Abstract
Paenibacillus popilliae contains vanF encoding a putative d-Ala:d-lactate (d-Lac) ligase, VanF, as part of the vanYFZFHFFXF cluster that is similar in structure to the enterococcal vanA and vanB clusters. Using growth curves, we demonstrated that vancomycin resistance in P. popilliae is inducible. Using degenerate oligonucleotides targeted at bacterial cell wall ligases, we identified a second ligase gene with features of a d-Ala:d-Ala ligase in both P. popilliae and the related, vancomycin-susceptible, Paenibacillus lentimorbus. The 3,380-bp region upstream of vanYFZFHFFXF in P. popilliae ATCC 14706 was sequenced and found to contain genes encoding a putative two-component regulator, VanRFSF, similar to VanRS but more closely related to a family of two-component regulators linked to VanY-like carboxypeptidases in several glycopeptide-susceptible Bacillus species. This upstream region also included a transposase similar to a transposase found in Bacillus halodurans and, in some strains, a 99-bp insertion of unknown function with 95% nucleotide identity to a portion of the Tn1546 transposase gene. Analysis of glycopeptide resistance-associated clusters from soil and/or insect-dwelling organisms may provide important clues to the molecular evolution of acquired glycopeptide resistance elements in human pathogens.
Acquired resistance to glycopeptides has increased dramatically among nosocomial pathogens in the United States over the past 15 years. Over 25% of enterococcal strains from intensive care units in the United States are now resistant to vancomycin, and acquired high-level vancomycin resistance has been recently described in several Staphylococcus aureus strains (8, 9, 10, 25). Acquired vancomycin resistance in enterococci is due predominantly to acquisition of the vanA or vanB glycopeptide resistance gene clusters (3, 14). These acquired glycopeptide resistance clusters can be transferred in vitro between enterococcal species, as well as from enterococci to other gram-positive pathogens including Listeria species, streptococci, and S. aureus (6, 26). The vanA and vanB clusters have been found in vivo in other nonpathogenic gram-positive organisms, including Bacillus circulans and Oerskovia species (15, 29). Based on differences in G+C content between the genes comprising the vanA and vanB clusters and the G+C content of other portions of the enterococcal genome, these resistance clusters are presumed to have originated in nonenterococcal, presumably environmental, organisms, with subsequent transfer to enterococci (27, 33). Gene clusters similar to vanHAX and vanHBBXB have been identified in several soil-dwelling glycopeptide-producing members of the bacterial order Actinomycetales. A complete vancomycin resistance system has also recently been identified in the non-glycopeptide-producing Streptomyces coelicolor (20). However, it is unlikely that these genetically unrelated organisms are the immediate source of glycopeptide resistance genes for enterococci (24). Other species more closely related to enterococci may have served as intermediate hosts for the flow of resistance genes from members of the bacterial order Actinomycetales into human pathogens. Genes homologous to enterococcal vanA and vanB have recently been identified in organisms of the soil-dwelling genera Paenibacillus and Rhodococcus (16).
We have previously described a vancomycin resistance gene cluster in Paenibacillus popilliae, a vancomycin-resistant environmental organism marketed as a biopesticide to suppress Japanese beetle populations by causing milky disease in Japanese beetle larvae (18, 28, 30, 31). The resistance cluster in P. popilliae includes vanF, encoding a putative d-Ala:d-Lac ligase homologous to VanA, as well as genes putatively encoding homologues of VanH, X, Y, and Z of the VanA cluster. All North American P. popilliae strains studied to date contain vanF, although the function of a glycopeptide resistance cluster that is so widely disseminated among P. popilliae remains unknown (18, 28, 30, 31). The goal of this study was to further characterize the vancomycin resistance cluster from P. popilliae by sequencing upstream of the previously characterized vanYFZFHFFXF elements to identify potential regulatory and transposase genes similar to those found associated with the vanA and vanB clusters. We also wished to further characterize the mode of expression of vanF and to search for an alternate ligase of the d-Ala:d-Ala type not associated with the vancomycin resistance cluster.
(Portions of this work were presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998, and at the 102nd General Meeting of the American Society for Microbiology, 2002.)
MATERIALS AND METHODS
Strains.
Strains used included P. popilliae ATCC 14706 and Bl17 (31) and 13 additional P. popilliae and Paenibacillus lentimorbus strains isolated from a variety of insect larvae. Strains were obtained from A. A. Yousten at Virginia Polytechnic Institute and State University, from D. Dingman at the Connecticut Agricultural Experiment Station, and from the Agricultural Research Service Culture Collection. Origins and glycopeptide susceptibility data for the strains used are shown in Table 1. Speciation of P. popilliae and P. lentimorbus strains was performed previously by DNA similarity analysis (18, 30, 31).
TABLE 1.
Source, glycopeptide susceptibilities, and results of PCR for vanF, P. popilliae ddl, vanRFSF, and the Tn1546 tnp-related insertional element for strains used in this study
| Strain | Species | MIC (μg/ml)
|
Sourcea | PCRb
|
||||
|---|---|---|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | vanF | ddl | vanRFSF | Tn1546 tnp | |||
| ATCC 14706 | P. popilliae | >1,000 | <0.5 | Yousten | + | + | + | + |
| BlPj1 | P. popilliae | >1,000 | <0.5 | Dingman | + | + | + | + |
| Ch1 | P. popilliae | >1,000 | <0.5 | Dingman | + | + | + | + |
| KLN4 | P. popilliae | >1,000 | <0.5 | Dingman | + | + | + | − |
| BpPj1 | P. popilliae | >1,000 | <0.5 | Dingman | + | + | + | + |
| BpPa | P. popilliae | >1,000 | <0.5 | Dingman | + | + | + | − |
| NRRL B-2524 | P. popilliae | >1,000 | <0.5 | Dingman | + | + | + | − |
| NRRL B-2043 | P. popilliae | >1,000 | <0.5 | ARS | + | + | + | + |
| NRRL B-4145 | P. popilliae | >1,000 | <0.5 | ARS | + | + | + | + |
| NRRL B-4154 | P. popilliae | >1,000 | <0.5 | ARS | + | + | + | − |
| NRRL B-2522 | P. lentimorbus | <1 | <0.5 | ARS | − | + | − | − |
| ATCC 14707 | P. lentimorbus | <1 | <0.5 | Dingman | − | + | − | − |
| KLN2 | P. lentimorbus | <1 | <0.5 | Dingman | − | + | − | − |
| Cp1 | P. lentimorbus | <1 | <0.5 | Dingman | − | + | − | − |
| BpPj1-Tr | P. popilliae | >1,000 | 32 | This study | ND | ND | + | ND |
| Bl 17 | P. popilliae | >1,000 | <0.5 | Yousten | + | ND | + | ND |
ARS, Agricultural Research Service.
ND, not done.
Phenotypic characterization of glycopeptide resistance.
All studies were performed with strains grown in either brain heart infusion (BHI) broth or agar or in MYPGP broth or agar (11). Susceptibility testing was performed by broth dilution and Etest with results read at 24 h or at 48 h for slower-growing strains. Growth rates were determined in BHI with or without supplemental vancomycin (10 μg/ml) or teicoplanin (10 μg/ml). For induction experiments, strains grown to early exponential growth phase in antibiotic-free medium were diluted 1/50 in medium with or without supplemental vancomycin. For characterization of growth in the presence of d-Ala:d-Ala ligase inhibition, P. popilliae strains were seeded onto BHI plates containing inhibitory concentrations of d-cycloserine (50 to 100 μg/ml). Disks containing either 30 μg of vancomycin or teicoplanin or 50 mM d-alanine-d-alanine were then added. Growth around the disks was recorded after 24 to 48 h.
Assay for inducible d-alanine-d-alanine dipeptidase activity.
Overnight cultures of P. popilliae were grown in BHI broth with or without supplemental vancomycin (10 μg/ml). One-milliliter aliquots of cells were resuspended in 500 μl of Tris-EDTA buffer and lysed with 10 U of mutanolysin (Sigma Chemical Co., St. Louis, Mo.) and Triton X-100 (final concentration, 1%). The suspension was centrifuged at 14,000 rpm for 30 min, and the supernatant was removed and assayed for release of d-alanine from d-alanine-d-alanine as previously described (1). Assay reagents included horseradish peroxidase, type I (Sigma Chemical Co., St. Louis, Mo.), d-amino acid oxidase (ICN Biomedical Research, Costa Mesa, CA), ortho-dianisidine (ICN Biomedical Research, Costa Mesa, CA), and d-alanine-d-alanine (Sigma Chemical Co., St. Louis, Mo.). Assays were performed in microtiter wells using 50 μl of supernatant and 150 μl of assay mixture; optical density at A460 was monitored over time in a Biotek EL312 Biokinetic reader (Bio-Tek Instruments, Inc., Winooski, VT). Comparative dd-dipeptidase activity was determined from calculation of the maximal rate of change in optical density units over time during the linear portion of the assay per milligram of protein, determined by the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
Selection of teicoplanin-resistant derivatives of P. popilliae.
Teicoplanin-resistant derivatives of vancomycin-resistant P. popilliae strains Ch1 and BpPj1 were selected by overnight growth on BHI agar supplemented with both vancomycin (10 μg/ml) and teicoplanin (1 μg/ml), followed by plating onto medium containing 10 μg/ml of teicoplanin. These strains were selected due to their rapid growth rates and large colony morphology, which facilitated selection of resistant derivatives. Teicoplanin-resistant derivatives were also selected by plating strains onto BHI agar with d-cycloserine, followed by incubation for 48 to 72 h. Individual cycloserine-resistant colonies were screened for teicoplanin resistance by the ability to grow on 10 μg/ml teicoplanin.
DNA extraction.
DNA was extracted from P. popilliae using DNA-STAT60 (Tel-Test, Inc., Friendswood, TX) or by extraction from washed cells by incubation in mutanolysin (50 units/ml) and 0.2% sodium dodecyl sulfate, followed by phenol-chloroform extraction and precipitation of DNA.
Amplification of unknown ligases.
Degenerate primers V1 and V2 targeted at d-Ala:d-X ligases including d-Ala:d-Ala ligases were used to amplify DNA from P. popilliae and P. lentimorbus as previously described (12). PCR conditions were as follows: denaturation at 94°C for 2 min, annealing at 45°C for 1 min, and amplification at 75°C for 2 min for 40 cycles. Amplified DNA of the predicted 630-bp size from three separate strains was purified and sequenced. Primers BVG-1 and BVG-2 were then used to amplify the related d-Ala:d-Ala ligase sequences in whole-cell suspensions of P. popilliae and P. lentimorbus strains using an annealing temperature of 55°C.
Sequencing of the upstream region of the vanF cluster.
Restriction site PCR was used to extend upstream of the published sequence as described previously (28, 32), using outward-facing primers in vanYF as the initial PCR tether in strains ATCC 14706 and Bl17. For sequencing, 9 μl of the PCR product was mixed with 1 μl of exonuclease (1 U/μl) (United States Biochemical, Cleveland, OH) and shrimp alkaline phosphatase (1 U/μl) (Boehringer Mannheim, Mannheim, Germany) and incubated at 37°C for 30 min, followed by incubation at 80°C for 15 min. Then, 1 μl of dimethyl sulfoxide and 1 μl of the sequencing primer (3.2 μM) were added. The DNA sequence was determined in both the 5′ to 3′ and 3′ to 5′ directions with a Taq dideoxy terminator cycle sequencing kit and a 373A DNA sequencer (Applied Biosystems, Foster City, CA) using a series of sequencing primers selected from the preliminary sequence data to ensure complete coverage of the entire amplified upstream region. Elements similar to those in strains ATCC 14706 and Bl17 were identified in other P. popilliae strains by using specific PCR primers internal to the vanRF, vanSF, Tn1546 tnp, and P. popilliae tnp genes. Primers used in this study are shown in Table 2. Sequences were analyzed with Sequencher 3.0 (Gene Codes Corp., Ann Arbor, MI).
TABLE 2.
Oligonucleotide primers used
| Primer | Sequence (5′→3′) | Location or functiona | Source |
|---|---|---|---|
| V1 | GGIGA(AG)GA(TC)GGI(TA)(CG)I(TCA)TICA(AG)GG | Degenerate ligase primer (with V2; 630 bp) | 11 |
| V2 | TG(AG)AAICCIGGIA(TAG)IGT(AG)TT | Degenerate ligase primer (with V1) | 11 |
| BVG-1 | CGTATGTTGGAGCAGGGGTG | P. popilliae Ddlb; bp 20-39 (with BVG-1; 334 bp) | This study |
| BVG-2 | CTTCCACTTCCCGCGCGTCAA | P. popilliae Ddlb; bp 354-333 (with BVG-2) | This study |
| TnpUP | TCTATTCGGCCCATCTCACG | Tn1546 insertion element; bp 1395-1413 | This study |
| TnpDOWN | CGTGAGATGGGCCGAATAGA | Tn1546 insertion element; bp 1413-1395 | This study |
| 1546U9/7 | TGGGCTTTATTCATGGCATC | Tn1546 tnpc; bp 1063-1082 | This study |
| 1546D9/7 | GCTATCACTTGCACGCTTAGA | Tn1546 tnpc; bp 1389-1380 | This study |
| VANR6/7 | CATGTTGCTGTCTAGCGCCA | vanSF; bp 2256-2275 | This study |
| VANR6/16 | CCATAACGGTATTCCCACCTT | vanRF; bp 2109-2089 | This study |
| VANYPOP | CGATCCTCTACTTTATCTTG | vanYF; bp 3508-3489 | This study |
| VANSEND | TAATCTTCTTAGGAATCCGC | vanSF; bp 3305-3296 | This study |
| BPOP FRF | GGTGCCTCGGTACTT | P. popilliae tnp; bp 1242-1257 | This study |
| Ppop-535F | CGCTCTCATTCTAGCTCACC | P. popilliae tnp; bp 506-487 | This study |
| Ppop-479R | GATCCTAAACAGCCAGCACC | Upstream of P. popilliae tnp 70-89 | This study |
| BPOP-159R | TACTAACGCCATCCTCTT | First stage primer for RS-PCR | This study |
| BPOP-67R | TACCCCTTTGTCAATTCT | Second stage primer for RS-PCR | This study |
| Ppop-339R | TTCTTGCCCATCCGATACC | First stage primer for RS-PCR | This study |
| Ppop-315F | CGCTTGGAAACGAAATATGG | First stage primer for RS-PCR | This study |
| Ppop-471F | GTTGAAGTGCCACATACTCC | Second stage primer for RS-PCR after 315F | This study |
| Ppop-334F | GCAACATACGGATGAACTGG | First stage primer for RS-PCR | This study |
| Ppop-464F | CTGGCTGTTTAGGATCACAAG | Second stage primer for RS-PCR after 334F | This study |
Nucleotide sequence accession numbers.
The nucleotide sequences of the proposed two-component regulatory clusters of P. popilliae ATCC 14706 and Bl17 (partial sequence) have been submitted to the GenBank database (accession no. AF155139 and AF432144, respectively). The partial nucleotide sequence of the putative d-Ala:d-Ala ligase has also been submitted to the GenBank database (accession no. AF098802).
RESULTS
Phenotypic characterization of vancomycin resistance in P. popilliae.
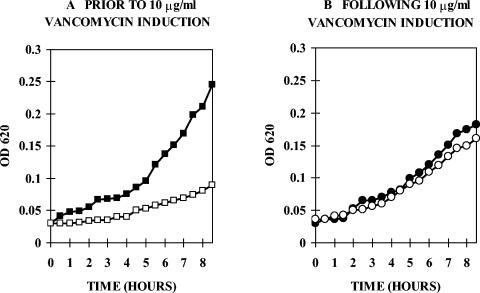
Ten reference strains of P. popilliae demonstrated inducible, high-level resistance to vancomycin with MICs of >500 to 1,000 μg/ml but were susceptible to teicoplanin (Table 1). A representative growth curve for strain Ch1 is shown in Fig. 1. Ch1 was grown overnight in antibiotic-free BHI medium and then diluted in medium with or without 10 μg/ml vancomycin. Growth in vancomycin lagged several hours behind growth in antibiotic-free medium (Fig. 1A). For comparison, when Ch1 was grown overnight in vancomycin and then diluted into medium with or without vancomycin, growth rates were similar after dilution into antibiotic-free or antibiotic-containing medium (Fig. 1B). These growth curves are typical of organisms showing inducible glycopeptide resistance (21).
FIG. 1.
(A) P. popilliae Ch1 grown overnight in antibiotic-free BHI medium and diluted 1/50 into medium without (solid squares) or with (open squares) vancomycin (10 μg/ml). Growth in vancomycin lagged several hours behind growth in antibiotic-free medium. (B) P. popilliae Ch1 grown overnight in vancomycin-supplemented medium (10 μg/ml) and then diluted 1/50 into medium without (solid circles) or with (open circles) vancomycin (10 μg/ml). Growth rates are similar in medium with or without vancomycin.
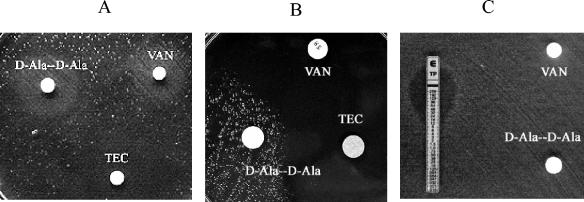
Figure 2A shows growth of strain BpPj1 streaked on BHI agar containing 100 μg/ml d-cycloserine. d-Cycloserine competitively inhibits d-Ala:d-Ala ligases but has a 10- to 100-fold lower effect on d-Ala:d-Lac ligases such as VanA (7). d-Cycloserine inhibition can be bypassed by exogenous d-alanine-d-alanine. Growth after 24 to 48 h of incubation was only observed around disks containing vancomycin and d-alanine-d-alanine, demonstrating that under normal growth conditions a d-cycloserine-susceptible d-Ala:d-Ala ligase is expressed but that growth following vancomycin exposure is not inhibited by d-cycloserine. Teicoplanin exposure, unlike vancomycin exposure, did not result in cycloserine-resistant growth. Figure 2C shows growth of a teicoplanin-resistant derivative (teicoplanin MIC, 16 μg/ml) of BpPj1, BpPj1-Tr, on cycloserine-BHI agar. For this strain, growth in the presence of cycloserine did not require induction by vancomycin, indicating constitutive expression of the cycloserine-resistant ligase.
FIG. 2.
(A) Growth of P. popilliae BpPj1 on BHI agar with 100 μg/ml cycloserine. Growth is observed only around vancomycin-containing disks and d-alanine-d-alanine containing disks but not around teicoplanin-containing disks. (B) Growth of P. lentimorbus Cp1 on BHI agar with 100 μg/ml d-cycloserine. Growth is observed only around disks containing d-alanine-d-alanine but not around vancomycin- or teicoplanin-containing disks. (C) Growth of BpPj1-Tr on BHI agar with 100 μg/ml cycloserine. Growth is observed independent of vancomycin induction.
Comparison of d-alanine-d-alanine dipeptidase activity in induced and uninduced strains.
Relative dd-dipeptidase activity as determined by release of free d-alanine, which has previously been used as a measure of expression of VanX-like activity (1), was 8.6-fold greater in P. popilliae BpPj1 grown overnight in 10 μg/ml vancomycin than in BpPj1 grown in antibiotic-free medium. dd-Dipeptidase activity of noninduced BpPj1 was similar to that of vancomycin-susceptible P. lentimorbus strains ATCC 14707 and Cp1.
Identification of a d-Ala:d-Ala ligase gene.
Using degenerate primers V1 and V2, a 630-bp sequence was amplified from P. popilliae Ch1. This sequence demonstrated 60 to 61% amino acid identity with B. subtilis d-Ala:d-Ala ligase as well as the d-Ala:d-Ala ligases of B. cereus and B. thuringiensis (data not shown) and >50% amino acid identity with d-Ala:d-Ala ligases of the Ddl group from a wide range of gram-positive pathogens including S. aureus, Listeria monocytogenes, and many enterococcal species. However, it demonstrated much lower similarity with the enterococcal vancomycin resistance d-Ala:d-Lac ligases VanA or VanB or the putative P. popilliae ligase VanF (13, 22, 31). Using PCR oligonucleotide primers internal to this sequence (Table 2), this sequence was amplified in all of the nine additional strains of P. popilliae tested (Table 1). A nucleotide fragment of identical size was also amplified from all of four strains tested of the closely related but vancomycin-susceptible organism P. lentimorbus. The fragment from P. lentimorbus ATCC 14707 was sequenced and shown to be 95% identical at the nucleotide level to the sequence from P. popilliae, suggesting that the putative Ddl from P. popilliae is not linked to expression of vancomycin resistance.
Sequencing of the region upstream of the vanYF carboxypeptidase gene.
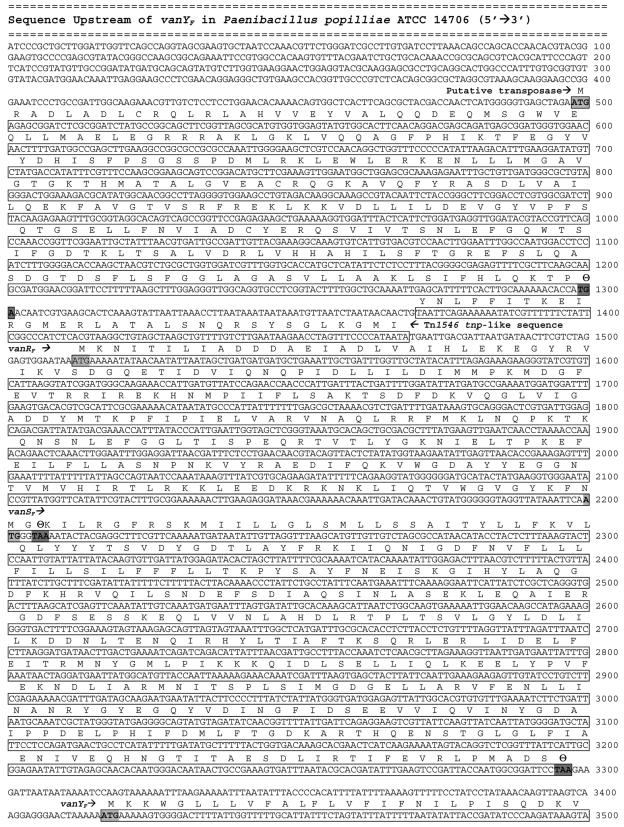
Using restriction site PCR, 3,380 bp of new sequence were obtained directly upstream from the previously published sequence of the gene encoding the putative carboxypeptidase, VanYF, from P. popilliae ATCC 14706 (28). Analysis of this sequence revealed open reading frames that included a 1,098-bp putative sensor histidine kinase, a 696-bp putative response regulator, and an 804-bp putative transposase (Fig. 3). The amino acid sequences of the putative sensor histidine kinase, VanSF, and the putative response regulator, VanRF, are similar to a wide variety of other bacterial two-component regulators, including those regulating expression of vanA, vanB, and other enterococcal glycopeptide resistance clusters. Specifically, VanSF and VanRF demonstrate 33% and 45% amino acid identity to VanS and VanR, respectively, from Enterococcus faecium BM4147 (2). However, VanSF and VanRF demonstrate even higher similarity to a family of two-component regulators found in the recently sequenced genomes of several Bacillus species, including Bacillus halodurans C-125 (GenBank BA000004), Bacillus cereus ATCC 14579 (GenBank AE017013), Bacillus anthracis strain Ames (GenBank AE017039), and Bacillus thuringiensis (GenBank AE017355). The greatest similarity is found with the two-component regulator of B. halodurans; putative VanSF and VanRF amino acid sequences are, respectively, 71% and 77% identical to their homologues in B. halodurans (Fig. 4). In the genomes of all four Bacillus species, the vanSF and vanRF homologues are adjacent to a vanY-like carboxypeptidase gene. The amino acid sequences of the putative Bacillus carboxypeptidases are 55 to 60% identical to the putative VanYF as well as to VanY from E. faecium BM4147. All of these Bacillus species are glycopeptide susceptible; a search of their published genomes revealed no homologues of any other structural elements of the VanF glycopeptide resistance cluster, with the exception of the presence of distant homologues of the vanZF gene in all of these species.
FIG. 3.
Nucleotide sequence of the region upstream of the previously sequenced vanYF gene including open reading frames encoding a putative transposase (bp 498 to 1301), the putative response regulator VanRF (bp 1512 to 2205), and the putative histidine kinase sensor VanSF (bp 2200 to 3297). The sequence from bp 1371 to 1469 has 95% identity to bp 436 to 535 of the Tn1546 tnp.
FIG. 4.
Comparison of the P. popilliae ATCC 14706 putative VanSF with its homologues in B. halodurans (GenBank BAB05528 [alternative start codon from annotated GenBank sequence]; 71% identical) and with VanS of E. faecium BM4147 (33% identical).
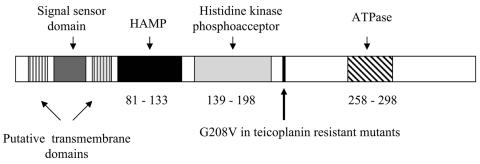
The overall structure of the putative VanSF protein was analyzed using the Conserved Domain Database of the Entrez system (23). VanSF includes the characteristic features of many histidine kinase-type sensor proteins such as a dimerization/phosphoacceptor domain and an ATP binding domain (Fig. 5). The N-terminal portion of the putative VanSF protein also contains two potential transmembrane domains flanking a 39-amino-acid sequence. This sequence, representing a potential signal sensor domain, demonstrates minimal similarity to the N-terminal regions of the enterococcal glycopeptide resistance VanS homologues. However, this domain shares conserved motifs with similar domains within the B. halodurans, B. cereus, and B. thurigiensis VanS homologues, with highest similarity (63% amino acid identity) to the comparable N-terminal region of the VanS homologue of B. halodurans (Fig. 4). Using PCR primers internal to vanRF and vanSF, the vanRF and vanSF genes were found in all 10 vancomycin-resistant P. popilliae strains tested but were not found in any of four strains of the closely related vancomycin-susceptible P. lentimorbus. In strain Bl17, vanSF exhibited 99% nucleotide identity to the gene in ATCC 14706, and a 114-bp fragment of vanRF exhibited 98% nucleotide identify to the same fragment in ATCC 14706.
FIG. 5.
Structure of the putative P. popilliae sensor histidine kinase showing conserved HAMP (histidine kinase, adenylyl cyclase, methyl-accepting chemotaxis protein, and phosphatase), histidine kinase photoacceptor and ATPase domains, putative transmembrane and signal sensor domains, and location of G→V substitution identified in two of three constitutively teicoplanin-resistant mutants.
We sequenced vanSF from three independently selected teicoplanin-resistant derivatives of strain BpPj1. All had teicoplanin MICs of 16 to 32 μg/ml and showed constitutive expression of glycopeptide resistance by growth curve and d-cycloserine plate assay. Two of these contained a point mutation resulting in substitution of valine for glycine at amino acid 208 of the putative VanSF sensor. The sequence of vanSF from the third teicoplanin-resistant strain did not differ from that of the parent BpPj1 strain.
Immediately upstream of vanR in P. popilliae 14706, we found a 99-bp fragment with extremely high nucleotide identity (95%) to a portion of the transposase gene from the vanA-associated resistance transposon Tn1546 of E. faecium BM4147 (GenBank M97297) (3). In P. popilliae 14706, this fragment is not part of an open reading frame. PCR directed at additional portions of the Tn1546 transposase gene found no other evidence of such sequences elsewhere in the P. popilliae genome. By PCR with a set of internal primers for the Tn1546-related insertional element, 6 of 10 unrelated strains of P. popilliae contained this fragment (Table 1).
The region upstream of the Tn1546 fragment contained an open reading frame encoding a protein with structural features of a complete transposase. The putative P. popilliae transposase is distinct from the Tn1546 transposase, is only distantly related to other transposases found in association with glycopeptide resistance genes, but is similar (32% amino acid identity) to a transposase found in the genome of B. halodurans (GenBank BAP07718). The B. halodurans transposase is not linked to the B. halodurans vanRS-like homologue or to the B. halodurans carboxypeptidase genes. The function of this transposase in B. halodurans has not been characterized. The G+C content of the vanF-associated transposase gene is 51%, higher than that of vanRFSF or the other structural genes of the vanF complex.
DISCUSSION
By sequencing upstream of the previously identified vanYF gene of the P. popilliae resistance cluster, we have identified a proposed complete glycopeptide resistance cluster with structural and functional homology to the enterococcal vanA and vanB resistance clusters (Fig. 6). We have also found a gene encoding a putative second ligase, in addition to the putative d-Ala:d-Lac ligase, VanF. The newly identified ligase has the sequence of a Ddl-type d-Ala:d-Ala ligase (13, 22) and is homologous to the only ligase we identified in glycopeptide-susceptible P. lentimorbus. Growth of P. popilliae in vancomycin-containing media demonstrated growth lag consistent with an inducible resistance cluster. Growth following vancomycin exposure, unlike growth in antibiotic-free medium, was d-cycloserine resistant, indicating that vancomycin-induced growth in P. popilliae is not dependent on a d-Ala:d-Ala ligase. The marked increase in dd-dipeptidase activity in P. popilliae following vancomycin exposure suggests that expression of VanXF, the postulated d-alanine-d-alanine dipeptidase in the VanF cluster, is also induced by vancomycin.
FIG. 6.
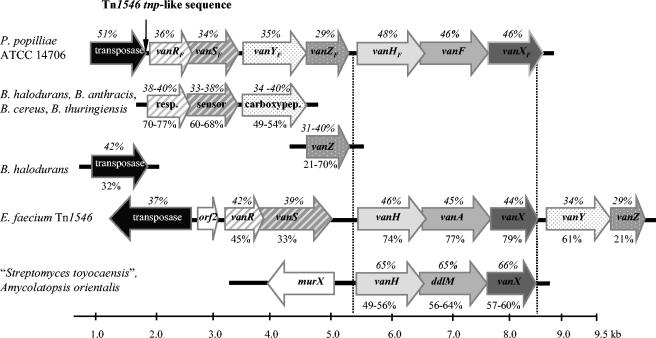
Alignment of the P. popilliae glycopeptide resistance gene cluster with the E. faecium transposon Tn1546 VanA glycopeptide resistance gene cluster, the “Streptomyces toyocaensis” and Amycolatopsis orientalis glycopeptide resistance gene clusters, and sequences from Bacillus species. The percent amino acid identity to P. popilliae is shown below the respective gene (i.e., below the arrows). The percent G+C content of each gene is shown above the genes (i.e., above the arrows). resp., response regulator; sensor, sensor histidine kinase; carboxypep., carboxypeptidase.
Identification of genes encoding a VanRS-like two-component regulator immediately upstream of the previously identified vanYFZFHFFXF portion of the resistance cluster suggests that this regulatory element may control expression of the structural genes of the resistance cluster. Although the putative proteins encoded by vanRFSF are structurally homologous to a wide variety of other bacterial two-component regulatory elements, including VanRS and VanRBSB, they are most similar to a family of two-component regulators associated with a carboxypeptidase gene in glycopeptide-susceptible Bacillus species (Fig. 6). This lends credence to the hypothesis that the genes encoding the carboxypeptidase and two-component regulator of P. popilliae may be of different origin than the vanHFFXF component of the resistance cluster. Similar arguments have been proposed regarding the origins of the vanA and vanB clusters, based on wide differences in G+C content of the diverse elements comprising these complex resistance clusters (3). Soil-dwelling Bacillus-like organisms such as P. popilliae may provide a unique evolutionary milieu where d-Lac dehydrogenase-d-Ala:d-Lac ligase-dd-dipeptidase clusters from glycopeptide-producing members of the order Actinomycetales can combine with the two-component regulators and associated carboxypeptidase genes of glycopeptide-susceptible Bacillus species.
The structure of the putative VanSF glycopeptide sensor shares the general features of a sensor histidine kinase with other two-component glycopeptide sensors including VanS and VanSB (4, 5, 14). The N-terminal region of the putative protein encoded by vanSF contains two apparent transmembrane domains that flank a sequence of 39 amino acids that may represent an extramembrane sensing element, similar to that in the proposed structure of VanSB (5). However, the amino acid sequence encoded by this postulated signal sensor in vanSF contains little homology to comparable sequences of VanS, VanSB, or the sensors of any other currently identified inducible glycopeptide resistance clusters. The broad diversity of the sequences in this family of sensor proteins in domains presumed to contribute the signal recognition specificity suggests that there may be several different paths by which the presence of glycopeptides in the external environment can directly or indirectly interact with the sensor protein to induce expression of the remainder of the resistance cluster. The specific signal that activates transcription of these resistance clusters remains in dispute (4). Although VanSF and VanSB likely have in common the ability to respond to vancomycin but not teicoplanin, this functional similarity is not reflected in the structures of their signal sensor sequences. The signal sensor region from VanSF does have some amino acid similarity to the carboxypeptidase-linked VanS homologues of B. halodurans, B. cereus, and B. thuringiensis, This suggests that the same signal that triggers expression of carboxypeptidase in glycopeptide-susceptible organisms may be directly or indirectly responsible for turning on expression of the P. popilliae VanF cluster. It is unlikely that the signal for induction of these carboxypeptidases is a glycopeptide.
Teicoplanin-resistant derivatives of vancomycin-resistant P. popilliae were readily selected by teicoplanin exposure. Analogous to the majority of in vivo and in vitro teicoplanin-resistant derivatives of VanB enterococci (5, 19), these strains appeared to constitutively express the d-Ala:d-Lac ligase as demonstrated by loss of d-cycloserine susceptibility and absence of growth lag in vancomycin-containing medium. Two of three independently selected teicoplanin-resistant derivatives of strain BpPj1 contained the same point mutation resulting in substitution of valine for glycine at amino acid 208 of the putative VanSF. This site does not correspond to mutations in the sequence of VanSB associated with constitutive expression of resistance (3, 5); thus, the specific mechanism of constitutive expression of glycopeptide resistance in these P. popilliae strains remains unclear.
We have previously shown that a strain of P. popilliae preserved as a hemolymph smear since 1945 is resistant to vancomycin (31). This isolate is from well before the first reports of vancomycin-resistant enterococci, indicating that vancomycin resistance in P. popilliae preceded acquired vancomycin resistance in enterococci. The role of the VanF glycopeptide resistance cluster in an environmental organism such as P. popilliae is unknown. The finding in this study of a putative transposase in association with the vanF cluster and the identification of a putative d-Ala:d-Ala ligase in both P. popilliae and the related vancomycin-susceptible P. lentimorbus, as well as our previous results showing that all U.S. and European strains of P. popilliae are resistant to vancomycin but that all Central and South American isolates are susceptible to vancomycin (18), strongly suggest that vancomycin resistance in P. popilliae is acquired.
The presence of a short sequence of DNA with high homology to the enterococcal Tn1546 transposase gene within the glycopeptide resistance cluster of several different strains of P. popilliae provides a tantalizing clue as to the origins of the P. popilliae cluster and to that of the vanA cluster. This sequence may represent a “footprint” of a Tn1546-like element that was necessary for an intermediate combinational step in the molecular evolution of the P. popilliae cluster and is additional evidence for the possible environmental exchange of resistance genes between enterococci and Bacillus-like organisms. Various Enterococcus species exist in the gut of insects; the potential transfer of vancomycin resistance genes from P. popilliae or similar species to Enterococcus species could have occurred in soil or, alternatively, in the gut of an insect. Of note, other species of soil-dwelling Paenibacillus and Rhodococcus have recently been found to contain vanA and vanB-like glycopeptide resistance genes (16). Glycopeptide-resistant enterococci of multiple species have also been recovered from a variety of environmental soil samples, including nonagricultural samples, in the apparent absence of vancomycin exposure, raising further questions about the possible environmental origins of enterococcal glycopeptide resistance elements (17). Several of our P. popilliae strains lacked this Tn1546 element although they contained all the other genes of the vanF cluster; the regions upstream of vanRFvanSF in these strains are currently being analyzed. Despite finding another complete putative transposase gene immediately adjacent to the P. popilliae cluster, all our efforts to date to move the resistance cluster from P. popilliae to P. lentimorbus, B. subtilis, or B. halodurans by conjugation or transformation have been unsuccessful.
Study of the antimicrobial resistance clusters of organisms such as P. popilliae can provide invaluable insight into the origin and function of similar resistance genes in human pathogens. In this study we show further evidence that the mechanism of vancomycin resistance in P. popilliae is similar to that of acquired vancomycin resistance in enterococci and that, as in enterococci, vancomycin resistance in P. popilliae was likely acquired.
Acknowledgments
We thank Edward Eirikis for technical support for studies performed for the identification of the P. popilliae d-Ala:d-Ala ligase and Kerryl E. Piper for technical support for the sequencing studies.
REFERENCES
- 1.Arthur, M., F. Depardieu, P. Reynolds, and P. Courvalin. 1996. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol. Microbiol. 21:33-44. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., C. Molinas, and P. Courvalin. 1992. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 174:2582-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin, 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur, M., and R. Quintiliani, Jr. 2001. Regulation of VanA- and VanB-type glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 45:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baptista, M., F. Depardieu, P. Reynolds, P. Courvalin, and M. Arthur. 1997. Mutations leading to increased levels of resistance to glycopeptide antibiotics in VanB-type enterococci. Mol. Microbiol. 25:93-105. [DOI] [PubMed] [Google Scholar]
- 6.Biavasco, F., E. Giovanetti, A. Miele, C. Vignaroli, B. Facinelli, and P. E. Varaldo. 1996. In vitro conjugative transfer of VanA vancomycin resistance between enterococci and Listeriae of different species. Eur. J. Clin. Microbiol. Infect. Dis. 15:50-59. [DOI] [PubMed] [Google Scholar]
- 7.Bugg, T. D., G. D. Wright, S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30:10408-10415. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin-United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 10.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin for the Vancomycin-Resistant Staphylococcus aureus Investigative Team. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 11.Dingmann, D. W., and D. P. Stahly. 1983. Medium promoting sporulation of Bacillus larvae and metabolism of medium components. Appl. Environ. Microbiol. 46:860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutka-Malen, S., C. Molinas, M. Arthur, and P. Courvalin. 1992. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a d-alanine:d-alanine ligase-related protein necessary for vancomycin resistance. Gene 112:53-58. [DOI] [PubMed] [Google Scholar]
- 13.Evers, S., B. Casadewall, M. Charles, S. Dutka-Malen, M. Galimand, and P. Courvalin. 1996. Evolution of structure and substrate specificity in d-alanine: d-alanine ligases and related enzymes. J. Mol. Evol. 42:706-712. [DOI] [PubMed] [Google Scholar]
- 14.Evers, S., and P. Courvalin. 1996. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR (B) two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 178:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana, R., M. Ligozzi, C. Pedrotti, E. M. Padovani, and G. Cornaglia. 1997. Vancomycin-resistant Bacillus circulans carrying the vanA gene responsible for vancomycin resistance in enterococci. Eur. J. Clin. Microbiol. Infect. Dis. 16:473-474. [DOI] [PubMed] [Google Scholar]
- 16.Guardabassi, L., H. Christensen, H. Hasman, and A. Dalsgaard. 2004. Members of the genera Paenibacillus and Rhodococcus harbor genes homologous to enterococcal glycopeptide resistance genes vanA and vanB. Antimicrob. Agents Chemother. 48:4915-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guardabassi, L., and A. Dalsgaard. 2004. Occurrence, structure and mobility of Tn1546-like elements in environmental isolates of vancomycin-resistant enterococci. Appl. Environ. Microbiol. 70:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison, H., R. Patel, and A. A. Yousten. 2000. Paenibacillus associated with milky disease in Central and South American scarabs. J. Invertebr. Pathol. 76:169-175. [DOI] [PubMed] [Google Scholar]
- 19.Hayden, M. K., G. M. Trenholme, J. E. Schultz, and D. F. Sahm. 1993. In vivo development of teicoplanin resistance in a VanB Enterococcus faecium isolate. J. Infect. Dis. 167:1224-1227. [DOI] [PubMed] [Google Scholar]
- 20.Hong, H. J., M. I. Hutchings, J. M. Neu, G. D. Wright, M. S. Paget, M. J. Buttner. 2004. Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Mol. Microbiol. 52:1107-1121. [DOI] [PubMed] [Google Scholar]
- 21.Leclercq, R., E. Derlot, M. Weber, J. Duval, and P. Courvalin. 1989. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 33:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lessard, I. A., V. L. Healy, I. S. Park, and C. T. Walsh. 1999. Determinants for differential effects on d-Ala-d-lactate vs d-Ala-d-Ala formation by the VanA ligase from vancomycin-resistant enterococci. Biochemistry 38:14006-14022. [DOI] [PubMed] [Google Scholar]
- 23.Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall, C. G., I. A. Lessard, I. Park, and G. D. Wright. 1998. Glycopeptide antibiotic resistance genes in glycopeptide-producing organisms. Antimicrob. Agents Chemother. 42:2215-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NNIS System. 2003. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 31:481-498. [DOI] [PubMed] [Google Scholar]
- 26.Noble, W. C., Z. Virani, and R. G. Cree. 1992. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 72:195-198. [DOI] [PubMed] [Google Scholar]
- 27.Patel, R. 2000. Enterococcal-type glycopeptide resistance genes in non-enterococcal organisms. FEMS Microbiol. Lett. 185:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Patel, R., K. Piper, F. R. Cockerill III, J. M. Steckelberg, and A. A. Yousten. 2000. The biopesticide Paenibacillus popilliae has a vancomycin resistance gene cluster homologous to the enterococcal VanA vancomycin resistance gene cluster. Antimicrob. Agents Chemother. 44:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power, E. G. M., Y. H. Abdulla, H. G. Talsania, W. Spice, S. Aathithan, and G. L. French. 1995. vanA genes in vancomycin-resistant isolates of Oerskovia turbata and Arcanobacterium (Corynebacterium) haemolyticum. J. Antimicrob. Chemother. 36:595-606. [DOI] [PubMed] [Google Scholar]
- 30.Rippere, K., M. T. Tran, A. A. Yousten, K. H. Hilu, and M. G. Klein. 1998. Bacillus popilliae and Bacillus lentimorbus bacteria causing milky disease in Japanese beetles and related scarab larvae. Int. J. Syst. Bacteriol. 48:395-402. [Google Scholar]
- 31.Rippere, K., R. Patel, J. R. Uhl, K. E. Piper, J. M. Steckelberg, B. C. Kline, F. R. Cockerill, and A. A. Yousten. 1998. DNA sequence resembling vanA and vanB in the vancomycin-resistant biopesticide Bacillus popilliae. J. Infect. Dis. 178:584-588. [DOI] [PubMed] [Google Scholar]
- 32.Weber, K. L., M. E. Bolander, and G. Sarkar. 1998. Rapid acquisition of unknown DNA sequence adjacent to a known segment by multiplex restriction site PCR. BioTechniques 25:415-419. [DOI] [PubMed] [Google Scholar]
- 33.Woodford, N. 2001. Epidemiology of the genetic elements responsible for acquired glycopeptide resistance in enterococci. Microb. Drug Resist. 7:229-236. [DOI] [PubMed] [Google Scholar]