Abstract
The colonization and resistance dynamics of aerobic gram-negative bacteria in the intestinal and oropharyngeal microfloras of patients admitted to intensive care units (ICU) and general wards were investigated during and after hospitalization. A total of 3,316 specimens were obtained from patients upon admission, once weekly during hospitalization, at discharge from the ICU, at discharge from the hospital, and 1 and 3 months after discharge from the hospital. Five colonies per specimen were selected for identification and susceptibility testing. In both patient populations, the gram-negative colonization rates in oropharyngeal specimens increased during hospitalization and did not decrease in the 3 months after discharge. In rectal specimens, colonization rates decreased during hospitalization and increased after discharge. There was a change in species distribution among the dominant microfloras during hospitalization. Klebsiella spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa were isolated more often, whereas the frequency of Escherichia coli declined. The percentage of ICU patients colonized with ampicillin- and/or cephalothin-resistant fecal E. coli was significantly increased at discharge from the hospital and did not change in the 3 months after discharge. The emergence of multidrug resistance was observed for E. coli during patient stays in the ICU. Resistance frequencies in E. coli significantly increased with the length of stay in the ICU. For the general ward population, no significant changes in resistance frequencies were found during hospitalization. From a population perspective, the risk of dissemination of resistant gram-negative bacteria into the community through hospitalized patients appears to be low for general ward patients but is noticeably higher among ICU patients.
Hospitals are considered particularly important for the containment of antimicrobial resistance. The combination of seriously ill patients, the intensive use of antibiotics, and cross-contamination has resulted in nosocomial infections with highly resistant bacterial pathogens (7, 12, 17). Infections caused by antibiotic-resistant microorganisms are associated with higher mortality and morbidity rates and higher costs than are antibiotic-sensitive bacterial infections (8, 10, 20). The prevalence of resistance for nearly all important microorganism/antibiotic combinations is generally higher among isolates from patients hospitalized in intensive care units (ICU) than that among non-ICU inpatients (2, 33, 35). Enterobacteriaceae and Pseudomonas aeruginosa have emerged as major causes of nosocomial infections and account for approximately 30% and 5%, respectively, of all bloodstream infections (16, 38). Antibiotic resistance surveillance programs have demonstrated an increase in resistance among these gram-negative pathogens (16, 35, 38).
Bacterial colonization is often a first step in the pathogenesis of nosocomial infections (5). Therefore, the choice of empirical antibiotic therapy depends, at least partly, on the colonization and resistance dynamics of the normal microflora.
Different mechanisms may lead to the colonization of hospitalized patients with resistant strains (3, 29). First, these strains may enter the hospital upon the admission of patients already colonized with resistant strains. Secondly, during hospitalization, susceptible bacteria may develop resistance due to genetic mutations or through the transfer of resistance genes. Thirdly, resistance may emerge through the induction of genes that are already present in susceptible bacterial subpopulations. A lack of hygiene and infection control may facilitate the spread of these resistant bacteria from patient to patient (5).
After discharge, patients may remain colonized with resistant bacteria acquired in the hospital, and these may subsequently spread into the community. Whether resistant strains will survive and replicate depends largely on the selection pressure exerted by antibiotics. In the late 1960s and 1970s, numerous colonization studies were performed to clarify the epidemiology of nosocomial infections with resistant bacteria (9, 11, 21-24, 39-41). These studies may now be of limited value due to major changes in antibiotic and patient characteristics. Other colonization studies of hospitalized patients have been based on single cultures from individual patients, without longitudinal follow-up during and after hospitalization (27, 28, 36). Moreover, isolates were often not identified to the species level and were described as coliforms or aerobic gram-negative bacteria (11, 27, 28, 41). In these studies, an observed increase in resistance in Enterobacteriaceae may in fact have been the result of a shift in the species distribution (36).
We therefore decided to design a prospective observational study with the aim to accurately assess the epidemiology of aerobic gram-negative bacterial colonization and antibiotic resistance in the oropharyngeal and intestinal microfloras of hospitalized patients from admission up to 3 months after discharge.
MATERIALS AND METHODS
Patients and study design. (i) Setting.
Erasmus MC is a 1,200-bed university referral hospital in Rotterdam, The Netherlands. The study was conducted at two ICUs (surgical and neurosurgical) and five general wards, including the departments of internal medicine, pulmonology, neurosurgery, urology, and gastroenterology. Patients were enrolled into the study between November 2000 and July 2003. The study was approved by the Medical Ethics Committee, and informed consent was provided before participation in the study.
(ii) Inclusion and exclusion criteria.
Patients were eligible for the study if they met all of the following criteria: ≥18 years of age, the length of stay was expected to be ≥5 days, and informed consent was given by the patient or his/her representative. The exclusion criteria for patients on general wards were as follows: preceding admission to an ICU or another general ward during the same hospitalization, the presence of an ileostoma, and a diagnosis of human immunodeficiency virus infection, tuberculosis, or cystic fibrosis. The same criteria were applicable for ICU patients except that preceding admission to a general ward was allowed.
(iii) Collection of specimens.
Rectal swabs (or stool specimens) and oropharyngeal swabs were obtained within 48 h of admission, once a week if the length of stay was more than 7 days, at discharge from the ICU (ICU patients only), and at discharge from the hospital. At discharge from the hospital and 1 and 3 months after discharge, patients were contacted by mail to send in a stool specimen and an oropharyngeal swab. A reminder was sent to nonresponders 1 week later. Persistent nonresponders were contacted again at subsequent times 1 and 3 months after discharge.
(iv) Data collection.
The following data were collected: age; gender; reason for admission; severity of acute illness upon admission, graded according to the simplified acute physiologic score for ICU patients (25); comorbidity upon admission according to the definitions of the Dutch National Intensive Care Evaluation (http://www.stichting-nice.nl); residential status 48 h before admission; hospitalization history in the 3 months prior to admission; and the consumption of antibiotics during hospitalization. Community pharmacies were asked for information about the use of antibiotics in the 3 months prior to and after hospitalization. Patients provided written informed consent for the acquisition of these medication lists.
Antibiotic use during hospitalization was expressed as the number of defined daily doses (DDD) per 100 bed days, whereas the use before and after hospitalization was expressed as the number of DDDs per 100 inhabitant days. We used the DDDs of antibiotics for systemic use listed in the Anatomical Therapeutic Chemical Classification System 2003 (47).
Microbiological methods. (i) Isolation of gram-negative bacteria.
All patient specimens were collected and analyzed at the microbiological laboratory of the Erasmus MC. Stool specimens were collected in plastic containers, and oropharyngeal and rectal swabs were transported in Amies transport medium. Stool specimens were diluted 1:10 in physiological saline containing 20% glycerol and stored at −20°C. After thawing, 10−2 and 10−4 dilutions in physiological saline were inoculated onto chromogenic plates (Chromagar Orientation; Becton Dickenson, Heidelberg, Germany).
Swabs were diluted in 1 ml of Stuart transport medium and stored at −80°C until assayed. After thawing, the samples were diluted further. Fifty microliters of the undiluted suspension and 50 μl of a 10−1 dilution in physiological saline were inoculated onto chromogenic plates. The plates were incubated aerobically at 37°C and examined after 18 to 24 h for growth and colony characteristics. The dilution with a countable number of colonies (≤100) was used for the selection of colonies. If both dilutions yielded countable numbers of colonies, the dilution with the most variety among different colonies was chosen. If no difference existed between the two dilutions, the lowest dilution was chosen. Five colonies were selected for further identification. An algorithm was developed for the selection of these five colonies. This method led to proportional sampling of the microorganisms present at concentrations above the detection limit; e.g., if 60 pink colonies and 40 blue colonies grew on the chromogenic agar, then the proposed algorithm resulted in the selection of three pink and two blue colonies. When no differences in color and/or morphology were observed, five colonies were randomly selected.
(ii) Identification.
In a previous study, we concluded that Chromagar Orientation allows the accurate identification of Escherichia coli by colony color (15). Pink colonies were therefore directly identified as E. coli. The identification of other colonies was done with the VITEK 2 system (bioMerieux, Marcy l'Etoile, France) and proprietary data management software (version VT2-R02.03) by using ID-GNI cards after subculturing on Colombia blood agar.
(iii) Determination of susceptibility.
MICs were determined with the VITEK 2 system using AST-N010/020 cards. The breakpoints of the National Committee for Clinical Laboratory Standards (NCCLS) were applied (34). The Advanced Expert System of the VITEK 2 system (version AES.R02.00N) was used for all readings and interpretations of susceptibility results. E. coli (ATCC 25922 and ATCC 35218) and P. aeruginosa (ATCC 27853) were used as reference strains.
Data analysis.
Patients were considered eligible for analysis when at least one consecutive sample was taken following the sample taken upon admission and when the length of stay was ≥5 days. Samples collected once weekly during hospital stays were categorized as “during stay.” When no sample was taken on the day of discharge from the ICU, the last sample taken during stay was considered representative of the colonization and resistance status at discharge and was therefore categorized as such. When patients died during stay on the ICU, the last sample taken was also categorized as “during stay.”
The percentage of patients colonized with resistant bacteria at different times was calculated for each antibiotic. We selected one isolate per patient with the most resistant result for that particular antibiotic. To test for differences in resistance frequencies between the different times, we used the chi-square test or Fisher's exact test (in cases of low [≤5] cell numbers).
Multidrug resistance was defined as oropharyngeal and/or rectal colonization with at least one isolate that was resistant to three or more of the antibiotics tested. The chi-square test was used to test for differences in multidrug resistance.
We used logistic regression analysis to assess whether resistance frequencies in bacteria were associated with the length of stay in the ICU. P values of <0.05 were considered significant. The data were analyzed by using SPSS 11.0 for Windows (SPSS Inc., Chicago, Ill.) and GraphPad Prism, version 3.0, for Windows (GraphPad Software, San Diego, Calif.).
RESULTS
Inclusion and follow-up of study population.
A total of 200 ICU and 319 general ward (GW) patients were included in this prospective study. The data for 17 ICU and 91 GW patients were not analyzed. The reasons for withdrawal from the analyses were a length of stay of less than 5 days (for the ICU group, n = 9; for the GW group, n = 52) and no collection of a second specimen following admission (for the ICU group, n = 8; for the GW group, n = 39).
Table 1 shows the characteristics of the two enrolled populations. The numbers of ICU and GW patients analyzed at the different times are presented in Table 2. Patients were lost to follow-up as a result of death, transfers, and withdrawal from the study.
TABLE 1.
Patient characteristics upon admission
| Characteristic | Value for patients in indicated areas
|
|
|---|---|---|
| Intensive care units (n = 183) | General wards (n = 228) | |
| Age (mean ± SD) (yrs) | 55.9 ± 15.6 | 57.1 ± 14.0 |
| Men (n [%]) | 120 (65.6) | 135 (59.2) |
| Reason for admission (n [%]) | ||
| Surgery | ||
| Abdominal | 53 (29.0) | 2 (0.9) |
| Neurosurgery | 32 (17.5) | 67 (29.4) |
| Transplantation | 14 (7.7) | |
| Urologic surgery | 46 (20.2) | |
| Other surgery | 20 (10.9) | |
| Trauma | 29 (15.8) | 1 (0.4) |
| Medical | ||
| Abdominal | 20 (8.8) | |
| Neurologic disorder | 13 (7.1) | 1 (0.4) |
| Obstructive pulmonary disease | 5 (2.7) | 54 (23.7) |
| Other medical disorder | 17 (9.3) | 37 (16.2) |
| Residential status 48 h before admission (n [%]) | ||
| Community | 145 (79.2) | 228 (100) |
| General ward at Erasmus MC | 29 (15.8) | |
| General ward at other hospital | 7 (3.8) | |
| Other | 2 (1.1) | |
| SAPS II on admission (mean [SD])a | 33.5 (13.9) | |
| Admissions with comorbidityb (no. [%]) | 54 (29.5) | 84 (36.8) |
| Hospitalization 3 mos prior to admission (n [%]) | 101 (55.2) | 52 (23) |
| Use of antibiotics 3 mos prior to admission (n [%]) | 42 (23) | 57 (25) |
Simplified acute physiology score (25).
According to the definitions of the Dutch National Intensive Care Evaluation (www.stichting-nice.nl). Comorbidity was defined as at least one of the following disorders: decreased immunity, respiratory insufficiency class IV according to the New York Heart Association (NYHA), cardiovascular insufficiency class IV according to NYHA, cirrhosis, hematologic malignancy, AIDS, neoplasm with metastases, or chronic renal failure.
TABLE 2.
Frequencies of aerobic gram-negative bacteria in the intestinal microflora during and after hospitalizationa
| Characteristic | Value for patients in ICU
|
Value for patients in general ward
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Admission | Discharge from ICU | Discharge from hospital | 1 mo after discharge from hospital | 3 mos after discharge from hospital | Admission to general ward | Discharge from hospital | 1 mo after discharge from hospital | 3 mos after discharge from hospital | |
| No. of patients | 183 | 150 | 62 | 82 | 73 | 228 | 205 | 185 | 167 |
| Samples with detectable aerobic gram-negative bacteria (n [%]) | 67 (37) | 51 (34) | 42 (67) | 66 (81) | 61 (84) | 112 (49) | 113 (55) | 131 (71) | 125 (75) |
| No. of isolates | 335 | 254 | 206 | 332 | 304 | 524 | 562 | 639 | 586 |
| Gram-negative bacteria (%) | |||||||||
| Enterobacter spp. | 2.4 | 3.1 | 1.5 | 3.6 | 2.3 | 3.4 | 2.7 | 2.5 | 3.6 |
| Escherichia coli | 83.9 | 68.5b | 84.0 | 79.2 | 85.2 | 89.5 | 83.8c | 85.6 | 87.7 |
| Klebsiella spp. | 9.0 | 20.1b | 5.8 | 10.5d | 7.6 | 3.1 | 9.3c | 6.3d | 3.1e |
| Pseudomonas spp. | 6.3b | 2.4c | d | e | 0.6 | 0.2 | 0.5 | ||
| Other | 4.7 | 2.0 | 6.3 | 6.7 | 4.9 | 3.4 | 4.0 | 5.6 | 5.1 |
The relative frequencies were compared in defined pairs, using the chi-square test or Fisher's exact test as applicable (P values of < 0.05 were considered significant).
Significant difference for discharge from ICU versus admission to participating ICU.
Significant difference for discharge from hospital versus admission to participating ICU or general ward.
Significant difference for 1 month after discharge from hospital versus discharge from hospital.
Significant difference for 3 months after discharge from hospital versus discharge from hospital.
Twenty-six patients (19.6%) were already hospitalized on a GW before admission to the ICU (Table 1). The median length of stay on the participating units was 12 days (range, 5 to 119 days) for the ICU patients (n = 183) and 10 days (range, 5 to 116 days) for the GW population (n = 228) (Table 1). Patients who died during stay on the ICU had a median length of stay of 22 days (range, 7 to 119 days; n = 25). The median length of stay in the hospital after discharge from the ICU was 15 days (range, 0 to 281 days; n = 158).
Bacterial strains.
In total, 3,316 specimens from 411 patients were collected. From these specimens, 6,069 gram-negative bacteria (3,941 E. coli, 755 Klebsiella spp., 497 Enterobacter spp., 243 Pseudomonas spp., 249 Serratia marcescens, and 384 other Enterobacteriaceae isolates) were isolated, identified, and tested for antimicrobial resistance.
Species distribution during and after hospitalization. (i) ICU population.
A total of 1,580 specimens, 788 of which were fecal and 792 of which were oropharyngeal, were studied. The number of fecal samples with detectable aerobic gram-negative bacteria varied from 27% for samples collected during stay on the ICU to 84% for samples collected 3 months after discharge from the hospital. A significant increase in the frequencies of Klebsiella spp. (P = 0.001) and P. aeruginosa (P < 0.001) and a significant decrease in the frequency of E. coli (P < 0.001) were found in intestinal microfloras at discharge from the ICU. At discharge from the hospital, the frequencies of the different species were the same as those upon admission to the ICU, with the exception of P. aeruginosa.
Upon admission of patients to the ICU, 18% of the oropharyngeal samples yielded gram-negative bacteria. For samples collected during stay in the ICU, at discharge from the ICU, at discharge from the hospital, and 1 and 3 months after discharge from the hospital, these percentages were 43, 27, 42, 31, and 33%, respectively. During hospitalization, the frequency of E. coli in oropharyngeal samples decreased significantly, from 43.1% upon admission to 26.9% at discharge from the ICU (P = 0.001) and finally to 13.0% (P < 0.001) at discharge from the hospital. One month after discharge from the hospital, the frequency of E. coli had already increased to 28.3% (P = 0.006). The frequency of P. aeruginosa in oropharyngeal samples increased significantly during hospitalization, from 6.0% upon admission to 13.2% at discharge from the ICU (P = 0.02), but did not decrease during the first 3 months after discharge from the hospital. The frequency of Serratia marcescens increased significantly during hospitalization, from 1.0% at discharge from the ICU to 14.6% at discharge from the hospital (P < 0.001), but decreased to 4.1% in the 3 months after discharge from the hospital (P = 0.004).
(ii) General ward population.
A total of 1,736 specimens, 881 of which were fecal and 855 of which were oropharyngeal, were studied. The number of fecal samples with detectable aerobic gram-negative bacteria varied from 43.8% for samples collected weekly during stay on the general ward to 74.8% for samples collected 3 months after discharge from the hospital (Table 2). A significant increase in the frequency of Klebsiella spp. and a significant decrease in the frequency of E. coli were found in intestinal microfloras at discharge from the general wards (Table 2). However, 3 months after discharge from the hospital, the frequencies of the different species were almost the same as those upon admission.
Upon admission of patients to the general ward, 1.1% of the oropharyngeal samples yielded detectable gram-negative bacteria, whereas for samples collected during stay in the hospital, at discharge from the hospital, and 1 and 3 months after discharge from the hospital, these percentages were 3.4, 12.4, 19.4, and 20.3%, respectively. From these oropharyngeal samples, 57 E. coli isolates, 348 other Enterobacteriaceae isolates, and 42 P. aeruginosa isolates were cultured. The number of isolates from the oropharyngeal samples was too small to allow a comparison of the species distributions over time.
Percentage of patients colonized with resistant E. coli during and after hospitalization.
The percentage of ICU patients colonized with ampicillin- and/or cephalothin-resistant fecal E. coli was significantly increased at discharge from the hospital and did not return to baseline in the 3 months after discharge (Table 3). Trimethoprim-sulfamethoxazole resistance fluctuated remarkably, with the lowest resistance rate after discharge from the ICU and the highest rate 3 months after discharge from the hospital.
TABLE 3.
Percentages of patients colonized with resistant Escherichia coli in the intestinal microflora during and after hospitalizationabc
| Antibiotic(s) | % Colonized ICU patients
|
% Colonized general ward patients
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Admission (n = 183) | Discharge from ICU (n = 150) | Discharge from hospital (n = 62) | 1 mo after discharge from hospital (n = 82) | 3 mos after discharge from hospital (n = 73) | Admission to general ward (n = 228) | Discharge from hospital (n = 205) | 1 mo after discharge from hospital (n = 185) | 3 mos after discharge from hospital (n = 167) | |
| Ampicillin | 10.5 | 12.7 | 22.6d | 18.1 | 22.2 | 14.6 | 13.7 | 21.1 | 17.8 |
| Piperacillin | 5.0 | 6.7 | 9.7 | 6.0 | 8.3 | 9.0 | 8.3 | 9.4 | 8.3 |
| Amoxicillin-clavulanic acid | 1.1 | 2.7 | 3.2 | 2.4 | 1.4 | 1.9 | 0.5 | 0.6 | 1.9 |
| Piperacillin-tazobactam | 0.6 | 0.7 | 0 | 2.4 | 0 | 0.5 | 0.5 | 0 | 0 |
| Meropenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cephalothin | 2.2 | 4.0 | 8.1d | 6.0 | 6.9 | 4.7 | 2.9 | 5.0 | 8.9 |
| Cefuroxime sodium | 0.6 | 2.0 | 1.6 | 2.4 | 2.8 | 0.5 | 1.0 | 1.1 | 1.0 |
| Ceftazidime | 0.6 | 1.3 | 0 | 1.2 | 0 | 0.5 | 0.5 | 0 | 0 |
| Gentamicin | 0.6 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 1.7 | 1.3 |
| Ciprofloxacin | 0.6 | 0 | 0 | 0 | 0 | 0.5 | 1.0 | 1.1 | 2.5 |
| Trimethoprim-sulfamethoxazole | 4.4 | 2.0 | 8.1 | 10.8 | 19.4 | 8.5 | 8.3 | 16.1e | 10.8 |
NCCLS breakpoints were applied.
One isolate per patient was included, giving the most resistant result for each antibiotic.
The resistance frequencies were compared in pairs, using the chi-square test or Fisher's exact test as applicable (P values of < 0.05 were considered significant). Significant difference for discharge from hospital versus admission to participating ICU.
Significant difference for 1 month after discharge from hospital versus discharge from hospital.
For the general ward population, no significant differences in resistance frequencies were found during hospitalization (Table 3). However, the percentage of patients colonized with trimethoprim-sulfamethoxazole-resistant E. coli increased significantly, from 8.3% at discharge from the hospital to 16.1% 1 month later. For both populations, the numbers of E. coli isolated from oropharyngeal samples at the different times were too small to allow a comparison of resistance frequencies over time.
Effect of length of stay on antibiotic resistance in Escherichia coli during hospitalization.
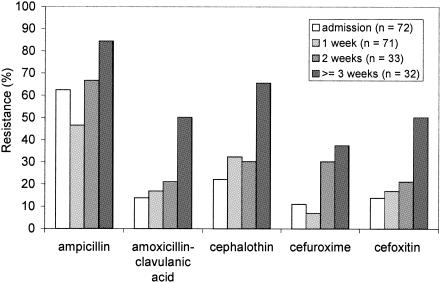
Resistance in E. coli strains isolated from oropharyngeal swabs was significantly associated with the length of stay in the ICU (by logistic regression, the P values were <0.001 for ampicillin, amoxicillin-clavulanic acid, cephalothin, cefuroxime, and cefoxitin) (Fig. 1). Similar results were found for E. coli strains isolated from rectal swabs that were taken weekly. Additionally, for rectal E. coli, the length of stay was also significantly associated with resistance to ceftazidime. However, we did not notice a significant trend of increasingly resistant E. coli strains with increasing lengths of stay among general ward patients.
FIG. 1.
Effect of length of stay on antibiotic resistance in Escherichia coli isolates from the oropharynxes of patients admitted to the intensive care unit.
Multidrug resistance of E. coli upon admission and during stay in ICU and general wards.
Upon admission to the ICU, 25 (14%) patients were colonized with at least one E. coli isolate that was resistant to one or more antibiotics. During stay in the ICU, the number increased to 50 (28%) patients. Resistance was most often observed against ampicillin, ampicillin and trimethoprim-sulfamethoxazole, or ampicillin and piperacillin (Table 4). Multidrug resistance (resistance to three or more antibiotics) was observed in five (3%) patients. Five E. coli strains, all from one patient, were resistant to all penicillins and cephalosporins and were producers of extended-spectrum beta-lactamases. During stay in the ICU, the number of patients colonized with multidrug-resistant E. coli increased significantly, to 28 (15%) (P < 0.001).
TABLE 4.
Frequency of common coresistance patterns in E. coli strains isolated on admission and during hospitalizationab
| Antibiotic(s) | Resistance in ICU (no. of patients [%])
|
Resistance in general ward (no. of isolates [%])
|
||
|---|---|---|---|---|
| Upon admission (n = 183) | During stay in ICUc (n = 183) | Upon admission (n = 228) | During stay on general wardc (n = 228) | |
| AMP | 12 (6.6) | 17 (9.3) | 10 (4.4) | 15 (6.6) |
| CEF | 3 (1.6) | |||
| SXT | 3 (1.6) | 4 (2.2) | 3 (1.3) | 3 (1.3) |
| AMP PIP | 5 (2.7) | 9 (4.9) | 7 (3.1) | 9 (3.9) |
| AMP SXT | 8 (4.4) | 10 (5.5) | 5 (2.2) | 14 (6.1) |
| AMP CEF | 2 (1.1) | 3 (1.6) | ||
| AMP CIP | 2 (1.1) | |||
| AMP PIP SXT | 2 (1.1) | 7 (3.8) | 8 (3.5) | 11 (4.8) |
| AMP PIP CEF | 2 (1.1) | 3 (1.3) | ||
| AMP AMC CEF FOX | 3 (1.6) | |||
| AMP AMC CEF FOX CXM | 5 (2.7) | |||
| AMP AMC CEF FOX CXM PIP | 2 (1.1) | |||
| AMP AMC CEF FOX CXM CAZ | 2 (1.1) | |||
For each location and time, resistance patterns are presented that occurred in at least 1% of the patients.
The antibiotics tested were ampicillin (AMP), piperacillin (PIP), amoxicillin-clavulanic acid (AMC), piperacillin-tazobactam (TZP), meropenem (MEM), cephalothin (CEF), cefuroxime (CXM), cefoxitin (FOX), ceftazidime (CAZ), cefepime (FEP), gentamicin (GEN), ciprofloxacin (CIP), and trimethoprim-sulfamethoxazole (SXT).
Including samples at discharge.
Upon admission to the general ward, 37 (16%) patients were colonized with at least one E. coli isolate that was resistant to one or more antibiotics. During stay on the general ward, the number increased to 44 (19%) patients. Resistance was most often observed against ampicillin, ampicillin and piperacillin, ampicillin and trimethoprim-sulfamethoxazole, or ampicillin, piperacillin, and trimethoprim-sulfamethoxazole (Table 4). Multidrug resistance was found in 17 (7%) patients and did not change during hospitalization.
Multidrug resistance of Enterobacter spp., Klebsiella spp., and P. aeruginosa during stay in the ICU and general wards.
During stay in the ICU, five (3%) patients were colonized with at least one Enterobacter sp. that was resistant to three or more antibiotics. An analysis of susceptibility patterns showed that almost half of these Enterobacter isolates showed combined resistance to cefuroxime, piperacillin, ceftazidime, piperacillin/tazobactam, and ciprofloxacin. Multidrug resistance in Klebsiella spp. was detected in two patients, whereas four patients carried multidrug-resistant P. aeruginosa.
During stay on the general wards, resistance towards three or more antibiotics was only detected for three Klebsiella spp. isolated from one patient.
Antibiotic use before, during, and after hospitalization.
The percentages of ICU and general ward patients receiving antibiotics during the 3 months preceding hospitalization were 23 and 25%, respectively (Table 1). Before hospitalization, the ICU and general ward population used 7 and 5 DDD/100 inhabitant days, respectively. The total use of antibiotics during stay in the ICU was 180 DDD/100 bed days. The use of ampicillin and piperacillin, amoxicillin/clavulanic acid and piperacillin/tazobactam, cephalosporins, expanded-spectrum cephalosporins, carbapenems, fluoroquinolones, and aminoglycosides represented 8%, 53%, 5%, 2%, 3%, 11%, and 5% of total consumption, respectively, in the ICU. After discharge from the ICU, 71 DDD/100 bed days was used in the general ward. The general ward population used 67 DDD/100 bed days during stay on the general ward. During the 3 months after discharge from the hospital, the general ward population consumed 7 DDD/100 inhabitant days, whereas the ICU population used 5 DDD/100 inhabitant days.
DISCUSSION
This study documents several interesting features of the colonization and resistance dynamics of aerobic gram-negative bacteria in the intestinal and oropharyngeal microfloras of patients admitted to ICUs and general wards in a tertiary care hospital.
Intestinal colonization rates.
Large differences in the prevalence of intestinal colonization by gram-negative bacteria were observed at different times. The lowest colonization rate was observed for patients during hospital stays both in the ICU and in the general ward population. Colonization rates increased after discharge from the hospital. Several factors may have contributed to the differences in gram-negative colonization rates. First, the use of antibiotics might have suppressed the gram-negative bacteria to concentrations below the detection limit (42). In that case, the results would reflect hospital practice. This hypothesis is supported by the fact that the colonization rates at different times and between the two populations were inversely related to the measured levels of antibiotic use. Thus, the higher the level of antibiotics used, the lower the gram-negative colonization rate. Secondly, either fresh stool specimens or rectal swabs were collected, and a bias may have been introduced at this point. For the ICU population, all patient samples upon admission to and at discharge from the ICU were collected with rectal swabs, whereas at discharge from the hospital, stool specimens were collected. For the general ward population, the percentages of cultures that were taken as rectal swabs upon admission and at discharge from the hospital were 77 and 45%, respectively. For both populations, stool specimens were collected 1 and 3 months after patients were discharged from the hospital. We collected these different specimens on the assumption that no differences in recovery exist between the two sampling methods (4). In a pilot study that was performed on healthy individuals prior to the present investigation, no differences in the predominant floras recovered from both types of specimens were found (data not shown). Although clear instructions for the collection of swabs from the rectum were given, one cannot exclude that swabs may occasionally have been taken from the perineum. Thirdly, the recovery of gram-negative bacteria from frozen specimens might have been suboptimal. However, the observations of Bonten et al. (4) do not support this theory. Other studies have also reported low colonization rates of gram-negative or gram-positive bacteria in hospitalized patients (6, 14, 30).
Oropharyngeal colonization rates.
For both patient populations, the prevalence of oropharyngeal colonization by gram-negative bacteria increased during hospitalization, and these rates were still increased 3 months after discharge from the hospital. This phenomenon has not been reported previously. We are unaware of any studies that have documented colonization rates with gram-negative bacteria after the discharge of patients from intensive care units and general wards. The very low colonization rate upon admission in general ward patients is consistent with the results of a recent study demonstrating that healthy individuals rarely carry oropharyngeal gram-negative bacteria (32). Previously, the severity of underlying disease, mechanical ventilation, and the presence of nasogastric feeding tubes were associated with oropharyngeal gram-negative bacterial colonization (26, 31, 44). Differences in colonization rates between the ICU and general ward populations and the high colonization rate in specimens obtained during stay in the ICU confirm these earlier findings.
Species distribution.
A change in species distribution among the dominant microfloras was observed during hospitalization. Suppression of the normal intestinal flora was more pronounced in the ICU population than in the general ward population. The normal intestinal and oropharyngeal microflora was frequently replaced with Klebsiella spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa. Previous studies have also found that hospitalization has an impact on the species distribution of the aerobic gram-negative fecal flora (23, 24, 39, 40).
Prevalence of resistance.
For this study, we analyzed the resistance data from a population perspective. A limited number of changes in the prevalence of resistance were found among the different time points. The percentage of ICU patients colonized with ampicillin- and/or cephalothin-resistant fecal E. coli was significantly increased at discharge from the hospital. These frequencies did not change during the 3 months after discharge. In The Netherlands, resistance rates to most antibiotics are among the lowest in Europe (13). Ampicillin/amoxicillin, piperacillin, cephalosporins, and trimethoprim-sulfamethoxazole are commonly used antibiotics in the hospital and/or the community (18, 43). Amoxicillin is the most frequently used first-line antibiotic in Dutch primary health care (43). The cephalosporin cefazolin is the drug of choice for surgical prophylaxis (46). The persistence of ampicillin and cephalothin resistance in fecal E. coli strains in ICU patients might have consequences for the empirical regimens and surgical prophylaxis used by these patients up to 3 months after discharge. For the general ward population, no significant differences in resistance frequencies were found during hospitalization. In a previous study, we assessed the prevalence of resistance of E. coli in the intestinal floras of surgical patients upon admission to the hospital, at discharge, and 1 and 6 months after discharge. We found no changes between the different time points (6). As far as we know, no other recent study has measured antibiotic resistance in the intestinal or oropharyngeal microflora during and after hospitalization.
It might be interesting to look for changes in resistance patterns at the level of individual patients. The observed increase in multidrug resistance during stay in the ICU and the increase in resistance with increasing lengths of stay for these patients are supportive of this approach. If changes in resistance do occur at the level of individual patients, then an examination of risk factors for the acquisition of antibiotic resistance can be performed (37). It is likely that the more frequent use of antibiotics in the ICU population results in the development of resistance at the level of individual patients (19).
Selection bias.
For the present study, different numbers of patients were present at the various time points examined. There is some risk of bias due to the differential loss of follow-up of sicker individuals. In a previous study, it was demonstrated that as the length of the ICU stay increased, the incidence of nosocomial infections and antibiotic resistance also increased (1). The observed increasing resistance with increasing length of stay in the ICU may therefore be due to the selection of sicker individuals who use more antibiotics. Three months after discharge, specimens were collected from 61% of patients with an ICU stay shorter than 12 days and from 38% of patients with a stay of more than 12 days. Moreover, 44 ICU patients and 133 general ward patients could be tracked through the entire project (all specimens were taken appropriately). We recalculated the resistance rates for these subpopulations. For both populations, the same “trends” were observed as those for the entire study population. Therefore, the selection bias appears to be limited.
External validity.
Whether the results of this study are representative of the conditions in other hospitals mainly depends on the studied population and the effectiveness of infection control measures taken to prevent cross-colonization. We suppose that most of the observed trends will be universal and can be extrapolated to other hospitals. However, we expect changes to be more extreme with higher rates of consumption of antibiotics. Our findings suggest only modest changes, but this may be due to the stringent usage of antibiotics and intensive infection control measures used in our hospital and in Dutch hospitals in general (18, 43, 45).
For the present study, only patients with a length of stay of 5 days or longer were included, since we hypothesized that these patients were at risk for the acquisition of resistant strains. In addition, patients with human immunodeficiency virus infection, tuberculosis, and cystic fibrosis were excluded. It is known that these patients use large volumes of antibiotics and are at high risk for the development of resistant microorganisms. The attribution of this relatively small group of patients to the dissemination of resistant stains into the community by the total hospital population was not taken into account.
In summary, the results of this study show that in patients in the ICU as well as in general ward patients, intestinal colonization rates with gram-negative bacteria are low during hospitalization and increase after discharge from the hospital. Suppression of the normal intestinal microflora is more pronounced for ICU patients than for general ward patients and is reversible. Oropharyngeal colonization rates increase during hospitalization and remain high during the 3 months after discharge from the hospital. From a population perspective, the risk of dissemination of resistant gram-negative bacteria in the community through hospitalized patients appears to be low for general ward patients but is noticeably higher among ICU patients. Antimicrobial resistance that emerges during the hospitalization of ICU patients may persist after discharge for at least 3 months. Whether bacterial, host, or community determinants are responsible for this phenomenon remains to be determined.
Acknowledgments
This study was financially supported by a grant received from the Revolving Fund from the Medical Research Advisory Board of the Erasmus MC.
We thank all patients who participated in this study and the medical staffs and nurses of the participating units who made this study possible. We thank Roel Verkooyen and Wil Goessens for their useful comments and Gonny van Eijken for technical assistance.
REFERENCES
- 1.Alberti, C., C. Brun-Buisson, H. Burchardi, C. Martin, S. Goodman, A. Artigas, A. Sicignano, M. Palazzo, R. Moreno, R. Boulme, E. Lepage, and R. Le Gall. 2002. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 28:108-121. [DOI] [PubMed] [Google Scholar]
- 2.Archibald, L., L. Phillips, D. Monnet, J. E. McGowan, Jr., F. Tenover, and R. Gaynes. 1997. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin. Infect. Dis. 24:211-215. [DOI] [PubMed] [Google Scholar]
- 3.Austin, D. J., and R. M. Anderson. 1999. Studies of antibiotic resistance within the patient, hospitals and the community using simple mathematical models. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:721-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonten, M. J., C. Nathan, and R. A. Weinstein. 1997. Recovery of nosocomial fecal flora from frozen stool specimens and rectal swabs: comparison of preservatives for epidemiological studies. Diagn. Microbiol. Infect. Dis. 27:103-106. [DOI] [PubMed] [Google Scholar]
- 5.Bonten, M. J., and R. A. Weinstein. 1996. The role of colonization in the pathogenesis of nosocomial infections. Infect. Control Hosp. Epidemiol. 17:193-200. [DOI] [PubMed] [Google Scholar]
- 6.Bruinsma, N., P. M. Filius, A. E. van den Bogaard, S. Nys, J. Degener, H. P. Endtz, and E. E. Stobberingh. 2003. Hospitalization, a risk factor for antibiotic-resistant Escherichia coli in the community? J. Antimicrob. Chemother. 51:1029-1032. [DOI] [PubMed] [Google Scholar]
- 7.Bukholm, G., T. Tannaes, A. B. Kjelsberg, and N. Smith-Erichsen. 2002. An outbreak of multidrug-resistant Pseudomonas aeruginosa associated with increased risk of patient death in an intensive care unit. Infect. Control Hosp. Epidemiol. 23:441-446. [DOI] [PubMed] [Google Scholar]
- 8.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127-1132. [DOI] [PubMed] [Google Scholar]
- 9.Cooke, E. M., S. Ewins, and R. A. Shooter. 1969. Changing faecal population of Escherichia coli in hospital medical patients. Br. Med. J. 4:593-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove, S. E., and Y. Carmeli. 2003. The impact of antimicrobial resistance on health and economic outcomes. Clin. Infect. Dis. 36:1433-1437. [DOI] [PubMed] [Google Scholar]
- 11.Datta, N. 1969. Drug resistance and R factors in the bowel bacteria of London patients before and after admission to hospital. Br. Med. J. 2:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorsey, G., H. T. Borneo, S. J. Sun, J. Wells, L. Steele, K. Howland, F. Perdreau-Remington, and D. R. Bangsberg. 2000. A heterogeneous outbreak of Enterobacter cloacae and Serratia marcescens infections in a surgical intensive care unit. Infect. Control Hosp. Epidemiol. 21:465-469. [DOI] [PubMed] [Google Scholar]
- 13.EARSS. Accessed 18 March 2005. [Online.] http://www.earss.rivm.nl.
- 14.Endtz, H. P., N. van den Braak, A. van Belkum, J. A. Kluytmans, J. G. Koeleman, L. Spanjaard, A. Voss, A. J. Weersink, C. M. Vandenbroucke-Grauls, A. G. Buiting, A. van Duin, and H. A. Verbrugh. 1997. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in The Netherlands. J. Clin. Microbiol. 35:3026-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filius, P. M. G., D. van Netten, P. J. Roovers, A. G. Vulto, I. C. Gyssens, H. A. Verbrugh, and H. P. Endtz. 2003. Comparative evaluation of three chromogenic agars for detection and rapid identification of aerobic gram-negative bacteria in the normal intestinal microflora. Clin. Microbiol. Infect. 9:912-918. [DOI] [PubMed] [Google Scholar]
- 16.Fluit, A. C., M. E. Jones, F. J. Schmitz, J. Acar, R. Gupta, and J. Verhoef. 2000. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin. Infect. Dis. 30:454-460. [DOI] [PubMed] [Google Scholar]
- 17.Gastmeier, P., K. Groneberg, K. Weist, and H. Ruden. 2003. A cluster of nosocomial Klebsiella pneumoniae bloodstream infections in a neonatal intensive care department: identification of transmission and intervention. Am. J. Infect. Control 31:424-430. [DOI] [PubMed] [Google Scholar]
- 18.Goossens, H., M. Ferech, R. Vander Stichele, and M. Elseviers. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579-587. [DOI] [PubMed] [Google Scholar]
- 19.Harbarth, S., A. D. Harris, Y. Carmeli, and M. H. Samore. 2001. Parallel analysis of individual and aggregated data on antibiotic exposure and resistance in gram-negative bacilli. Clin. Infect. Dis. 33:1462-1468. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 21.Johanson, W. G., Jr., A. K. Pierce, J. P. Sanford, and G. D. Thomas. 1972. Nosocomial respiratory infections with gram-negative bacilli. The significance of colonization of the respiratory tract. Ann. Intern. Med. 77:701-706. [DOI] [PubMed] [Google Scholar]
- 22.Johanson, W. G., A. K. Pierce, and J. P. Sanford. 1969. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N. Engl. J. Med. 281:1137-1140. [DOI] [PubMed] [Google Scholar]
- 23.LeFrock, J. L., C. A. Ellis, and L. Weinstein. 1979. The impact of hospitalization on the aerobic fecal microflora. Am. J. Med. Sci. 277:269-274. [DOI] [PubMed] [Google Scholar]
- 24.LeFrock, J. L., C. A. Ellis, and L. Weinstein. 1979. The relation between aerobic fecal and oropharyngeal microflora in hospitalized patients. Am. J. Med. Sci. 277:275-280. [DOI] [PubMed] [Google Scholar]
- 25.Le Gall, J. R., S. Lemeshow, and F. Saulnier. 1993. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957-2963. [DOI] [PubMed] [Google Scholar]
- 26.Leibovitz, A., M. Dan, J. Zinger, Y. Carmeli, B. Habot, and R. Segal. 2003. Pseudomonas aeruginosa and the oropharyngeal ecosystem of tube-fed patients. Emerg. Infect. Dis. 9:956-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leistevuo, T., P. Toivonen, M. Osterblad, M. Kuistila, A. Kahra, A. Lehtonen, and P. Huovinen. 1996. Problem of antimicrobial resistance of fecal aerobic gram-negative bacilli in the elderly. Antimicrob. Agents Chemother. 40:2399-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy, S. B., B. Marshall, S. Schluederberg, D. Rowse, and J. Davis. 1988. High frequency of antimicrobial resistance in human fecal flora. Antimicrob. Agents Chemother. 32:1801-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipsitch, M., and B. R. Levin. 1997. The population dynamics of antimicrobial chemotherapy. Antimicrob. Agents Chemother. 41:363-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.London, N., R. Nijsten, A. E. van den Bogaard, and E. Stobberingh. 1993. Antibiotic resistance of faecal Enterobacteriaceae isolated from healthy volunteers, a 15-week follow-up study. J. Antimicrob. Chemother. 32:83-91. [DOI] [PubMed] [Google Scholar]
- 31.Mobbs, K. J., H. K. van Saene, D. Sunderland, and P. D. Davies. 1999. Oropharyngeal gram-negative bacillary carriage in chronic obstructive pulmonary disease: relation to severity of disease. Respir. Med. 93:540-545. [DOI] [PubMed] [Google Scholar]
- 32.Mobbs, K. J., H. K. van Saene, D. Sunderland, and P. D. Davies. 1999. Oropharyngeal gram-negative bacillary carriage: a survey of 120 healthy individuals. Chest 115:1570-1575. [DOI] [PubMed] [Google Scholar]
- 33.Monnet, D. L., L. K. Archibald, L. Phillips, F. C. Tenover, J. E. McGowan, Jr., and R. P. Gaynes. 1998. Antimicrobial use and resistance in eight U.S. hospitals: complexities of analysis and modeling. Intensive Care Antimicrobial Resistance Epidemiology Project and National Nosocomial Infections Surveillance System Hospitals. Infect. Control Hosp. Epidemiol. 19:388-394. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. Fourteenth informational supplement. NCCLS document M100-S14. NCCLS, Wayne, Pa.
- 35.NNIS System. 2003. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 31:481-498. [DOI] [PubMed] [Google Scholar]
- 36.Osterblad, M., A. Hakanen, R. Manninen, T. Leistevuo, R. Peltonen, O. Meurman, P. Huovinen, and P. Kotilainen. 2000. A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora. Antimicrob. Agents Chemother. 44:1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson, D. L. 2002. Looking for risk factors for the acquisition of antibiotic resistance: a 21st-century approach. Clin. Infect. Dis. 34:1564-1567. [DOI] [PubMed] [Google Scholar]
- 38.Pfaller, M. A., R. N. Jones, G. V. Doern, and K. Kugler. 1998. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997). Antimicrob. Agents Chemother. 42:1762-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack, M., P. Charache, R. E. Nieman, M. P. Jett, J. A. Reimhardt, and P. H. Hardy, Jr. 1972. Factors influencing colonisation and antibiotic-resistance patterns of gram-negative bacteria in hospital patients. Lancet ii:668-671. [DOI] [PubMed] [Google Scholar]
- 40.Rose, H. D., and J. Schreier. 1968. The effect of hospitalization and antibiotic therapy on the gram-negative fecal flora. Am. J. Med. Sci. 255:228-236. [DOI] [PubMed] [Google Scholar]
- 41.Shaw, E. J., N. Datta, G. Jones, F. M. Marr, and W. J. Froud. 1973. Effect of stay in hospital and oral chemotherapy on the antibiotic sensitivity of bowel coliforms. J. Hyg. (London) 71:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 43.SWAB. 2004. NethMap 2004—consumption of antibiotic agents and antibiotic resistance among medically important bacteria in The Netherlands. [Online.] http://www.swab.nl.
- 44.Thuong, M., K. Arvaniti, R. Ruimy, P. de la Salmoniere, A. Scanvic-Hameg, J. C. Lucet, and B. Regnier. 2003. Epidemiology of Pseudomonas aeruginosa and risk factors for carriage acquisition in an intensive care unit. J. Hosp. Infect. 53:274-282. [DOI] [PubMed] [Google Scholar]
- 45.van den Broek, P. J. 1999. National guidelines for infection control in The Netherlands. J. Hosp. Infect. 43(Suppl.):S297-S299. [DOI] [PubMed] [Google Scholar]
- 46.van Kasteren, M. E., I. C. Gyssens, B. J. Kullberg, H. A. Bruining, E. E. Stobberingh, and R. J. Goris. 2000. Optimizing antibiotics policy in the Netherlands. V. SWAB guidelines for perioperative antibiotic prophylaxis. Foundation Antibiotics Policy Team. Ned. Tijdschr. Geneeskd. 144:2049-2055. [PubMed] [Google Scholar]
- 47.W.H.O. Collaborating Centre for Drug Statistics Methodology (Norway) (2002). 2003. Guidelines for ATC-classification and DDDs assignment. W.H.O. Collaborating Centre, Oslo, Norway.