Abstract
The biological activity of a new intravenous (i.v.) preparation of human vaccinia immune globulin (VIGIV) was evaluated in two mouse models of vaccinia virus (VV) infection. In a mouse tail lesion model, female CD-1 mice were inoculated i.v. with 7 × 104 PFU of VV to produce >10 lesions per tail 8 days later. In a mouse lethality model, female severe combined immunodeficient (SCID) mice were inoculated i.v. with 3 × 104 PFU of VV to produce 100% mortality within 45 days. The ability of VIGIV to reduce tail lesion formation in CD-1 mice and mortality in SCID mice was determined by (i) pretreatment of a lethal VV dose with VIGIV prior to i.v. inoculation into SCID mice and (ii) i.v. administration of VIGIV to CD-1 and SCID mice the day before and up to 8 days after VV infection. VIGIV reduced the proportion of CD-1 mice with >10 tail lesions in a dose-related manner when VIGIV was given 1 day before and up to 1 day after VV inoculation. The pretreatment of VV with VIGIV prolonged survival and decreased mortality. VIGIV (100 and 400 mg/kg) prolonged survival when given up to 4 days after VV inoculation, and the 400-mg/kg dose reduced the mortality rate by 80% when given the day before or immediately after VV inoculation. The biological activity of VIGIV was demonstrated in both the immunocompetent and immunocompromised murine models. The timing of treatment relative to VV inoculation appeared to be important for the demonstration of VIGIV's biological activity.
The renewed smallpox vaccination program for Department of Defense (DoD) personnel, first-responders, and healthcare and laboratory workers has increased the demand for the availability of therapeutic agents for the treatment of possible severe adverse reactions to smallpox vaccination. Adverse reactions to the currently licensed smallpox vaccine, Dryvax, have been well established and include accidental implantation involving extensive lesions, eczema vaccinatum, generalized vaccinia, and progressive vaccinia. The characteristics of these complications have been reviewed extensively (4). Individuals with eczema or atopic dermatitis and those undergoing immunosuppressive therapy are especially vulnerable to complications of smallpox vaccination and are generally excluded from vaccination unless they are exposed to the disease (27). The Centers for Disease Control (CDC), the Food and Drug Administration (FDA), state health departments, and the DoD are continually monitoring individuals for adverse reactions to the smallpox vaccine (6). New, potentially safer smallpox vaccines are currently being developed to mitigate this problem. However, while these new vaccines are being developed, individuals receiving the current smallpox vaccine, especially those with eczema or atopic dermatitis, cardiac myopathies, or immunosuppression or women who are pregnant, continue to be at risk (13).
Vaccinia immune globulin (VIG) and cidofovir are currently the only two investigational products recommended by the CDC for the treatment of severe complications related to smallpox vaccination (12, 27). Cidofovir is only recommended when VIG is not efficacious. An intramuscular (i.m.) preparation of VIG (VIGIM) has been available since the 1950s; however, relatively large dose volumes (42 ml for a 70-kg human) are required for treatment, so it must be delivered in divided doses over an extended time period. Although VIGIM is used extensively, its effectiveness has not been evaluated in a controlled study. The product has reverted to investigational new drug status due to an unexplained color change associated with long-term storage (4). Additionally, the need to replenish dwindling supplies has renewed efforts to develop new preparations of VIG with improved tolerabilities. To this end, the DoD has developed an intravenous (i.v.) preparation of human VIG (VIGIV) to mitigate potential adverse reactions following smallpox vaccination.
The new VIGIV product is a sterile liquid human immunoglobulin containing a high titer of neutralizing antibody to vaccinia virus (VV). It offers the advantage of i.v. administration, along with potential improvements in stability and pharmacokinetic properties. Safety and pharmacokinetic evaluations of VIGIV have been investigated in a phase 1 clinical trial (12); however, since the incidence of adverse reactions to smallpox vaccination is low and the deliberate exposure of human subjects to potentially health-threatening levels of VV is unethical, a direct evaluation of the VV-neutralizing activity of VIGIV in humans was not possible. Therefore, an evaluation of the biological activity of VIGIV in suitable animal models was required.
Although there are currently no animal models that completely mimic the complications associated with smallpox vaccination in humans, multiple animal models of orthopoxvirus infections have been developed for a variety of species. These models have been used extensively to demonstrate the modulation of VV infection by several antiviral compounds (7, 8, 19-21, 24). Mice are used most frequently, although rabbit and nonhuman primate models have also been developed. The most extensively used murine models are the mouse tail lesion model (MTLM) and the mouse lethality model (MLM). The MTLM developed by Boyle et al. (3) exhibits a self-limiting, nonlethal infection characterized by the formation of lesions over the entire tail following the administration of VV via the tail vein. The MLM is based on a lethal infection of severe combined immunodeficient (SCID) mice with VV. A 100% incidence of mortality is generally observed within 30 days of VV infection in these mice. Several antiviral agents have been shown to reduce tail lesion numbers or prolong survival in these models (19-21). The MLM model was also previously used to demonstrate the in vivo VV-neutralizing activities of licensed intravenous immunoglobulin products and an FDA Center for Biologics Evaluation and Research interim reference VIG (10). These models offer several advantages for the purposes of the present investigation, including (i) a demonstrated responsiveness to antiviral agents, (ii) the ability to assess VIGIV's biological activity in both immunocompetent and immunocompromised systems, and (iii) the presence of quantifiable end points (tail lesion counts and mortality), which allow for statistical evaluations of the results.
For the present study, we used in vivo and in vitro treatment regimens to evaluate the biological activity of VIGIV in murine models of VV infection. The results of these studies were used in conjunction with pharmacokinetic and safety data generated in human trials to support the approval of a Biologics Licensure Application from the FDA. The ability of VIGIV to significantly reduce the tail lesion number or mortality rate compared to that observed in controls at specific dose levels and administration times was determined with the immunocompetent and immunocompromised murine models, respectively. While this study is not a direct predictor of human efficacy, the results demonstrate the ability of VIGIV to neutralize VV activity when administered in vivo and provide evidence for the likelihood of success of VIGIV as an effective treatment for severe adverse reactions to smallpox vaccination.
MATERIALS AND METHODS
Mice.
Female CD-1 mice purchased from Charles River Laboratories (Portage, Mich.) were used for the evaluation of VIGIV's biological activity in the MTLM. At the time of dosing, the mice were 21 to 26 days old and weighed between 10 and 17 g. They were quarantined for 4 days prior to dosing and were housed in groups of five per cage over corncob bedding in conventional polycarbonate cages with wire tops for the duration of the study. All animals were provided Harlan Teklad Certified Rodent Diet 2018C and fresh tap water ad libitum.
Female SCID mice purchased from Charles River Laboratories (Raleigh, N.C.) were used for the evaluation of VIGIV's biological activity in the MLM. At the time of dosing, the mice were approximately 6 weeks old and weighed between 14 and 21 g. The animals were acclimated for 7 days prior to dosing. SCID mice were provided Harlan Teklad Irradiated Rodent Diet T.2918, sterilized by the supplier by the use of irradiation, ad libitum. Sterilized bottled water was supplied ad libitum. The mice were housed in groups of five per cage over sterilized shredded paper bedding in Tecniplast Sealsafe cages supplied with HEPA-filtered air for the duration of the study.
General procedures for animal care and housing were done in accordance with the Guide for the Care and Use of Laboratory Animals (16a). All animals were housed in environmentally controlled rooms with at least 10 air changes per hour. The temperature was maintained at 18 to 23°C, and the relative humidity was generally 50% ± 20%, with a 12-hour light/dark cycle. The Midwest Research Institute Institutional Animal Care and Use Committee and the United States Army Medical Research and Materiel Command Animal Care and Use Review Office approved the use of mice in this study. At the end of the designated study periods, surviving mice were euthanized by CO2 asphyxiation.
Virus.
The Dryvax vaccine manufactured by Wyeth Laboratories (lot no. 4008284) was kindly provided by the CDC. Dryvax was chosen as the source of VV since it is currently the only licensed product available for smallpox vaccination and thus serves as the de facto standard for vaccinia virus-related studies. The vaccine was reconstituted in Hanks balanced salt solution (HBSS) and diluted to provide estimated dose levels in a volume of 0.2 ml per dose. The estimated dose was based on the stated titer of 1.0 × 108 PFU/ml. Since the stability of Dryvax prepared in this manner was unknown, exploratory studies were performed to assess its temporal viability following reconstitution. Titer confirmations performed in conjunction with model development suggested a decline in VV viability over time (data not shown). Therefore, except for during model development, reconstitution and dilutions were performed each day of inoculation. Prepared doses were maintained in sterile vials and stored at 2 to 8°C until use.
VIGIV.
VIGIV (DVC lot no. VIG-4) was provided as a sterile, preservative-free, isotonic solution containing 50 mg/ml immunoglobulin stabilized with 5% sucrose and 1% human serum albumin. The process for the production of VIGIV was identical to that for the production of two other commercial human immunoglobulin products, cytomegalovirus immune globulin for i.v. use and respiratory syncytial virus immune globulin for i.v. use, except for an additional nanofiltration step to remove potential viral contaminants. Licensed plasmapheresis centers collected source plasma from U.S. military personnel between 2 and 78 weeks after a booster vaccination with Dryvax (Wyeth Laboratories, Inc.) (12). The VIGIV lot used for these experiments had a 50% plaque reduction neutralization titer of 4,954. VIGIV was diluted in sterile HBSS to provide dose levels of approximately 400 and 100 mg/kg of body weight in a volume of 0.2 ml. Control solutions were prepared by diluting a sterile solution containing 5% sucrose and 1% human serum albumin in HBSS in the same manner as the high-dose level of VV. Doses were transferred to sterile vials and stored at 2 to 8°C until use.
Evaluation of VIGIV's biological activity.
An initial series of studies were conducted to develop and determine the reproducibility of the MTLM and MLM chosen for the evaluation of VIGIV's biological activity. The design and results of these studies are described in Results. Based on the results of these studies, for the evaluation of the biological activity of VIGIV, we used female CD-1 mice weighing 10 to 17 g and inoculated i.v. with 7 × 104 PFU as the qualified MTLM model and approximately 6-week-old female SCID mice weighing 15 to 21 g and inoculated i.v. with 3 × 104 PFU VV as the qualified MLM model.
In vivo activity in the MTLM.
The MTLM was used to determine the ability of VIGIV to reduce the number of tail lesions caused by VV. Groups of 25 mice were inoculated with 7 × 104 PFU VV on day 0. Mice in designated groups were administered a single tail vein injection of VIGIV or control solution on day −1 (1 day before), 0 (immediately following), 1, 2, 4, or 6 relative to VV inoculation. Approximate dose levels of 100 mg/kg and 400 mg/kg VIGIV were administered by the tail vein injection of 1.6 mg or 6.4 mg of VIGIV in a volume of 0.2 ml. VIGIV dosing was based on an assumed body weight of 16 g; dosing was not adjusted for individual variations in mouse body weights. The calculated average dosages for mice used in the mouse tail lesion model were 115 ± 9 and 462 ± 37 mg/kg for the 100- and 400-mg/kg target dosage groups, respectively. On day 8, the tails were stained with a mixture of 1.0% fluorescein and 0.5% methylene blue to enhance the visibility of lesions and thereby facilitate counting.
In vitro activity in the MLM.
To more fully assess the biological activity of VIGIV, it was important that we evaluate its direct virus-neutralizing potential; therefore, we tested the ability of VIGIV to neutralize VV in vitro in the MLM. Various amounts of VIGIV (0, 0.5, 1.5, and 5.0 mg) were incubated with 3 × 104 PFU VV for 1 h at room temperature. The 0-mg dose was prepared by diluting control material with HBSS in the same manner as the 5.0-mg VIGIV dose. Preparations of the same amounts of VIGIV incubated in HBSS served as controls. A preparation of 3 × 104 PFU diluted in HBSS was used as the virus control cohort. At the end of the 1-h incubation period, groups of five SCID mice were injected via the tail vein with the VIGIV/VV, VIGIV/HBSS, or virus control mixture. The mice were observed daily for death or morbidity for 56 days following VV inoculation. During this period, the day of death was recorded for each mouse that died.
In vivo activity in the MLM.
The ability of VIGIV to reduce the mortality associated with VV administration to SCID mice was evaluated by using the MLM. Groups of five SCID mice were inoculated with a lethal dose of VV (3 × 104 PFU) on day 0. Mice were given a single i.v. injection of VIGIV or control solution on day −1 (1 day before), 0 (immediately following), 1, 2, 4, 6, or 8 relative to VV inoculation. Approximate dose levels of 100 mg/kg and 400 mg/kg VIGIV were administered by the injection of 2.0 mg or 8.0 mg of VIGIV in a volume of 0.2 ml. VIGIV dosing was based on a mean body weight of 20 g; dosing was not adjusted for individual variations in mouse body weights. The mice were observed daily for death or morbidity for 49 days following VV inoculation. During this period, the day of death was recorded for each mouse that died.
Statistics.
For the MTLM, the proportion of mice with >10 tail lesions 8 days after VV inoculation was used as the outcome measure for statistical assessments of differences between the groups by chi-square analysis. Survival analysis was used to determine if VIGIV treatment resulted in a survival benefit in the MLM. The statistics were based on the day of death for the groups at each dose level and on the time of VIGIV administration compared to the day of death recorded for the control group with the same dose timing. The product-limit (Kaplan-Meier) method of survival analysis was used to determine mean survival times and to test for differences between treatment and control groups. The day of death for those animals surviving throughout the study was assumed to be the termination day of the study. The proportion of mice that died during the observation period after VV inoculation was also used as an outcome measure for statistical analyses. Chi-square analysis and Fisher's exact test were used to determine statistically significant differences. The Fisher's exact test probability levels are reported since this test is more appropriate when sample sizes are small. A statistical criterion of probability (P) of <0.05 was used to indicate significant differences in the proportions of deaths between the treated and control groups.
RESULTS
MTLM development and qualification.
An initial series of studies was conducted to develop and qualify the MTLM. Early development studies evaluated the dose of VV required to produce approximately 15 to 20 lesions per tail 8 days after i.v. inoculation of CD-1 mice via the tail vein. Groups of 25 mice, weighing 20 to 29 g each, were inoculated with 101, 102, 103, 104, or 105 PFU VV in 0.2 ml of HBSS. A group of control mice were inoculated with 0.2 ml of HBSS via i.v. injection. The appearance of the tail lesions and the absolute tail lesion counts, up to and including 28 lesions per tail, were recorded for each mouse on days 6, 7, 8, 9, and 10 following inoculation with VV. Higher counts were considered too numerous to count. General observations indicated that tail lesions began to appear approximately 6 days after VV inoculation, with lesion formation progressing through day 8. Thereafter, tails developed a scaly appearance and the enumeration of lesions was difficult. No lesions were observed in the control group. Very few mice inoculated with 104 PFU VV or less developed >10 lesions per tail. In contrast, mice inoculated with 105 PFU VV developed lesions by day 6 that were too numerous to count. Thus, the desired target end point of 15 to 20 lesions per tail was not achieved at any VV dose level. However, the results suggested that this end point might be achieved by using doses between 104 and 105 PFU.
A second model development study was conducted using VV inocula clustered around the 104 to 105 PFU dose range. Groups of 25 mice, weighing 21 to 29 g each, were inoculated i.v. with 5 × 103, 1 × 104, 2.5 × 104, 5 × 104, or 7.5 × 104 PFU VV. A group of control mice was given 0.2 ml of HBSS via i.v. injection. The mice were observed for 10 days. Again, the desired number of 15 to 20 lesions per tail was not achieved at any dose level. Most mice failed to display >10 lesions per tail at any dose level. No lesions were observed in the control group.
Since previously published studies suggested that body weight influences tail lesion formation (3), a third study was conducted using smaller mice. Groups of 10 mice, weighing 10 to 16 g each, were inoculated i.v. with 1 × 103, 1 × 104, 3 × 104, or 7 × 104 PFU VV. Eight days after inoculation, mice in the groups receiving 3 × 104 and 7 × 104 PFU had average lesion numbers within the desired target range of 15 to 20 lesions per tail. A caveat to this observation was that multiple mice in these groups could not be included in the calculation because they had lesion numbers that were too numerous to count (>28). As a result, the calculated average tail lesion number was artificially low. To mitigate this problem, we used the number of animals with tail lesions numbering above and below a certain threshold as an alternative end point. With an arbitrary threshold of 10 lesions per tail and the inclusion of mice with tail lesions too numerous to count, 90% and 70% of the mice in the cohorts receiving 3 × 104 and 7 × 104 PFU, respectively, had >10 lesions per tail. Only 40% of the mice in the cohort receiving 104 PFU had >10 lesions per tail, and no mice in the cohort receiving 103 PFU had >10 lesions per tail.
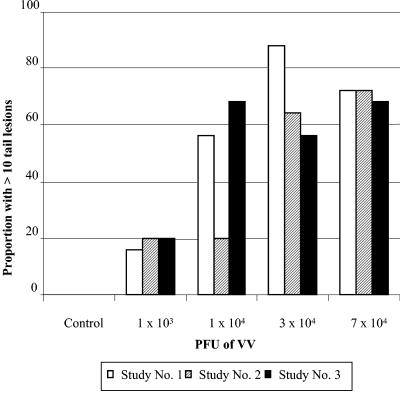
Finally, three repetitions were performed to determine the final parameters and reproducibility of the MTLM that would be used to evaluate VIGIV's biological activity. For each repetition, female CD-1 mice weighing 10 to 16 g were inoculated i.v. in groups of 25 with 1 × 103, 1 × 104, 3 × 104, or 7 × 104 PFU VV. A group of control mice was given 0.2 ml of HBSS via i.v. tail vein injection. No lesions were observed in the control group. Tail lesions were counted for each mouse on day 8. Since many of the mice developed lesions that were too numerous to count, a determination of the average number of lesions per tail was not used as an end point because the average could not be calculated accurately. Therefore, the proportion of mice with >10 tail lesions was used as the outcome measure for a statistical analysis to test for differences in the occurrence of tail lesions based on dose level and study repetition. Figure 1 shows the relationship between dose level and study repetition in terms of the occurrence of >10 tail lesions on day 8. A dose-related increase in the proportion of mice with >10 tail lesions was observed (P < 0.001). On day 8, there were no significant differences between the three study repetitions in the proportions of mice with >10 tail lesions given 7 × 104 PFU. The 7 × 104 PFU dose was used for subsequent evaluations of VIGIV's biological activity.
FIG. 1.
Effect of VV dose on tail lesion formation in CD-1 mice. Tail lesions were counted 8 days after inoculation with VV. A dose-related increase in the proportion of mice with >10 tail lesions was observed (P < 0.001 by chi-square analysis). There were no significant differences among the three study repetitions in the proportions of mice given 7 × 104 PFU VV that developed >10 tail lesions.
MLM development and qualification.
The initial study for MLM development used groups of five female 6-week-old SCID mice inoculated intraperitoneally (i.p.) with 1 × 101, 5 × 101, 1 × 102, 5 × 102, 1 × 103, 5 × 103, 1 × 104, or 1 × 105 PFU VV. A control cohort was inoculated i.p. with 0.2 ml of sterile HBSS. The mice were observed for 49 days, at which time surviving mice were euthanized. No animals died by day 49 in this study. Therefore, a second study was conducted to evaluate the lethality of i.p. administration at a higher VV dose as well as following i.v. administration. Groups of five female SCID mice were inoculated i.p. with 1 × 106 PFU VV or i.v. with 5 × 102, 1 × 103, or 1 × 104 PFU VV. The mice were observed daily for death or morbidity for 35 days following VV inoculation. During this period, the day of death was recorded for each mouse that died. The remaining mice were euthanized on day 35. The 106 PFU i.p. inoculum resulted in 60% mortality by day 35. The 102, 103, and 104 PFU i.v. inocula resulted in 40%, 40%, and 60% mortality, respectively, by day 35. Deaths occurred between days 25 and 35.
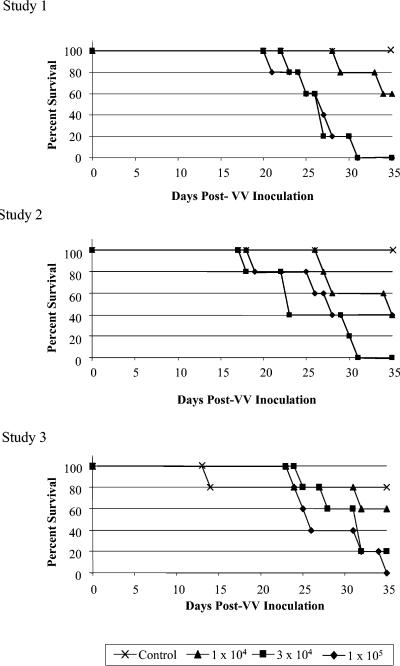
Three repeat studies were performed to determine the final parameters and reproducibility of the MLM that would be used to evaluate VIGIV's biological activity. Groups of five female 6-week-old SCID mice were inoculated i.v. with 1 × 104, 3 × 104, or 1 × 105 PFU VV. Mice in the control group received 0.2 ml of HBSS. For each study repetition, the mice were observed daily for death or morbidity for 35 days following VV inoculation. During this period, the day of death was recorded for each mouse that died. One mouse in a control cohort for one of the repetitions died unexpectedly on day 14, due to unknown causes. Statistical analyses were conducted to determine whether there were differences in mortality between both the dose levels and the studies. The proportions of mice that died in each group differed significantly among the four dose levels (P < 0.001). However, a comparison of the proportions of mice that died after inoculation with the two highest dose levels indicated that there were no significant differences either between the two dose levels or across the three studies (Fig. 2). The 3 × 104 PFU dose was used for subsequent evaluations of VIGIV's biological activity in the MLM.
FIG. 2.
Effect of VV dose on mortality in SCID mice. Mice were inoculated i.v. with VV on day 0, and mortality was assessed for a period of 35 days. A comparison of the proportions of mice that died from the groups receiving 3 × 104 and 1 × 105 PFU VV indicated that there were no significant differences, either between the two dose levels or across the three studies.
In vivo activity in the mouse tail lesion model.
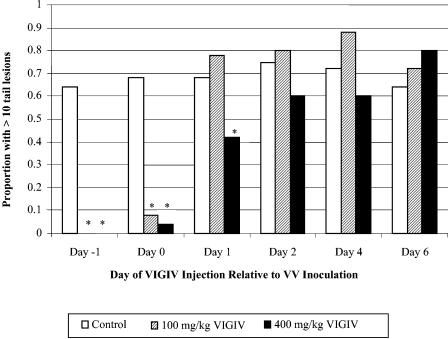
Both doses of VIGIV significantly reduced the proportion of mice with >10 lesions per tail when given the day before (day 1) or immediately after (day 0) VV inoculation compared to that for control groups. Treatment with the 400-mg/kg dose of VIGIV on day 1 also reduced the proportion of mice with >10 lesions per tail (Fig. 3). The proportion of mice with >10 lesions per tail was not reduced in animals receiving VIGIV on days 2 to 6.
FIG. 3.
In vivo VIGIV biological activity in the mouse tail lesion model. Tail lesions were counted 8 days after inoculation with VV. *, P ≤ 0.05 versus controls (chi-square analysis); n = 25 per group.
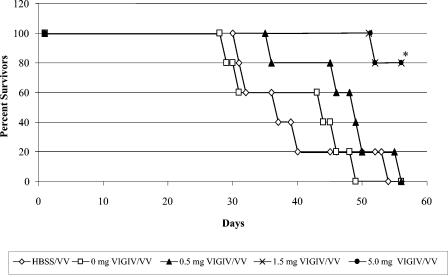
In vitro activity in the mouse lethality model.
Incubating the lethal dose of VV in vitro with either 1.5 or 5.0 mg of VIGIV before administering the inoculum to mice significantly prolonged their survival compared to that of untreated VV control groups (Fig. 4). The pretreatment of VV with 1.5 or 5.0 mg of VIGIV also resulted in an 80% (chi-square test; P ≤ 0.05) decrease in mortality compared to the untreated VV group.
FIG. 4.
In vitro VIGIV biological activity in the mouse lethality model. Various amounts of VIGIV (0, 0.5, 1.5, and 5.0 mg) were incubated with 3 × 104 PFU VV for 1 hour at room temperature prior to i.v. injection via the tail vein of SCID mice. Mortality was assessed for a period of 56 days. *, P ≤ 0.05 versus controls (Kaplan-Meier analysis); n = 5 per group.
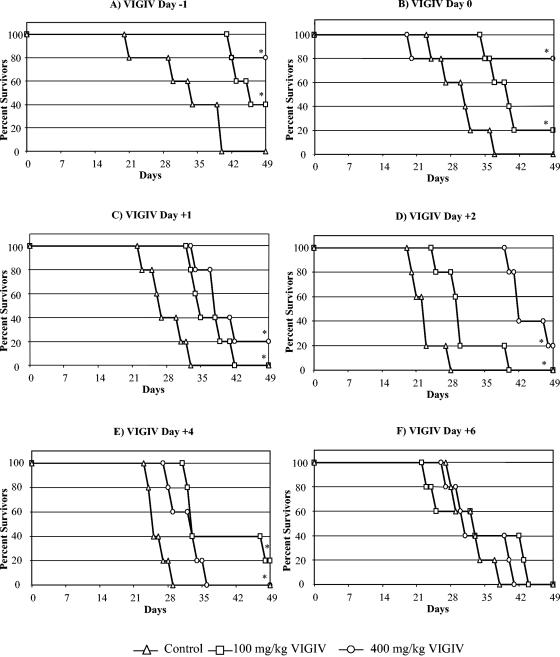
In vivo activity in the mouse lethality model.
A survival analysis was used to determine if VIGIV treatment resulted in a survival benefit. The results of the survival analyses for VIGIV are shown in Fig. 5A to F. The administration of both dose levels of VIGIV resulted in significantly prolonged survival (P ≤ 0.05) compared to that of controls when administered from day 1 to day 4 following VV inoculation. No differences in survival were observed if VIGIV was administered on day 6 (Fig. 5F) or day 8 (data not shown) following VV inoculation.
FIG. 5.
In vivo VIGIV biological activity in the mouse lethality model. SCID mice were inoculated i.v. with VV on day 0 and treated with VIGIV on day −1, 0, 1, 2, 4, or 6, and mortality was assessed for a period of 49 days. *, P ≤ 0.05 versus controls (Kaplan-Meier analysis); n = 5 per group.
A statistical analysis of the effect of VIGIV on the proportion of deaths that occurred by the end of the observation period indicated that the administration of the 400-mg/kg dose of VIGIV either the day before (day 1) or immediately after (day 0) VV inoculation reduced mortality by 80% (P ≤ 0.05 by chi-square test). Other dose levels or timing combinations did not significantly reduce the number of deaths.
DISCUSSION
Clinical reports published between 1956 and 1975 established the use of VIGIM as an effective treatment of adverse reactions to smallpox vaccination (11, 14, 23, 25). These reports suggested a role for antibodies in the immune response to VV infection (4). Even under circumstances such as progressive vaccinia, a condition now known to result from deficient cell-mediated immune function (4), VIGIM proved to have beneficial effects (15-18). Although the efficacy of VIGIV has not been tested in humans, the safety and pharmacokinetics of this product have been evaluated in a clinical trial (12). This clinical study demonstrated that the i.v. administration of VIGIV was well tolerated and resulted in a more favorable pharmacokinetic profile than that of VIGIM. Since a direct evaluation of the VV-neutralizing activity of VIGIV in humans was not possible, evaluations of the biological activity of VIGIV in suitable animal models are required. The primary utility of these in vivo models may be to provide additional assurance regarding the neutralizing activity of VIGIV (10). Specific forms of VV believed to be responsible for viral dissemination are only produced in vivo, and thus the most relevant measure of VIGIV-neutralizing activity can only be assessed by the use of in vivo assays.
VIGIV was effective at improving the outcome of the MLM when given up to 4 days after VV infection. However, VIGIV did not protect SCID mice when it was given at later times. Both cell-mediated and humoral immunity are defective in the SCID mouse (22). The results suggest that once VV infections are established in mice, the supplementation of humoral immunity alone is insufficient to abolish infection, and some form of cell-mediated immune response is required. It is interesting that an antiviral treatment produced a similar response and that when the antiviral treatment was discontinued, SCID mice rapidly succumbed to viral infection (19-21). A recent study by Belyakov et al. (2) demonstrated that antibody and cell-mediated responses share responsibility in the resolution of VV infection. Their study also suggested that the mechanism of resistance to VV infection differed in naïve mice from that in mice immunized against VV. Cell-mediated responses appeared to play the primary role in viral clearance in naive mice, whereas antibodies were sufficient for protection in immunized mice and cytotoxic T lymphocytes were not required. In the present study, the early administration of VIGIV appeared to produce protection analogous to the mechanism of resistance to viral challenge offered by immunization. The MTLM used fully immunocompetent mice. The highest dose of VIGIV tested (400 mg/kg) was effective in the MTLM only when given up to 1 day after VV infection. This finding suggests that once a local infection is established, cell-mediated immunity is the primary protective response. Infection in the MTLM is self-limiting, and tail lesions resolve without treatment 4 to 5 days after the appearance of initial lesions. Thus, native cell-mediated and humoral immunity appears to be sufficient for resolving infection over a relatively short time course in the MTLM. The augmentation of native immune responses by VIGIV treatment may not be necessary or demonstrable with the MTLM.
While the MTLM and MLM represent valuable tools for assessing VIGIV's biological activity, it is important to recognize that these models do not serve as predictors of VIGIV's efficacy in human disease. The MTLM and MLM were not developed to mimic conditions associated with severe adverse reactions to smallpox vaccination in humans. For example, infection in the murine models was initiated by i.v. administration, whereas most human complications would probably arise through viral contact with the skin. When considering the possible relevance of findings of the MLM to human complications, it is important to bear in mind that both arms of adaptive immunity are defective in the SCID mouse. Consequently, this condition is not identical to all cases of human immunodeficiency. There are clear differences in the severity of complications, particularly progressive vaccinia, depending on the disorder of immune function (5). Additionally, because of potential immune responses to the human antibody, the pharmacokinetics of VIGIV in mice may be quite different from those observed in humans. Therefore, the therapeutic regimen of VIGIV that might be efficacious in humans cannot be predicted from these models. Furthermore, there are many examples of biological drugs that showed promising effects in mice but failed to have similar success in human trials (26). In general, the immunological response of mice to VV may be substantially different from the human response.
Bray (4) has emphasized the need for the development of appropriate animal models for assessments of potential therapeutic agents for the treatment of smallpox vaccination-associated complications. Suggested models include the NC/Nag and NOA mouse models of atopic dermatitis, transgenic mice that constitutively overexpress interleukin-4 in the skin for evaluations of eczema vaccinatum, and nonhuman primate models rendered immunodeficient through retroviral infection, irradiation, or treatment with antimetabolites or antilymphocyte serum for evaluations of progressive vaccinia (4). A recently developed simian model of human immunodeficiency virus type 1 infection suggested that antibodies play an important role in limiting complications associated with smallpox vaccination (9). Determinations of the effectiveness of VIGIV in such models might add further validity to its potential efficacy in humans, but even the predictive value of nonhuman primate models will be limited by the potential allogeneic immune response to human VIGIV.
Whereas the intended use of VIGIV is the treatment of adverse reactions to smallpox vaccination, these mouse studies also support a possible role for VIGIV as a postexposure prophylactic against intentional uses of smallpox as a biological weapon. There are currently no plans for a large-scale smallpox vaccination program, and therefore much effort is being exerted to develop effective and safe antiviral agents that can be used as supplemental treatments to vaccination if a smallpox outbreak/attack occurs (1, 4). One of the primary impediments to the approved use of these antiviral agents is their potential toxicity (4). The results of the present animal studies, in conjunction with VIGIV's favorable safety profile in humans (12), suggest that VIGIV could be considered for use as a postexposure prophylactic. Clearly, additional animal studies will be required to evaluate the effectiveness of VIGIV against different classes of orthopoxviruses (e.g., variola virus and monkeypox virus). Ultimately, the evaluation of VIGIV activity in a nonhuman primate model of lethal orthopoxvirus infection would be required for a more relevant demonstration of its efficacy.
In summary, two murine models of VV infection demonstrated the biological activity of VIGIV. Reduced tail lesion formation in immunocompetent mice and decreased mortality and prolonged survival in immunocompromised mice were demonstrated. The U.S. Food and Drug Administration recently approved the VIGIV evaluated in these studies for human use. In conjunction with the pharmacokinetic and safety data generated in human trials, the demonstration of in vivo VIGIV-neutralizing activity was instrumental for obtaining this approval.
Acknowledgments
This work was funded by the Joint Vaccine Acquisition Program, Department of Defense (DoD) contract DAMD 17-98-C-8024.
This work does not represent official DoD positions, policies, or decisions.
REFERENCES
- 1.Baker, R. O., M. Bray, and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle, J. J., R. F. Haff, and R. C. Stewart. 1966. Evaluation of antiviral compounds by suppression of tail lesions in vaccinia-infected mice. Antimicrob. Agents Chemother. 6:536-539. [PubMed] [Google Scholar]
- 4.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 58:101-114. [DOI] [PubMed] [Google Scholar]
- 5.Bray, M., and M. E. Wright. 2003. Progressive vaccinia. Clin. Infect. Dis. 36:766-774. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2003. Update: cardiac and other adverse events following civilian smallpox vaccination—United States, 2003. Morb. Mortal. Wkly. Rep. 52:639-642. [PubMed] [Google Scholar]
- 7.De Clercq, E., and P. De Somer. 1968. Effect of interferon, polyacrylin acid, and polymethacrylic acid on tail lesions on mice infected with vaccinia virus. Appl. Microbiol. 16:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Clercq, E., M. Luczak, D. Shugar, P. F. Torrence, J. A. Waters, and B. Witkop. 1976. Effect of cytosine, arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc. Soc. Exp. Biol. Med. 151:487-490. [DOI] [PubMed] [Google Scholar]
- 9.Edghill-Smith, Y., D. Venzon, T. Karpova, J. McNally, J. Nacsa, W. P. Tsai, E. Tryniszewska, M. Moniuszko, J. Manischewitz, L. R. King, S. J. Snodgrass, J. Parrish, P. Markham, M. Sowers, D. Martin, M. G. Lewis, J. A. Berzofsky, I. M. Belyakov, B. Moss, J. Tartaglia, M. Bray, V. Hirsch, H. Golding, and G. Franchini. 2003. Modeling a safer smallpox vaccination regimen, for human immunodeficiency virus type 1-infected patients, in immunocompromised macaques. J. Infect. Dis. 188:1181-1191. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith, J. C., N. Eller, M. Mikolajczyk, J. Manischewitz, H. Golding, and D. E. Scott. 2004. Intravenous immunoglobulin products contain neutralizing antibodies to vaccinia. Vox Sanguinis 86:125-129. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein, J. A., J. M. Neff, J. M. Lane, and J. P. Koplan. 1975. Smallpox vaccination reactions, prophylaxis, and therapy of complications. Pediatrics 55:342-347. [PubMed] [Google Scholar]
- 12.Hopkins, R. J., W. Kramer, W. Blackwelder, M. Ashtekar, L. Hague, S. D. Winker-La Roche, G. Berezuk, D. Smith, and P. T. Leese. 2004. Safety and pharmacokinetic evaluation of intravenous vaccinia immune globulin in healthy volunteers. Clin. Infect. Dis. 39:759-766. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine. 1999. Assessment of future scientific needs for live variola virus. National Academic Press, Washington, D.C. [PubMed]
- 14.Kempe, C. H., T. Berge, and B. England. 1956. Hyperimmune vaccinial gamma globulin: source, evaluation and use in prophylaxis and therapy. Pediatrics 18:177-188. [PubMed] [Google Scholar]
- 15.Lane, J. M., F. L. Ruben, E. Abrutyn, and J. D. Millar. 1970. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. JAMA 212:441-444. [PubMed] [Google Scholar]
- 16.Lane, J. M., F. L. Ruben, J. M. Neff, and J. D. Millar. 1970. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J. Infect. Dis. 122:303-309. [DOI] [PubMed] [Google Scholar]
- 16a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academic Press, Washington, D.C.
- 17.Neff, J. M., J. M. Lane, J. H. Pert, R. Moore, J. D. Millar, and D. A. Henderson. 1967. Complications of smallpox vaccination. I. National survey in the United States, 1963. N. Engl. J. Med. 276:125-132. [DOI] [PubMed] [Google Scholar]
- 18.Neff, J. M., R. H. Levine, J. M. Lane, E. A. Ager, H. Moore, B. J. Rosenstein, J. D. Millar, and D. A. Henderson. 1967. Complications of smallpox vaccination United States 1963. II. Results obtained by four statewide surveys. Pediatrics 39:916-923. [PubMed] [Google Scholar]
- 19.Neyts, J., and E. De Clercq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 20.Neyts, J., and E. De Clercq. 2001. Efficacy of 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine for treatment of vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 45:84-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neyts, J., E. Verbeken, and E. De Clercq. 2002. Effect of 5-iodo-2′-deoxyuridine on vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 46:2842-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole, T. W. 1996. Discovery, development, and history of the SCID mouse. Lab. Animal 25:29-32. [Google Scholar]
- 23.Sharp, J. C., and W. B. Fletcher. 1973. Experience of anti-vaccinia immunoglobulin in the United Kingdom. Lancet i:656-659. [DOI] [PubMed] [Google Scholar]
- 24.Smee, D. F., and R. W. Sidwell. 2003. A review of compounds exhibiting anti-orthopoxvirus activity in animal models. Antiviral Res. 57:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sussman, S., and M. Grossman. 1965. Complications of smallpox vaccination. Effects of vaccinia immune globulin therapy. J. Pediatr. 1965:1168-1173. [Google Scholar]
- 26.'t Hart, B. A., S. Amor, and M. Jonker. 2004. Evaluating the validity of animal models for research into therapies for immune-based disorders. Drug Discov. Today 9:517-524. [DOI] [PubMed] [Google Scholar]
- 27.Wharton, M., R. A. Strikas, R. Harpaz, L. D. Rotz, B. Schwartz, C. G. Casey, M. L. Pearson, and L. J. Anderson. 2003. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:1-16. [PubMed] [Google Scholar]