Abstract
Objective
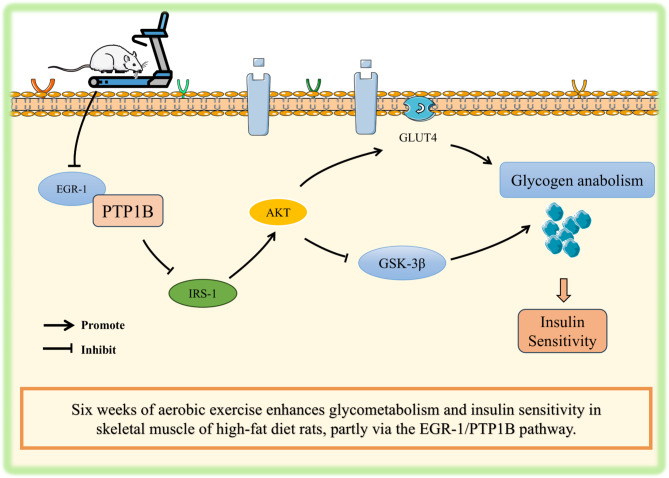
Impaired skeletal muscle glycogen synthesis contributes to insulin resistance (IR). Aerobic exercise reported to ameliorate IR by augmenting insulin signaling, however the detailed mechanism behind this improvement remains unclear. This study investigated whether aerobic exercise enhances glycogen anabolism and insulin sensitivity via EGR-1/PTP1B signaling pathway in skeletal muscle of rats.
Methods
Sprague-Dawley rats fed a high-fat diet (HFD), and performed treadmill exercise training for 6-week. Oral glucose tolerance test was conducted to confirm the IR. Periodic Acid-Schiff (PAS) staining and anthrone colorimetry were used to assess the skeletal muscle glycogen. RT-qPCR, western blot, and immunofluorescence were used to detect the EGR-1/PTP1B pathway and associated signaling molecules.
Results
We found that exercise training significantly decreased blood glucose, insulin, and homeostasis model assessment for IR (HOMA-IR) against HFD-induced elevation. Decreased muscle glycogen content due to HFD was significantly restored after exercise training. Exercise training promoted mRNA expressions of Irs1, Akt, and Glut4, while inhibited Gsk-3β expression against HFD. Next, the decreased IRS1 (phosphorylated/total), AKT (phosphorylated/total), and GLUT4, and increased GSK-3β proteins with HFD were significantly reversed by exercise. Furthermore, HFD-induced overexpression of EGR-1 and PTP1B evidenced by mRNA, protein, and immunofluorescence intensity, were substantially inhibited by exercise, which may contribute to promote insulin sensitivity and glycogen anabolism.
Conclusions
Aerobic exercise training promotes insulin sensitivity and skeletal muscle glycogen synthesis in HFD-fed rats. The beneficial effects of exercise might be mediated by EGR-1/PTP1B signaling pathway in skeletal muscle, however further studies are necessary to confirm this mechanism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-024-00888-8.
Keywords: Glycometabolism, Insulin sensitivity, Treadmill running, Glycogen synthesis, GLUT4, IRS1
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-024-00888-8.
Introduction
Chronic high-fat diet (HFD) consumption is associated with increased risk of developing obesity and insulin resistance (IR) [1], and subsequent triggering of type 2 diabetes mellitus (T2DM) [2]. Skeletal muscle, the largest energy-metabolizing organ of the body, plays a crucial role in preventing or alleviating the IR through its ability to uptake and utilize the glucose. Skeletal muscle also involved in regulation of insulin signaling pathways, and glycogen synthesis capacity [3–5].
Early growth response factor-1 (EGR-1), a zinc-finger transcription factor activated by various cytokines, growth factors and hormones, plays a key role in regulation of cell proliferation, apoptosis, and cellular metabolism [6–8]. Loss of EGR-1 function has been shown to improve IR in adipose tissue and liver [9, 10]. A recent study indicated that elevated EGR-1 inhibits postprandial insulin signaling and glucose uptake in skeletal muscle, and thereby decrease insulin sensitivity [11]. Therefore, EGR-1 is considered an ideal target protein in regulation of IR. Additionally, inhibition of EGR-1/protein tyrosine phosphatase 1B (PTP1B) activity enhances skeletal muscle insulin sensitivity and postprandial glucose stability [11]. The potential mechanism underlying these beneficial effects may be associated with downregulation of PTP1B activity, phosphorylation of insulin receptor substrate (IRS), inhibition of glycogen synthase kinase-3β (GSK-3β) activity, enhancing of glycogen synthesis, and improving of insulin sensitivity [11–13]. The insulin signaling pathway plays a crucial role in regulation of glucose homeostasis and metabolism. Upon insulin binding to its receptor, IRS is activated, and initiate two major pathways, including protein kinase B (PKB/AKT)-mediated translocation of glucose transporter type 4 (GLUT4), and inhibition of GSK-3β [14, 15]. In the first pathway, activated IRS promotes the phosphorylation of phosphatidylinositol-3-kinase (PI3K), leading to the activation of AKT [16]. Activated AKT promotes translocation of GLUT4 to the cell membrane, and thereby increase glucose uptake by the cell [17]. In the second pathway, AKT phosphorylates and inhibits GSK-3β, a negative regulator of glycogen synthesis. The inhibition of GSK-3β allows glycogen synthase to remain active, and thereby enhances glycogen storage [18]. These coordinated actions of the IRS-AKT pathway are essential for maintaining of blood glucose levels, and ensuring efficient energy storage and utilization.
Aerobic exercise, as a non-pharmacological intervention, not only improves skeletal muscle contractility in obese individuals [19], but also enhances insulin sensitivity [20]. Glycogen synthesis and its content in skeletal muscle reflect the metabolic balance between glucose availability and insulin sensitivity. Although exercise has been shown to improve IR through skeletal muscle glycogen anabolism [21], the role of EGR-1/PTP1B signaling pathway in this beneficial effect remains unclear. Therefore, the purpose of this study was to examine the effect of aerobic exercise training on skeletal muscle EGR-1/PTP1B pathway in response to a HFD. We further explored whether exercise improve skeletal muscle glycogen anabolism and insulin sensitivity in rats fed a HFD through EGR-1/PTP1B signaling pathway. Exercise-associated beneficial effects may provide new strategies for preventing and treating the IR.
Materials and methods
Experimental animals
Twenty-four male Sprague-Dawley (SD) rats (6-week-old; weighing 257 ± 10 g) were housed in a specific pathogen-free environment. Two rats per cage were maintained in a temperature-controlled room (23 ± 2 °C) with a 12-h light and 12-h dark cycle. Food (standard diet or high-fat diet) and water were provided ad libitum. Daily food intake was recorded, while body weights were collected for every 3-day once. All procedures complied with the National Research Council Guidelines for the Care and Use of laboratory animals. This study was conducted according to the animal management regulations of the Ministry of Health of China, and was approved by the Animal Ethics Committee of Zhejiang Normal University (No. ZSDW2022027).
Animal grouping and treatment
After a one-week adaptation period, the rats were randomly assigned into three groups, including control (CON, n = 8), high-fat diet (HFD, n = 8) and HFD plus aerobic exercise (HE, n = 8), and treated for a period of 6-week. Rats in the control group fed a normal diet obtained from the Research Diets (Research Diets Inc., NJ, USA). The rats in HFD and HE groups fed a 60% high-fat diet (D12492, Research Diets Inc., NJ, USA). Following HFD feeding, the rats in HE group performed an aerobic exercise training for a period of 6-week, as described in the protocol below.
Treadmill exercise protocol
Prior to the actual exercise session, rats were familiarized with the treadmill environment. For familiarization, rats in the HE group ran on a treadmill at low running speed (16 m/min) for 20-minute on day 1. Then the running speed (2 m/min/day) and running time (10-min/day) were gradually increased to reach the target speed (24 m/min) and time (60-min) [22]. The final exercise training protocol consisted of 60-min/day, 5 days/week for a period of 6-week. The exercise load comprised a running of 15 m/min for the first 5-minute, 20–25 m/min for the next 50-minute, and 15 m/min for the last 5-minute, without incline. The exercise program was scheduled from Monday to Friday between 5:00 and 7:00 p.m. The exercise intensity ranged from approximately 60–70% of VO2max [23]. We adopted this exercise protocol based on our previous study, which reported to be effective in producing the physiological changes in SD rats [22].
Oral glucose tolerance test (OGTT) and insulin sensitivity
OGTT was performed twice in our study following a 12-hour fasting period. The first OGTT was performed prior to the intervention (pre or baseline), and the second OGTT was performed after the intervention (post). Following the exercise training, the animals rested for 12-hour (while feeding), and then fasted for 12-hour before the OGTT. Blood samples were collected from the tail of each rat at 0-, 30-, 60-, 90-, and 120-minute after an oral administration of glucose (2 g/kg body weight). The changes in blood glucose levels were measured using a glucometer (Sinocare, Changsha, China), and recorded for three times to calculate the average value. The glucose area under the curve (AUC) was calculated and presented as histograms [24].
Serum insulin levels were measured using the rat insulin ELISA kit (Sangon Biotech, Shanghai, China), according to the manufacturer’s instructions. The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated as follows:
HOMA-IR = ((fasting plasma insulin in µIU/mL) × (fasting plasma glucose in mg/dL))/405 [25].
Sample collection and biochemical assays
Forty-eight hours after the last exercise session (6-week), rats were anesthetized with urethane (2 g/kg, intraperitoneally) following a 12-hour fasting period. The fasting time was the same in CON and HFD groups to ensure the similar conditions in all groups. Blood samples (5 mL) were collected, and centrifuged at 3000 rpm for 15-minute. Then, the portions of the quadriceps were excised for different analyses: one portion was embedded in the optimal cutting temperature compound (OCT, Sakura, Tokyo, Japan), and frozen in isopentane, while the other portion was frozen in liquid nitrogen, and stored at -80 °C. The quadriceps muscle was selected for its balanced distribution of type I and type II muscle fibers, ease of access for reliable sampling, and its relevance to metabolic outcomes [26, 27].
In addition, the subcutaneous fat was carefully dissected beneath the dermis, ensuring minimal contamination from surrounding tissues. Following this, epididymal fat was excised from around the epididymis. Special care was taken to preserve its structural integrity. Both fat tissue samples were weighed on an analytical balance (Sartorius, BSA224S, Germany). The weight of each fat depot was recorded immediately to determine the total fat mass for subsequent analysis.
Periodic acid-schiff (PAS) staining and measurement of skeletal muscle glycogen content
For histological analysis, muscle tissue Sect. (10 μm thickness) were prepared using a cryostat (Leica CM1860, Germany). The sections were mounted on glass slides, and fixed with 4% paraformaldehyde for 10-minute at room temperature before undergoing PAS staining. The slides were immersed in periodic acid solution for 10-minute, washed three times with distilled water, and incubated with Schiff solution at 37 °C for 45-minute. Finally, the slides were washed with distilled water for 5-minute, and dehydrated using graded alcohols.
To measure the glycogen levels in the quadriceps, the glycogen assay kit (BC0345, Solarbio, China) was used, and performed according to the manufacturer’s instructions. Briefly, snap-frozen quadriceps (80 mg) were homogenized, and diluted with 1 mL distilled water. The homogenate was boiled at 100℃ for 20-minute, centrifuged at 8000 g for 10-minute at 25℃, and then the supernatant was collected. Glycogen was extracted by a strong alkaline extract solution, and the glycogen content was determined using anthrone chromogen under strong acidic conditions [28].
Quantitative real-time polymerase chain reaction (qPCR)
Quadriceps muscle tissue (50 mg) was lysed with TRIzol reagent to extract total RNA. The concentration and purity of the extracted RNA were assessed with a NanoDrop spectrophotometer (Multiskan SkyHigh, Thermo Fisher Scientific, Waltham, USA). The RNA purity was determined by measuring the absorbance ratios at 260/280 nm and 260/230 nm on a NanoDrop spectrophotometer (Multiskan SkyHigh, Thermo Fisher Scientific, Waltham, USA), ensuring the ratios were above 1.8 and 2.0, respectively, to confirm high purity. For cDNA synthesis, 1 µg of total extracted RNA from each sample was used with the PrimeScript RT Master Mix (Takara, Shiga, Japan). The qPCR assays were performed using PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Waltham, USA) to ensure specific and sensitive detection of target gene expression. All qPCR analyses were conducted on the CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, USA). The reaction conditions comprised an initial pre-denaturation step at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 10 s and annealing/extension at 60 °C for 30 s. Gene expression levels were quantified using the 2 − ΔΔCt method with GAPDH serving as the internal reference [22]. The primer sequences are provided in Table 1.
Table 1.
Primers for qPCR used in this study
| Gene | Forward | Reverse |
|---|---|---|
| Egr-1 | ATTTCCTATTCAAAGTCCGAGAGTCAG | TCCATCAGTAAGAGGCAGGTGTC |
| Ptp1b | CGGACCACCTCAACTCAACCAATC | CAACACCACCTTGTCGTACTCGTC |
| Glut4 | GCTGGGCGACGGACACTC | GGACACATAACTCATGGATGGAACC |
| Gsk-3β | CCACCATCCTTATCCCTCCTCAC | TGTCCACGGTCTCCAGCATTAG |
| Irs1 | CAAGCCTGTCCTCTCCTACTACTC | GCAGTTGCGGTATAGCGAAGG |
| Akt | TGTGGCAAGATGTGTATGAGAAGAAG | AGGCGGCGTGATGGTGATC |
| Gapdh | AAGTTCAACGGCACAGTCAAGG | GACATACTCAGCACCAGCATCAC |
Western blot
Total protein was extracted from rat quadriceps muscle using the commercially available RIPA lysis buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP40, 1% sodium deoxycholate, and 0.1% SDS, Thermo Fisher Scientific, 89901USA) with protease and phosphatase inhibitors (Thermo Fisher Scientific, 78440, USA). The protein concentration was determined using the BCA protein assay kit (Thermo Fisher Scientific, 23235, USA). Equal amounts of protein along with a protein ladder (Bio-Rad, 1610374, USA), were subjected to SDS-PAGE for electrophoresis. Electrophoresis was conducted at a constant voltage of 70–100 V until the dye front reached an appropriate distance. Following separation, proteins were transferred to polyvinylidene fluoride (PVDF) membranes using a wet transfer system (Bio-Rad) at 80 V for 2 h. The transfer buffer consisted of 25 mM Tris, 192 mM glycine, and 20% methanol. the PVDF membranes were then blocked in Tris-buffered saline with Tween® 20 (TBST, 50 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH 7.4) containing 5% skimmed milk at room temperature for 1 h. The membranes were then incubated overnight at 4 °C with primary antibodies specific for EGR-1 (Proteintech, 22008-1-AP), PTP1B (Santa Cruz, sc-133259), GSK-3β (Proteintech, 67329-1-Ig), phospho-AKT Ser473 (p-AKT, Proteintech, 66444-1-Ig), AKT (CST, 9272), phospho-IRS1 Ser307 (p-IRS1, CST, 2381), IRS1 (Proteintech, 17509-1-AP) and GLUT4 (Proteintech, 66846-1-Ig), all diluted at a ratio of 1:2000. After washing the membranes three times with TBST, they were incubated at room temperature for 2 h with appropriate secondary antibodies: goat anti-mouse (Proteintech, SA00001-1) or goat anti-rabbit (CST, #7074), also diluted at 1:2000. Detection of protein bands was performed using an enhanced chemiluminescence kit (Thermo Fisher Scientific, Waltham, USA) and visualized with a gel imaging system (Bio-Rad, Hercules, USA). The optical density values of the target proteins were quantified relative to that of GAPDH using Fiji software [29].
Immunofluorescence
Muscle samples were collected, and immediately frozen in OCT. Using a cryostat (Leica CM1860, Germany), 10 μm thick sections were cut, and mounted on glass slides. The slides were fixed with 4% paraformaldehyde for 10-minute at room temperature. After three washes with PBS (5-minute each), slides were permeabilized with 0.5% Triton X-100 in PBS for 20-minute, followed by three additional washes with PBS. Subsequently, the slides were blocked with 1% BSA in PBST (PBS + 22.52 mg/mL Glycine + 0.1% Tween-20) for 30-minute at room temperature. Primary antibodies, EGR-1 and PTP1B, were incubated overnight at 4℃. Slides were rinsed three times with PBS, and incubated with fluorescent secondary antibodies (Alexa Fluor 488-conjugated goat anti-mouse, Abcam, AB150113 or Alexa Fluor 594-conjugated goat anti-rabbit, AB150080) at a ratio of 1:500 in antibody dilution buffer for one-hour at room temperature in the dark. Finally, the cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (Beyotime Institute of Biotechnology, China). The stained tissue was observed, and photographed using a fluorescence microscope (Leica, Wetzlar, Germany) [29].
Statistical analysis
Data were statistically analyzed using IBM SPSS Statistics 20 (Chicago, USA), and image analysis and mapping were performed using Fiji software and GraphPad Prism 9 (San Diego, USA). The Pearson correlation coefficient of the selected images was analyzed using Fiji software. All data were summarized as mean ± SEM. Comparisons between multiple groups were conducted using a one-way analysis of variance (ANOVA), with post hoc analysis by Tukey’s multiple comparisons test. A significance level of P < 0.05 was considered statistically significant.
Results
Exercise decreases body weight and fat weight in rats fed a high-fat diet
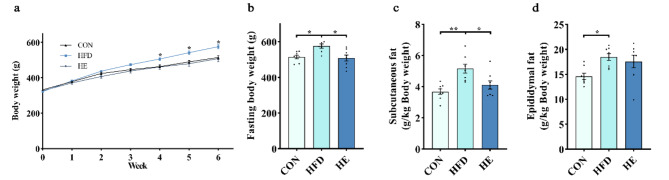
During the intervention, we recorded the body weight changes in all groups. We found significantly increased body weights in the HFD group compared with that of in control group after 6-week (Fig. 1a-b). However, the increased body weight was effectively decreased by exercise training in the HE group (Fig. 1a-b). Our analysis further revealed a significant reduction in subcutaneous fat depot in the HE group compared to that of in HFD group (Fig. 1c). Conversely, no significant difference was observed in epididymal fat weight between HFD and HE groups (Fig. 1d). These results suggest that aerobic exercise training preferentially mobilizes the subcutaneous fat rather than the visceral fat for energy.
Fig. 1.
Aerobic exercise decreases body weight and fat weight in rats fed a high-fat diet. (a) Body weight changes during the intervention. (b) Body weight at the end of the intervention. (c, d) Subcutaneous fat and epididymal fat weight. All data are presented as mean ± SEM (n = 8 per group); *P < 0.05, ** P < 0.01, *** P < 0.001
Aerobic exercise restores insulin sensitivity in rats fed a high-fat diet
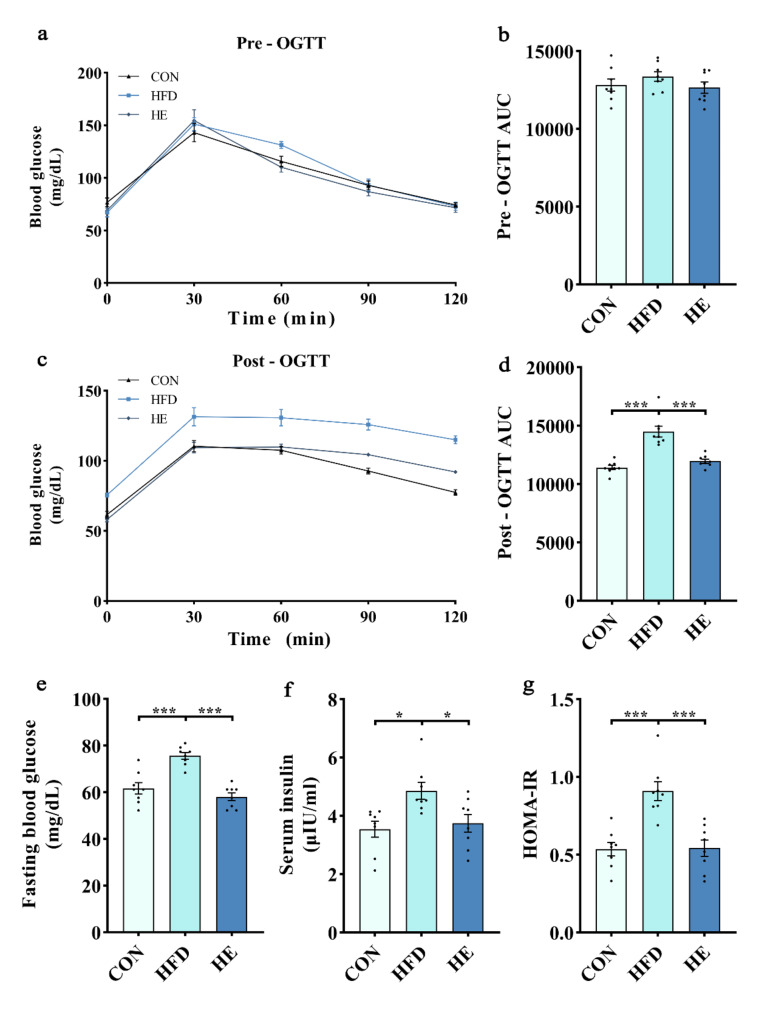
To evaluate the glucose homeostasis and insulin sensitivity in response to a HFD, we conducted pre- and post-intervention OGTT. There were no significant differences in pre-intervention OGTT results among the groups (Fig. 2a-b). Post-intervention OGTT results suggesting the impaired glucose tolerance in rats fed a HFD, as we found higher glucose AUC for the HFD group compared with that of the control (Fig. 2c-d). However, the impaired glucose tolerance was significantly ameliorated by exercise training, as we noticed lower glucose AUC in HE group compared with HFD group. In addition, HFD for 6-week significantly elevated fasting blood glucose, insulin, and HOMA-IR compared to the control diet (Fig. 2e-g). These elevations were significantly decreased in HE group (Fig. 2e-g), underscoring the beneficial effects of aerobic exercise on insulin sensitivity against HFD.
Fig. 2.
Aerobic exercise restored insulin sensitivity in rats fed a high-fat diet. (a-d) Blood glucose concentrations and the area under the curve (AUC) in response to OGTT before and after intervention. Changes in (e) fasting blood glucose, (f) serum insulin, and (g) HOMA-IR after intervention. All data are presented as mean ± SEM (n = 8 per group); * P < 0.05, ** P < 0.01, *** P < 0.001
Exercise restores skeletal muscle glycogen content against HFD
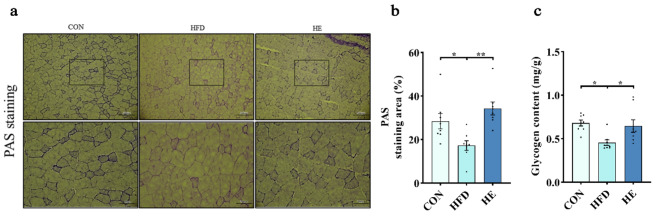
Skeletal muscle glycogen content was determined by PAS staining and glycogen content detection kits. Both protocols consistently showed a substantial reduction in glycogen content in HFD compared to control diet (Fig. 3a-c). The decreased glycogen content indicates potential adversities of HFD on glycogen anabolism in the skeletal muscle. This impact may be due to the reduced insulin sensitivity and/or decreased glucose uptake with chronic HFD. However, 6-week aerobic exercise restored skeletal muscle glycogen content, as evidenced by both PAS staining and glycogen content assay results (Fig. 3a-c).
Fig. 3.
Aerobic exercise restored glycogen content in skeletal muscle of rats fed a high-fat diet. (a-c) PAS staining and quadriceps glycogen content. Scale bars, 200 μm and 100 μm; Upper panel: image of PAS staining. Lower panel: zoomed-in view of the frames indicated in the upper panel. Five regions were randomly selected from each sample, and quantified (n = 8 per group); * P < 0.05, ** P < 0.01, *** P < 0.001
Exercise restores glycogen anabolism in skeletal muscle of rats fed a high-fat diet
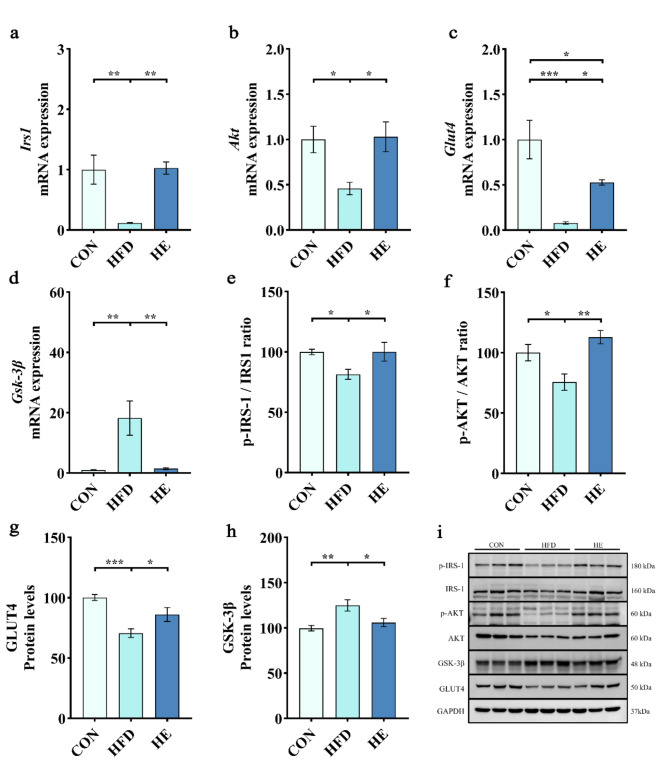
To elucidate the molecular mechanism underlying the improvement of glycogen anabolism through exercise, we investigated the mRNA expressions and protein levels of molecules involved in this phenomenon. We found HFD significantly suppressed the mRNA expressions of Irs1, Akt, and Glut4 in quadriceps (Fig. 4a-c). Correspondingly, HFD decreased the ratios of p-IRS1/IRS1 and p-AKT/AKT, and protein levels of GLUT4 compared to normal diet (Fig. 4e-g). However, aerobic exercise significantly restored the mRNA expressions (Fig. 4a-c), and protein levels (Fig. 4e-g) of all signaling molecules. In addition, overexpression of Gsk-3β mRNA and elevation of GSK-3β protein levels with HFD were completely reversed by exercise training (Fig. 4d and h). The results showed that HFD adversely affected the skeletal muscle glycogen anabolism, and exercise training restored these adverse effects by promoting the p-IRS1/IRS1, p-AKT/AKT, GLUT4, and GSK-3β signaling molecules.
Fig. 4.
Aerobic exercise restored skeletal muscle glycogen anabolism in rats fed a high-fat diet. (a-d) mRNA expression of Irs1, Akt, Glut4, and Gsk-3β. (e-f) Phosphorylated and total protein levels of IRS1 and AKT represented as p-IRS1/IRS1 and p-AKT/AKT ratios. (g-h) Protein levels of GLUT4 and GSK-3β. (i) Representative western blot images of p-IRS1, IRS1, p-AKT, AKT, GSK-3β, GLUT4, and internal control GAPDH in quadriceps. For western blot, we selected 3 animals from each group, and assessments were repeated for 3 times, which gives 9 protein bands per group (n = 3 per group). All data are presented as mean ± SEM; * P < 0.05, ** P < 0.01, *** P < 0.001
Exercise inhibits EGR-1/PTP1B mRNA and protein against HFD and restores glycogen anabolism
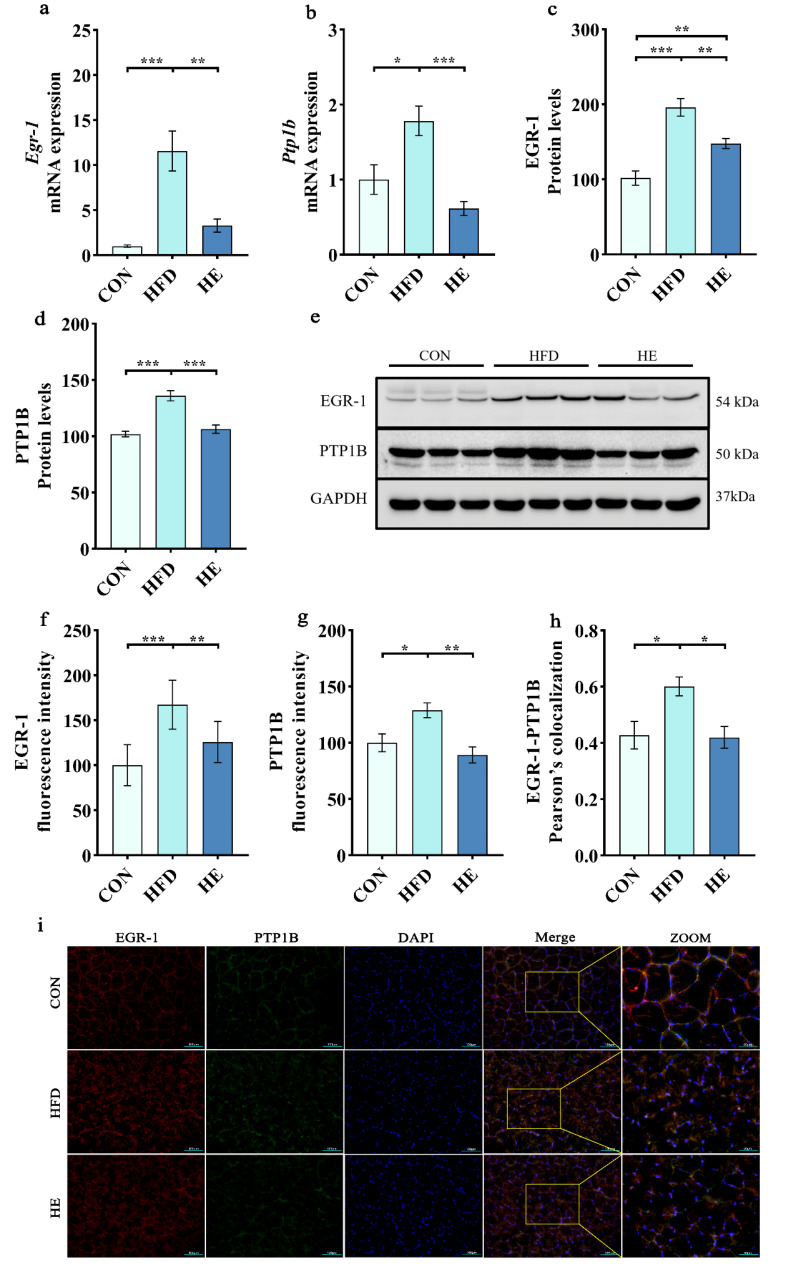
We further investigated the underlying mechanism on exercise-induced restoration of glycogen anabolism by assessing the mRNA expressions and protein levels of EGR-1 and PTP1B in quadriceps. Pearson correlation coefficient analysis was also performed to assess the correlation between EGR-1 and PTP1B across the experimental groups. The Pearson correlation coefficient quantifies the strength and direction of linear association between two variables, ranging from − 1 (perfect negative correlation) to 1 (perfect positive correlation) [30]. We found that the mRNA expressions of Egr-1 and Ptp1b (Fig. 5a and b), and protein levels of EGR-1 and PTP1B (Fig. 5c and d) were significantly elevated with HFD. Furthermore, colocalization of EGR-1 and PTP1B was also significantly higher in the HFD, as evidenced by immunofluorescence images (Fig. 5i). However, the overexpressed mRNA of Egr-1 and Ptp1b, correspondingly increased protein levels, and increased immunofluorescent intensities with HFD were markedly decreased by exercise training (Fig. 5a-i). These findings suggest that aerobic exercise can enhance insulin sensitivity and skeletal muscle glycogen anabolism probably through the inhibition of EGR-1/PTP1B signaling.
Fig. 5.
Aerobic exercise inhibited EGR-1/PTP1B mRNA and protein expressions in rats fed a high-fat diet. (a-b) mRNA expression of Egr1 and Ptp1b. (c-d) Protein levels of EGR-1 and PTP1B. (e) Representative western blot images of EGR-1 and PTP1B. (f, g) Fluorescence intensities of EGR-1 and PTP1B. (h) Colocalization of EGR-1-PTP1B. (i) Representative fluorescence images of EGR-1 and PTP1B. Scale bars, 100 μm and 50 μm. All data are presented as mean ± SEM. For western blot, we selected 3 animals from each group, and assessments were repeated 3 times, which gives 9 protein bands per group (n = 3 per group). Five regions were randomly selected from each sample, and quantified (n = 8 per group); * P < 0.05, ** P < 0.01, *** P < 0.001
Discussion
In this study, we demonstrated the beneficial effects of aerobic exercise training against HFD-induced insulin resistance and glycometabolism impairments in rats. HFD-induced impaired insulin sensitivity and decreased muscle glycogen content were reversed by exercise training. These beneficial effects of exercise were supported a significant upregulation skeletal muscle IRS1, AKT, and GLUT4 mRNA expressions and protein levels against HFD-induced downregulation. Furthermore, HFD-induced overexpression of EGR-1/PTP1B mRNA and protein levels were effectively suppressed by exercise training. Our findings revealed that 6-week aerobic exercise training can improve the insulin signaling possibly by mediating and promoting the skeletal muscle glycogen anabolism through the EGR-1/PTP1B signaling pathway in HFD-fed rats.
Pancreatic beta cells play a crucial role in the regulation of glucose homeostasis by secreting the insulin, and facilitating the storage of approximately 20% and 30% of glycogen in liver and skeletal muscle, respectively [31, 32]. Previous studies have emphasized the close connection between glycogen anabolism and diabetes pathogenesis with a reduction of glycogen content in liver and skeletal muscle of patients with diabetes [33–35]. Impaired skeletal muscle glycogen anabolism and glucose transport represents early metabolic abnormalities in pathogenesis of T2DM [35–38]. Skeletal muscle glycogen anabolism, coordinated by GSK-3β, is impaired in patients with obesity or T2DM, which then lead to reduce insulin sensitivity [39–41]. GSK-3β is a key protein kinase in glycogen metabolism that phosphorylates and inhibits glycogen synthase, a key enzyme in glycogen synthesis, and thereby contribute to decrease glycogen storage in the muscle [42–44]. Elevated levels of GSK-3β in skeletal muscle result in an imbalance in glycogen metabolism, and impaired glucose tolerance [45]. Numerous studies have shown that long-term exercise training-induced physiological adaptation in skeletal muscle could favorably modulate GSK-3β activity, and that could improve insulin sensitivity and increase glycogen content [46–48]. Our findings revealing the capability of 6-week aerobic exercise training in the restoration of muscle glycogen content through the inhibition of GSK-3β mRNA expression and protein in HFD-fed rats.
GLUT4 plays a vital role in regulation of insulin-responsive glucose transport in skeletal muscle, which increase correlates with enhanced glucose transport and increased glycogen content [49, 50]. GLUT4 deficiency skeletal muscle is implicated in impaired insulin-stimulated glycogen synthesis in patients with diabetes [50, 51]. Evidence from a GLUT4 knockout mice model demonstrated insulin resistance and glucose intolerance that are resembling a diabetic phenotype [52, 53]. Aerobic exercise reported to upregulate the GLUT4, and further contribute to lower the fasting and postprandial blood glucose levels [54–56]. This effect possibly mediated through increased insulin sensitivity, and activation of key signaling pathways, such as AMP-activated protein kinase (AMPK) and PI3K/AKT cascade [57]. Aerobic exercise training also reported to augment GLUT4 gene expression through the transcription factors, myocyte enhancer factor 2 (MEF2) and peroxisome proliferator-activated receptor-1α (PGC-1α), facilitates glucose delivery, and thereby improves overall glucose metabolism [58, 59]. Elevated GLUT4 activity not only augments glucose uptake but also supports glycogen synthesis, which is crucial for maintaining the muscle energy reserves and functions [60]. Our findings demonstrated that increased mRNA expression and activated AKT activity, as well as decreased mRNA and protein levels of GSK-3β with exercise training might have contributed to promote anabolic pathways and glycogen storage in skeletal muscle. The role of AKT is essential in facilitating the GLUT4 translocation and glycogen synthase activity for efficient glucose utilization and storage [61]. In our study, exercise intervention effectively ameliorated the HFD-induced insulin resistance by modulating the gene expressions, phosphorylation and protein levels of key signaling molecules involved in glycogen anabolism. These insights underscore the intricate interplay of glycogen anabolism in responses suggesting the potential therapeutic avenues for the effective management of insulin resistance and blood glucose.
IRS1 is a key signaling protein in muscle, which activates intracellular signaling cascades that promote insulin response to aerobic exercise [62, 63]. Activation of IRS1 promotes AKT, and regulates various cellular processes, such as glucose metabolism, apoptosis, and cell proliferation [64, 65]. Therefore, IRS1/AKT pathway is one of the important signaling pathways regulating the glucose homeostasis, and its activation is critical for IR in skeletal muscle. Furthermore, GSK-3β which is capable of phosphorylating the IRS1, was found to decrease with exercise, and subsequently represented by restored p-IRS1/IRS ratio. It is also shown that the reduction of EGR-1 in adipose tissue can augment the IRS1 tyrosine phosphorylation, and restore insulin sensitivity through the PI3K/AKT and ERK/MAPK pathways [9]. PTP1B is widely expressed in the endoplasmic reticulum of insulin-targeted tissues, including liver, muscle, and adipose tissue [66]. PTP1B negatively regulates the insulin signaling by dephosphorylating the IRS or insulin receptor (INSR) [67–69]. A study on PTP1B-deficient mice showed improved insulin sensitivity, lower blood glucose levels, and resistance to HFD-induced obesity [70], emphasizing the pivotal regulatory role of PTP1B in metabolic disorders. Conversely, overexpression of PTP1B in skeletal muscle impairs insulin signal transduction, diminishes glucose uptake, and induces IR [71]. Human study indicated a negative correlation between skeletal muscle PTP1B gene expression and insulin sensitivity [69]. Exercise reported to decrease PTP1B activity and expression in rats, leading to enhanced skeletal muscle insulin sensitivity through increased tyrosine phosphorylation of insulin receptors and reduced PTP1B binding [72]. Another study showed that short-term strength training can improve the hepatic insulin sensitivity by reducing the liver PTP1B in obese mice [73]. Evidence from our study revealed that 6-week aerobic exercise training can reduce the skeletal muscle EGR-1/PTP1B expressions, and contribute to restore the insulin sensitivity against HFD in rats.
Limitations
Although our findings provide valuable insights into the effects of aerobic exercise on glycometabolism and insulin sensitivity in HFD-fed rats, there are few limitations that should be acknowledged. The absence of exercise-only group in our study, constrains the capacity to demonstrate the independent effects of exercise on studied biomarkers. Next, the six-week exercise duration may not comprehensively capture the beneficial effects or adaptations that are associated with prolonged exercise. Moreover, the absence of gene knockout models perhaps imprecise to demonstrate the molecular evidence particularly the role of EGR-1 and PTP1B in mediating the beneficial effects. Future studies with a broader range of experimental conditions, extended intervention duration, and advanced genetic models could further deepen the understanding of exercise-induced beneficial effects and metabolic health.
Conclusion
Our findings demonstrated that 6-week aerobic exercise training is beneficial on enhancing the insulin sensitivity and promoting the glycogen anabolism in HFD-fed rats. These beneficial effects possibly mediated through the EGR-1/PTP1B signaling pathway in skeletal muscle. Further confirmatory studies are necessary to define the detailed molecular mechanism involved in this phenomenon.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Wei Li and Ting Li: Conceptualization, Methodology, Project administration and Funding acquisition. Liangzhi Zhang and Xiaojie Liu: Investigation and Visualization. Mallikarjuna Korivi, Jing Hu, and Helong Quan: Software, Formal analysis, Data Curation and Supervision. Liangzhi Zhang and Wei Li: Writing - Original Draft and Methodology. Sang Ki Lee, Lifeng Wang and Ting Li: Validation, Resources and Writing - Review & Editing. All authors read and approved the final paper.
Funding
This work was financial supported by Science and Technology Bureau of Jinhua City, grant number 2022-3-136, 2022-4-058. This study also supported by the Zhejiang Provincial Natural Science Foundation of China, grant number LY19C110002.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liangzhi Zhang and Xiaojie Liu contributed equally to this work.
Contributor Information
Ting Li, Email: tingli@zjnu.edu.cn.
Wei Li, Email: ty1986@zjnu.edu.cn.
References
- 1.Kubota N, Terauchi Y, Miki H, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance[J]. Mol Cell. 1999;4(4):597–609. [DOI] [PubMed] [Google Scholar]
- 2.Aras M, Tchang BG, Pape J. Obesity and Diabetes[J]. Nurs Clin North Am. 2021;56(4):527–41. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes[J]. Diabetes Care. 2009;32(Suppl 2):S157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: review of the underlying molecular mechanisms[J]. J Cell Physiol. 2019;234(6):8152–61. [DOI] [PubMed] [Google Scholar]
- 5.Jensen J, Rustad PI, Kolnes AJ, et al. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise[J]. Front Physiol. 2011;2:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman ES, Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology[J]. Am J Pathol. 1999;154(3):665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavrier P, Zerial M, Lemaire P, et al. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells[J]. EMBO J. 1988;7(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang RP, Liu C, Fan Y, et al. Egr-1 negatively regulates human tumor cell growth via the DNA-binding domain[J]. Cancer Res. 1995;55(21):5054–62. [PubMed] [Google Scholar]
- 9.Yu X, Shen N, Zhang ML, et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice[J]. EMBO J. 2011;30(18):3754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Tao W, Bu D, et al. Egr-1 transcriptionally activates protein phosphatase PTP1B to facilitate hyperinsulinemia-induced insulin resistance in the liver in type 2 diabetes[J]. FEBS Lett. 2019;593(21):3054–63. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Tao WW, Chong DY, et al. Early growth response-1 negative feedback regulates skeletal muscle postprandial insulin sensitivity via activating Ptp1b transcription[J]. FASEB J. 2018;32(8):4370–9. [DOI] [PubMed] [Google Scholar]
- 12.Fukada T, Tonks NK. The reciprocal role of Egr-1 and sp family proteins in regulation of the PTP1B promoter in response to the p210 bcr-abl oncoprotein-tyrosine kinase[J]. J Biol Chem. 2001;276(27):25512–9. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Nam K, Ju JH, et al. S100A4 negatively regulates beta-catenin by inducing the Egr-1-PTEN-Akt-GSK3beta degradation pathway[J]. Cell Signal. 2014;26(10):2096–106. [DOI] [PubMed] [Google Scholar]
- 14.De Meyts P. The Insulin Receptor and Its Signal Transduction Network[J]. 2000. [PubMed]
- 15.Hanke S, Mann M. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2[J]. Mol Cell Proteom. 2009;8(3):519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation[J]. Biochimie. 2005;87(1):99–109. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Somwar R, Bilan PJ, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts[J]. Mol Cell Biol. 1999;19(6):4008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikoulina SE, Ciaraldi TP, Mudaliar S, et al. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle[J]. Diabetes. 2002;51(7):2190–8. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Barroso M, Vargas-Vargas MA, Pena-Montes DJ et al. Comparative effect of three different Exercise intensities in Combination with Diazoxide on Contraction Capacity and oxidative stress of skeletal muscle in obese Rats[J]. Biology (Basel), 2022,11(9). [DOI] [PMC free article] [PubMed]
- 20.Dube JJ, Amati F, Stefanovic-Racic M, et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited[J]. Am J Physiol Endocrinol Metab. 2008;294(5):E882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price TB, Perseghin G, Duleba A, et al. NMR studies of muscle glycogen synthesis in insulin-resistant offspring of parents with non-insulin-dependent diabetes mellitus immediately after glycogen-depleting exercise[J]. Proc Natl Acad Sci U S A. 1996;93(11):5329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Jiang X, Hu J, et al. Exercise attenuates high-fat diet-induced PVAT dysfunction through improved inflammatory response and BMP4-regulated adipose tissue browning[J]. Front Nutr. 2024;11:1393343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisloff U, Helgerud J, Kemi OJ, et al. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy[J]. Am J Physiol Heart Circ Physiol. 2001;280(3):H1301–10. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Matsuda M, Balas B, et al. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test[J]. Diabetes Care. 2007;30(1):89–94. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man[J]. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 26.Young A. The relative isometric strength of type I and type II muscle fibres in the human quadriceps[J]. Clin Physiol. 1984;4(1):23–32. [DOI] [PubMed] [Google Scholar]
- 27.Sahinis C, Kellis E, Galanis N, et al. Intra- and inter-muscular differences in the cross-sectional area of the quadriceps muscles assessed by extended field-of-view ultrasonography[J]. Med Ultrason. 2020;22(2):152–8. [DOI] [PubMed] [Google Scholar]
- 28.Roe JH, Dailey RE. Determination of glycogen with the anthrone reagent[J]. Anal Biochem. 1966;15(2):245–50. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Lin H, Yang X, et al. Effects of dapagliflozin monotherapy and combined aerobic exercise on skeletal muscle mitochondrial quality control and insulin resistance in type 2 diabetes mellitus rats[J]. Biomed Pharmacother. 2023;169:115852. [DOI] [PubMed] [Google Scholar]
- 30.Paul N, Raymond J, Lumbreras S, et al. Activation of the glucocorticoid receptor rapidly triggers calcium-dependent serotonin release in vitro[J]. CNS Neurosci Ther. 2021;27(7):753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor R, Magnusson I, Rothman DL, et al. Direct assessment of liver glycogen storage by 13 C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects[J]. J Clin Invest. 1996;97(1):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor R, Price TB, Katz LD, et al. Direct measurement of change in muscle glycogen concentration after a mixed meal in normal subjects[J]. Am J Physiol. 1993;265(2 Pt 1):E224–9. [DOI] [PubMed] [Google Scholar]
- 33.Kasuga M, Ogawa W, Ohara T. Tissue glycogen content and glucose intolerance[J]. J Clin Invest. 2003;111(9):1282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnusson I, Rothman DL, Katz LD, et al. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13 C nuclear magnetic resonance study[J]. J Clin Invest. 1992;90(4):1323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shulman GI, Rothman DL, Jue T, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13 C nuclear magnetic resonance spectroscopy[J]. N Engl J Med. 1990;322(4):223–8. [DOI] [PubMed] [Google Scholar]
- 36.DeFronzo RA, Lilly. lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM[J]. Diabetes, 1988,37(6):667–687. [DOI] [PubMed]
- 37.Solis-Herrera C, Triplitt C, Cersosimo E et al. Pathogenesis of Type 2 Diabetes Mellitus[J]. 2000.
- 38.Zhang P, Li T, Wu X, et al. Oxidative stress and diabetes: antioxidative strategies[J]. Front Med. 2020;14(5):583–600. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome[J]. Proc Natl Acad Sci U S A. 2007;104(31):12587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan AS, Li G, McMillin S et al. Pathways in skeletal muscle: protein signaling and insulin sensitivity after Exercise training and weight loss interventions in Middle-aged and older Adults[J]. Cells, 2021,10(12). [DOI] [PMC free article] [PubMed]
- 41.Pedersen AJ, Hingst JR, Friedrichsen M, et al. Dysregulation of muscle glycogen synthase in recovery from exercise in type 2 diabetes[J]. Diabetologia. 2015;58(7):1569–78. [DOI] [PubMed] [Google Scholar]
- 42.MacAulay K, Woodgett JR. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of type 2 diabetes[J]. Expert Opin Ther Targets. 2008;12(10):1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McManus EJ, Sakamoto K, Armit LJ, et al. Role that phosphorylation of GSK3 plays in insulin and wnt signalling defined by knockin analysis[J]. EMBO J. 2005;24(8):1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorburn AW, Gumbiner B, Bulacan F, et al. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus[J]. J Clin Invest. 1991;87(2):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce NJ, Arch JR, Clapham JC, et al. Development of glucose intolerance in male transgenic mice overexpressing human glycogen synthase kinase-3beta on a muscle-specific promoter[J]. Metabolism. 2004;53(10):1322–30. [DOI] [PubMed] [Google Scholar]
- 46.Markuns JF, Wojtaszewski JF, Goodyear LJ. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle[J]. J Biol Chem. 1999;274(35):24896–900. [DOI] [PubMed] [Google Scholar]
- 47.Manabe Y, Gollisch KS, Holton L, et al. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content[J]. FEBS J. 2013;280(3):916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aschenbach WG, Ho RC, Sakamoto K, et al. Regulation of dishevelled and beta-catenin in rat skeletal muscle: an alternative exercise-induced GSK-3beta signaling pathway[J]. Am J Physiol Endocrinol Metab. 2006;291(1):E152–8. [DOI] [PubMed] [Google Scholar]
- 49.Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease[J]. Pflugers Arch. 2020;472(9):1273–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsao TS, Burcelin R, Katz EB, et al. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle[J]. Diabetes. 1996;45(1):28–36. [DOI] [PubMed] [Google Scholar]
- 51.Cline GW, Petersen KF, Krssak M, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes[J]. N Engl J Med. 1999;341(4):240–6. [DOI] [PubMed] [Google Scholar]
- 52.Stenbit AE, Tsao TS, Li J, et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes[J]. Nat Med. 1997;3(10):1096–101. [DOI] [PubMed] [Google Scholar]
- 53.Kim JK, Zisman A, Fillmore JJ, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4[J]. J Clin Invest. 2001;108(1):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity[J]. Annu Rev Med. 1998;49:235–61. [DOI] [PubMed] [Google Scholar]
- 55.Richter EA, Hargreaves M, Exercise. GLUT4, and skeletal muscle glucose uptake[J]. Physiol Rev. 2013;93(3):993–1017. [DOI] [PubMed] [Google Scholar]
- 56.Houmard JA, Shinebarger MH, Dolan PL, et al. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men[J]. Am J Physiol. 1993;264(6 Pt 1):E896–901. [DOI] [PubMed] [Google Scholar]
- 57.Maarbjerg SJ, Sylow L, Richter EA. Current understanding of increased insulin sensitivity after exercise - emerging candidates[J]. Acta Physiol (Oxf). 2011;202(3):323–35. [DOI] [PubMed] [Google Scholar]
- 58.Holmes B, Dohm GL. Regulation of GLUT4 gene expression during exercise[J]. Med Sci Sports Exerc. 2004;36(7):1202–6. [DOI] [PubMed] [Google Scholar]
- 59.Ferrari F, Bock PM, Motta MT, et al. Biochemical and molecular mechanisms of glucose uptake stimulated by Physical Exercise in insulin resistance state: role of Inflammation[J]. Arq Bras Cardiol. 2019;113(6):1139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren JM, Semenkovich CF, Gulve EA, et al. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle[J]. J Biol Chem. 1994;269(20):14396–401. [PubMed] [Google Scholar]
- 61.Wang C, Deng Y, Yue Y, et al. Glutamine enhances the hypoglycemic effect of insulin in L6 cells via Phosphatidylinositol-3-Kinase (PI3K)/Protein kinase B (AKT)/Glucose transporter 4 (GLUT4) signaling Pathway[J]. Med Sci Monit. 2018;24:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chibalin AV, Yu M, Ryder JW, et al. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin-receptor substrates 1 and 2[J]. Proc Natl Acad Sci U S A. 2000;97(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirwan JP, Del AL, Hernandez JM, et al. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle[J]. J Appl Physiol (1985). 2000;88(2):797–803. [DOI] [PubMed] [Google Scholar]
- 64.Cong LN, Chen H, Li Y, et al. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells[J]. Mol Endocrinol. 1997;11(13):1881–90. [DOI] [PubMed] [Google Scholar]
- 65.Kim YB, Nikoulina SE, Ciaraldi TP, et al. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes[J]. J Clin Invest. 1999;104(6):733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tonks NK. PTP1B: from the sidelines to the front lines![J]. FEBS Lett. 2003;546(1):140–8. [DOI] [PubMed] [Google Scholar]
- 67.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome[J]. Nature. 2001;414(6865):821–7. [DOI] [PubMed] [Google Scholar]
- 68.Goldstein BJ, Bittner-Kowalczyk A, White MF, et al. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein[J]. J Biol Chem. 2000;275(6):4283–9. [DOI] [PubMed] [Google Scholar]
- 69.Stull AJ, Wang ZQ, Zhang XH, et al. Skeletal muscle protein tyrosine phosphatase 1B regulates insulin sensitivity in African Americans[J]. Diabetes. 2012;61(6):1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene[J]. Science. 1999;283(5407):1544–8. [DOI] [PubMed] [Google Scholar]
- 71.Zabolotny JM, Haj FG, Kim YB, et al. Transgenic overexpression of protein-tyrosine phosphatase 1B in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action[J]. J Biol Chem. 2004;279(23):24844–51. [DOI] [PubMed] [Google Scholar]
- 72.Ropelle ER, Pauli JR, Prada PO, et al. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation[J]. J Physiol. 2006;577(Pt 3):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Da CRK, Martins PR, Peruca GF et al. Short-term strength Exercise reduces hepatic insulin resistance in obese mice by reducing PTP1B content, regardless of changes in Body Weight[J]. Int J Mol Sci, 2021,22(12). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.