Abstract
Tenofovir disoproxil fumarate (TDF) is a nucleotide analogue approved for treatment of human immunodeficiency virus (HIV) infection. TDF also has been shown in vitro to inhibit replication of wild-type hepatitis B virus (HBV) and lamivudine-resistant HBV mutants and to inhibit lamivudine-resistant HBV in patients and HBV in patients coinfected with the HIV. Data on the in vivo efficacy of TDF against wild-type virus in non-HIV-coinfected or lamivudine-naïve chronic HBV-infected patients are lacking in the published literature. The antiviral effect of oral administration of TDF against chronic woodchuck hepatitis virus (WHV) infection, an established and predictive animal model for antiviral therapy, was evaluated in a placebo-controlled, dose-ranging study (doses, 0.5 to 15.0 mg/kg of body weight/day). Four weeks of once-daily treatment with TDF doses of 0.5, 1.5, or 5.0 mg/kg/day reduced serum WHV viremia significantly (0.2 to 1.5 log reduction from pretreatment level). No effects on the levels of anti-WHV core and anti-WHV surface antibodies in serum or on the concentrations of WHV RNA or WHV antigens in the liver of treated woodchucks were observed. Individual TDF-treated woodchucks demonstrated transient declines in WHV surface antigen serum antigenemia and, characteristically, these woodchucks also had transient declines in serum WHV viremia, intrahepatic WHV replication, and hepatic expression of WHV antigens. No evidence of toxicity was observed in any of the TDF-treated woodchucks. Following drug withdrawal there was prompt recrudescence of WHV viremia to pretreatment levels. It was concluded that oral administration of TDF for 4 weeks was safe and effective in the woodchuck model of chronic HBV infection.
Chronic infection with the hepatitis B virus (HBV) is a major public health problem and is responsible for 1.2 million deaths per year worldwide (49). It is estimated that more than 2 billion people throughout the world have serological evidence of previous or current HBV infection, with over 350 million chronic carriers of HBV (49). Carriers of HBV are at high risk of developing chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Although safe and effective prophylactic vaccines against HBV are available, the use of drug and/or immunotherapeutic strategies for treatment of chronically infected human patients is limited. Interferon therapy and nucleoside analogues, alone and in combination, can be effective against HBV, but not all patients respond and severe side effects of interferon and emergence of nucleoside-resistant mutants often limit their use (1, 7, 16, 40).
Until recently, lamivudine was the only nucleoside analogue licensed for treatment of chronic HBV infection. Although safe and effective against HBV, the therapeutic efficacy of lamivudine is limited by the frequent development of drug-resistant HBV mutants (22, 30). Recently, the nucleotide analogue adefovir dipivoxil (ADV) was licensed for treatment of HBV infection, and clinical studies have shown that it is effective in inhibiting the replication of wild-type and lamivudine-resistant HBV mutants (19, 32, 39, 50, 52). While experience with ADV for treatment of chronic HBV infection is more limited than with lamivudine, ADV drug resistance appears to develop less frequently than with lamivudine (1, 15, 39, 48, 51).
Tenofovir disoproxil fumarate [TDF; bis(isopropyloxycarbonyloxymethyl)-(R)-9-(2-phosphonyl-methoxypropyl)adenine;also called bis(POC)-PMPA], an oral prodrug of tenofovir, is an acyclic nucleotide analogue closely related to ADV and has been approved for treatment of human immunodeficiency virus (HIV) infection (2, 14, 31). Tenofovir also has potent antiviral activity against wild-type HBV and duck hepatitis B virus, lamivudine-resistant HBV mutants in cell culture (53-55), and lamivudine-resistant HBV infection in vivo (3, 5, 17, 29, 36, 37, 41, 45-47). Since TDF is licensed for the treatment of HIV infection, there is increasing interest in examining TDF alone or in combination with other antiviral drugs for treatment of chronic HBV infection.
Woodchuck hepatitis virus (WHV) and its natural host, the Eastern woodchuck (Marmota monax), is a well-characterized mammalian model available for basic and therapeutic research on HBV. The woodchuck model has been useful in studies of the pathogenesis of acute, self-limited, and chronic HBV infection and in the preclinical evaluation of efficacy and, importantly, safety of drug candidates for treatment of chronic HBV infection (11, 27, 33, 35) and for prevention of associated diseases, including hepatocellular carcinoma (43). Results of drug efficacy studies in the woodchuck characteristically have been similar to those observed in patients chronically infected with HBV (27).
The objective of this study was to determine the dose range for antiviral activity of TDF in a placebo-controlled study in woodchucks. The results indicate that TDF is effective in suppressing viral replication in chronic infection with wild-type WHV. This will enable further experimental modeling of TDF at optimal doses for treatment of laboratory-derived, lamivudine-resistant mutants of WHV (23) and for the preclinical assessment of drug combinations.
MATERIALS AND METHODS
Woodchucks.
The woodchucks used in this study were born to WHV-negative females and reared in environmentally controlled laboratory animal facilities at Cornell University. Woodchucks used for treatment with TDF were inoculated at 3 days of age with 5 million woodchuck infectious doses of a standardized WHV inoculum (cWHV7P1 or WHV7P2) (9). Woodchucks selected for use developed WHV surface antigen (WHsAg) serum antigenemia and became chronic WHV carriers. The chronic carrier status of these woodchucks was confirmed prior to initiation of drug treatment, and all were free of hepatocellular carcinoma at the beginning of the study, based on hepatic ultrasound examination and serum γ-glutamyl transferase (GGT) activity. All experimental procedures involving woodchucks were performed under protocols approved by the Cornell University Institutional Animal Care and Use Committee.
Drug.
Tenofovir and TDF were provided by Gilead Sciences, Inc. (Foster City, CA). For the antiviral study in chronic WHV carrier woodchucks, sufficient TDF was weighed each week to treat the woodchucks of the various groups for 1 week. The drug was dissolved in isotonic saline solution and then suspended in a semisynthetic liquid diet (Dyets Inc., Bethlehem, PA) to ensure complete consumption. Volumes of drug sufficient to treat each individual woodchuck were withdrawn from the stock solution each day, mixed with liquid diet immediately prior to administration, and administered orally by dose syringe once daily for 28 days (42). The liquid diet alone was administered daily to the control group of woodchucks.
Pharmacokinetic and oral bioavailability study.
A total of 18 WHV-negative, adult woodchucks were assigned to two treatment groups. Each treatment group was subdivided into three groups, A, B, and C, consisting of three woodchucks/sex/group. With treatment 1, groups A, B, and C were dosed with tenofovir at 2.5, 7.5, and 12.5 mg/kg of body weight via a single bolus intravenous injection. After a 1-week washout period, the same 18 woodchucks received treatment 2, in which groups A, B, and C were dosed with TDF orally at 5.0, 15.0, and 25.0 mg/kg. Blood samples were collected prior to dosing and at 0.25, 0.5, 1, 1.5, 2, 6, 8, 12, 24, and 48 h postdose. Concentrations of tenofovir were determined in plasma using a high-performance liquid chromatographic mass spectrometric method (24), and noncompartmental pharmacokinetic analysis was performed on the plasma tenofovir data.
Antiviral study.
Twenty adult chronic WHV carrier woodchucks were stratified equally by age, sex, body weight, and serum GGT activity into five treatment groups consisting of four animals each: (i) TDF at 15.0 mg/kg once per day, (ii) TDF at 5.0 mg/kg/day, (iii) TDF at 1.5 mg/kg/day, (iv) TDF at 0.5 mg/kg/day, and (v) a placebo control. The woodchucks were treated daily for 4 weeks and observed for an additional 12 weeks following cessation of drug treatment.
Blood samples (4.0 ml) were obtained for WHV DNA analysis and serological testing under general anesthesia (ketamine at 50 mg/kg and xylazine at 5 mg/kg) 2 weeks prior to drug administration, on the first day of treatment prior to drug administration (day zero or week zero), at 1/2, 1, 3, 5, and 7 days of drug treatment, and then weekly through the 4 weeks of drug treatment. Thereafter, samples were obtained at 1, 3, 5, 7, and 14 days after termination of drug treatment and then every 2 weeks for a total of 8 weeks following drug withdrawal, and a final sample was obtained at 12 weeks following the end of treatment. The woodchucks were weighed each time they were anesthetized and bled, and the body weights of drug-treated groups were compared to those of placebo controls. Drug dosages for individual woodchucks were adjusted based on the most recent body weight.
Complete blood counts were performed prior to treatment, at the end of treatment (week 4), and at 4 and 12 weeks following drug withdrawal. Serum biochemical measurements were made 2 weeks prior to drug administration, at time zero and after 4 weeks of treatment, and at 4 and 12 weeks following drug withdrawal. Serum chemistry measurements included serum GGT, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase (AST), and sorbitol dehydrogenase (SDH), total serum bilirubin, serum albumin, blood urea nitrogen, creatinine, Na, K, Cl, and bicarbonate. Iron status was evaluated by determining total serum iron levels, iron binding capacity, and percent iron saturation (42).
Serum WHV DNA concentrations were measured before treatment, during treatment, and during the posttreatment follow-up period until week 16 of the study at frequent intervals as described above. WHV viremia in serum samples was assessed by two different quantitative methods depending on the concentration of WHV DNA: (i) dot blot hybridization using three replicate volumes (10 μl) of undiluted serum (sensitivity, 1.0 × 107 WHV genome equivalents per ml [WHVge/ml]) compared with a standard dilution series of WHV recombinant DNA plasmid (pWHV8) (9), and (ii) real time PCR assay (Taqman PCR system; Applied Biosystems, Foster City, CA) using three replicate samples of WHV DNA extracted from 200 μl of serum (QIAamp DNA blood kit; QIAGEN, Valencia, CA) and direct comparisons with parallel PCR assays of standard dilutions of pWHV8 and of the standardized WHV7 inoculum, which has a known content of WHV DNA (9) (sensitivity, 1.0 × 103 WHVge/ml). The WHV target amplicon spanning core region nucleotides 507 to 573 was amplified using WHV-specific forward and reverse primer sets and a WHV-specific probe (forward primer, 5′-GAGCTTCTAGGTCCCCCAGAA-3′; reverse primer, 5′-CGACGCGGTGATTGAGATCT-3′; probe, 5′-ACGCACTCCCTCTCCTCGCAGGA-3; Applied Biosystems). WHsAg and antibodies to WHV core antigen (anti-WHc) and WHsAg (anti-WHs) were determined before treatment, during treatment, and during the posttreatment follow-up period until week 16 of the study at frequent intervals as described above, using WHV-specific enzyme immunoassays (10).
Liver biopsies were obtained 2 weeks prior to drug administration, at the end of the 4-week treatment period, and at 4 and 12 weeks following drug withdrawal. Biopsies were obtained under general anesthesia (ketamine at 50 mg/kg and xylazine at 5 mg/kg) using 16-gauge Bard Biopty-Cut disposable biopsy needles directed by ultrasound imaging (38, 42). Aliquots of biopsy specimens were fixed in phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histopathological analysis (i.e., portal hepatitis, lobular hepatitis, bile duct proliferation, steatosis, and liver cell dysplasia) (38, 42). Sections of these tissues also were stained for intrahepatic WHV nucleocapsid antigen (WHcAg) and WHsAg detection using immunohistochemical methods (38, 42). A second aliquot of liver was placed immediately in liquid nitrogen and stored at −70°C until nucleic acid analyses were performed. Levels of intrahepatic WHV nucleic acids (WHV DNA, WHV RNA, and WHV DNA monomers) were quantitatively determined by Southern and Northern blot hybridizations as previously described (27, 42).
Statistics.
The antiviral effect of oral TDF administration on WHV replication was determined in two ways. First, the geometric mean serum WHV DNA concentrations of drug-treated groups of woodchucks were determined, and the concentrations before, during, and following treatment were compared to that of the placebo-treated control group of woodchucks. Second, the mean concentrations of WHV nucleic acids in hepatic biopsy tissue were determined before treatment, at the end of treatment, and at 4 and 12 weeks posttreatment, and the respective values for drug-treated and control woodchucks were compared. Statistical comparisons of the concentrations of WHV nucleic acids in serum and liver tissues between groups at each sampling date were performed using Student's t test (two-tailed) or the Mann-Whitney U test (two-tailed, exact value; SPSS 11.5 program; SSPS Inc., Chicago, IL). P values of <0.05 were considered statistically significant.
RESULTS
Pharmacokinetic study.
In the intravenous dose groups the mean concentrations of tenofovir in plasma decreased with time over a 24-h period. In the oral dose groups, mean tenofovir plasma concentrations increased for 0.5 to 1.0 h and then gradually decreased over the course of 24 h (data not shown).
Mean values of pharmacokinetic parameters were calculated and the intravenous and oral treatment groups compared. In the intravenous group, the mean values for maximum concentration of drug in serum (Cmax) were 8.49 ± 5.33, 8.55 ± 4.74, and 47.5 ± 37.6 μg/ml for the 2.5-, 7.5-, and 12.5-mg/kg dose groups, respectively, and the corresponding mean values for the area under the concentration-time curve for 0 h to infinity (AUC0-∞) were 4.21 ± 1.68, 5.39 ± 2.47, and 21.0 ± 3.04 μg · h/ml. The half-life of the terminal elimination phase, 0.693/elimination rate constant (t1/2λz), ranged from 0.858 to 9.33 h. In the oral dose groups, the mean values for Cmax were 0.171 ± 0.0687, 0.377 ± 0.217, and 0.524 ± 0.229 μg/ml for the 5.0-, 15.0-, and 25.0-mg/kg dose groups, respectively, and the corresponding mean AUC0-∞ values were 0.635 ± 0.162, 1.42 ± 0.413, and 1.85 ± 0.687 μg · h/ml. The t1/2λz ranged from 2.48 to 7.29 h.
When Cmax and AUC0-∞ were dose normalized for the intravenous and oral dose groups, the slopes showed no statistically significant differences from zero, indicating that the Cmax and AUC0-∞ increased in a dose proportional manner with linear pharmacokinetics (data not shown). No significant differences were observed between genders.
The mean bioavailability of the oral dose groups treated with 5.0, 15.0, and 25.0 mg/kg of TDF were 20.6 ± 12.9, 32.4 ± 16.2, and 10.0 ± 4.16%, respectively. The apparent reduction in tenofovir bioavailability in the 25.0-mg/kg group may indicate saturation of a critical mechanism with increasing doses of TDF.
Antiviral study.
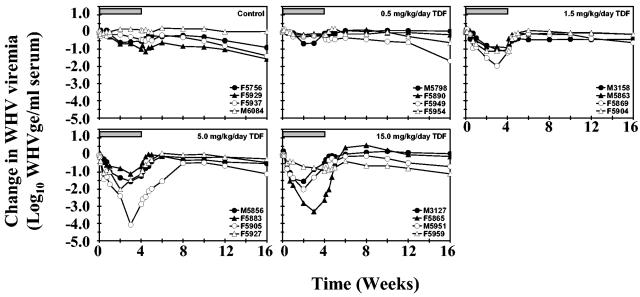
Oral administration of TDF at doses of 0.5, 1.5, or 5.0 mg/kg/day induced a dose-dependent decline in serum viremia in woodchucks chronically infected with WHV (Fig. 1 and Table 1). The lowest dose of TDF used in this study (0.5 mg/kg/day) reduced the average serum WHV DNA concentration slightly but significantly compared to pretreatment levels by 0.2 log at the end of the 4-week treatment period (P < 0.01). Oral administration of 1.5 mg of TDF per kg/day produced a gradual and progressive reduction in the average concentration of WHV viremia during the 4 weeks of treatment. At the end of the treatment period, the average concentration of WHV DNA was reduced significantly compared to pretreatment levels by 1.1 logs (P < 0.01). Serum WHV DNA also was significantly reduced compared to pretreatment levels at the end of weeks 1, 2, and 3 (P < 0.01) and compared to placebo at the end of weeks 1, 2, 3, and 4 (P < 0.05).
FIG. 1.
Antiviral effect of oral TDF administration on serum WHV DNA in chronic WHV carrier woodchucks. Horizontal bars denote the 4-week treatment period. Log10 changes in WHV viremia from baseline at week zero prior to TDF administration for individual woodchucks in each treatment group are displayed. WHVge, WHV genomic equivalents (virion or WHV DNA-containing virus particles).
TABLE 1.
Antiviral effects of oral TDF administration on serum WHV DNA concentrations in woodchucks chronically infected with WHV
| Treatment and time | WHV viremia (log10 WHVge/ml of serum)a
|
|
|---|---|---|
| Mean | Mean change | |
| Control (placebo) | ||
| Pretreatment (wk 0) | 10.0 ± 0.1 (9.9-10.2) | 0 |
| 1 wk treatment (wk 1) | 9.9 ± 0.3 (9.6-10.2) | −0.1 ± 0.1 (−0.3-0.0) |
| 2 wk treatment (wk 2) | 9.7 ± 0.5 (9.2-10.4) | −0.3 ± 0.4 (−0.7-0.2) |
| 3 wk treatment (wk 3) | 9.7 ± 0.5 (9.2-10.3) | −0.3 ± 0.4 (−0.7-0.2) |
| End of treatment (wk 4) | 9.5 ± 0.6 (8.9-10.3) | −0.5 ± 0.5 (−1.0-0.1) |
| 1 wk posttreatment (wk 5) | 9.6 ± 0.5 (9.0-10.2) | −0.4 ± 0.4 (−0.9-0.0) |
| 2 wk posttreatment (wk 6) | 9.8 ± 0.5 (9.2-10.4) | −0.2 ± 0.4 (−0.6-0.2) |
| 4 wk posttreatment (wk 8) | 9.7 ± 0.5 (9.0-10.4) | −0.3 ± 0.4 (−0.8-0.2) |
| 12 wk posttreatment (wk 16) | 9.0 ± 0.8 (8.3-10.2) | −1.0 ± 0.7 (−1.6-0.0)b |
| TDF (0.5 mg/kg/day) | ||
| Pretreatment (wk 0) | 10.1 ± 0.5 (9.8-10.8) | 0 |
| 1 wk treatment (wk 1) | 10.0 ± 0.5 (9.6-10.7) | −0.1 ± 0.1 (−0.3-0.0) |
| 2 wk treatment (wk 2) | 9.8 ± 0.6 (9.1-10.6) | −0.3 ± 0.2 (−0.7-−0.1)b |
| 3 wk treatment (wk 3) | 9.8 ± 0.6 (9.1-10.6) | −0.3 ± 0.2 (−0.6-−0.1) |
| End of treatment (wk 4) | 9.9 ± 0.5 (9.6-10.6) | −0.2 ± 0.1 (−0.2-−0.1)c |
| 1 wk posttreatment (wk 5) | 10.0 ± 0.7 (9.4-11.0) | −0.1 ± 0.3 (−0.4-0.2) |
| 2 wk posttreatment (wk 6) | 10.0 ± 0.5 (9.5-10.7) | −0.1 ± 0.2 (−0.3-0.1) |
| 4 wk posttreatment (wk 8) | 10.0 ± 0.5 (9.4-10.7) | −0.1 ± 0.2 (−0.4-0.1) |
| 12 wk posttreatment (wk 16) | 9.5 ± 0.9 (8.2-10.2) | −0.6 ± 0.8 (−1.7-0.1) |
| TDF (1.5 mg/kg/day) | ||
| Pretreatment (wk 0) | 10.0 ± 0.2 (9.9-10.3) | 0 |
| 1 wk treatment (wk 1) | 9.4 ± 0.4 (9.0-9.9) | −0.6 ± 0.2 (−0.9-−0.3)cd |
| 2 wk treatment (wk 2) | 8.9 ± 0.5 (8.4-9.5) | −1.1 ± 0.3 (−1.5-−0.8)cd |
| 3 wk treatment (wk 3) | 8.8 ± 0.6 (7.9-9.2) | −1.2 ± 0.5 (−2.0-−0.9)cd |
| End of treatment (wk 4) | 8.9 ± 0.2 (8.7-9.1) | −1.1 ± 0.1 (−1.2-−0.9)cd |
| 1 wk posttreatment (wk 5) | 9.8 ± 0.1 (9.7-9.9) | −0.2 ± 0.2 (−0.4-0.0) |
| 2 wk posttreatment (wk 6) | 9.9 ± 0.1 (9.8-10.0) | −0.1 ± 0.2 (−0.4-0.1) |
| 4 wk posttreatment (wk 8) | 9.8 ± 0.1 (9.8-9.9) | −0.2 ± 0.2 (−0.4-0.0) |
| 12 wk posttreatment (wk 16) | 9.7 ± 0.2 (9.3-9.8) | −0.3 ± 0.2 (−0.6-−0.1)b |
| TDF (5.0 mg/kg/day) | ||
| Pretreatment (wk 0) | 10.1 ± 0.2 (9.9-10.4) | 0 |
| 1 wk treatment (wk 1) | 9.0 ± 0.4 (8.7-9.6) | −1.1 ± 0.5 (−1.7-−0.7)ce |
| 2 wk treatment (wk 2) | 8.5 ± 0.7 (7.9-9.5) | −1.6 ± 0.7 (−2.4-−0.8)cd |
| 3 wk treatment (wk 3) | 8.1 ± 1.2 (6.3-9.1) | −2.0 ± 1.4 (−4.1-−1.1)b |
| End of treatment (wk 4) | 8.6 ± 0.9 (7.5-9.6) | −1.5 ± 1.0 (−2.9-−0.7)b |
| 1 wk posttreatment (wk 5) | 9.4 ± 0.8 (8.4-10.3) | −0.7 ± 0.9 (−2.0-0.0) |
| 2 wk posttreatment (wk 6) | 9.7 ± 0.6 (8.8-10.2) | −0.4 ± 0.8 (−1.5-0.1) |
| 4 wk posttreatment (wk 8) | 9.9 ± 0.2 (9.6-10.1) | −0.2 ± 0.2 (−0.5-0.0) |
| 12 wk posttreatment (wk 16) | 9.5 ± 0.3 (9.3-9.8) | −0.6 ± 0.4 (−1.1-−0.2)b |
| TDF (15.0 mg/kg/day) | ||
| Pretreatment (wk 0) | 10.3 ± 0.6 (9.8-11.1) | 0 |
| 1 wk treatment (wk 1) | 9.1 ± 1.2 (8.0-10.6) | −1.2 ± 0.6 (−1.8-−0.4)ce |
| 2 wk treatment (wk 2) | 8.5 ± 1.4 (7.0-10.4) | −1.8 ± 0.9 (−2.8-−0.7)cd |
| 3 wk treatment (wk 3) | 8.7 ± 1.6 (6.5-10.3) | −1.6 ± 1.2 (−3.3-−0.8)b |
| End of treatment (wk 4) | 9.1 ± 1.4 (7.1-10.1) | −1.2 ± 1.0 (−2.6-−0.5) |
| 1 wk posttreatment (wk 5) | 9.9 ± 0.5 (9.3-10.3) | −0.4 ± 0.3 (−0.7-−0.1)b |
| 2 wk posttreatment (wk 6) | 10.3 ± 0.4 (9.8-10.7) | 0.0 ± 0.3 (−0.4-0.4) |
| 4 wk posttreatment (wk 8) | 10.3 ± 0.3 (9.9-10.5) | 0.0 ± 0.5 (−0.6-0.6) |
| 12 wk posttreatment (wk 16) | 9.8 ± 0.1 (9.7-10.0) | −0.5 ± 0.5 (−1.1-0.1) |
There were four woodchucks in each group. Values are means ± standard deviations, and overall ranges of values for WHV nucleic acids are presented in parentheses.
Difference from pretreatment value is statistically significant (P < 0.05).
Difference from pretreatment value is statistically significant (P < 0.01).
Difference from placebo control is statistically significant (P < 0.05).
Difference from placebo control is statistically significant (P < 0.01).
Administration of 5.0 mg of TDF per kg/day induced a more rapid decline in the average concentration of serum WHV DNA during the 4 weeks of treatment. At the end of the treatment period, the average concentration of WHV viremia was reduced compared to pretreatment levels by 1.5 logs, and this reduction was almost threefold greater than that observed at the 1.5-mg/kg/day dose level of TDF. Serum WHV DNA was significantly reduced compared to pretreatment levels at the end of weeks 1, 2 (P < 0.01), 3, and 4 (P < 0.05). WHV viremia also was significantly reduced compared to placebo at weeks 1 (P < 0.01) and 2 (P < 0.05).
Administration of the highest dose of TDF (15.0 mg/kg/day) induced a rapid decline in the average concentration of WHV viremia, and the reduction in serum WHV DNA during the initial 2 weeks of treatment was comparable to that observed with the 5.0-mg/kg/day dose of TDF. A high degree of individual variation in antiviral response was observed in this group of woodchucks. No change in WHV viremia was observed in one of the four woodchucks (F5959), while reductions in serum WHV DNA were observed in two (M3127 and M5951) and a greater reduction was noted in a fourth woodchuck (F5865). Due to this variable antiviral response, the average concentration of serum WHV DNA in this group of woodchucks was reduced by only 1.2 logs at the end of therapy, and the differences between pretreatment levels or placebo-treated control woodchucks were not statistically significant.
No significant changes in the average concentration of serum WHV DNA were observed in the placebo-treated control group during the study period except for the last week of the study (Table 1). After cessation of oral TDF administration, WHV viremia returned to pretreatment levels within 1 to 4 weeks in all treated woodchucks (Fig. 1).
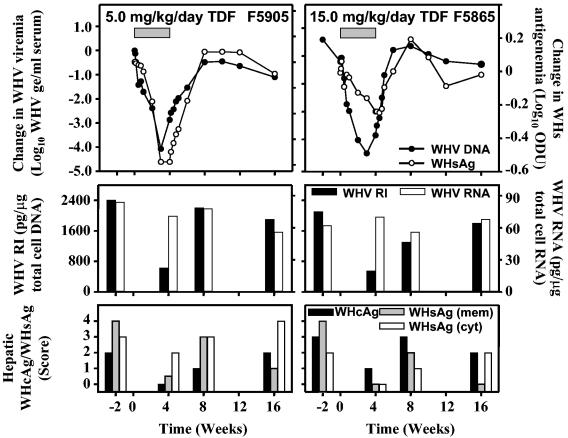
No significant changes were observed in the average levels of serum WHsAg in woodchucks from TDF-treated groups (data not shown). WHsAg serum antigenemia correlated with declines in serum WHV DNA observed during treatment of individual woodchucks. In three woodchucks, one each of which was treated with the 0.5 mg/kg/day (M5798), 5.0 mg/kg/day (F5905), or 15.0 mg/kg/day dose of TDF (F5865), transient declines in WHsAg serum antigenemia (3- to 18-fold) were closely associated with the changes observed in WHV viremia (Fig. 2).
FIG. 2.
Antiviral effect of oral TDF administration on serum WHV DNA and WHsAg, intrahepatic WHV replication and WHV RNA, and hepatic expression of WHcAg and of membranous and cytoplasmic WHsAg in individual chronic WHV carrier woodchucks. Woodchuck F5905 received TDF at a dose of 5.0 mg/kg/day for 4 weeks (left panels), whereas woodchuck F5865 was treated for 4 weeks with 15.0 mg/kg/day of TDF (right panels). Horizontal bars in the top panels denote the 4-week treatment period. Log10 changes in WHV viremia and WHsAg antigenemia from baseline at week zero prior to TDF administration are shown in the top panels. WHVge, WHV genomic equivalents (virion or WHV DNA-containing virus particles); ODU, optical density units; WHV RI, hepatic WHV DNA replicative intermediates; WHsAg (mem), hepatic membranous WHsAg; WHsAg (cyt), hepatic cytoplasmic WHsAg. Levels of hepatic cellular DNA and RNA were quantified by hybridization to a commercial β-actin gene probe (Oncor, Inc., Gaithersburg, MD) by Southern or Northern blot hybridization techniques, respectively.
No significant changes in the average levels of anti-WHc or anti-WHs antibodies in the serum of TDF-treated groups were observed (data not shown). In the placebo-treated control group, no significant changes in the levels of WHsAg serum antigenemia or of WHV-specific antibodies were observed.
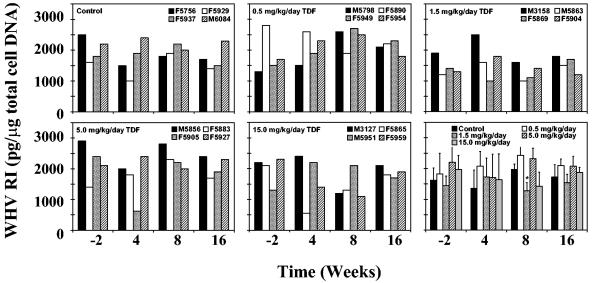
No changes in the hepatic concentration of WHV replication intermediates were observed in the placebo control group (Fig. 3 and Table 2). The average concentration of intrahepatic WHV replication intermediates was not reduced by any dose of TDF after 4 weeks of treatment, consistent with the moderate and sometimes variable changes observed in serum WHV DNA (Fig. 1 and 3; Table 2). Finally, no effects of TDF treatment on the average concentrations of intrahepatic WHV RNA were observed (Table 2).
FIG. 3.
Antiviral effect of oral TDF administration on intrahepatic WHV replication in chronic WHV carrier woodchucks. Values for individual woodchucks in each treatment group are displayed in first five panels. Mean values for all of the experimental groups are compared in the last panel (vertical lines denote standard deviations). Each experimental group contained four woodchucks. The asterisk within the last panel indicates that the mean value of intrahepatic WHV replicative intermediates for woodchucks treated with TDF at a dose of 1.5 mg/kg/day was statistically different from placebo-treated control woodchucks at week 8 (P < 0.01). WHV RI, hepatic WHV DNA replicative intermediates. Levels of hepatic cellular DNA were quantified by hybridization to a commercial β-actin gene probe (Oncor, Inc., Gaithersburg, MD) by Southern blot hybridization techniques as described in the text.
TABLE 2.
Antiviral effects of oral TDF administration on hepatic WHV replicative intermediates and WHV RNA in woodchucks chronically infected with WHV
| Treatment and time | Mean RI (pg/μg of total cell DNA)a | Mean RNA (pg/μg of total cell RNA) |
|---|---|---|
| Control (placebo) | ||
| Pretreatment (wk −2) | 1,820 ± 403 (1,600-2,500) | 55 ± 12 (56-83) |
| End of treatment (wk 4) | 1,361 ± 594 (1,000-2,400) | 59 ± 20 (50-92) |
| 4 wk posttreatment (wk 8) | 1,975 ± 171 (1,800-2,200) | 81 ± 11 (68-91) |
| 12 wk posttreatment (wk 16) | 1,725 ± 403 (1,400-2,300) | 77 ± 15 (60-95) |
| TDF (0.5 mg/kg/day) | ||
| Pretreatment (wk −2) | 1,825 ± 670 (1,300-2,800) | 78 ± 14 (62-96) |
| End of treatment (wk 4) | 2,075 ± 479 (1,500-2,600) | 75 ± 14 (54-85) |
| 4 wk posttreatment (wk 8) | 2,425 ± 359 (1,900-2,700) | 79 ± 10 (69-93) |
| 12 wk posttreatment (wk 16) | 2,100 ± 216 (1,800-2,300) | 75 ± 12 (60-88) |
| TDF (1.5 mg/kg/day) | ||
| Pretreatment (wk −2) | 1,450 ± 311 (1,200-1,900) | 66 ± 7 (56-72) |
| End of treatment (wk 4) | 1,725 ± 618 (1,000-2,500) | 73 ± 13 (60-88) |
| 4 wk posttreatment (wk 8) | 1,275 ± 275 (1,000-1,600)b | 69 ± 14 (54-81) |
| 12 wk posttreatment (wk 16) | 1,550 ± 265 (1,200-1,800) | 77 ± 11 (67-92) |
| TDF (5.0 mg/kg/day) | ||
| Pretreatment (wk −2) | 2,200 ± 627 (1,400-2,900) | 75 ± 18 (55-94) |
| End of treatment (wk 4) | 1,705 ± 765 (620-2,400) | 73 ± 16 (52-89) |
| 4 wk posttreatment (wk 8) | 2,325 ± 340 (2,000-2,800) | 80 ± 7 (71-87) |
| 12 wk posttreatment (wk 16) | 2,075 ± 330 (1,700-2,400) | 66 ± 10 (56-78) |
| TDF (15.0 mg/kg/day) | ||
| Pretreatment (wk −2) | 1,975 ± 457 (1,300-2,300) | 62 ± 8 (51-70) |
| End of treatment (wk 4) | 1,638 ± 844 (550-2,400) | 71 ± 7 (63-80) |
| 4 wk posttreatment (wk 8) | 1,425 ± 457 (1,100-2,100) | 72 ± 11 (56-80) |
| 12 wk posttreatment (wk 16) | 1,875 ± 171 (1,700-2,100) | 74 ± 15 (55-90) |
There were four woodchucks in each group. Values are means ± standard deviations, and overall ranges of values for WHV nucleic acids are presented in parentheses. Concentrations of hepatic intracellular WHV DNA replicative intermediates (RI) and total WHV RNA were normalized for total cellular DNA or RNA by using the relative levels of β-actin DNA or RNA (β-actin hybridization probe purchased from Oncor, Inc., Gaithersburg, MD).
Difference from placebo control group is statistically significant, P < 0.01.
No differences between experimental groups were observed histologically in biopsy specimens of the liver obtained prior to treatment. Portal hepatitis, lobular hepatitis, bile duct proliferation, steatosis, and liver cell dysplasia in all groups initially were mild in degree (median scores of 1 to 1.5 in a 0-to-4 scoring system). At the end of the 4-week treatment period, there were no remarkable differences in the histological appearance of biopsy specimens from TDF-treated and placebo-treated woodchucks, and no differences between groups were observed in the biopsy specimens obtained at 4 and 12 weeks posttreatment (data not shown). Similarly, no remarkable differences were observed between TDF-treated groups of woodchucks and placebo-treated control woodchucks in the immunohistochemical staining of either intrahepatic WHcAg or WHsAg in biopsy specimens obtained at the end of the treatment period or at 4 or 12 weeks posttreatment (data not shown). Two woodchucks, however, one in each of the 5.0-mg/kg/day (F5905) and 15.0-mg/kg/day TDF dose groups (F5865), had reductions in the hepatic expression of WHcAg and of membranous and cytoplasmic WHsAg at the end of the treatment period (Fig. 2). Declines in both viral markers were transient. These two woodchucks also had transient reductions in serum WHV DNA, in WHsAg serum antigenemia, and in intrahepatic WHV replication intermediates (Fig. 1 to 3).
No clinical signs of toxicity were observed in TDF-treated woodchucks at any of the dose levels used. Changes in body weights of drug-treated groups of woodchucks were similar to those of placebo-treated control woodchucks. No drug- or dose-related changes in bilirubin, in total serum protein, in serum albumin, or in amylase were apparent in TDF-treated groups compared to the placebo-treated control group (data not shown). Similarly, no treatment-related changes were observed in comparison with controls regarding blood urea nitrogen, creatinine, blood glucose, cholesterol, serum Na, K, Cl, calcium, phosphorus, iron, iron binding capacity, or percent iron saturation. Hematological parameters in all TDF-treated groups were similar to those of the placebo-treated control group, including erythrocyte count and hematocrit and the total leukocyte and neutrophil counts.
Three of the woodchucks treated with TDF doses of 5.0 or 15.0 mg/kg/day (M5856, F5905, and F5865) had serum activities of SDH and AST that were elevated prior to the start of treatment compared to all the other woodchucks treated with TDF (in the three woodchucks, the average SDH level was 468.0 ± 166.3 IU/liter and the average AST level was 154.3 ± 27.0 IU/liter versus 183.2 ± 108.8 IU/liter and 85.2 ± 28.4 IU/liter in all the other TDF-treated woodchucks; P < 0.01). After 4 weeks of treatment, the average log10 change in SDH level from baseline at week zero for woodchucks M5856, F5905, and F5865 demonstrated a decrease of 0.193 that was significantly different from the increase of 0.119 observed in all the other TDF-treated woodchucks (P < 0.01). Importantly, woodchucks M5856, F5905, and F5865 had the greatest reductions in WHV viremia (Fig. 1) and in hepatic expression of WHcAg and WHsAg (Fig. 2 [data shown for F5905 and F5865]) at the end of TDF treatment compared to all the other TDF-treated woodchucks. Woodchucks F5905 and F5865 also had the greatest reduction in WHsAg serum antigenemia (Fig. 2) and in intrahepatic WHV replication intermediates (Fig. 3) after 4 weeks of TDF treatment.
A slight, but statistically significant, elevation in the average count of platelets was observed in the 15.0-mg/kg/day TDF dose group at the end of the treatment period compared to the control group (985.5 × 103 ± 145.7 × 103 versus 761.1 × 103 ± 74.0 × 103; P < 0.05). Increases in the average platelet count from pretreatment levels also were noted at the end of drug treatment in the other TDF dose groups and in the placebo-treated control group, but these differences were not statistically significant. The average platelet count continued to increase further in all experimental groups (except in the 5.0-mg/kg/day TDF dose group) until 4 weeks following drug withdrawal, with no significant differences between TDF-treated groups and placebo-treated controls. Thrombocytosis was reversible, and the average platelet counts decreased in all groups with no significant differences between TDF-treated and control groups (data not shown).
DISCUSSION
In this report, the antiviral activity of the nucleotide analogue TDF was assessed against WHV in chronically infected woodchucks versus placebo controls in a dose-finding study. In the woodchuck model of chronic HBV infection, we show that TDF is an antiviral against wild-type hepadnaviruses that significantly suppresses serum viremia in vivo compared to placebo-treated controls and without significant toxicity during a 4-week period of daily oral administration.
TDF at doses of 1.5 and 5.0 mg/kg/day for 4 weeks inhibited WHV replication significantly in chronic WHV carrier woodchucks as demonstrated by 1.1- to 1.5-log reductions in serum WHV DNA concentrations compared to pretreatment levels (P < 0.05 to 0.01) (Fig. 1; Table 1). Reduced antiviral activity was observed with decreasing dose of TDF, although at the highest dose used (15.0 mg/kg/day) the high variability of antiviral responses observed in individual woodchucks seemed to indicate diminished activity. However, by excluding woodchuck F5959, the one woodchuck of this group that appeared nonresponsive to TDF treatment (Fig. 1), a 1.3-log reduction in the average concentration of serum WHV viremia was observed at the end of treatment (data not shown).
The results are consistent with those of in vitro studies on the efficacy of TDF against wild-type HBV and duck HBV in cell cultures (53-55). The relative reduction in serum WHV viremia in this study was similar to those previously reported for other antiviral drugs after 4 weeks of administration to chronic WHV carrier woodchucks (e.g., adefovir dipivoxil, β-l-2′-deoxyadenosine [L-dA], famciclovir, interferon alpha, lamivudine) (6, 12, 25-28). Four-week treatment of chronic WHV carrier woodchucks with 1-O-hexadecylpropanediol-3-phospho-acyclovir, clevudine (L-FMAU), emtricitabine, entecavir, telbivudine (L-dT, β-l-thymidine), or β-l-2′-deoxycytidine (L-dC), however, induced greater antiviral effects on serum WHV viremia than those observed with TDF in this study (6, 8, 13, 21, 28, 34, 38).
A significant percentage of patients infected with HIV are coinfected with HBV and are at risk of developing HBV-associated liver diseases. Many patients coinfected with HIV and HBV have received lamivudine as part of their combination therapy for HIV. Antiviral activity against HBV in such patients is characteristically transient because of the development of lamivudine resistance (4, 17, 20, 44). In several recent studies, HIV patients with lamivudine-resistant HBV infection have been treated with TDF, and a significant inhibition of HBV replication has been demonstrated (3, 5, 17, 36, 37, 41). Since TDF is used clinically in combination with other nucleosides to treat HIV infection, there is interest in examining TDF alone or in combination with other antiviral drugs for treatment of chronic HBV infection.
The results thus far assessing the efficacy of TDF against HBV in HIV-coinfected patients after 24 weeks of treatment demonstrate that HBV DNA concentrations decreased by approximately 4 to 5 logs on average (3, 17, 36, 37, 41, 46, 47). After 4 weeks of daily treatment with TDF the reduction in the average HBV DNA concentrations in these patients was between 1 and 3 logs (36, 46, 47). Furthermore, daily TDF treatment of patients infected with lamivudine-resistant HBV mutants for 12 months demonstrated a similar reduction in HBV DNA concentrations as those seen in HBV/HIV-coinfected patients of 4.5 to 5.5 logs on average (29, 46, 47).
The rapid rebound of WHV replication following termination of therapy, the lack of an effect on hepatic WHV RNA in all TDF-treated woodchucks, the lack of reduction on serum WHsAg, and hepatic expression of viral antigens in most woodchucks are likely due to the failure of 4 weeks of TDF treatment to reduce WHV replication to levels low enough to reduce the levels of covalently closed circular WHV DNA (WHV cccDNA) in the liver. Hepadnaviral cccDNA genomes are the template for viral RNA transcription (18); however, there are currently no data available on the efficacy of TDF in reducing hepadnaviral cccDNA levels following short- or long-term treatment.
Four weeks of TDF therapy at oral doses of 0.5, 1.5, 5.0, and 15.0 mg/kg/day was well tolerated in WHV-infected woodchucks and produced no physical, biochemical, or hematological evidence of toxicity at any dose. No evidence of nephrotoxicity was observed in any of the treated woodchucks in this study.
In three woodchucks from the 5.0- and 15.0-mg/kg/day TDF dose groups (M5856, F5905, and F5865) in which serum activities of SDH and AST were significantly elevated prior to the start of treatment compared to all the other woodchucks treated with TDF, SDH levels were decreased by the end of the 4-week treatment period. These three woodchucks also had the greatest reductions in certain viral markers at the end of treatment compared to all the other TDF-treated woodchucks (Fig. 1 to 3). This observation on SDH levels in woodchucks M5856, F5905, and F5865 is similar to the majority of reports of serum enzyme normalization observed in TDF-treated patients that are coinfected with HBV and HIV (17, 36, 37, 41, 46, 47).
A statistically significant increase in the average platelet count was observed at the end of treatment in the group that received the highest dose of TDF (i.e., 15.0 mg/kg/day), but the effect was transient and within the normal range for woodchucks. Increases in the platelet counts have not been reported in preclinical studies and appear not to be a common observation during TDF treatment of patients with HBV/HIV coinfection, and one study reported that the number of platelets remained unaltered (47).
In summary, oral TDF was safe and effective in reducing concentrations of WHV DNA and WHV DNA replicative intermediates in serum and in liver in the woodchuck model of chronic HBV infection. Significant suppression of serum WHV viremia was seen at TDF doses of 0.5, 1.5, and 5.0 mg/kg/day, whereas the 15.0-mg/kg/day dosage appeared not to provide further benefit. This was related to the high degree of variation in the antiviral response of individual woodchucks and, perhaps, to the saturation of a critical mechanism with increasing doses of TDF that was suggested by the pharmacokinetic results. The antiviral activity and favorable safety profile observed in vivo in woodchucks support the continued clinical development of TDF alone and in combination with lamivudine or other antiviral drugs for treatment of chronic HBV infection.
Acknowledgments
This work was supported by contract NO1-AI-05399 (College of Veterinary Medicine, Cornell University) and contract NO1-AI-95390 (Division of Molecular Virology and Immunology, Georgetown University School of Medicine) from the National Institute of Allergy and Infectious Diseases.
We gratefully acknowledge the expert assistance of Mary Ascenzi, Betty Baldwin, Lou Ann Graham, Erin Graham, and Chris Bellezza (Cornell University) and Frances Wells (Georgetown University).
REFERENCES
- 1.Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. [DOI] [PubMed] [Google Scholar]
- 2.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhamou, Y., R. Tubiana, and V. Thibault. 2003. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N. Engl. J. Med. 348:177-178. [DOI] [PubMed] [Google Scholar]
- 4.Bessesen, M., D. Ives, L. Condreay, S. Lawrence, and K. E. Sherman. 1999. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin. Infect. Dis. 28:1032-1035. [DOI] [PubMed] [Google Scholar]
- 5.Bruno, R., P. Sacchi, C. Zocchetti, V. Ciappina, M. Puoti, and G. Filice. 2003. Rapid hepatitis B virus-DNA decay in co-infected HIV-hepatitis B virus ‘e-minus’ patients with YMDD mutations after 4 weeks of tenofovir therapy. AIDS 17:783-784. [DOI] [PubMed] [Google Scholar]
- 6.Bryant, M. L., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, P. Marion, E. Cretton-Scott, R. F. Schinazi, and J. P. Sommadossi. 2001. Antiviral l-nucleosides specific for hepatitis B virus infection. Antimicrob. Agents Chemother. 45:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreno, V., J. C. Porres, I. Mora, J. Gutiez, J. A. Quiroga, S. Ramon y Cajal, H. Oliva, C. Compernolle, and J. Bartolome. 1987. A controlled study of treatment with recombinant interferon alpha in chronic hepatitis B virus infection: induction and maintenance schedules. Antivir. Res. 8:125-137. [DOI] [PubMed] [Google Scholar]
- 8.Colonno, R. J., E. V. Genovesi, I. Medina, L. Lamb, S. K. Durham, M. L. Huang, L. Corey, M. Littlejohn, S. Locarnini, B. C. Tennant, B. Rose, and J. M. Clark. 2001. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J. Infect. Dis. 184:1236-1245. [DOI] [PubMed] [Google Scholar]
- 9.Cote, P. J., B. E. Korba, R. H. Miller, J. R. Jacob, B. H. Baldwin, W. E. Hornbuckle, R. H. Purcell, B. C. Tennant, and J. L. Gerin. 2000. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31:190-200. [DOI] [PubMed] [Google Scholar]
- 10.Cote, P. J., C. Roneker, K. Cass, F. Schodel, D. Peterson, B. Tennant, F. De Noronha, and J. Gerin. 1993. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 6:161-169. [DOI] [PubMed] [Google Scholar]
- 11.Cote, P. J., I. Toshkov, I. Nakamura, S. Menne, B. Korba, B. Tennant, and J. Gerin. 2002. Chronicity as an outcome of experimental neonatal woodchuck hepatitis virus infection results from a deficient type 1 immune response to acute infection, p. 280-285. In H. S. Margolis, M. J. Alter, T. J. Liang, and J. L. Dienstag (ed.), Viral hepatitis and liver diseases. Proceedings of the 10th International Symposium on Viral Hepatitis and Liver Disease. International Medical Press Ltd., Atlanta, Ga.
- 12.Cullen, J. M., D. H. Li, C. Brown, E. J. Eisenberg, K. C. Cundy, J. Wolfe, J. Toole, and C. Gibbs. 2001. Antiviral efficacy and pharmacokinetics of oral adefovir dipivoxil in chronically woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 45:2740-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen, J. M., S. L. Smith, M. G. Davis, S. E. Dunn, C. Botteron, A. Cecchi, D. Linsey, D. Linzey, L. Frick, M. T. Paff, A. Goulding, and K. Biron. 1997. In vivo antiviral activity and pharmacokinetics of (-)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 41:2076-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. O. Kahn. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaugerre, C., A. G. Marcelin, V. Thibault, G. Peytavin, T. Bombled, M. V. Bochet, C. Katlama, Y. Benhamou, and V. Calvez. 2002. Human immunodeficiency virus (HIV) type 1 reverse transcriptase resistance mutations in hepatitis B virus (HBV)-HIV-coinfected patients treated for HBV chronic infection once daily with 10 milligrams of adefovir dipivoxil combined with lamivudine. Antimicrob. Agents Chemother. 46:1586-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dienstag, J. L., R. P. Perrillo, E. R. Schiff, M. Bartholomew, C. Vicary, and M. Rubin. 1995. A preliminary trial of lamivudine for chronic hepatitis B infection. N. Engl. J. Med. 333:1657-1661. [DOI] [PubMed] [Google Scholar]
- 17.Dore, G. J., D. A. Cooper, A. L. Pozniak, E. DeJesus, L. Zhong, M. D. Miller, B. Lu, and A. K. Cheng. 2004. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J. Infect. Dis. 189:1185-1192. [DOI] [PubMed] [Google Scholar]
- 18.Ganem, D. 1996. Hepadnaviridae: the viruses and their replication, p. 2703-2737. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 19.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 20.Hoff, J., F. Bani-Sadr, M. Gassin, and F. Raffi. 2001. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of anti-human immunodeficiency virus regimens. Clin. Infect. Dis. 32:963-969. [DOI] [PubMed] [Google Scholar]
- 21.Hostetler, K. Y., J. R. Beadle, W. E. Hornbuckle, C. A. Bellezza, I. A. Tochkov, P. J. Cote, J. L. Gerin, B. E. Korba, and B. C. Tennant. 2000. Antiviral activities of oral 1-O-hexadecylpropanediol-3-phosphoacyclovir and acyclovir in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 44:1964-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain, M., and A. S. Lok. 1999. Mutations in the hepatitis B virus polymerase gene associated with antiviral treatment for hepatitis B. J. Viral Hepat. 6:183-194. [DOI] [PubMed] [Google Scholar]
- 23.Jacob, J. R., B. E. Korba, P. J. Cote, I. Toshkov, W. E. Delaney IV, J. L. Gerin, and B. C. Tennant. 2004. Suppression of lamivudine-resistant B-domain mutants by adefovir dipivoxil in the woodchuck hepatitis virus model. Antivir. Res. 63:115-121. [DOI] [PubMed] [Google Scholar]
- 24.Kiriakidis, M. 2001. The determination of GS-1278 (R-PMPA) in rhesus monkey plasma (EDTA) using a high performance liquid chromatographic mass spectrometric method. MDS Pharma Services, Montreal, Canada.
- 25.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. D. Gangemi, B. C. Tennant, and J. L. Gerin. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and alpha interferon against WHV replication in chronic carrier woodchucks. Antivir. Ther. 5:95-104. [PubMed] [Google Scholar]
- 26.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. L. Gerin, and B. C. Tennant. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and famciclovir against WHV replication in chronic WHV carrier woodchucks. Antivir. Res. 45:19-32. [DOI] [PubMed] [Google Scholar]
- 27.Korba, B. E., P. Cote, W. Hornbuckle, B. C. Tennant, and J. L. Gerin. 2000. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 31:1165-1175. [DOI] [PubMed] [Google Scholar]
- 28.Korba, B. E., R. F. Schinazi, P. Cote, B. C. Tennant, and J. L. Gerin. 2000. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob. Agents Chemother. 44:1757-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo, A., J. L. Dienstag, and R. T. Chung. 2004. Tenofovir disoproxil fumarate for the treatment of lamivudine-resistant hepatitis B. Clin. Gastroenterol. Hepatol. 2:266-272. [DOI] [PubMed] [Google Scholar]
- 30.Lau, D. T., M. F. Khokhar, E. Doo, M. G. Ghany, D. Herion, Y. Park, D. E. Kleiner, P. Schmid, L. D. Condreay, J. Gauthier, M. C. Kuhns, T. J. Liang, and J. H. Hoofnagle. 2000. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 32:828-834. [DOI] [PubMed] [Google Scholar]
- 31.Louie, M., C. Hogan, A. Hurley, V. Simon, C. Chung, N. Padte, P. Lamy, J. Flaherty, D. Coakley, M. Di Mascio, A. S. Perelson, and M. Markowitz. 2003. Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naive chronically HIV-1-infected individuals. AIDS 17:1151-1156. [DOI] [PubMed] [Google Scholar]
- 32.Marcellin, P., T. T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B E antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 33.Menne, S., and P. J. Cote. 2003. The woodchuck as an emerging animal model for immunopathogenesis and immunotherapy of human HBV infection. Recent Res. Dev. Virol. 5:117-141. [Google Scholar]
- 34.Menne, S., C. A. Roneker, B. E. Korba, J. L. Gerin, B. C. Tennant, and P. J. Cote. 2002. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-beta-l-arabinofuranosyl)-uracil (L-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J. Virol. 76:5305-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menne, S., and B. C. Tennant. 1999. Unraveling hepatitis B virus infection of mice and men (and woodchucks and ducks). Nat. Med. 5:1125-1126. [DOI] [PubMed] [Google Scholar]
- 36.Nelson, M., S. Portsmouth, J. Stebbing, M. Atkins, A. Barr, G. Matthews, D. Pillay, M. Fisher, M. Bower, and B. Gazzard. 2003. An open-label study of tenofovir in HIV-1 and Hepatitis B virus co-infected individuals. AIDS 17:F7-F10. [DOI] [PubMed] [Google Scholar]
- 37.Nunez, M., M. Perez-Olmeda, B. Diaz, P. Rios, J. Gonzalez-Lahoz, and V. Soriano. 2002. Activity of tenofovir on hepatitis B virus replication in HIV-co-infected patients failing or partially responding to lamivudine. AIDS 16:2352-2354. [DOI] [PubMed] [Google Scholar]
- 38.Peek, S. F., P. J. Cote, J. R. Jacob, I. A. Toshkov, W. E. Hornbuckle, B. H. Baldwin, F. V. Wells, C. K. Chu, J. L. Gerin, B. C. Tennant, and B. E. Korba. 2001. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 33:254-266. [DOI] [PubMed] [Google Scholar]
- 39.Perrillo, R., E. Schiff, E. Yoshida, A. Statler, K. Hirsch, T. Wright, K. Gutfreund, P. Lamy, and A. Murray. 2000. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129-134. [DOI] [PubMed] [Google Scholar]
- 40.Renault, P. F., and J. H. Hoofnagle. 1989. Side effects of alpha interferon. Semin. Liver Dis. 9:273-277. [DOI] [PubMed] [Google Scholar]
- 41.Ristig, M. B., J. Crippin, J. A. Aberg, W. G. Powderly, M. Lisker-Melman, L. Kessels, and P. Tebas. 2002. Tenofovir disoproxil fumarate therapy for chronic hepatitis B in human immunodeficiency virus/hepatitis B virus-coinfected individuals for whom interferon-alpha and lamivudine therapy have failed. J. Infect. Dis. 186:1844-1847. [DOI] [PubMed] [Google Scholar]
- 42.Tennant, B. C., B. H. Baldwin, L. A. Graham, M. A. Ascenzi, W. E. Hornbuckle, P. H. Rowland, I. A. Tochkov, A. E. Yeager, H. N. Erb, J. M. Colacino, C. Lopez, J. A. Engelhardt, R. R. Bowsher, F. C. Richardson, W. Lewis, P. J. Cote, B. E. Korba, and J. L. Gerin. 1998. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology 28:179-191. [DOI] [PubMed] [Google Scholar]
- 43.Tennant, B. C., I. A. Toshkov, S. F. Peek, J. R. Jacob, S. Menne, W. E. Hornbuckle, R. D. Schinazi, B. E. Korba, P. J. Cote, and J. L. Gerin. 2004. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 127(Suppl. 1):S283-S293. [DOI] [PubMed] [Google Scholar]
- 44.Thibault, V., Y. Benhamou, C. Seguret, M. Bochet, C. Katlama, F. Bricaire, P. Opolon, T. Poynard, and H. Agut. 1999. Hepatitis B virus (HBV) mutations associated with resistance to lamivudine in patients coinfected with HBV and human immunodeficiency virus. J. Clin. Microbiol. 37:3013-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Bommel, F., A. Schernick, U. Hopf, and T. Berg. 2003. Tenofovir disoproxil fumarate exhibits strong antiviral effect in a patient with lamivudine-resistant severe hepatitis B reactivation. Gastroenterology 124:586-587. [DOI] [PubMed] [Google Scholar]
- 46.Van Bommel, F., T. Wunsche, S. Mauss, P. Reinke, A. Bergk, D. Schurmann, B. Wiedenmann, and T. Berg. 2004. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology 40:1421-1425. [DOI] [PubMed] [Google Scholar]
- 47.Van Bommel, F., T. Wunsche, D. Schurmann, and T. Berg. 2002. Tenofovir treatment in patients with lamivudine-resistant hepatitis B mutants strongly affects viral replication. Hepatology 36:507-508. [DOI] [PubMed] [Google Scholar]
- 48.Villeneuve, J. P., D. Durantel, S. Durantel, C. Westland, S. Xiong, C. L. Brosgart, C. S. Gibbs, P. Parvaz, B. Werle, C. Trepo, and F. Zoulim. 2003. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J. Hepatol. 39:1085-1089. [DOI] [PubMed] [Google Scholar]
- 49.Vryheid, R. E., M. A. Kane, N. Muller, G. C. Schatz, and S. Bezabehd. 2000. Infant and adolescent hepatitis B immunization up to 1999: a global overview. Vaccine 19:1026-1037. [DOI] [PubMed] [Google Scholar]
- 50.Westland, C. E., H. Yang, W. E. Delaney IV, C. S. Gibbs, M. D. Miller, M. Wulfsohn, J. Fry, C. L. Brosgart, and S. Xiong. 2003. Week 48 resistance surveillance in two phase 3 clinical studies of adefovir dipivoxil for chronic hepatitis B. Hepatology 38:96-103. [DOI] [PubMed] [Google Scholar]
- 51.Xiong, S., H. Yang, C. E. Westland, W. E. Delaney, D. Colledge, A. Bartholomeusz, V. Thibault, Y. Benhamou, P. Angus, M. Wulfsohn, C. S. Gibbs, J. Fry, C. L. Brosgart, and S. Locarnini. 2003. Resistance surveillance of HBeAg-chronic hepatitis B (CHB) patients treated for two years with adefovir dipivoxil (ADV). J. Hepatol. 38(Suppl. 2):182. [Google Scholar]
- 52.Yang, H., C. E. Westland, W. E. Delaney IV, E. J. Heathcote, V. Ho, J. Fry, C. Brosgart, C. S. Gibbs, M. D. Miller, and S. Xiong. 2002. Resistance surveillance in chronic hepatitis B patients treated with adefovir dipivoxil for up to 60 weeks. Hepatology 36:464-473. [DOI] [PubMed] [Google Scholar]
- 53.Ying, C., E. De Clercq, and J. Neyts. 2000. Lamivudine, adefovir and tenofovir exhibit long-lasting anti-hepatitis B virus activity in cell culture. J. Viral Hepat. 7:79-83. [DOI] [PubMed] [Google Scholar]
- 54.Ying, C., E. De Clercq, W. Nicholson, P. Furman, and J. Neyts. 2000. Inhibition of the replication of the DNA polymerase M550V mutation variant of human hepatitis B virus by adefovir, tenofovir, L-FMAU, DAPD, penciclovir and lobucavir. J. Viral Hepat. 7:161-165. [DOI] [PubMed] [Google Scholar]
- 55.Yokota, T., K. Konno, E. Chonan, S. Mochizuki, K. Kojima, S. Shigeta, and E. de Clercq. 1990. Comparative activities of several nucleoside analogs against duck hepatitis B virus in vitro. Antimicrob. Agents Chemother. 34:1326-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]