Abstract
Background
Tuina is an effective treatment for the decrease of skeletal muscle atrophy after peripheral nerve injury. However, the underlying mechanism of action remains unclear. This study aimed to explore the underlying mechanisms of tuina in rats with sciatic nerve injury (SNI).
Methods
We established an SNI rat model. After Tuina intervention, curative effects were evaluated by behavioral assessment, nerve function index, and muscle atrophy index (MAI). Pathological changes were observed by transmission electron microscopy and immunofluorescence. Insulin-like growth factor 1 (IGF-1), forkhead box O (FoxO) and p-FoxO levels were detected using enzyme-linked immunosorbent assay. Western blotting was performed to detect the expression of proteins involved in the PI3K/AKT signaling pathway.
Result
Behavioral assessment, nerve function index, and MAI revealed that the tuina had significantly improved muscle atrophy after SNI compared with the SNI model group. Transmission electron microscopy showed that tuina improved muscle ultramicrostructure. CD31 immunofluorescence revealed that tuina improved microcirculation. Furthermore, we observed that tuina differentially regulated the levels of IGF-1, FoxO and p-FoxO, and the protein expression of p-Phosphoinositide 3-kinase (p-PI3K), p-AKT, and vascular endothelial growth factor in the anterior tibial muscle and soleus muscles.
Conclusion
Tuina could effectively inhibit skeletal muscle atrophy via the microcirculation pathway in a rat model of SNI by regulating the expression of IGF-1 and FoxO. The underlying mechanism of action may involve the PI3K/Akt signaling pathway.
Keywords: Peripheral nerve injury, Tuina, Muscle atrophy, Microcirculation, PI3K/AKT pathway
Background
Peripheral nerve injury (PNI) is a neurological condition that adversely affects human and animal patients [1]. It is associated with poor functional outcomes, insufficient nerve recovery, and the loss of motor function. Additionally, they are followed by a partial recovery, muscle atrophy, and profound weakness [2]. Changes in microvasculature after nerve injury is an important factor in muscle atrophy. Injury-induced loss of local blood vessels contributes to inflammation and ischemia, and thus, to the overall damage to the nerves and muscles that innervate [3]. The reversal of microvascular dysfunction may provide new approaches for the treatment and prevention of PNI.
Skeletal muscle atrophy is a debilitating consequence of denervation that contributes toward incomplete functional recovery [4]. A combination of factors, including increased proteolysis, decreased protein synthesis, and impaired regenerative capacity, contribute to skeletal muscle atrophy [5]. Activation of the phosphatidylinositol-3 kinase (PI3K)/AKT signaling pathway prevents muscle atrophy by inhibiting the activity of FoxO transcription factors and augmenting protein synthesis. PI3K/Akt signaling can also predominantly inhibit the effects of myostatin, causing an increase in skeletal muscle size [6]. Moreover, PI3K and its downstream target Akt are implicated in several cellular responses linked to angiogenesis, including endothelial cell migration and survival. Constitutively active PI3K or Akt overexpression promotes angiogenesis in vivo and increases vascular endothelial growth factor (VEGF) expression [7].
Tuina, a nonsurgical intervention, is an alternative medical therapy that is safe and has virtually no side effects. Several studies have reported its application in multiple diseases to improve disability and pain [8–10]. Additionally, owing to their anti-inflammatory and blood circulation-promoting effects, Tuina is widely used for myopathy recovery [11]. Tuina has a positive effect on the treatment of PNI through autophagy, synaptic plasticity, axon regeneration, and remyelination, ultimately achieving the purpose of restoring sensory and motor function [12–14].
Whether the mechanical effects of tuina on PNI inhibit skeletal muscle atrophy via the microcirculation pathway remains unknown. Further investigations are required to determine whether this process is regulated by the PI3K/AKT pathway deserves further exploration. To address these questions, we established a rat model of sciatic nerve injury and observed the effects of tuina on muscle function, muscle atrophy, microcirculation, and the PI3K/AKT pathway.
Materials and methods
Animals
Animal experiments were approved by the Animal Care and Use Committee of the Beijing University of Chinese Medicine (No. BUCM-2023032303-1119). All animal experiments were designed according to the principles of the 3Rs (Replacement, Reduction and Refinement) and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague–Dawley (SD) rats (6-week-old, 190–210 g) were purchased from Beijing SPF Biotechnology Co., Ltd. (Beijing, China), and housed in a pathogen-free environment with four animals per cage. The rats are fed in a 12-h light–dark cycle environment, temperature (25 ± 0.5) °C, humidity 40–50%, and had free access to diet and drinking water.
SNI model
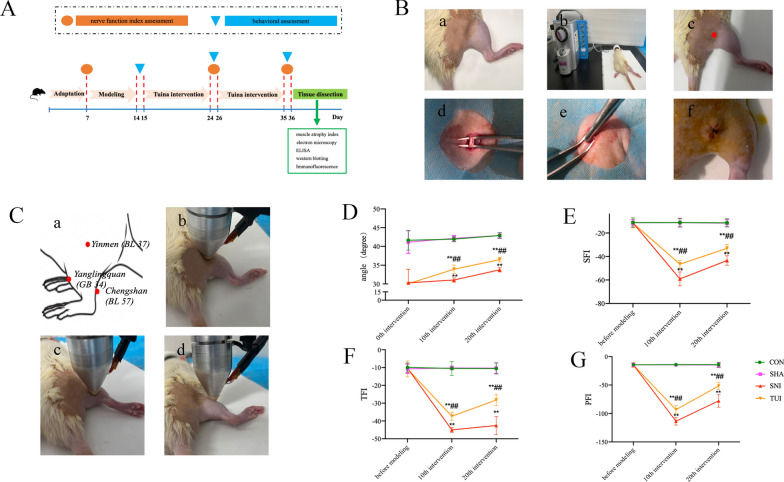
Thirty-six rats were randomly divided into four groups (n = 9 each): blank control (CON), sham model (SHA), sciatic nerve injury model (SNI), and Tuina (TUI) group. Pathological modeling was initiated one week after acclimatization. As SNI rat model was established as previously described [4]. After anesthetized, approximately 1 cm-long incision was made in the skin along the direction of the sciatic nerve, exposing the lower edge of the piriformis. For rats in the SNI and TUI groups, special hemostatic pliers were used to clamp 5 mm from the distal end of the sciatic nerve nodule for 5 s with full force (6 N), resulting in an injury point of approximately 2 mm (Fig. 1B). For the rats in the SHA group, only the sciatic nerve was only found, sterilized, sutured, and sterilized. For the rats in CON group, only routine feeding.
Fig. 1.
Tuina improved SNI rat behavioral performance and repair of nerve injury. A Animal experimental flow; B The process of modeling; a skin preparation; b anesthetization; c the position of incision; d exposure of the sciatic nerve trunk; e clamping; f suture. C Tuina intervention; a the schematic of the acupoint location, b BL 37 stimulus; c BL 57 stimulus; d GB 34 stimulus. D The angle of the inclined plate changes; E SFI; F TFI; G PFI. Results are presented as mean ± standard deviation. **P < 0.001 vs. SHA; ##P < 0.001 vs. SNI
Tuina intervention
The TUI group received “Three-Manipulation and Three-Acupoint” treatment, the procedure was as follows: The Tuina Manipulation Simulator (Self-developed machine, China invention patent number ZL202320511277.5) was set to stimulate with a force of 4 N, 60 times per minute. The stimulus rod was placed on Yinmen (BL 37), Chengshan (BL 57) and Yanglingquan (GB 34) of the surgical side, then finger pressing, plucking, and kneading manipulation were stimulated, respectively (Fig. 1C). Each method was used for 1 min at each acupoint, for a total of 9 min. Ten treatments were followed by one day rest and ten treatments were repeated; overall, twenty treatments were applied.
Grip restraint was performed in the SHA and SNI groups. The rats in the CON group were removed from the cage and returned. To reduce the animal stress response, the animals were petted and stroked for 9 min before the formal intervention every day.
Behavioral assessment
An electrically inclined plate tester was used to detect changes in hind limb muscle strength. Rats in each group were subjected to behavioral testing, and the test was performed on the day of 0th intervention (Day 15), 10th (Day 25) and 20th (Day 36) treatment. The heads of the rats were placed on the board toward the end, and the angle of the board was gradually increased after the rats calmed down. When the rats could not stay in this position for 5 s, the critical angle of the protractor was recorded, and the average of three measurements was taken.
Nerve function index
Nerve function index was collected using the DigiGait™ Imaging System and then analyzed by the DigiGait™ 15.0 analysis software (Mouse Specifics, Inc.; Quincy, MA, USA). The test was performed before modeling (Day 7), on the day of 10th (Day 25) and 20th (Day 36) treatment. The rats were acclimated to the apparatus 1 week before the experiment, and the treadmill belt was gradually accelerated to 10 cm/s.
Muscle atrophy index (MAI)
After the rats were sacrificed (Day 36), the anterior tibial and soleus muscles were removed and their wet weights were measured using an electronic balance. MAI was defined as the muscle weight divided by the body weight. Empty stomach weights of pre-dissected rats and their anterior tibial and soleus muscles (post-dissection) were measured.
Transmission electron microscopy
The anterior tibial and soleus muscles, and sciatic nerves were removed, fixed in pre-cooled 2.5% (w/v) glutaraldehyde for 3 h, and washed with 0.1 M PB. Subsequently, they were postfixed in a 1% (w/v) osmic acid solution for 1 h, washed in 0.1 M PB for 1 h, dehydrated (through a grade series of ethanol solutions), and embedded in Epon 812 epoxy resin. The segments were cut into 70 nm-thick ultrathin slices. The sections were then stained with saturated aqueous uranyl acetate (2%) and citrate and observed and analyzed by transmission electron microscopy.
Immunofluorescence
The anterior tibial muscle, soleus muscle, and sciatic nerve were fixed in 4% paraformaldehyde at 4 °C, dehydrated using a sucrose gradient, embedded in optimal cutting temperature compound, and cut into 4 μm sections. The paraffin sections were dewaxed, dehydrated, subjected to antigen retrieval, cleared of spontaneous fluorescence, and blocked with serum. Primary antibodies for CD 31 (1:500, abcam, USA) were incubated at 4 °C overnight. Sections were rinsed with phosphate-buffered saline (PBS) and incubated with secondary antibody Goat Anti-Rabbit IgG H&L (1:500, abcam, USA) at 37 °C for 30 min, and then rinsed with PBS. The sections were observed under a fluorescence microscope, and ImageJ software was used to analyze the Pearson coefficient of immunofluorescence co-localization and fluorescence intensity of each protein.
Enzyme-linked immunosorbent assay (ELISA)
Fifty milligrams of the gastrocnemius muscle and tibialis anterior muscle were weighed, and PBS was added at a weight (mg)/volume (μL) ratio of 1:10 for homogenization. The protein concentrations of the samples were determined using an ELISA kit. The test procedure was: standard dilution, sample addition, washing, color development, and reaction termination. The absorbance of each well was measured and finally calculated the concentration of forkhead box O (FoxO), p-FoxO, and insulin-like growth factor 1 (IGF-1) by drawing a standard curve.
Western blotting
The gastrocnemius, tibialis anterior muscles, and sciatic nerves were lysed in radio immunoprecipitation assay (RIPA) lysis buffer. A Bicinchoninic Acid (BCA) protein assay kit was used to measure the protein concentration. Briefly, 20 μg of total protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to poly (vinylidene fluoride) (PVDF) membranes at 400 mA for 30 min. Subsequently, the membranes were blocked with a blocking solution for 30 min at room temperature. The membranes were incubated overnight at 4 °C with the following primary antibodies: mouse anti-GAPDH (1:10,000, Ym3029, Immunoway), rabbit anti-p-PI3K (1:1000, AF3241, Affbiotech), rabbit anti-p-Akt (1:1000, bs-0867R, Bioss), and rabbit anti-VEGF (1:1000, bs-1665R, Bioss). After washing three times with tris-buffered saline with 0.1% Tween® 20 detergent (TBST), the secondary horseradish peroxidase (HRP)-conjugated antibodies were as follows: goat anti-rabbit IgG H&L (1:10,000, bs-0295G, Bioss) and goat anti-mouse IgG H&L (1:10,000, bs-0296G, Bioss). After washing three times with TBST, an enhanced chemiluminescence (ECL) kit was used to detect immunoactivity.
Statistical analysis
Data analysis was performed using SPSS Statistics software (version 26.0, IBM, Armonk, NY, USA). Data were presented as mean ± SD. Student’s t-test was used to compare the differences between the two groups. One-way ANOVA was used for comparisons between the groups, and the least significant difference (LSD) multiple comparison test was used for multiple comparisons. P < 0.05 was treated as statistically significant.
Results
Tuina improved behavioral performance
No redness or swelling of injury was observed in each group, and their health status was good after the modeling operation. Seven days after the SNI model was established, the knee joints of the rats were straightened with limited flexion and limp, the digits were retracted and fine motor function was greatly affected, indicating that the model was successfully prepared [15–17]. The model success rate tended to be 81%, and we also excluded the rats for gnawing toes which might influence behavioral tests.
For behavioral assessment, the angle of the inclined plate was used to evaluate muscle strength and motor function. We observed that the angle of the inclined plate of the rats in the CON and SHA groups after 10th and 20th intervention was not statistically different. Compared with the SHA group, the inclined plate angle of the rats in the SNI and TUI groups decreased significantly 7 days after modeling (0th intervention), but there was no significant difference between the SNI and TUI groups. After the 10th and 20th intervention, the inclined plate angle of the rats in the TUI group increased significantly compared with those in the SNI group; however, there were still significant differences compared with those in the SHA group. These results showed that Tuina intervention can effectively improve motor function in the lower limbs after nerve injury (Fig. 1D).
Tuina promoted nerve injury repair
The nerve function index was used to evaluate recovery of the injured nerve. The results of rats in each group showed that, compared with the baseline (before modeling), the sciatic function index (SFI), tibial function index (TFI), and peroneal function index (PFI) of rats in the SNI group and the TUI group decreased significantly; compared with the SNI group after the 10th and 20th interventions, the SFI, TFI, PFI of rats in the TUI group increased significantly. These results showed that Tuina intervention can effectively promote the recovery of fine movements in the hind limbs of rats and facilitate the recovery of motor function following nerve injury (Fig. 1E–G).
Tuina alleviated muscle atrophy
The muscle atrophy index (MAI) was used to evaluate muscle atrophy. The MAI of the anterior tibial and soleus muscles showed similar changes among the four groups. There were no statistically significant differences between the CON and SHA groups. Compared with the SHA group, the MAI of the rats in the SNI and TUI groups decreased significantly. Compared with the SNI group, the TUI group showed a significant increase; however, there were still significant differences compared with the SHA group. These results showed that Tuina intervention can effectively relieve muscle atrophy caused by nerve damage.
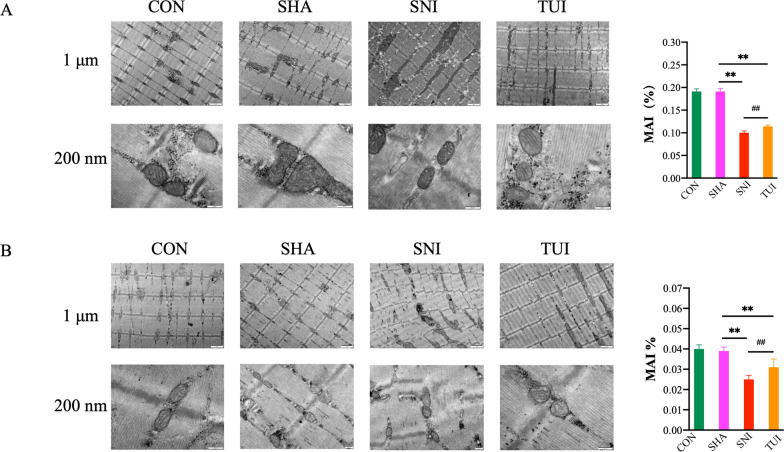
TEM was used to evaluate the ultrastructural changes in the muscles. The results of the anterior tibial and soleus muscle showed that in the CON and SHA groups, the myosin filaments were arranged neatly and the transverse lines were obvious. A band was formed by the interlacing and overlapping of heterotopic myosin and actin filaments. Only the actin filaments passed through the region, showing an isotropic I band. There were regular Z lines between I band and the sarcomeres between the Z line junctions. The M line represents the site of marked myosin thickening. Healthy mitochondria were observed among the muscle fibers. In the SNI group, the myofilaments were arranged in a disorderly fashion, and the horizontal lines disappeared. Typical skeletal muscle structures such as the A band, I band, Z line, and M line disappeared. The spaces between the muscle bundles were significantly enlarged. A large number of vacuolated mitochondria were found between muscle bundles and fibers. In the TUI group, the myofilaments were arranged neatly and horizontal lines appeared. Lines for the Z line, M line, A band, and I band were observed. The internal structure of mitochondria between the myofilaments and fascicles was not clear and vacuolated mitochondria were occasionally observed. These results showed that Tuina intervention effectively relieved the ultrastructural changes in muscle atrophy caused by nerve damage (Fig. 2).
Fig. 2.
Tuina alleviated SNI rat muscle atrophy. A Ultramicrostructure and MAI of the anterior tibial muscle; B ultramicrostructure and MAI of the soleus muscle. Results are presented as mean ± standard deviation. **P < 0.001 vs. SHA; ##P < 0.001 vs. SNI
Tuina enhanced microcirculation
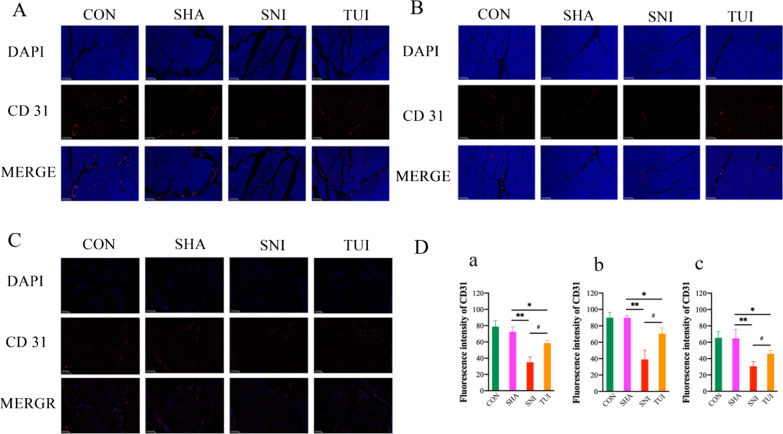
Immunofluorescence was used to evaluate microcirculation in the muscles and nerves. Figure 3 shows the results of the immunofluorescence staining for CD 31 in each group. The nuclei were stained blue with DAPI, and CD 31 was stained red and marked microvascular endothelial cells. The expression of CD 31 in the sciatic nerve, anterior tibial muscle, and soleus muscle showed similar changes among the four groups. Compared with CON and SHA groups, CD 31 expression was significantly reduced in the SNI group than in the CON and SHA group. Compared with that in the SHA group, fluorescence intensity of CD 31 in the SNI group was significantly decreased. CD 31 distribution was observed in the TUI group. Fluorescence intensity was significantly higher in the TUI group than in the SNI group. These results showed that Tuina intervention could effectively increase microvascular density after nerve injury in rats.
Fig. 3.
Tuina improved SNI rat microcirculation. A Immunofluorescence staining of sciatic nerve; B Immunofluorescence staining of anterior tibial muscle; C Immunofluorescence staining of soleus muscle; D Fluorescence intensity of CD 31; a sciatic nerve; b anterior tibial muscle; c soleus muscle. Scale bars: 100 μm. Results are presented as mean ± standard deviation. *P < 0.05 vs. SHA; **P < 0.001 vs. SHA; #P < 0.05 vs. SNI
Tuina regulated muscle atrophy associated with IGF-1 and FoxO
As shown in Fig. 4, expression levels of IGF-1 in the anterior tibial and soleus muscles were significantly lower in the SNI and TUI groups than in the SHA group. Conversely, the TUI group had increased expression levels compared to the SNI group. P-FoXO / FoXO was significantly lower in the SNI group than in the SHA group. The TUI group increased p-FoXO / FoXO compared to the SNI group. These outcomes suggested that tuina could alleviate muscle atrophy after SNI in association with IGF-1 and FoxO.
Fig. 4.
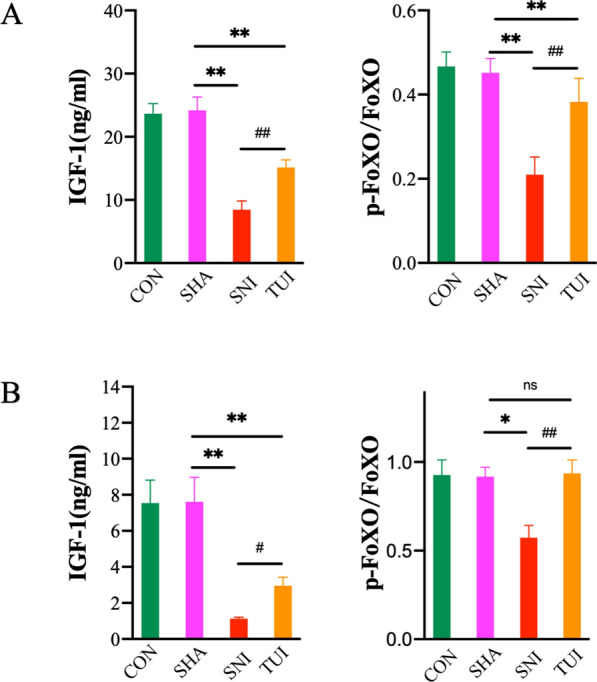
Tuina regulated muscle atrophy associated with IGF-1 and FoxO. A Relative protein expression of the anterior tibial muscle; B Relative protein expression of the soleus muscle; Results are presented as mean ± standard deviation. **P < 0.001 vs. SHA; #P < 0.05 vs. SNI; ##P < 0.001 vs. SNI
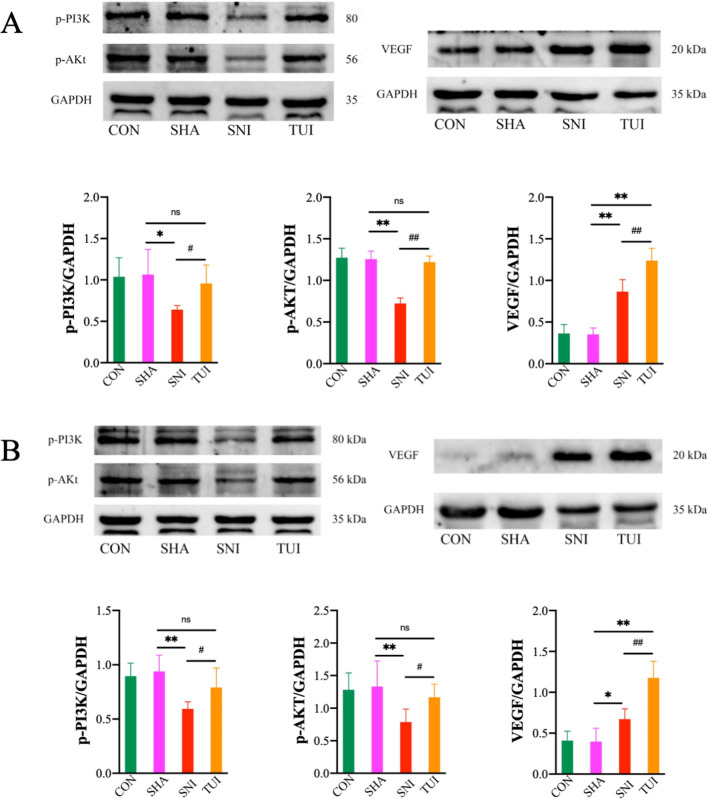
Tuina regulated factors associated with the PI3K/AKT pathway
Western blotting was performed to evaluate the changes in the anterior tibial and soleus muscles in the PI3K/AKT signaling pathway. The protein expression levels of p-PI3K and p-AKT significantly decreased in the SNI group. However, Tuina treatment enhanced this expression. The VEGF protein expression levels were significantly higher in the TUI group than in the SNI group (Fig. 5). This suggested that tuina affected the PI3K/AKT signaling pathway in the anterior tibial and soleus muscles of rats with SNI.
Fig. 5.
Tuina regulated factors associated with the PI3K/AKT pathway. A Relative protein expression of the anterior tibial muscle and quantification of specific signal intensities; B Relative protein expression of the soleus muscle and quantification of specific signal intensities; Results are presented as mean ± standard deviation. *P < 0.05 vs. SHA; **P < 0.001 vs. SHA; #P < 0.05 vs. SNI; ##P < 0.001 vs. SNI
Discussion
Tuina, a non-pharmacological therapy with few or no side effects, has been extensively used in clinical practice for the relief and treatment of diseases, such as low back pain, lumbar disc herniation, and sequelae in post-stroke. Under the guidance of traditional Chinese medicine and Western medicine anatomy and pathology, tuina acts on the body surface through various manipulations to regulate the physiological and pathological state to treat diseases. The benefits of Tuina therapy include increased blood flow, reduced pain and perceived fatigue, attenuation of inflammatory signals of muscle injury, improved muscle strength and self-perception of muscle injury, and reduced muscle tension [18–20]. Tuina has the advantages of not causing dependence, easier use, faster effect onset of effects, and increased popularity worldwide, including athletes. Related studies include case reports, meta-analyses, controlled clinical trials, and animal experiments showing its positive effects on all types of injury. In this study, we found that Tuina intervention improved hindlimb motor function in rats with SNI by promoting nerve repair and inhibiting muscle atrophy.
The SNI model was used to simulate the clinical PNI, which allowed for the evaluation of neuropathic changes and nerve regeneration. The “Three-Manipulation and Three-Acupoint” is a combination of manipulations and acupoints that we have studied and proven to be effective [4, 14–16, 21]. Yinmen (BL 37) is located in the body surface projection area of the sciatic nerve trunk at the biceps femoris muscle; Chengshan (BL 57) is located in the body surface projection area of the tibial nerve at the gastrocnemius muscle; and Yanglingquan (GB 34) is located in the body surface projection area of the common peroneal nerve at the tibialis anterior muscle. We found that after 20th intervention, the function and structure of muscles and nerve in the intervention area were significantly improved, and demonstrated significant effects of the “Three-Manipulation and Three-Acupoint.”
Sciatic damage was estimated to contribute to 90% inhibition of muscle mass. Moreover, the soleus muscles retained only 8% contractibility in 14-week denervated rats, and the tibial anterior region experienced 3–5% contraction in long-term denervation [15]. Tuina can effectively accelerate the recovery of muscle fibers, improve the structure and morphology of gastrocnemius muscle, and delay the atrophy after sciatic nerve transection [22, 23]. In this study, we found that the SNI model led to a decrease in the mass of the anterior tibial and soleus muscles and an ultrastructural change, after which the mass of the muscles increased significantly and the ultrastructure was restored.
Mitochondria play an important role in maintaining cellular homeostasis and skeletal muscle health, and mitochondrial dysfunction can lead to skeletal muscle atrophy. During muscle atrophy, mitochondrial degradation reduces mitochondrial quality and quantity, which is controlled by mitochondrial autophagy, mitochondrial fusion and fission kinetics [24]. Therefore, mitochondrial are the key entry point in the treatment of muscle atrophy. Crane et al. showed that tuina therapy is clinically beneficial for promoting mitochondrial biogenesis [25]. In this study, TEM showed that the structure and morphology of the mitochondrial were healthier in tuina-treated SNI rats than in SNI model rats.
The PI3K/Akt pathway is one of the most important pathways that regulates muscle atrophy. Muscle atrophy leads to the loss of muscle mass and function and is characterized by a reduction in muscle fiber size and mass, and an imbalance between protein synthesis and muscle degradation. Protein synthesis in the skeletal muscle is mainly regulated by the PI3K/Akt signaling axis. This pathway plays a critical role in myotube hypertrophy and Akt activation in rat muscles prevents denervation-induced atrophy [26]. Additionally, an increase in the activity of the PI3K/Akt signaling pathway is critical for autophagy suppression, and the formation of autophagosomes is stimulated by decreased levels of PI3K [27]. Skeletal muscle loses its contractile function after denervation, resulting in reduced blood perfusion and, thus, leading to nutritional and metabolic abnormalities in the target muscle, hyperactivation of inflammation after injury also promotes skeletal muscle atrophy and fibrosis. Activated AKT regulates vascular function and causes vasodilation, vascular remodeling and angiogenesis [28]. Furthermore, AKT activation induces the expression of high levels of HIF-1, which can upregulate the expression of VEGF, thereby promoting angiogenesis [29]. This study investigated the mechanism of inhibition of the muscle atrophy of the tuina in an SNI rat model. We observed that Tuina promoted SNI recovery in rats by regulating PI3K/Akt signaling to enhance VEGF expression. Therefore, the PI3K/Akt signaling pathway represents a promising target for exploring the pathogenesis and treatment mechanisms of PNI.
IGF-1 is a critical hormone that regulates muscle mass and proteostasis. IGF-1 activates the intracellular adaptor protein, insulin receptor substrate-1, and induces the downstream PI3K/Akt pathway. Moreover, IGF-1 treatment or Akt activation can negatively regulate FoxO transcription factors, resulting in the inhibition of the protein degradation pathway, ubiquitin–proteasome system, and autophagy-lysosome system. IGF-1 signaling cascades maintain muscle mass by suppressing of FoxO-mediated autophagy and protein degradation [30]. This study showed that IGF-1 and FoxO are major factors in the regulation of muscle atrophy by Tuina, providing a better understanding of the mechanism of action of Tuina in the treatment of PNI. However, the specific mechanisms by which tuina affects PNI remain to be elucidated.
This study had some limitations, as agonists or inhibitors of the pathways were not used for further verification. The current study was limited by the small number of experimental animals. Future studies with large animal groups would further deepen our understanding of these effects. Moreover, we did not clarify the mechanism of actions of Tuina in PNI. The emphasis we focused in this study is the effects of Tuina for muscle atrophy after SNI, but the most fundamental cause of various manifestations is nerve damage. For the next step, we will aim to explore whether the recovery of muscle atrophy can facilitate the injury-nerve repair or is there any positive or negative effects on nerve repair.
Conclusions
This study revealed that Tuina effectively inhibits skeletal muscle atrophy via the microcirculation pathway in a rat model of SNI. This was accomplished by regulating the expression of IGF-1 and FoxO in the anterior tibial and soleus muscles, leading to the onset and advancement of muscle atrophy. The underlying mechanism of action may be associated with the PI3K/Akt signaling pathway.
Acknowledgements
We would like to thank Li Wei of Beijing Jiaotong University for the technical help, and thank Editage (www.editage.cn) for English language editing.
Abbreviations
- PNI
Peripheral nerve injury
- SNI
Sciatic nerve injury
- IGF
Insulin-like growth factor 1
- PI3K
Phosphatidylinositol-3 kinase
- FoxO
Forkhead box O
- BL 37
Yinmen
- GB 34
Yanglingquan
- BL 57
Chengshan
- MAI
Muscle atrophy index
- H&E
Hematoxylin and eosin staining
- SFI
Sciatic function index
- TFI
Tibial function index
- PFI
Peroneal function index
- ELISA
Enzyme-linked immunosorbent assay
Author contributions
TY: study conception, design of the work. JL, JS, HZ, JY, JC: animal experiments and data acquisition. YX, NS: statistical analysis; YZ, HZ: analysis and data interpretation, drafting of the manuscript. YZ, HW: approval of the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 82274675, 82074573) and the Beijing Natural Science Foundation (No. 7232278).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The experimental designs and animal care were approved by the Ethics Committee for Animal Care and Use Committee of the Beijing University of Chinese Medicine (No. BUCM-2023032303-1119), and all procedures were conducted in strict accordance with the National Institutes of Health standards stated in the Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yingqi Zhang and Hanyu Zhang contributed as first authors.
Contributor Information
Hourong Wang, Email: wanghourong5142@bjhmoh.cn.
Tianyuan Yu, Email: yutianyuan@sina.com.
References
- 1.Mokarram N, Dymanus K, Srinivasan A, et al. Immunoengineering nerve repair. Proc Natl Acad Sci USA. 2017;114(26):E5077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oudega M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012;349(1):269–88. [DOI] [PubMed] [Google Scholar]
- 4.Zainul Z, Heikkinen A, Koivisto H, et al. Collagen XIII is required for neuromuscular synapse regeneration and functional recovery after peripheral nerve injury. J Neurosci. 2018;38(17):4243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Qi G, Wang K, et al. Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol. 2023;214: 115664. [DOI] [PubMed] [Google Scholar]
- 6.Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–78. [DOI] [PubMed] [Google Scholar]
- 7.Jia L, Zheng P, Wang H, et al. VEGF alleviates lower limb ischemia in diabetic mice by altering muscle fiber types. Exp Ther Med. 2022;23(4):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Kong L, Ren J, et al. Effect of traditional Chinese exercise combined with massage on pain and disability in patients with lumbar disc herniation: a multi-center, randomized, controlled, assessor-blinded clinical trial. Front Neurol. 2022;13: 952346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabanas-Valdes R, Calvo-Sanz J, Serra-Llobet P, et al. The effectiveness of massage therapy for improving sequelae in post-stroke survivors. A systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(9):4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu ZF, Zhang Y, Liu J, et al. Effect of traditional Chinese non-pharmacological therapies on knee osteoarthritis: a narrative review of clinical application and mechanism. Orthop Res Rev. 2024;16:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang B, Ruan L, Wang L, Xue H, Sun M, Duan M, Peng L. Mimicking ding's roll method on notexin-induced muscle injury in rats. J Vis Exp. 2023;(198). 10.3791/65820. [DOI] [PubMed]
- 12.Liu Z, Wang H, Yu T, et al. A review on the mechanism of tuina promoting the recovery of peripheral nerve injury. Evid Based Complement Alternat Med. 2021;2021:6652099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv TT, Mo YJ, Yu TY, et al. Using RNA-Seq to explore the repair mechanism of the three methods and three-acupoint technique on DRGs in sciatic nerve injured rats. Pain Res Manag. 2020;2020:7531409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyu T, Liu Z, Yu T, et al. Applying RNA sequencing technology to explore repair mechanism of Tuina on gastrocnemius muscle in sciatic nerve injury rats. Chin Med J (Engl). 2022;135(19):2378–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav A, Dabur R. Skeletal muscle atrophy after sciatic nerve damage: mechanistic insights. Eur J Pharmacol. 2024;970: 176506. [DOI] [PubMed] [Google Scholar]
- 16.Lv T, Mo Y, Yu T, et al. An Investigation into the rehabilitative mechanism of tuina in the treatment of sciatic nerve injury. Evid Based Complement Altern Med. 2020;2020:5859298. 10.1155/2020/5859298. [DOI] [PMC free article] [PubMed]
- 17.Savastano LE, Laurito SR, Fitt MR, et al. Sciatic nerve injury: a simple and subtle model for investigating many aspects of nervous system damage and recovery. J Neurosci Methods. 2014;227:166–80. [DOI] [PubMed] [Google Scholar]
- 18.Nunes GS, Bender PU, De Menezes FS, et al. Massage therapy decreases pain and perceived fatigue after long-distance Ironman Triathlon: a randomised trial. J Physiother. 2016;62(2):83–7. [DOI] [PubMed] [Google Scholar]
- 19.Shin MS, Sung YH. Effects of massage on muscular strength and proprioception after exercise-induced muscle damage. J Strength Cond Res. 2015;29(8):2255–60. [DOI] [PubMed] [Google Scholar]
- 20.Kang L, Liu P, Peng A, et al. Application of traditional Chinese therapy in sports medicine. Sports Med Health Sci Sports Med. 2021;3(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachula, Yang Z, Yu T, et al. Exploring the mechanism of immediate analgesia induced by tuina intervention on minor chronic constriction injury in rats using LC-MS. J Pain Res. 2024;17:321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma SJ, Zhang JP, Hua XY, et al. Tuina therapy promotes behavioral improvement and brain plasticity in rats with peripheral nerve injury and repair. Brain Behav. 2023;13(9):e3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan F, Yu TY, Wong S, et al. Chinese tuina downregulates the elevated levels of tissue plasminogen activator in sciatic nerve injured Sprague-Dawley rats. Chin J Integr Med. 2017;23(8):617–24. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Ji Y, Liu R, et al. Mitochondrial dysfunction: roles in skeletal muscle atrophy. J Transl Med. 2023;21(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crane JD, Ogborn DI, Cupido C, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4(119):119ra13. [DOI] [PubMed] [Google Scholar]
- 26.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi X, Tao J, Qian Y, et al. Morroniside ameliorates inflammatory skeletal muscle atrophy via inhibiting canonical and noncanonical NF-kappaB and regulating protein synthesis/degradation. Front Pharmacol. 2022;13:1056460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CZ, Wu SC, Chang CM, et al. Arctigenin, a potent ingredient of Arctium lappa L., induces endothelial nitric oxide synthase and attenuates subarachnoid hemorrhage-induced vasospasm through PI3K/Akt pathway in a rat model. BioMed Res Int. 2015;2015:490209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu F, Na L, Li Y, et al. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.O’Neill BT, Lee KY, Klaus K, et al. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J Clin Investig. 2016;126(9):3433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.