Abstract
Background
Prostate cancer is the most common diagnosed tumor and the fifth cancer related death among men in Europe. Although several genetic alterations such as ERG-TMPRSS2 fusion, MYC amplification, PTEN deletion and mutations in p53 and BRCA2 genes play a key role in the pathogenesis of prostate cancer, specific gene alteration signature that could distinguish indolent from aggressive prostate cancer or may aid in patient stratification for prognosis and/or clinical management of patients with prostate cancer is still missing. Therefore, here, by a multi-omics approach we describe a prostate cancer carrying the fusion of TMPRSS2 with ERG gene and deletion of 16q chromosome arm.
Results
We have observed deletion of KDM6A gene, which may represent an additional genomic alteration to be considered for patient stratification. The cancer hallmarks gene signatures highlight intriguing molecular aspects that characterize the biology of this tumor by both a high hypoxia and immune infiltration scores. Moreover, our analysis showed a slight increase in the Tumoral Mutational Burden, as well as an over-expression of the immune checkpoints. The omics profiling integrating hypoxia, ROS and the anti-cancer immune response, optimizes therapeutic strategies and advances personalized care for prostate cancer patients.
Conclusion
The here data reported can lay the foundation for predicting a poor prognosis for the studied prostate cancer, as well as the possibility of targeted therapies based on the modulation of hypoxia, ROS, and the anti-cancer immune response.
Keywords: Prostate cancer, KDM6A, Hypoxia, ROS, Immune response, Personalized medicine
Background
Prostate cancer (PC) is widely recognized for its elevated occurrence and genomic variability [1] resulting in significant gene alteration [2, 3]. In 2020, the reported number of new cases exceeded 1,414,000 worldwide, with over 375,300 related deaths, underscoring the substantial global impact and burden associated with this neoplasia (https://gco.iarc.fr/tomorrow). Approximately 80% of cases are initially localized, while 20% exhibit metastasis to regional lymph nodes (13%) or distant organs (6–7%) at the time of diagnosis. The presence of metastasis significantly impacts the 5-year survival rate, reaching 100% for localized cases and dropping to 30% for those with metastasis. PC exhibits an extended natural progression, categorized through various parameters including prostate-specific antigen (PSA) levels, histological features, TNM classification, and clinical conditions such as localized PC, advanced PC, and metastatic PC [4–6].
Treatment is currently depending on the stage of the tumor, evaluated both at clinical and histological level. Accordingly, the main therapeutical options include active surveillance, radical prostatectomy, or standalone external-beam radiation therapy [7]. Despite the slow growth of prostate lesions, metastatic formations typically emerge around a decade post-diagnosis, posing a substantial health challenge in patient management [2, 8]. The current clinical and histological prognostic parameters used for PC management are still in evolution. PSA levels used for early screening or recurrence detection, are influenced by several factors such as aging and inflammation [9]. The Gleason score, as well as its new Gleason Group classification, provides a more straightforward representation for clinicians and support in treatment decisions [10]. Nonetheless, several studies demonstrate that PC lesions with the same Gleason group can exhibit different biological behaviors and responses to treatment [11, 12]. In this context, the search of specific molecular profile for PC gains prominence. By integrating molecular markers, genomic profiling, and advanced technologies, researchers and clinicians aim to refine the stratification of PC. A molecular characterization not only holds the promise of improving prognostic accuracy but also opens new perspectives for the development of targeted therapies.
In recent years, molecules implicated in various signaling pathways have been suggested as targets for PC diagnosis, prognosis, and personalized therapies [13–15]. Specifically, germline mutations in DNA damage repair genes [16], pathways associated with cancer-related hypoxia [17], cell death [18], epithelial-to-mesenchymal transition (EMT) [19–21], proliferation, and anti-tumoral immune response have been proposed as main drivers of PC occurrence and progression. Tumor hypoxia, stemming from an irregular and dysfunctional vascular network within the tumor, represents a significant barrier to the effectiveness of immunotherapy [22]. Indeed, hypoxia gives rise to an immunosuppressive tumor microenvironment (TME) [23] by fostering processes such as angiogenesis, metabolic reprogramming [24, 25], remodeling of the extracellular matrix, the EMT, p53 inactivation [26–30], and evasion of the immune response [31–34]. Hence, mitigating cancer-associated hypoxia could potentially serve as an effective strategy to decrease PC proliferation, as well as enhance the effectiveness of various conventional and unconventional treatments, including hormone therapy or immune modulation. In particular, the possibility to modify the anti-tumoral immune response is becoming a therapeutic choice for patients with recurrent PC, as recent evidence has demonstrated the overexpression of immune checkpoint molecules such as PDL-1 and CTLA4 in PC [35–37].
The convergence of morphological and molecular evidence could provide a comprehensive understanding of PC, offering clinicians the tools they need to make more informed decisions regarding prognosis and personalized therapeutic interventions [38, 39]. This synthesis of traditional pathology and cutting-edge molecular biology represents a crucial step forward in advancing our capabilities to detect and cure PC.
To achieve this goal, comprehensive data related to the molecular characterization of specific PC cases are essential. In light of this, in this case report, we present a thorough morphological and multi-omics investigation of a PC sample from a 69-year-old patient. This analysis highlights intriguing molecular aspects that characterize the biology of this tumor such as immune checkpoints up-regulation and very high hypoxia and proliferation molecular signature scores. This paradigmatic case demonstrates the need to individual multi-omic analysis.
Methods
Collection of samples
Tumor tissues collection was performed using standardized protocol [40, 41]. Hematoxylin and Eosin (H&E) stained serial sections were used for pathological quality control (QC). Criteria for selecting tumor samples included a tumor content of at least 30%, necrosis less than or equal to 30%, and the presence of invasive tumor cells. Adjacent normal tissues were also procured. Protein lysate preparation and nucleic acid extraction were carried out using 10 mg of each tissue specimen [42–45]. Throughout the procedure, tissues remained frozen to maintain integrity.
Histological examination utilized serial sections from formalin-fixed and paraffin-embedded (FFPE) blocks [47–49]. Two independent pathologists conducted histological analysis on hematoxylin and eosin (H&E)-stained slides.
Nucleic acid extraction and quality assessment
As previously described, frozen tissue slices were used for nucleic acid extraction and quality assessment [50].
Library preparation and NGS sequencing
Libraries for whole genome sequencing (WGS) and whole transcriptome sequencing were performed as previously described [50].
NGS data processing
To align NGS data, Grch38 genome assembly was used as reference. As concern the normal samples, the Haplotype Caller from the Genome Analysis Toolkit (GATK) was used for both identification and annotation of short genomic variations. WGS somatic variations were called using a consensus of Mutect2 [51], Strelka [52], Varscan [53] and Somatic Sniper [54]. Structural variations were called using R packages TitanCNA [55], DellyCNV and DellyCall [56], as well as Manta [57]. RNA-Seq differential expression was based on normalized readcount data (TPM: transcripts per million).
Bioinformatical analyses
Mutational signatures were calculated using the R package MutationalPatterns [58–61]. MSI classification was done using R package MSIseq [63]. Metrices to define chromosomal instability were determined using R package CINmetrics [50] and CNHplus [64]. Aneuploidy events were analysed using ASCETS [65]. Aneuploidy event span more than 90% of the chromosome. Visualization of results was done in IGV [66]. TMB was calculated as the number of non-synonymous mutations of protein coding genes divided by exome size in Megabases.
Results and discussion
A 69-year-old patient underwent radical prostatectomy due to the presence of a suspicious nodule in the apical and left posterolateral region. Histological examination allowed the identification of several multifocal cancer lesions, the largest of which measured 2.3 cm. According to the WHO grade, the lesion was classified as an acinar prostate infiltrating carcinoma with a Gleason score of 9 (4 + 5), grade group 5, very aggressive PC. In the upper left lateral portion of the prostate, the carcinomatous lesion infiltrated the prostatic apex and the surrounding adipose tissue. No metastatic lymph nodes were detected. According to the TNM classification, the tumour was staged as pT2c pN0. The patient did not undergo previous therapy.
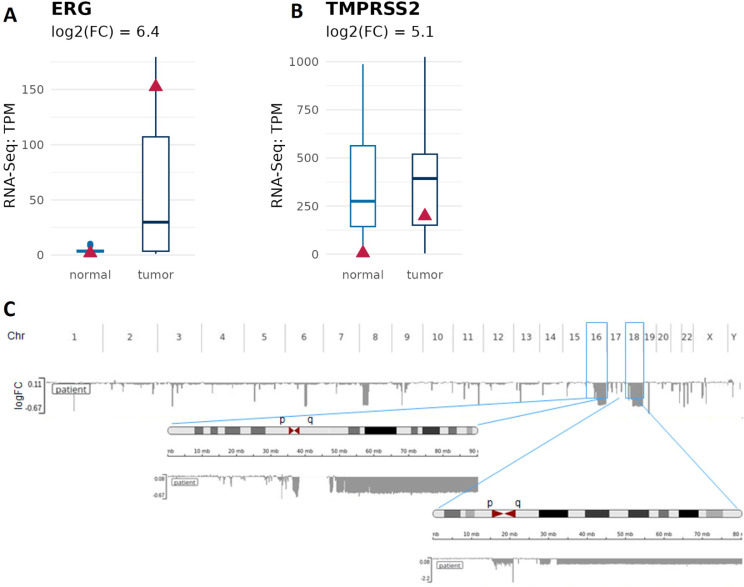
Genomic and transcriptomic profile of the prostate cancer revealed ERG-TMPRSS2 gene fusion [37] (Fig. 1). This genomic alteration was first described in 2005 [66] and is characterized by a chromosomal rearrangement that leads to the fusion of the promoter region of the transmembrane protease serine 2 (TMPRSS2) (locus 21q22.3) with locus 21q22.2 carrying the gene ERG, a member of the transcription factor erythroblastosis virus E26 transforming sequence family (ETS). In agreement with the literature [67], our transcriptomic analysis show that both genes are also highly up regulated in our patient of interest (log2FC: 6.4 and 5.1) (Fig. 1A and B). In addition, we have also found several chromosome rearrangements including large chromosomal deletions (> 90% of the chromosome arm) in chromosome arms 16q (cohort: 5.6%) and 18q (cohort: 19.4%), which have been already reported in prostate cancer [68, 69] (Fig. 1C). These alterations are predictors of poor prognosis. Indeed, they are associated with advanced tumor stage, high Gleason score and increased risk of biochemical recurrence [70, 71]. ERG fusion positive PC show a higher frequency of 3p13, 16q23, TP53 and PTEN deletion [72, 73]. In agreement, analyzed PC shows both deletion of 16q and ERG fusion [74].
Fig. 1.
Tumor isolated from our patient of interest carries ERG-TMPRSS2 gene fusion. A) ERG mRNA expression is higher in tumor tissue of our patient of interest when compared to the normal adjacent tissue. B) TMPRSS2 mRNA expression is higher in tumor tissue of our patient of interest when compared to the normal adjacent tissue. RNA levels were assessed by RNA sequencing; TPM = transcripts per million. C) Image shows aneuploidy of analyzed PC tissue. Boxplots indicate the values of prostate cancer background cohort, and the red triangle refers to our patient of interest
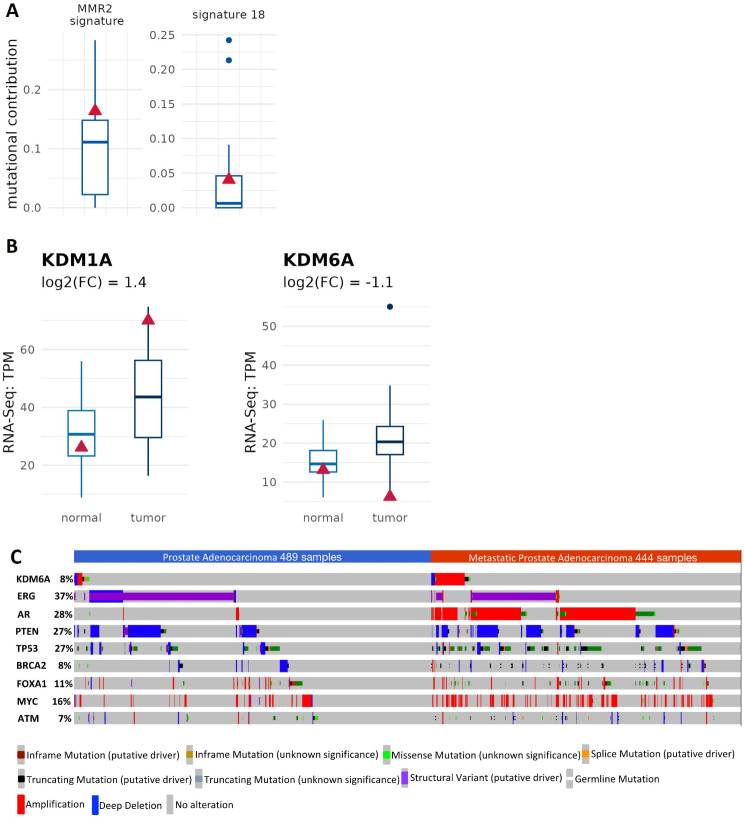
PC is associated with the accumulation of somatic mutations in the prostate epithelial involving mainly genes that modulate cell growth, cell proliferation, cell death and DNA damage response [75]. However, the mutational burden of prostate cancer is very low [76] and copy number changes and chromosomal rearrangement are the most characteristic genomic alterations displayed in PC [77]. In keeping, we did not find somatic mutations in cancer related genes. Nevertheless, we observed some germline mutations affecting genes involved the regulation of DNA damage response (Table 1). To further support this, the DNA mismatch repair (MMR) signature has a slightly increased frequency (Fig. 2A).
Table 1.
Germline variation in genes belonging to DNA damage response pathways in the patient of interest
| Symbol | Transcript | Effect | AA change |
|---|---|---|---|
| APEX1 | ENST00000216714.7 | missense variant | Asp148Glu |
| BARD1 | ENST00000432456.5 | splice region variant | |
| BRCA1 | ENST00000357654.7 | missense variant | Ser1613Gly |
| BRCA1 | ENST00000357654.7 | missense variant | Glu1038Gly, Pro871Leu |
| CHEK1 | ENST00000428830.6 | missense variant | Ile471Val |
| EME1 | ENST00000338165.8 | missense variant | Glu69Asp |
| ERCC2 | ENST00000391945.8 | missense variant | Lys751Gln, Asp312Asn |
| ERCC4 | ENST00000311895.7 | splice region variant, intron variant | |
| ERCC5 | ENST00000355739.8 | missense variant | Gly1053Arg, Gly1080Arg |
| ERCC6 | ENST00000355832.9 | splice region variant, intron variant | |
| EXO1 | ENST00000348581.9 | missense variant | Val458Met |
| FANCA | ENST00000567510.1 | frameshift variant | Glu77fs |
| FANCM | ENST00000267430.9 | missense variant | Asn1253Ser, Asn1876Ser |
| GEN1 | ENST00000317402.11 | missense variant | Ser92Thr |
| LIG4 | ENST00000356922.5 | missense variant | Ala3Val |
| PMS1 | ENST00000409985.5 | frameshift variant | Leu164fs |
| PMS2 | ENST00000265849.11 | missense variant | Gly857Ala |
| POLE | ENST00000320574.9 | missense variant | Pro1549Ala |
| POLE | ENST00000320574.9 | missense variant | Phe695Ile |
| POLM | ENST00000492605.5 | splice region variant, non coding transcript exon variant | |
| SEM1 | ENST00000606019.5 | splice region variant, intron variant | |
| TOPBP1 | ENST00000260810.9 | missense variant | Asn1042Ser |
Fig. 2.
MMR and ROS related signatures. (A) Prostate cancer isolated from the patient of interest display a prevalence in MMR2 signature and slightly elevated contribution to signature 18. (B) RNA-Seq analysis showing an increase in the KDM1A expression and a reduction in the KDM6A mRNA levels. (C) KDM6A genetic alterations in prostate adenocarcinoma and metastatic adenocarcinoma. An oncoPrint of individual patient tumours which are positive for genetic alterations in KDM6A compared to the most representative genes involved in the pathogenesis of prostate cancer. Patient cohorts (Prostate Adenocarcinoma: TCGA, PanCancer Atlas; Metastatic Prostate Adenocarcinoma: SU2C/PCF Dream Team, PNAS 2019). Boxplots indicate the values of prostate cancer background cohort, and the red triangle refers to our patient of interest
Relevant studies revealed genetic abnormalities affecting DNA repair mechanisms in almost 20–30% of advanced castration-resistant PCs, some of which are inherited and present in the germline [78]. Phase II/III clinical trial findings, along with additional clinical evidence, endorse the exploration of PARP inhibitors and DNA-damaging agents in this specific molecular PC subgroup, building upon successes observed in other cancer types [79–82]. These investigations present a promising avenue for enhancing patient care through tailored therapeutic approaches. The increase in MMR signature suggests the potential for these innovative therapies for patients of our interest.
Compared to a cohort of 40 pancreatic cancer patients, the analysed PC sample also exhibited elevated reference signature 18 (Fig. 2A). According to the Human Genome Variation Society, this signature is linked to C > A a mutation resulting from ROS-induced guanine oxidation, indicating high levels ROS in the tumor environment. ROS seems indeed associated with poor prognosis, cancer progression, and importantly, resistance to common treatments such as chemotherapy [83, 84]. Therefore, reducing ROS levels could represent a strategy for improving the success of anti-cancer treatments in patients with high reference signature 18. Of note, in our case we have also detected the deletion of the gene coding for lysine demethylases 6 (KDM6A) (Fig. 2B). In PC several epigenetic alterations have been described [85–87], including overexpression of histone lysine demethylases LSD1/KDM1A [88, 89] which is associated with an increased proliferation. To our best knowledge, here for the first time is described a deletion of KDM6A in human PC. To further characterize KDM6A genetic alterations in PC, we took advantage of the online tool cBioPortal for cancer genomics [90–92] for searching genetic alterations across both prostate adenocarcinoma and metastatic prostate adenocarcinoma. As shown in Fig. 2C, the variation frequency of KDM6A is about 8% (cBioPortal), frequency that is comparable with two relevant genes in prostate cancer such as BRCA2 [93] and ATM [94]. The most representative alterations are amplification and deep deletion. Moreover, in few patients several mutations have been identified, including truncating, splice and missense mutations. Among them, truncating mutations of KDM6A have been described mainly in bladder cancer, where are considered likely oncogenic [95]. In particular, those mutations lead to increased cell proliferation and migration. There are also evidences that loss of KDM6A may have a role in resistance to chemotherapy in acute myeloid leukaemia [96]. Moreover, our bioinformatic analysis highlights a subset of patients in which KDM6A gene is amplified, suggesting a possible oncogenic function. Therefore, further investigation is needed to understand whether KDM6A acts either as tumor suppressor gene or oncogene in PC and whether it can potentially play a role as biomarkers for predicting patient prognosis and/or a possible pharmacological target. Beside the classification of tumor subtype by genomic alterations that may assist in predicting prognosis and to guide treatment decision [97], gene expression signature may be a complementary approach to identify biomarkers for a better management of cancer patients. Our RNA-Seq analysis indicates that tumor isolated from our patient of interest is characterized by a high hypoxia (Fig. 3A) and immune infiltration scores (Fig. 3B), which is associated with worse prognosis [98, 99]. The RNA-Seq analysis conducted on PC unveils a striking increase in the expression of hypoxia-related genes. This suggests a profound hypoxic state within the tumor microenvironment, likely stemming from inadequate oxygen supply due to rapid tumor growth and aberrant vasculature [100–102].
Fig. 3.
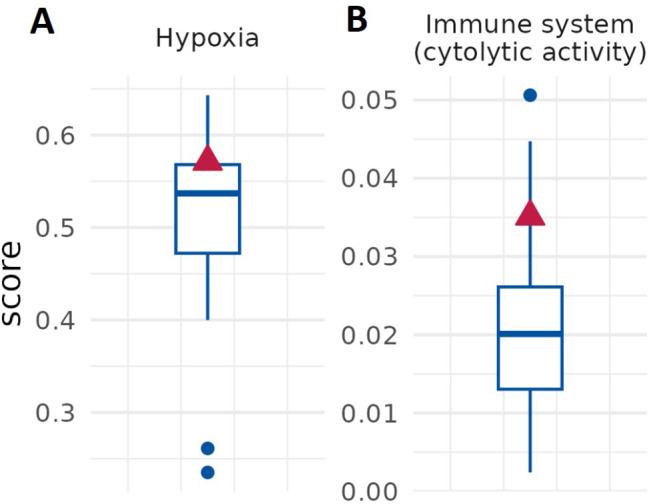
Cancer gene expression signature. Elevated hypoxia (a) and immune system signature (b) scores are detected in patient’s cancer. Red triangle refers to our patient of interest, boxplots to the prostate cohort
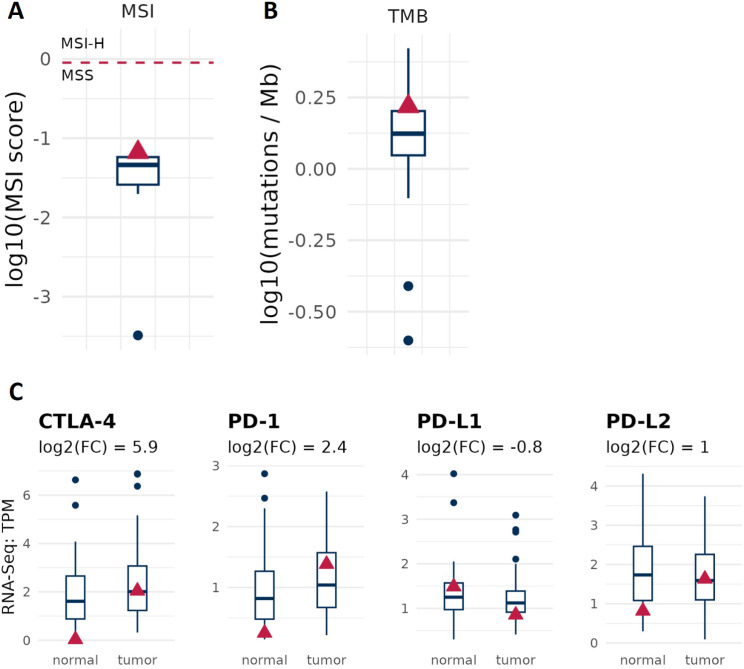
Hypoxia is currently considered a hallmark feature of aggressive cancers, including PC, and is closely associated with poor prognosis [103]. Specifically, hypoxia plays a multifaceted role in shaping the tumor microenvironment and promoting cancer progression. By inducing angiogenesis, metabolic reprogramming, extracellular matrix remodeling, epithelial-mesenchymal transition, p53 inactivation, and immune evasion, hypoxia creates a hostile environment that facilitates tumor growth and metastasis [17, 26, 104]. One notable consequence of hypoxia is the suppression of anti-tumor immune responses within the tumor microenvironment [105, 106]. Therefore, we asked whether immunotherapy may be an option for treating patients that share the same profile of genomic alterations found in our case. To do so, we assessed the specific immunotherapy markers that may hold clinical relevance such as TMB, MSI and immune checkpoint gene expression [107]. As shown in Fig. 4A and B, tumor isolated from our patient of interest show a slight increase in TMB, low MSI and is also chromosome stable. Gene expression profile of PC displays an upregulation of immune checkpoints as CTLA-4, PD-1 and PD-L2 (Fig. 4C). Overall, these biomarkers indicate that in this patient the immunotherapy with checkpoint inhibitors would be recommended.
Fig. 4.
Immunotherapy markers in our patient. (A) Tumor isolated from the patient show elevated tumor mutational burden (TMB). (B) microsatellite instability (MSI) compared to the prostate cohort. (C) RNA-Seq expression levels of immune checkpoint genes in the patient. CTLA-4, PD-1 and PD-L2 mRNA expression is increased in tumor tissue when compared to the adjacent normal tissue. Boxplots indicate the values of prostate cancer background cohort, and the red triangle refers to our patient of interest
In this context, vascular normalization, a therapeutic approach aimed at restoring the structure and function of tumor blood vessels, has emerged as a promising strategy to counteract the effects of hypoxia in cancer [108]. By improving oxygen delivery and reversing hypoxia-induced signaling pathways, vascular normalization can alleviate the hypoxic conditions within tumors. This, in turn, could elevate the efficacy of cancer immunotherapy by creating a more favorable tumor microenvironment for immune cell infiltration and activation. According to this, we also observed the downregulation of miR-224-5p (Fig. 5A). In fact, this miRNA is involved in the complex network that link hypoxia and anti-cancer immune response [109]. Indeed, the miR-224-5p capability to affect the anti-cancer activity of NK cells on PC is regulated by HIF-1α via NCR1/NKp46 pathway. In light of this, the investigated PC tumor was characterized by the presence of few infiltrated NKs (Fig. 5B).
Fig. 5.
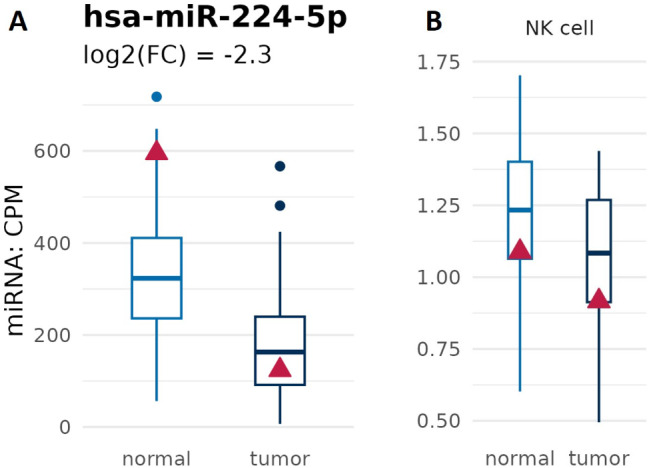
has-miR-224-5p expression and NK cells tumoral infiltration. (A) Graph shows downregulation of has-miR-224-5p in the analyzed PC tissue. (B) Few NK cells in the PC. Boxplots indicate the values of prostate cancer background cohort, and the red triangle refers to our patient of interest
Overall, strategies that target hypoxia and promote vascular normalization hold great potential for improving cancer treatment outcomes, particularly in the context of immunotherapy. By addressing the immunosuppressive effects of hypoxia and enhancing anti-tumor immune responses, these approaches offer new avenues for enhancing patient survival.
Conclusion
The presented case underscores the crucial importance of molecular profiling in prostate carcinomas, emphasizing the shift towards personalized medicine [25, 105, 110, 111]. By identifying specific genomic aberrations such as the ERG-TMPRSS2 gene fusion, deletion of KDM6A, and elevated immune checkpoint expression, we gain invaluable insights into the underlying mechanisms of tumor development and progression. Furthermore, the detection of the MMR signature and ROS-associated reference signature 18 suggests the potential for targeted therapeutic interventions tailored to the individual patient’s molecular profile. These findings emphasize the importance of comprehensive molecular characterization in guiding treatment decisions and improving patient outcomes [112–114]. Moving forward, integrating molecular profiling into clinical practice holds great promise for optimizing therapeutic strategies and advancing personalized care for PC patients. Specifically, the data reported here lay the foundation for predicting a poor prognosis for the studied PC, as well as the possibility of targeted therapies based on the modulation of hypoxia, ROS, and the anti-cancer immune response.
Acknowledgements
Not applicable.
Author contributions
MA, GM, JB, AM and PB conceived the project, MA, GM, JB, MS, YS, GS, EC, GC, JH and FL wrote the manuscript; EG, VR, FS, JB, MC, VI and MS prepared figures. All of the Authors have approved this submitted version.
Funding
This work has been supported by the MUR-PNRR M4C2I1.3 PE6 project PE00000019 Heal Italia (CUP: E83C22004670001) to GM, MA, AM, GS; Associazione Italiana per la Ricerca contro il Cancro (AIRC) to GM (IG 2022 ID 27366; 2023–2027) to EC (IG#22206; 2019–2023).
Data availability
Data will be made available on reasonable request.
Declarations
Ethical approval
All the procedures carried out in the research with participation of humans were in compliance with the ethical standards of the institutional and/or national ethics committee and with the Helsinki Declaration of 1964 and its subsequent changes or with comparable ethics standards. Informed voluntary consent was obtained from every participant of the study: Approval on 09-2019, number 96 − 19. It is not a clinical trial.
Consent for publication
Not applicable.
Competing interests
GM is editor in Biology Direct.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alessandro Mauriello, Email: alessandro.mauriello@uniroma2.it.
Pierluigi Bove, Email: pierluigi.bove@uniroma2.it.
References
- 1.Martínez-Jiménez F, Movasati A, Brunner SR, Nguyen L, Priestley P, Cuppen E, Van Hoeck A. Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature. 2023;618(7964):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Lei Z, Liu W, Xiong J, Shi Y, Yang L, Gao Q, Le K, Zhang B. RCC2 promotes prostate cancer cell proliferation and migration through Hh/GLI1 signaling pathway and cancer stem-like cells. Biol Direct. 2023;18(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Q, Lan Z, Zhang S, Ren H, Wang S, Wang P, Feng L, Li D, Wang C, Bai X, Zhang J. Overexpression of ZNF488 supports pancreatic cancer cell proliferation and tumorigenesis through inhibition of ferroptosis via regulating SCD1-mediated unsaturated fatty acid metabolism. Biol Direct. 2023;18(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Netto GJ, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, Menon S, Raspollini MR, Rubin MA, Srigley JR, Hoon Tan P, Tickoo SK, Tsuzuki T, Turajlic S, Cree I, Moch H. The 2022 World Health Organization Classification of Tumors of the urinary system and male genital organs-Part B: prostate and urinary tract tumors. Eur Urol. 2022;82(5):469–82. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of Tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–19. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40(2):244–52. [DOI] [PubMed]
- 7.Graham LS, Lin JK, Lage DE, Kessler ER, Parikh RB, Morgans AK. Management of prostate Cancer in older adults. Am Soc Clin Oncol Educ Book. 2023;43:e390396. [DOI] [PubMed] [Google Scholar]
- 8.Le Hars M, Castro-Vega LJ, Rajabi F, Tabatadze D, Romero M, Pinskaya M, Groisman I. Pro-tumorigenic role of lnc-ZNF30-3 as a sponge counteracting mir-145-5p in prostate cancer. Biol Direct. 2023;18(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merriel SWD, Pocock L, Gilbert E, Creavin S, Walter FM, Spencer A, Hamilton W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022;20(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, Tombal B, Gillessen S, ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–34. [DOI] [PubMed] [Google Scholar]
- 11.Swanson GP, Trevathan S, Hammonds KAP, Speights VO, Hermans MR. Gleason score evolution and the effect on prostate Cancer outcomes. Am J Clin Pathol. 2021;155(5):711–7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Dong Y, Zhang K. Gene expression analysis reveals a pitfall in the molecular research of prostate tumors relevant to Gleason score. J Bioinform Comput Biol. 2020;18(5):2050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montanaro M, Agostini M, Anemona L, Bonanno E, Servadei F, Finazzi Agrò E, Asimakopoulos AD, Ganini C, Cipriani C, Signoretti M, Bove P, Rugolo F, Imperiali B, Melino G, Mauriello A, Scimeca M. ZNF750: A Novel Prognostic Biomarker in Metastatic Prostate Cancer. Int J Mol Sci. 2023;24(7):6519. ZNF750: A Novel Prognostic Biomarker in Metastatic Prostate Cancer. Int J Mol Sci. 2023;24(7). [DOI] [PMC free article] [PubMed]
- 14.Scimeca M, Montanaro M, Bonfiglio R, Anemona L, Agrò EF, Asimakopoulos AD, Bei R, Manzari V, Urbano N, Giacobbi E, Servadei F, Bonanno E, Schillaci O, Mauriello A. The ETS homologous factor (EHF) represents a useful immunohistochemical marker for Predicting prostate Cancer Metastasis. Diagnostics (Basel). 2022;12(4). [DOI] [PMC free article] [PubMed]
- 15.Mapelli SN, Albino D, Mello-Grand M, Shinde D, Scimeca M, Bonfiglio R, Bonanno E, Chiorino G, Garcia-Escudero R, Catapano CV, Carbone GM. A novel prostate cell type-specific gene signature to interrogate prostate tumor differentiation status and monitor therapeutic response (running title: phenotypic classification of prostate tumors). Cancers (Basel). 2020;12(1). [DOI] [PMC free article] [PubMed]
- 16.Lozano R, Castro E, Aragón IM, Cendón Y, Cattrini C, López-Casas PP, Olmos D. Genetic aberrations in DNA repair pathways: a cornerstone of precision oncology in prostate cancer. Br J Cancer. 2021;124(3):552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan P, Zhang N, Candi E, Agostini M, Piacentini M, TOR C, Shi Y, Huang Y, Melino G. Alleviating hypoxia to improve cancer immunotherapy. Oncogene. 2023;42(49):3591–604. [DOI] [PubMed] [Google Scholar]
- 18.Vitale I, Pietrocola F, Guilbaud E, Aaronson SA, Abrams JM, Adam D, et al. Apoptotic cell death in disease-current understanding of the NCCD 2023. Cell Death Differ. 2023;30(5):1097–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonfiglio R, Sisto R, Casciardi S, Palumbo V, Scioli MP, Giacobbi E, Servadei F, Melino G, Mauriello A, Scimeca M. Aluminium bioaccumulation in colon cancer, impinging on epithelial-mesenchymal-transition and cell death. Sci Total Environ. 2024;908:168335. [DOI] [PubMed] [Google Scholar]
- 20.Odero-Marah V, Hawsawi O, Henderson V, Sweeney J. Epithelial-mesenchymal transition (EMT) and prostate Cancer. Adv Exp Med Biol. 2018;1095:101–10. [DOI] [PubMed] [Google Scholar]
- 21.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, Muller PA, Dötsch V, Kehrloesser S, Sayan BS, Giaccone G, Lowe SW, Takahashi N, Vandenabeele P, Knight RA, Levine AJ, Melino G. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A. 2012;109(38):15312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem Sci. 2015;40(8):425–34. [DOI] [PubMed] [Google Scholar]
- 23.Amelio I, Mancini M, Petrova V, Cairns RA, Vikhreva P, Nicolai S, Marini A, Antonov AA, Le Quesne J, Baena Acevedo JD, Dudek K, Sozzi G, Pastorino U, Knight RA, Mak TW, Melino G. p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression. Proc Natl Acad Sci U S A. 2018;115(46):E10869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30(4):876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappello A, Tosetti G, Smirnov A, Ganini C, Yang X, Shi Y, Wang Y, Melino G, Bernassola F, Candi E. p63 orchestrates serine and one carbon metabolism enzymes expression in head and neck cancer. Biol Direct. 2023;18(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Pastrana I, Birli E, Coutts AS. p53-dependent DNA repair during the DNA damage response requires actin nucleation by JMY. Cell Death Differ. 2023;30(7):1636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pant V, Sun C, Lozano G. Tissue specificity and spatio-temporal dynamics of the p53 transcriptional program. Cell Death Differ. 2023;30(4):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priami C, Montariello D, De Michele G, Ruscitto F, Polazzi A, Ronzoni S, Bertalot G, Binelli G, Gambino V, Luzi L, Mapelli M, Giorgio M, Migliaccio E, Pelicci PG. Aberrant activation of p53/p66Shc-mInsc axis increases asymmetric divisions and attenuates proliferation of aged mammary stem cells. Cell Death Differ. 2022;29(12):2429–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panatta E, Butera A, Celardo I, Leist M, Melino G, Amelio I. p53 regulates expression of nuclear envelope components in cancer cells. Biol Direct. 2022;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butera A, Roy M, Zampieri C, Mammarella E, Panatta E, Melino G, D’Alessandro A, Amelio I. p53-driven lipidome influences non-cell-autonomous lysophospholipids in pancreatic cancer. Biol Direct. 2022;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scimeca M, Bonfiglio R, Urbano N, Cerroni C, Anemona L, Montanaro M, Fazi S, Schillaci O, Mauriello A, Bonanno E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urologic Oncology: Seminars Original Investigations. 2019;37(5):e29719–31. [DOI] [PubMed] [Google Scholar]
- 32.Peng Q, Shi X, Li D, Guo J, Zhang X, Zhang X, Chen Q. SCML2 contributes to tumor cell resistance to DNA damage through regulating p53 and CHK1 stability. Cell Death Differ. 2023 Jul;30(7):1849–67. [DOI] [PMC free article] [PubMed]
- 33.Yi J, Li H, Chu B, Kon N, Hu X, Hu J, Xiong Y, Kaniskan HU, Jin J, Gu W. Inhibition of USP7 induces p53-independent tumor growth suppression in triple-negative breast cancers by destabilizing FOXM1. Cell Death Differ. 2023 Jul;30(7):1799–810. [DOI] [PMC free article] [PubMed]
- 34.Miller P, Akama-Garren EH, Owen RP, Demetriou C, Carroll TM, Slee E, Al Moussawi K, Ellis M, Goldin R, O’Neill E, Lu X. p53 inhibitor iASPP is an unexpected suppressor of KRAS and inflammation-driven pancreatic cancer. Cell Death Differ. 2023;30(7):1619–1635. p53 inhibitor iASPP is an unexpected suppressor of KRAS and inflammation-driven pancreatic cancer. Cell Death Differ. 2023;30(7):1619–1635. [DOI] [PMC free article] [PubMed]
- 35.Bonfiglio R, Nardozi D, Scimeca M, Cerroni C, Mauriello A, Bonanno E. PD-L1 in immune-escape of breast and prostate cancers: from biology to therapy. Future Oncol. 2017;13(24):2129–31. [DOI] [PubMed] [Google Scholar]
- 36.Ness N, Andersen S, Khanehkenari MR, Nordbakken CV, Valkov A, Paulsen EE, Nordby Y, Bremnes RM, Donnem T, Busund LT, Richardsen E. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget. 2017;8(16):26789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Wang Y, Zhang J, Hu Q, Zhi F, Zhang S, Mao D, Zhang Y, Liang H. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16(4):5450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawlina S, Chowdhury HH, Smrkolj T, Zorec R. Dendritic cell-based vaccine prolongs survival and time to next therapy independently of the vaccine cell number. Biol Direct. 2022;17(1):5doi. 10.1186/s13062-022-00318-w. PMID: 35197090; PMCID: PMC8864901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dou R, Kang S, Yang H, Zhang W, Zhang Y, Liu Y, Ping Y, Pang B. Identifying the driver miRNAs with somatic copy number alterations driving dysregulated ceRNA networks in cancers. Biol Direct. 2023;18(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Smirnov A, Buonomo OC, Mauriello A, Shi Y, Bischof J, Woodsmith J, Melino G, Candi E, Bernassola F, TOR CENTRE. A primary luminal/HER2 negative breast cancer patient with mismatch repair deficiency. Cell Death Discov. 2023;9(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonfiglio R, Galli F, Varani M, Scimeca M, Borri F, Fazi S, Cicconi R, Mattei M, Campagna G, Schönberger T, Raymond E, Wunder A, Signore A, Bonanno E. Extensive histopathological characterization of Inflamed Bowel in the Dextran Sulfate Sodium mouse model with emphasis on clinically relevant biomarkers and targets for Drug Development. Int J Mol Sci. 2021;22(4):2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melino S, Paci M. Progress for dengue virus diseases. Towards the NS2B-NS3pro inhibition for a therapeutic-based approach. FEBS J. 2007;274(12):2986–3002. [DOI] [PubMed] [Google Scholar]
- 43.Aceto A, Dragani B, Melino S, Allocati N, Masulli M, Di Ilio C, Petruzzelli R. Identification of an N-capping box that affects the alpha 6-helix propensity in glutathione S-transferase superfamily proteins: a role for an invariant aspartic residue. Biochem J. 1997;322(Pt 1):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabelli R, Iorio E, De Martino A, Podo F, Ricci A, Viticchiè G, Rotilio G, Paci M, Melino S. Rhodanese-thioredoxin system and allyl sulfur compounds. FEBS J. 2008;275(15):3884–99. [DOI] [PubMed] [Google Scholar]
- 45.Melino G, Memmi EM, Pelicci PG, Bernassola F. Maintaining epithelial stemness with p63. Sci Signal. 2015;8(387):re9. [DOI] [PubMed] [Google Scholar]
- 46.Servadei F, Anemona L, Cardellini M, Scimeca M, Montanaro M, Rovella V, Di Daniele F, Giacobbi E, Legramante IM, Noce A, Bonfiglio R, Borboni P, Di Daniele N, Ippoliti A, Federici M, Mauriello A. The risk of carotid plaque instability in patients with metabolic syndrome is higher in women with hypertriglyceridemia. Cardiovasc Diabetol. 2021;20(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nepravishta R, Sabelli R, Iorio E, Micheli L, Paci M, Melino S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. FEBS J. 2012;279(1):154–67. [DOI] [PubMed] [Google Scholar]
- 48.Vitali A, Botta B, Delle Monache G, Zappitelli S, Ricciardi P, Melino S, Petruzzelli R, Giardina B. Purification and partial characterization of a peroxidase from plant cell cultures of Cassia didymobotrya and biotransformation studies. Biochem J. 1998;331(Pt 2):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han Y, Rovella V, Smirnov A, Buonomo OC, Mauriello A, Perretta T, Shi Y, Woodmsith J, Bischof J, TOR CENTRE, Melino G, Candi E, Bernassola F. A BRCA2 germline mutation and high expression of immune checkpoints in a TNBC patient. Cell Death Discov. 2023;9(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Luo W, Jones K, Bian X, Williams R, Higson H, Wu D, Hicks B, Yeager M, Zhu B. SomaticCombiner: improving the performance of somatic variant calling based on evaluation tests and a consensus approach. Sci Rep. 2020;10(1):12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Scheffler K, Halpern AL, Bekritsky MA, Noh E, Källberg M, Chen X, Kim Y, Beyter D, Krusche P, Saunders CT. Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods. 2018;15(8):591–4. [DOI] [PubMed] [Google Scholar]
- 52.Koboldt DC, Chen K, Wylie T, Larson DE, McLellan MD, Mardis ER, Weinstock GM, Wilson RK, Ding L. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics. 2009;25(17):2283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larson DE, Harris CC, Chen K, Koboldt DC, Abbott TE, Dooling DJ, Ley TJ, Mardis ER, Wilson RK, Ding L. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics. 2012;28(3):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha G, Roth A, Khattra J, Ho J, Yap D, Prentice LM, Melnyk N, McPherson A, Bashashati A, Laks E, Biele J, Ding J, Le A, Rosner J, Shumansky K, Marra MA, Gilks CB, Huntsman DG, McAlpine JN, Aparicio S, Shah SP. TITAN: inference of copy number architectures in clonal cell populations from tumor whole-genome sequence data. Genome Res. 2014;24(11):1881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rausch T, Zichner T, Schlattl A, Stütz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28(18):i333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Schulz-Trieglaff O, Shaw R, Barnes B, Schlesinger F, Källberg M, Cox AJ, Kruglyak S, Saunders CT. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32(8):1220–2. [DOI] [PubMed] [Google Scholar]
- 57.Manders F, Brandsma AM, de Kanter J, Verheul M, Oka R, van Roosmalen MJ, van der Roest B, van Hoeck A, Cuppen E, van Boxtel R. MutationalPatterns: the one stop shop for the analysis of mutational processes. BMC Genomics. 2022;23(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellomaria A, Barbato G, Melino G, Paci M, Melino S. Recognition mechanism of p63 by the E3 ligase Itch: novel strategy in the study and inhibition of this interaction. Cell Cycle. 2012; 11(19):3638-48. 10.4161/cc.21918. PMID: 22935697. [DOI] [PMC free article] [PubMed]
- 59.Melino S, Leo S, Toska Papajani V. Natural hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in type 2 diabetes Prevention and Therapy. Nutrients. 2019;11(7):1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sunzini F, De Stefano S, Chimenti MS, Melino S. Hydrogen Sulfide as Potential Regulatory Gasotransmitter in Arthritic Diseases. Int J Mol Sci. 2020; 21(4):1180. 10.3390/ijms21041180. PMID: 32053981. [DOI] [PMC free article] [PubMed]
- 61.Huang MN, McPherson JR, Cutcutache I, Teh BT, Tan P, Rozen SG. MSIseq: Software for assessing microsatellite instability from catalogs of somatic mutations. Sci Rep. 2015;5:13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oza VH, Fisher JL, Darji R, Lasseigne BN. CINmetrics: an R package for analyzing copy number aberrations as a measure of chromosomal instability. PeerJ. 2023;11:e15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grendár M, Martínek P, Loderer D, Ondič O. CNHplus: the chromosomal copy number heterogeneity which respects biological constraints. BioRxiv. 2022. 10.1101/2022.09.30.510279. [Google Scholar]
- 64.Spurr LF, Touat M, Taylor AM, Dubuc AM, Shih J, Meredith DM, Pisano WV, Meyerson ML, Ligon KL, Cherniack AD, Li YY, Beroukhim R. Quantification of aneuploidy in targeted sequencing data using ASCETS. Bioinformatics. 2021;37(16):2461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–8. [DOI] [PubMed] [Google Scholar]
- 67.Kulda V, Topolcan O, Kucera R, Kripnerova M, Srbecka K, Hora M, Hes O, Klecka J, Babuska V, Rousarova M, Benson V, Pesta M. Prognostic significance of TMPRSS2-ERG Fusion Gene in prostate Cancer. Anticancer Res. 2016;36(9):4787–93. [DOI] [PubMed] [Google Scholar]
- 68.Kluth M, Graunke M, Möller-Koop C, Hube-Magg C, Minner S, Michl U, Graefen M, Huland H, Pompe R, Jacobsen F, Hinsch A, Wittmer C, Lebok P, Steurer S, Büscheck F, Clauditz T, Wilczak W, Sauter G, Schlomm T, Simon R. Deletion of 18q is a strong and independent prognostic feature in prostate cancer. Oncotarget. 2016;7(52):86339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kluth M, Jung S, Habib O, Eshagzaiy M, Heinl A, Amschler N, Masser S, Mader M, Runte F, Barow P, Frogh S, Omari J, Möller-Koop C, Hube-Magg C, Weischenfeldt J, Korbel J, Steurer S, Krech T, Huland H, Graefen M, Minner S, Sauter G, Schlomm T, Simon R. Deletion lengthening at chromosomes 6q and 16q targets multiple tumor suppressor genes and is associated with an increasingly poor prognosis in prostate cancer. Oncotarget. 2017;8(65):108923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crundwell MC, Chughtai S, Knowles M, Takle L, Luscombe M, Neoptolemos JP, Morton DG, Phillips SM. Allelic loss on chromosomes 8p, 22q and 18q (DCC) in human prostate cancer. Int J Cancer. 1996;69(4):295–300. Allelic loss on chromosomes 8p, 22q and 18q (DCC) in human prostate cancer. Int J Cancer. 1996;69(4):295–300. [DOI] [PubMed]
- 71.Ueda T, Komiya A, Emi M, Suzuki H, Shiraishi T, Yatani R, Masai M, Yasuda K, Ito H. Allelic losses on 18q21 are associated with progression and metastasis in human prostate cancer. Genes Chromosomes Cancer. 1997;20(2):140–7. [DOI] [PubMed] [Google Scholar]
- 72.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kluth M, Harasimowicz S, Burkhardt L, Grupp K, Krohn A, Prien K, Gjoni J, Haß T, Galal R, Graefen M, Haese A, Simon R, Hühne-Simon J, Koop C, Korbel J, Weischenfeld J, Huland H, Sauter G, Quaas A, Wilczak W, Tsourlakis MC, Minner S, Schlomm T. Clinical significance of different types of p53 gene alteration in surgically treated prostate cancer. Int J Cancer. 2014;135(6):1369–80. [DOI] [PubMed] [Google Scholar]
- 74.Demichelis F, Setlur SR, Beroukhim R, Perner S, Korbel JO, Lafargue CJ, Pflueger D, Pina C, Hofer MD, Sboner A, Svensson MA, Rickman DS, Urban A, Snyder M, Meyerson M, Lee C, Gerstein MB, Kuefer R, Rubin MA. Distinct genomic aberrations associated with ERG rearranged prostate cancer. Genes Chromosomes Cancer. 2009;48(4):366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cancer Genome Atlas Research Network. The Molecular Taxonomy of primary prostate Cancer. Cell. 2015;163(4):1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hieronymus H, Schultz N, Gopalan A, Carver BS, Chang MT, Xiao Y, Heguy A, Huberman K, Bernstein M, Assel M, Murali R, Vickers A, Scardino PT, Sander C, Reuter V, Taylor BS, Sawyers CL. Copy number alteration burden predicts prostate cancer relapse. Proc Natl Acad Sci U S A. 2014;111(30):11139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schiewer MJ, Knudsen KE. DNA damage response in prostate Cancer. Cold Spring Harb Perspect Med. 2019;9(1):a030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, Procopio G, de Menezes J, Girotto G, Arslan C, Mehra N, Parnis F, Brown E, Schlürmann F, Joung JY, Sugimoto M, Virizuela JA, Emmenegger U, Navratil J, Buchschacher GL, Poehlein C, Harrington EA, Desai C, Kang J, Saad F. Abiraterone and Olaparib for metastatic castration-resistant prostate Cancer. NEJM Evid. 2022;1(9):EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- 80.Saad F, Clarke NW, Oya M, Shore N, Procopio G, Guedes JD, Arslan C, Mehra N, Parnis F, Brown E, Schlürmann F, Joung JY, Sugimoto M, Sartor O, Liu YZ, Poehlein C, Barker L, Del Rosario PM, Armstrong AJ. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094–108. [DOI] [PubMed] [Google Scholar]
- 81.Antonarakis ES, Park SH, Goh JC, Shin SJ, Lee JL, Mehra N, McDermott R, Sala-Gonzalez N, Fong PC, Greil R, Retz M, Sade JP, Yanez P, Huang YH, Begbie SD, Gafanov RA, De Santis M, Rosenbaum E, Kolinsky MP, Rey F, Chiu KY, Roubaud G, Kramer G, Sumitomo M, Massari F, Suzuki H, Qiu P, Zhang J, Kim J, Poehlein CH, Yu EY. Pembrolizumab Plus Olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate Cancer: the randomized, Open-Label, phase III KEYLYNK-010 trial. J Clin Oncol. 2023;41(22):3839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chi KN, Rathkopf D, Smith MR, Efstathiou E, Attard G, Olmos D, Lee JY, Small EJ, Pereira de Santana Gomes AJ, Roubaud G, Saad M, Zurawski B, Sakalo V, Mason GE, Francis P, Wang G, Wu D, Diorio B, Lopez-Gitlitz A, Sandhu S. MAGNITUDE principal investigators. Niraparib and Abiraterone acetate for metastatic castration-resistant prostate Cancer. J Clin Oncol. 2023;41(18):3339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jelic MD, Mandic AD, Maricic SM, Srdjenovic BU. Oxidative stress and its role in cancer. J Cancer Res Ther. 2021 Jan-Mar;17(1):22–8. [DOI] [PubMed]
- 84.Nakamura H, Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112(10):3945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jarrard DF, Bova GS, Ewing CM, Pin SS, Nguyen SH, Baylin SB, Cairns P, Sidransky D, Herman JG, Isaacs WB. Deletional, mutational, and methylation analyses of CDKN2 (p16/MTS1) in primary and metastatic prostate cancer. Genes Chromosomes Cancer. 1997;19(2):90–6. [PubMed] [Google Scholar]
- 86.Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58(2):204–9. [PubMed] [Google Scholar]
- 87.López J, Añazco-Guenkova AM, Monteagudo-García Ó, Blanco S. Epigenetic and epitranscriptomic control in prostate Cancer. Genes (Basel). 2022;13(2). [DOI] [PMC free article] [PubMed]
- 88.Gao S, Chen S, Han D, Wang Z, Li M, Han W, Besschetnova A, Liu M, Zhou F, Barrett D, Luong MP, Owiredu J, Liang Y, Ahmed M, Petricca J, Patalano S, Macoska JA, Corey E, Chen S, Balk SP, He HH, Cai C. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat Genet. 2020;52(10):1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AH, Günther T, Buettner R, Schüle R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–9. [DOI] [PubMed] [Google Scholar]
- 90.de Bruijn I, Kundra R, Mastrogiacomo B, Tran TN, Sikina L, Mazor T, et al. Analysis and visualization of longitudinal genomic and clinical data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023;83(23):3861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, Elemento O, Rubin MA, Robinson D, Lonigro R, Hussain M, Chinnaiyan A, Vinson J, Filipenko J, Garraway L, Taplin ME, AlDubayan S, Han GC, Beightol M, Morrissey C, Nghiem B, Cheng HH, Montgomery B, Walsh T, Casadei S, Berger M, Zhang L, Zehir A, Vijai J, Scher HI, Sawyers C, Schultz N, Kantoff PW, Solit D, Robson M, Van Allen EM, Offit K, de Bono J, Nelson PS. Inherited DNA-Repair gene mutations in men with metastatic prostate Cancer. N Engl J Med. 2016;375(5):443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. 2017;541(7637):359–64. [DOI] [PubMed] [Google Scholar]
- 95.Ler LD, Ghosh S, Chai X, Thike AA, Heng HL, Siew EY, Dey S, Koh LK, Lim JQ, Lim WK, Myint SS, Loh JL, Ong P, Sam XX, Huang D, Lim T, Tan PH, Nagarajan S, Cheng CW, Ho H, Ng LG, Yuen J, Lin PH, Chuang CK, Chang YH, Weng WH, Rozen SG, Tan P, Creasy CL, Pang ST, McCabe MT, Poon SL, Teh BT. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci Transl Med. 2017;9:378. [DOI] [PubMed] [Google Scholar]
- 96.Stief SM, Hanneforth AL, Weser S, Mattes R, Carlet M, Liu WH, Bartoschek MD, Domínguez Moreno H, Oettle M, Kempf J, Vick B, Ksienzyk B, Tizazu B, Rothenberg-Thurley M, Quentmeier H, Hiddemann W, Vosberg S, Greif PA, Metzeler KH, Schotta G, Bultmann S, Jeremias I, Leonhardt H, Spiekermann K. Loss of KDM6A confers drug resistance in acute myeloid leukemia. Leukemia. 2020;34(1):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuzick J, Berney DM, Fisher G, Mesher D, Møller H, Reid JE, Perry M, Park J, Younus A, Gutin A, Foster CS, Scardino P, Lanchbury JS, Stone S, Transatlantic Prostate Group. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106(6):1095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer. 2010;102(2):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest. 2022;132(11):e159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kong P, Wang X, Gao YK, Zhang DD, Huang XF, Song Y, Zhang WD, Guo RJ, Li H, Han M. RGS5 maintaining vascular homeostasis is altered by the tumor microenvironment. Biol Direct. 2023;18(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, Zhu WY, Shen SY, Shen JH, Chen XD. Biological roles of RNA m7G modification and its implications in cancer. Biol Direct. 2023;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohamed OAA, Tesen HS, Hany M, Sherif A, Abdelwahab MM, Elnaggar MH. The role of hypoxia on prostate cancer progression and metastasis. Mol Biol Rep. 2023;50(4):3873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanellis DC, Zisi A, Skrott Z, Lemmens B, Espinoza JA, Kosar M, Björkman A, Li X, Arampatzis S, Bartkova J, Andújar-Sánchez M, Fernandez-Capetillo O, Mistrik M, Lindström MS, Bartek J. Actionable cancer vulnerability due to translational arrest, p53 aggregation and ribosome biogenesis stress evoked by the disulfiram metabolite CuET. Cell Death Differ. 2023;30(7):1666–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu P, Zhao L, Kroemer G, Kepp O. Conventional type 1 dendritic cells (cDC1) in cancer immunity. Biol Direct. 2023;18(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang X, Wang Y, Rovella V, Candi E, Jia W, Bernassola F, Bove P, Piacentini M, Scimeca M, Sica G, Tisone G, Mauriello A, Wei L, Melino G, Shi Y. Aged mesenchymal stem cells and inflammation: from pathology to potential therapeutic strategies. Biol Direct. 2023;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng R, Li F, Li F, Gong A. Targeting tumor vascularization: promising strategies for vascular normalization. J Cancer Res Clin Oncol. 2021;147(9):2489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen CH, Li SX, Xiang LX, Mu HQ, Wang SB, Yu KY. HIF-1α induces immune escape of prostate cancer by regulating NCR1/NKp46 signaling through miR-224. Biochem Biophys Res Commun. 2018;503(1):228–34. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Chen XN, Zhang H, Wen JK, Gao HT, Shi B, Wang DD, Han ZW, Gu JF, Zhao CM, Xue WY, Zhang YP, Qu CB, Yang Z. CDK13 promotes lipid deposition and prostate cancer progression by stimulating NSUN5-mediated m5C modification of ACC1 mRNA. Cell Death Differ. 2023;30(12):2462–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuang Z, Liu X, Zhang N, Dong J, Sun C, Yin M, Wang Y, Liu L, Xiao D, Zhou X, Feng Y, Song D, Deng H. USP2 promotes tumor immune evasion via deubiquitination and stabilization of PD-L1. Cell Death Differ. 2023;30(10):2249–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng Y, Liu J, Wang Z, Cui C, Zhang T, Zhang S, Gao P, Hou Z, Liu H, Guo J, Zhang J, Wen Y, Wei W, Zhang L, Liu J, Long J. Prostate-specific oncogene OTUD6A promotes prostatic tumorigenesis via deubiquitinating and stabilizing c-Myc. Cell Death Differ. 2022;29(9):1730–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan Y, Hou T, Dan W, Zhu Y, Liu B, Wei Y, Wang Z, Gao Y, Zeng J, Li L. ERK1/2 inhibits Cullin 3/SPOP-mediated PrLZ ubiquitination and degradation to modulate prostate cancer progression. Cell Death Differ. 2022;29(8):1611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Q, Jin X, Zhang P, Li Q, Lv Z, Ding Y, He H, Wang Y, He Y, Zhao X, Zhao SM, Li Y, Gao K, Wang C. SPOP mutations promote p62/SQSTM1-dependent autophagy and Nrf2 activation in prostate cancer. Cell Death Differ. 2022;29(6):1228–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request.