Abstract
We have studied the interference of roxithromycin with NADPH oxidase, the key enzymatic system for oxidant production by human neutrophils. Roxithromycin alters the reconstitution of an active enzyme and impairs the translocation to the outer membrane of the cytosolic components p47-phox and p67-phox. Interestingly, in resting cells roxithromycin directly triggers the translocation of these factors without stimulating the oxidative burst.
Oxidant production by polymorphonuclear neutrophils (PMN) plays an important role in host defenses against invading microorganisms. NADPH oxidase, the enzymatic complex that catalyzes superoxide anion formation, is a multicomponent enzyme system composed of at least five subunits including cytosolic components (p47-phox [phox for phagocyte oxidase], p67-phox, and a GTP-binding protein, p21-rac) and membrane-associated components (a flavocytochrome, cytochrome b558, and a GTP-binding protein, rap-1A) (2). PMN activation by appropriate stimuli is accompanied by phosphorylation and translocation of cytosolic factors to the membrane and their binding to cytochrome b. Inappropriate or excessive PMN activation can result in an exaggerated and destructive oxidative response, leading to host tissue injury (11). Modulation of NADPH oxidase activity is an interesting candidate strategy in the treatment of inflammatory diseases (3, 8). Macrolide antibiotics have recently gained interest in this field, owing to their nonantibiotic properties (5-7). We and others have previously reported that various erythromycin A-derived macrolides depress the oxidative burst of human neutrophils stimulated with various agonists and promote PMN exocytosis (1). The aim of this work was to obtain further insights into macrolide-induced modification of the NADPH oxidase system. Roxithromycin was chosen as the test drug.
All reagents, except where indicated, were from Sigma Chemical Co. Roxithromycin (Aventis, Romainville, France) was dissolved at 1 mg/30 μl of dimethyl sulfoxide (DMSO) and further diluted in Hanks buffered salt solution (HBSS). All antibiotics and reagents were endotoxin free, as shown by the Limulus amoebocyte lysate assay. PMN were obtained by 2% dextran sedimentation of heparinized venous blood, followed by centrifugation on Ficoll-Paque and subsequent osmotic lysis of erythrocytes. Neutrophils were washed and suspended in HBSS at 4°C until use (1). Cell viability was measured by the release of the cytoplasmic marker enzyme lactate dehydrogenase into the extracellular medium. Results are expressed as the mean plus the standard error of the mean of n experiments performed with neutrophils from different human volunteers. Experiments with roxithromycin were paired with controls (corresponding DMSO solutions). Analysis of variance was used for multiple comparisons. Paired normally distributed data were analyzed using Student's t test. Concentration dependence was analyzed by constructing regression curves. All statistical tests were run on the Statworks program, version 1.2 (Cricket software, 1985).
Effect of roxithromycin on NADPH oxidase activity and kinetic constants in particulate fractions prepared from PMA-stimulated neutrophils.
A cell-free system for assay of NADPH oxidase activity was obtained as follows. Neutrophils (108/ml) were stimulated for 5 min with phorbol-12 myristate-13 acetate (PMA; 2 μg/ml). The reaction was stopped by adding cold HBSS and centrifugation (10 min at 400 × g and 4°C). The pellet was recovered in 0.34 M sucrose solution containing 5 × 10−4 M phenylmethylsulfonyl fluoride and sonicated (4 × 10 s) with a Fisher sonic dismembranator. Unbroken cells were removed by centrifugation, and the supernatant was ultracentrifuged at 26,000 × g for 20 min at 4°C. The pellet containing NADPH oxidase was suspended in 0.34 M sucrose and stored at −70°C until use (12). Protein content was determined by the Bio-Rad technique. NADPH oxidase activity in the particulate fraction of stimulated neutrophils was determined by measuring the reduction of cytochrome c by 50 μg of protein. The reaction was started by adding 500 μM NADPH, and cytochrome c reduction was followed at 550 nm. Superoxide anion generated by this system was measured in terms of superoxide dismutase-inhibitable cytochrome c reduction in a Uvikon 860 spectrophotometer. To assess the effect of macrolides on NADPH oxidase activity, roxithromycin (100 mg/liter) or 0.3% DMSO (control) was added to the particulate fraction before measuring superoxide production (direct effect on enzyme activity) or neutrophils were incubated for 30 min in the presence of roxithromycin or DMSO before preparing the particulate fraction (effect on enzyme reconstitution). Kinetic constants for NADPH oxidase, i.e., the substrate concentration at which the rate is half-maximal (Km) and the reaction rate when enzyme activity is saturated (Vmax), were determined by graphic methods from Lineweaver-Burk plots of the Michaelis-Menten curves obtained by measuring the rate of cytochrome c reduction at NADPH concentrations ranging from 25 μM to 1 mM.
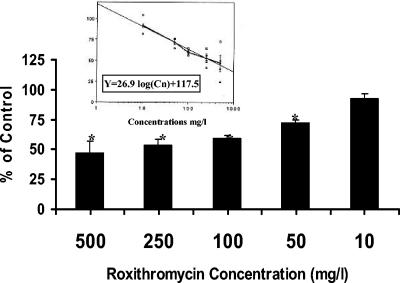
First, a roxithromycin concentration of 100 mg/liter was chosen because it produced maximal inhibition of oxidant production in the intact-cell system (1). When roxithromycin was added to the particulate fraction containing NADPH oxidase, neither the enzyme activity nor the kinetic constants were modified (Table 1). By contrast, roxithromycin markedly inhibited NADPH oxidase activity when added to cells before stimulation and enzyme preparation. It must be noted that control preparations obtained from PMN incubated for 30 min in HBSS, and to a lesser extent in DMSO, displayed a greater (although nonsignificant) activity, which may be due to the priming of PMN during the incubation time. However, the membrane preparations obtained from cells incubated in the presence of roxithromycin did not express a greater enzyme activity compared to those obtained from nonincubated PMN (about 30 nmol of cytochrome c reduced/min/mg protein), suggesting that roxithromycin may alter a pathway leading to priming. The inhibition was concentration dependent (Fig. 1). The concentration which inhibited 50% of NADPH oxidase activity was calculated as 323 mg/liter by constructing a logarithmic regression curve [y = −26.9 log(Cn) + 117.5; r = 0.994, P < 0.001]. This value was greater than that obtained with intact cells (1), but to achieve optimal NADPH oxidase reconstitution, we used a PMA concentration (2 μg/ml) far higher than that used with intact cells (100 ng/ml), which may have overcome the inhibitory effect of the drug.
TABLE 1.
Effect of roxithromycin on NADPH oxidase activity and kinetic constants
| Time of drug addition and parameter | HBSS | 0.3% DMSO | Roxithromycin (100 mg/liter) |
|---|---|---|---|
| After isolation of particulate fractions | |||
| Activitya | 30 ± 4.8 (5)d | 28 ± 3.15 (7) | 30 ± 3.5 (7) |
| Kmb | 95 ± 29.5 (4) | 129 ± 32.2 (7) | 131 ± 29.7 (7) |
| Vmaxc | 42 ± 10.1 (4) | 55 ± 15.4 (4) | 52 ± 9.3 (7) |
| 30 min before stimulation and isolation of particulate fractions | |||
| Activity | 64 ± 19.3 (7) | 48 ± 9.3 (12) | 31 ± 6.4 (12)e |
| Km | 64 ± 10.7 (5) | 115 ± 30.0 (12) | 81 ± 13.8 (12) |
| Vmax | 52 ± 8.4 (5) | 60 ± 7.8 (12) | 39 ± 4.5 (12)e |
Nanomoles of cytochrome c reduced/min/mg protein (500 μM NADPH).
Km, NADPH, μM.
Vmax, nanomoles of cytochrome c reduced/min/mg protein.
The number of experiments is in parentheses.
Significantly different from control (DMSO solution).
FIG. 1.
Effects of various roxithromycin concentrations on NADPH oxidase activation. PMN were incubated with the macrolide for 30 min before stimulation with PMA (2 μg/liter) and isolation of the particulate fraction containing NADPH oxidase. The mean and the standard error of the mean of five independent experiments are indicated by black columns. *, P < 0.01 versus control (100%). The logarithmic concentration-dependent effect is shown in the inset.
In addition (Table 1), the Km for NADPH was not significantly different whatever the experimental conditions used, whereas the Vmax fell significantly (to about 60% of the control) when, and only when, the drug was added to cells before stimulation and NADPH oxidase preparation. These data suggest that the presence of roxithromycin during cell preparation led to the production of a reduced quantity of active enzyme with normal substrate affinity. Similar results have been obtained with chloroquine and other weak bases which, like roxithromycin, are concentrated within PMN granules (12).
Effect of macrolides on PKC activity.
As protein kinase C (PKC) is a key enzyme in p47-phox and p67-phox phosphorylation, a step required for NADPH oxidase reconstitution, we further analyzed the effect of roxithromycin on PKC activity. To measure PKC activity, neutrophils (107/ml) were suspended in lysis buffer, sonicated (4 × 10 s) at 4°C, and then centrifuged (100,000 × g for 60 min at 4°C) in a Beckman TL ultracentrifuge (4). The resulting supernatant (cytosol) was used as the source of PKC. PKC was assayed by using histone-III-S as the substrate in a reaction mixture (0.1 ml) containing 20 mM Tris-HCl, 10 μM MgCl2, 20 μg of histone-III-S, 4 μg of phosphatidylserine, 0.9 μg of diolein, 0.6 mM CaCl2, 10 mM ATP (containing 0.5 μCi of [γ-32P]ATP [30 to 40 Ci/mmol; Dupont New England Nuclear, Boston, MA]), and 20 μg of cytosolic protein in the presence of roxithromycin (100 mg/liter) or 0.3% DMSO (control). After incubation for 10 min at 30°C, the reaction was stopped by adding 400 μl of ice-cold 20% trichloroacetic acid and 100 μl of bovine serum albumin (2.5 mg/ml) as a carrier. After centrifugation at 10,000 × g for 10 min, the pellet was dissolved in 0.5 N NaOH and further washed after adding ice-cold trichloroacetic acid. Four milliliters of Dimilume scintillation fluid was added to the resulting pellet. PKC activity was measured as pellet-associated radioactivity (pmol ATP/min/mg protein) after subtracting nonspecific activity measured in the absence of phosphatidylserine-diolein and calcium. Roxithromycin (100 mg/liter) did not modify the phosphorylation of histone-III-S (1,482 ± 120.0 versus 1,432 ± 114.1 pmol ATP/min/mg protein) or that of endogenous proteins used as substrates (results not shown).
Effect of roxithromycin on p47-phox and p67-phox translocation.
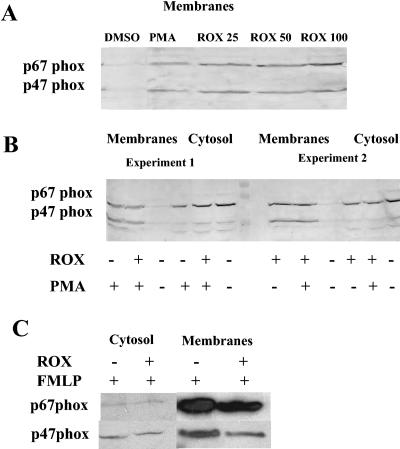
Reconstitution of an active oxidase complex requires phosphorylated p47-phox and p67-phox to be translocated to the membrane and to combine with cytochrome b555. To assess translocation of cytosolic components, cells (108/ml) were treated for 30 min with roxithromycin (to achieve complete inhibition of superoxide anion production) or DMSO (control) and then with or without PMA (1 μg/ml, 8 min). Cytosol and membranes were isolated after sonication (4 × 10 s, 0°C) and centrifugation on a sucrose gradient. Proteins were subjected to sodium dodecyl sulfate-13% polyacrylamide gel electrophoresis in Laemmli buffer. The separated proteins were electrophoretically transferred to nitrocellulose sheets, blocked as described in reference 10, and probed with anti-p47-phox and anti-p67-phox antibodies (a generous gift from B. M. Babior, The Scripps Research Institute, La Jolla, CA) at respective dilutions of 1:5,000 and 1:1,000. The p47-phox and p67-phox bands were visualized with a secondary horseradish peroxidase-conjugated swine anti-rabbit immunoglobulin G in the ECL detection system (Amersham). It is well known that cell stimulation with PMA or N-formyl-l-methionyl-leucyl-phenylalanine (fMLP) induces translocation of cytosolic factors. Interestingly, PMN pretreatment for 30 min with roxithromycin, in the absence of stimulation, induced spontaneous translocation of p47-phox and p67-phox from the cytosol to the membrane (Fig. 2A and B), and this effect was evidenced at a concentration of 25 μg/ml and slightly stronger at 100 μg/ml (Fig. 2A). When added prior to enzyme activation by PMA, roxithromycin slightly impaired the translocation of p47-phox and, to a lesser extent, p67-phox (Fig. 2B); however, there was a strong interindividual variability of this effect: the PMN from some volunteers were susceptible to this inhibitory action (Fig. 2B, experiment 1), while for other PMN (Fig. 2B, experiment 2) the inhibition was less marked and the inhibitory effect was mainly evident in the cytosol fraction. By contrast, when PMN were stimulated with fMLP, roxithromycin impaired the translocation of the two cytosolic factors in all PMN samples (Fig. 2C). The impairment of the assembly of the oxidase components by roxithromycin may correlate with the inhibition of oxidant production in cells stimulated with PMA and fMLP. In particular, this drug displays a greater inhibitory effect when the cells are stimulated with fMLP than when PMA is the stimulus (1), and the interindividual susceptibility of PMN to the inhibitory effect of roxithromycin (and other macrolides) on oxidant production has been previously reported. A very unexpected finding was the direct stimulation of p47-phox and p67-phox translocation by roxithromycin. However, this was not sufficient to induce NADPH oxidase activation, as roxithromycin did not directly trigger superoxide formation in resting cells, even after long incubation times (30 to 120 min; results not shown). Previous studies by our group (1) have shown that in resting PMN, erythromycin A-derived macrolides directly stimulate phospholipase D (PLD) activity (and thus promote PMN exocytosis) while in stimulated cells, they inhibit PLD and phosphatidate phosphohydrolase (PPH) activities, an effect likely responsible for the impairment of oxidant production, as reported for other PPH inhibitors (9). Although roxithromycin does not directly inhibit PKC activity, the decreased production of diacylglycerol (as a result of PPH inhibition) may result in reduced activity of PKC (or certain PKC subsets); this might generate a different phosphorylation profile of the cytosolic components of NADPH oxidase and inhibit their translocation to the PMN membrane. How roxithromycin directly promotes p47-phox and p67-phox translocation in resting PMN was not clarified here. As this translocation does not result in oxidase activation, it is likely that the cytosolic factors are not correctly phosphorylated and are thus unable to assemble properly with the membrane constituents. In particular, because roxithromycin activates PLD and blocks PPH, there is a large increase in phosphatidic acid in resting cells pretreated with this macrolide. The role of phosphatidic acid-dependent kinases in the phosphorylation of the cytosolic components of NADPH oxidase should now be explored.
FIG. 2.
Effect of roxithromycin (ROX) on p47-phox and p67-phox translocation. PMN were preincubated for 30 min with 0.3% DMSO or roxithromycin. Membrane and cytosol fractions were then prepared, and p47-phox and p67-phox were detected as described in the text. All samples contained the same quantity (10 μg) of protein. (A) PMN were preincubated for 30 min with 0.3% DMSO or roxithromycin (25 to 100 mg/liter). One experiment representative of three different experiments is shown. (B) PMN were preincubated for 30 min with 0.3% DMSO (ROX −) or 100 mg/liter roxithromycin (ROX +). They were further stimulated with PMA at 1 μg/ml for 8 min (PMA +) or left unstimulated (PMA −). Two experiments representative of four different experiments are shown. (C) PMN were preincubated for 30 min with 0.3% DMSO (control; lanes marked ROX −) or 100 mg/liter roxithromycin (lanes marked ROX +). They were further stimulated with fMLP (10−6 M, 1 min). One experiment representative of four different experiments is shown.
REFERENCES
- 1.Abdelghaffar, H., D. Vazifeh, and M. T. Labro. 1997. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase D-phosphatidate phosphohydrolase transduction pathway: l-cladinose is involved both in alterations of neutrophil functions and modulation of this transductional pathway. J. Immunol. 159:3995-4005. [PubMed] [Google Scholar]
- 2.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 3.Cross, A. R. 1990. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic. Biol. Med. 8:71-93. [DOI] [PubMed] [Google Scholar]
- 4.El Benna, J., J. Hakim, and M. T. Labro. 1992. Inhibition of human neutrophil protein kinase C activity by the antimalarial drug mefloquine. Biochem. Pharmacol. 43:527-532. [DOI] [PubMed] [Google Scholar]
- 5.Labro, M. T. 1998. Immunological effects of macrolides. Curr. Opin. Infect. Dis. 11:681-688. [DOI] [PubMed] [Google Scholar]
- 6.Labro, M. T. 2004. Cellular and molecular effects of macrolides. Curr. Pharm. Design 10:3067-3080. [DOI] [PubMed] [Google Scholar]
- 7.Labro, M. T. 2004. Macrolide antibiotics: current and future uses. Exp. Opin. Pharmacother. 5:541-550. [DOI] [PubMed] [Google Scholar]
- 8.Miesel, R., D. Sanocka, M. Kurpisz, and H. Kröger. 1995. Antiinflammatory effects of NADPH oxidase inhibitors. Inflammation 19:347-362. [DOI] [PubMed] [Google Scholar]
- 9.Perry, D. K., W. L. Hand, D. E Edmonson, and J. D. Lambeth. 1992. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase. Inhibition by phosphatidic acid phosphohydrolase inhibitors. J. Immunol. 149:2749-2758. [PubMed] [Google Scholar]
- 10.Schwarz, H. P., M. J. Heeb, J. D. Wencel-Drake, and J. H. Griffin. 1985. Identification and quantitation of protein S in human platelets. Blood 66:1452-1455. [PubMed] [Google Scholar]
- 11.Smith, J. A. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 56:672-686. [DOI] [PubMed] [Google Scholar]
- 12.Styrt, B., and M. S. Klempner. 1986. Inhibition of neutrophil oxidative metabolism by lysosomotropic weak bases. Blood 67:334-342. [PubMed] [Google Scholar]