillin-binding domain. The complete penicillin-binding domain of PBP 1a from isolate 5031 had been introduced. The first two substitutions, Q281L and D311N, of the penicillin-binding domain of PBP 2x were missing from the three transformed clones. The first E333G mutation in the penicillin-binding domain of PBP 2b was also missing from one of the selected clones. These are indications that these substitutions are not important determinants of β-lactam resistance.
PBP expression levels.
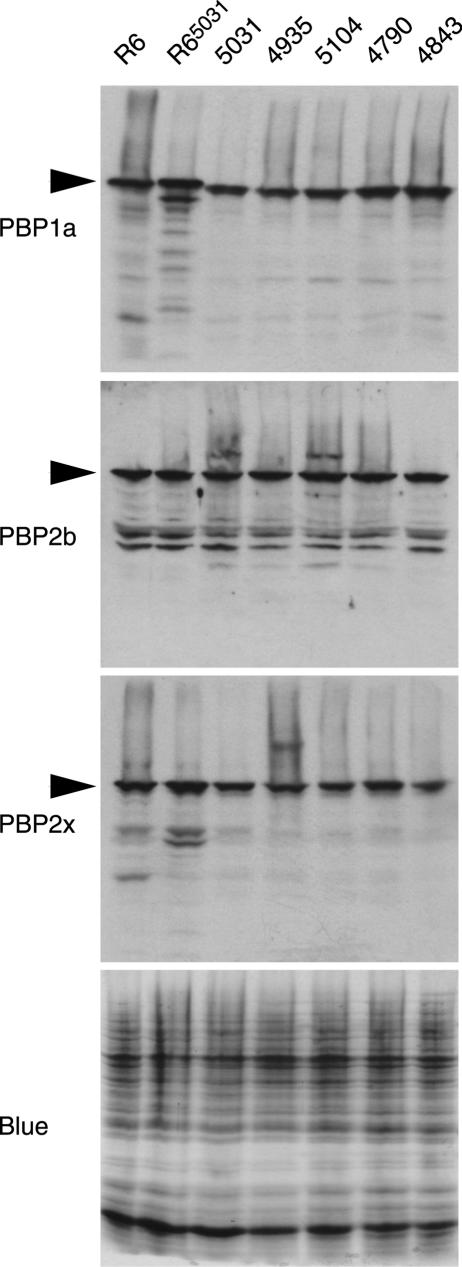
The expression levels of PBP 1a, PBP 2b, and PBP 2x in strains R6, R65031, 5031, 4935, 5104, 4790, and 4883 were compared by immunoblotting (Fig. 1). No significant differences that could be correlated with the resistance level were observed. The R6 and R65031 strains appeared to express slightly more PBP 1a and possibly more PBP 2x than the wild strains. The electrophoretic mobility of PBP 1a from the wild resistant strains appeared to be slightly increased compared to those of the PBP 1a proteins from strains R6 and R65031. This variation, already noted in early studies (18), is presumably due to differences in amino acid compositions, as the proteins have the same size.
FIG. 1.
Comparison by immunoblotting of the expression of PBP 1a, PBP 2b, and PBP 2x in strains R6, R65031, 5031, 4935, 5104, 4790, and 4843. The Coomassie blue-stained gel shows that equivalent amounts of proteins were analyzed for each strain. The intense band at the bottom is the lyzosyme added to the lysis buffer.
DISCUSSION
The main finding from our study is that S. pneumoniae clinical isolates with identical penicillin-binding domains for the six PBPs and identical MurM proteins can have different levels of β-lactam resistance. The range of MICs for penicillin G and cefotaxime covered by our five isolates with identical PBPs and MurM is clinically relevant, as isolate 5031 is “intermediate” and isolate 4883 is “resistant,” according to the classification guide lines in use in France (4). Therefore, molecular diagnostic tools based solely on the sequences of the pbps and murM genes could fail to accurately predict the level of β-lactam resistance of an isolate.
Laboratory strain R6 has generally been considered a standard of susceptibility. Transfer of the relevant genes from a resistant strain to R6 would usually confer to the latter the resistance level of the donor strain (1, 22, 28). When an R6 strain transformed with the pbp genes from a clinical strain fails to reach the level of β-lactam resistance of the donor strain, it is assumed that an additional resistance factor is required. In this paper, we report that the transfer of the three mosaic pbp genes from a clinical isolate to R6 results in a higher level of resistance than that of the donor strain. This result demonstrates that the genetic background modulates the level of resistance conferred by the PBPs and that some wild strains are less prone to resistance than laboratory strain R6. If the R6 genome is defined as the reference background that allows expression of the standard resistance of a given set of mosaic PBPs, wild genetic factors not only can increase but also can decrease the resistance level. An experimental example of such a negative factor is given by the null allele of murM, which suppresses completely the resistance conferred by the PBPs (9).
The nature of the positive or negative factors that modulate the resistance of isolates 5031, 4935, 5104, 4790, 4843, and R65031 is a matter of speculation. It must be noted here that the five clinical isolates belong to the same global clone (Spain 23F-1), as determined by MLST. The relative quantification of PBP 1a, PBP 2b, and PBP 2x presented in Fig. 1 rules out variation of expression as a major cause of resistance differences. Although the sequences of the various penicillin-binding domains were identical, we do not know if this identity extends to the other domains of the proteins. We cannot exclude the possibility that substitutions in non-penicillin-binding domains may alter the resistance. It has been suggested that sequence regions outside the active site permit a more or less optimal functioning of the PBPs, depending on their compatibility with interacting partners in putative multienzymatic complexes that synthesize the peptidoglycan (2, 10).
The nature of the capsule may conceivably affect the MICs for various antibiotics by altering the diffusion of the small molecules toward the bacterial cell wall. However, isolates 4843, 4790, 5104, and 5031 are of the same serotype, serotype 23. Therefore, their capsules must be very similar, if not identical, and are probably not responsible for modulating the β-lactam resistance.
Besides murM, several non-pbp genes were found to affect β-lactam resistance. Point mutations in the ciaH gene, which encodes a histidine protein kinase, were selected in the laboratory upon selection with cefotaxime (12), whereas cpoA mutants (cpoA encodes a putative glycosyltransferase) were selected with piperacillin (11).
The above examples are of single genes that influence β-lactam resistance. It is also possible that multiple genes may subtly affect the MICs for various antibiotics or even that the overall fitness of a strain can modify the level of PBP-based resistance. Therefore, a complete understanding of the resistance mechanism would require the genome-wide comparison of many strains with different or similar resistance profiles, followed by experimental testing through gene transfers. Meanwhile, an imperfect sequence-based method able to rapidly qualify an isolate in the categories susceptible, reduced susceptibility, or likely to be highly resistant (6, 8, 14-16, 30) may already be developed and provide some benefit in clinical settings.
Acknowledgments
We thank Dominique Champelovier and Benoit Gallet for technical assistance, as well as Hervé Duborjal and Jean-François Mouret at GenomeExpress for DNA sequencing.
We acknowledge the use of the pneumococcal MLST database, which is located at Imperial College London and which is funded by the Wellcome Trust. This work was supported by a fellowship to L.C. and a grant from the Région Rhône Alpes and was partially funded by the 6th European Framework Program (COBRA LSHM-CT-2003-503335).
REFERENCES
- 1.Barcus, V. A., K. Ghanekar, M. Yeo, T. J. Coffey, and C. G. Dowson. 1995. Genetics of high-level penicillin resistance in clinical isolates of Streptococcus pneumoniae. FEMS Microbiol. Lett. 126:299-303. [DOI] [PubMed] [Google Scholar]
- 2.Chesnel, L., L. Pernot, D. Lemaire, D. Champelovier, J. Croize, O. Dideberg, T. Vernet, and A. Zapun. 2003. The structural modifications induced by the M339F substitution in PBP2x from Streptococcus pneumoniae further decreases the susceptibility to beta-lactams of resistant strains. J. Biol. Chem. 278:44448-44456. [DOI] [PubMed] [Google Scholar]
- 3.Coffey, T. J., C. G. Dowson, M. Daniels, and B. G. Spratt. 1995. Genetics and molecular biology of beta-lactam-resistant pneumococci. Microb. Drug Resist. 1:29-34. [DOI] [PubMed] [Google Scholar]
- 4.Comité de l'Antibiogramme de la Société Française de Microbiologie. 2004. Communiqué 2004. Société Française de Microbiologie Janvier 2004. [Online.] http://www.sfm.asso.fr/doc/casfm/download.php?fichier=Comm2004.pdf. Accessed 30 September 2004.
- 5.Dessen, A., N. Mouz, E. Gordon, J. Hopkins, and O. Dideberg. 2001. Crystal structure of PBP2x from a highly penicillin-resistant Streptococcus pneumoniae clinical isolate: a mosaic framework containing 83 mutations. J. Biol. Chem. 276:45106-45112. [DOI] [PubMed] [Google Scholar]
- 6.du Plessis, M., A. M. Smith, and K. P. Klugman. 1999. Application of pbp1A PCR in identification of penicillin-resistant Streptococcus pneumoniae. J. Clin. Microbiol. 37:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 8.Filipe, S. R., E. Severina, and A. Tomasz. 2000. Distribution of the mosaic structured murM genes among natural populations of Streptococcus pneumoniae. J. Bacteriol. 182:6798-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipe, S. R., and A. Tomasz. 2000. Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc. Natl. Acad. Sci. USA 97:4891-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grebe, T., and R. Hakenbeck. 1996. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob. Agents Chemother. 40:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grebe, T., J. Paik, and R. Hakenbeck. 1997. A novel resistance mechanism against beta-lactams in Streptococcus pneumoniae involves CpoA, a putative glycosyltransferase. J. Bacteriol. 179:3342-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guenzi, E., A. M. Gasc, M. A. Sicard, and R. Hakenbeck. 1994. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12:505-515. [DOI] [PubMed] [Google Scholar]
- 13.Hakenbeck, R., A. Konig, I. Kern, M. van der Linden, W. Keck, D. Billot-Klein, R. Legrand, B. Schoot, and L. Gutmann. 1998. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level beta-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J. Bacteriol. 180:1831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho, P. L., R. C. Wong, F. K. Chow, M. Y. Cheung, S. S. Wong, W. C. Yam, and T. L. Que. 2004. Application of a multiplex pbp2b and pbp2x PCR for prediction of penicillin resistance in Streptococcus pneumoniae. J. Antimicrob. Chemother. 53:890-891. [DOI] [PubMed] [Google Scholar]
- 15.Jalal, H., S. Organji, J. Reynolds, D. Bennett, E. O'Mason, Jr., and M. R. Millar. 1997. Determination of penicillin susceptibility of Streptococcus pneumoniae using the polymerase chain reaction. Mol. Pathol. 50:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearns, A. M., C. Graham, D. Burdess, J. Heatherington, and R. Freeman. 2002. Rapid real-time PCR for determination of penicillin susceptibility in pneumococcal meningitis, including culture-negative cases. J. Clin. Microbiol. 40:682-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauss, J., and R. Hakenbeck. 1997. A mutation in the d,d-carboxypeptidase penicillin-binding protein 3 of Streptococcus pneumoniae contributes to cefotaxime resistance of the laboratory mutant C604. Antimicrob. Agents Chemother. 41:936-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin, C., C. Sibold, and R. Hakenbeck. 1992. Relatedness of penicillin-binding protein 1a genes from different clones of penicillin-resistant Streptococcus pneumoniae isolated in South Africa and Spain. EMBO J. 11:3831-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morlot, C., A. Zapun, O. Dideberg, and T. Vernet. 2003. Growth and division of Streptococcus pneumoniae: localization of the high molecular weight penicillin-binding proteins during the cell cycle. Mol. Microbiol. 50:845-855. [DOI] [PubMed] [Google Scholar]
- 20.Mouz, N., A. M. Di Guilmi, E. Gordon, R. Hakenbeck, O. Dideberg, and T. Vernet. 1999. Mutations in the active site of penicillin-binding protein PBP2x from Streptococcus pneumoniae. Role in the specificity for beta-lactam antibiotics. J. Biol. Chem. 274:19175-19180. [DOI] [PubMed] [Google Scholar]
- 21.Mouz, N., E. Gordon, A. M. Di Guilmi, I. Petit, Y. Petillot, Y. Dupont, R. Hakenbeck, T. Vernet, and O. Dideberg. 1998. Identification of a structural determinant for resistance to beta-lactam antibiotics in gram-positive bacteria. Proc. Natl. Acad. Sci. USA 95:13403-13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz, R., C. G. Dowson, M. Daniels, T. J. Coffey, C. Martin, R. Hakenbeck, and B. G. Spratt. 1992. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 6:2461-2465. [DOI] [PubMed] [Google Scholar]
- 23.Nagai, K., T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 2002. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob. Agents Chemother. 46:1273-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagliero, E., L. Chesnel, J. Hopkins, J. Croize, O. Dideberg, T. Vernet, and A. M. Di Guilmi. 2004. Biochemical characterization of Streptococcus pneumoniae penicillin-binding protein 2b and its implication in beta-lactam resistance. Antimicrob. Agents Chemother. 48:1848-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pernot, L., L. Chesnel, A. Le Gouellec, J. Croize, T. Vernet, O. Dideberg, and A. Dessen. 2004. A PBP2x from a clinical isolate of Streptococcus pneumoniae exhibits an alternative mechanism for reduction of susceptibility to beta-lactam antibiotics. J. Biol. Chem. 279:16463-16470. [DOI] [PubMed] [Google Scholar]
- 26.Reichmann, P., A. Konig, A. Marton, and R. Hakenbeck. 1996. Penicillin-binding proteins as resistance determinants in clinical isolates of Streptococcus pneumoniae. Microb. Drug Resist. 2:177-181. [DOI] [PubMed] [Google Scholar]
- 27.Sanbongi, Y., T. Ida, M. Ishikawa, Y. Osaki, H. Kataoka, T. Suzuki, K. Kondo, F. Ohsawa, and M. Yonezawa. 2004. Complete sequences of six penicillin-binding protein genes from 40 Streptococcus pneumoniae clinical isolates collected in Japan. Antimicrob. Agents Chemother. 48:2244-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, A. M., and K. P. Klugman. 2001. Alterations in MurM, a cell wall muropeptide branching enzyme, increase high-level penicillin and cephalosporin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2393-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, A. M., and K. P. Klugman. 2003. Site-specific mutagenesis analysis of PBP 1A from a penicillin-cephalosporin-resistant pneumococcal isolate. Antimicrob. Agents Chemother. 47:387-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ubukata, K., Y. Asahi, A. Yamane, and M. Konno. 1996. Combinational detection of autolysin and penicillin-binding protein 2B genes of Streptococcus pneumoniae by PCR. J. Clin. Microbiol. 34:592-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber, B., K. Ehlert, A. Diehl, P. Reichmann, H. Labischinski, and R. Hakenbeck. 2000. The fib locus in Streptococcus pneumoniae is required for peptidoglycan crosslinking and PBP-mediated beta-lactam resistance. FEMS Microbiol. Lett. 188:81-85. [DOI] [PubMed] [Google Scholar]