Abstract
Background
The proliferation capacity of adult cardiomyocytes is very limited in the normal adult mammalian heart. Previous studies implied that cardiomyocyte proliferation increases after injury stimulation, but the result is controversial partly due to different methodologies. We aim to evaluate whether myocardial infarction (MI) stimulates cardiomyocyte proliferation in adult mice.
Methods
A comprehensive literature search was conducted through PubMed/Medline, Embase, and Web of Science databases from 1 January 2000 to 21 December 2023. The SYRCLE’s Risk of Bias tool for animal experiments was used to evaluate the quality of the literature by two independent reviewers. Twenty-six studies with cell cycle indicators (Ki67+, PH3+, BrdU/EdU+, and AurkB+) to evaluate cycling cardiomyocytes were collected for a meta-analysis. Another 10 studies with genetic reporter/tracing systems to evaluate cardiomyocyte proliferation were collected for a systematic review.
Results
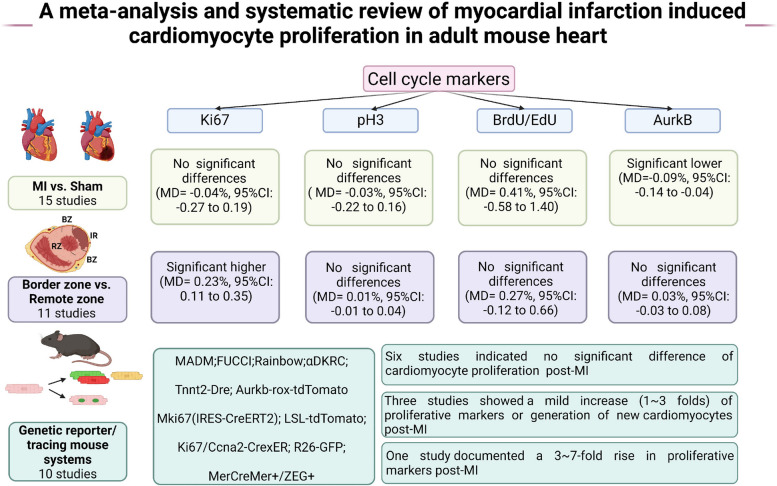
Evaluating cardiomyocyte proliferation by immunostaining of the cell cycle indicators on heart tissue, the meta-analysis showed that differences of Ki67+, PH3+, and BrdU/EdU+ cycling cardiomyocytes between MI and Sham groups were not statistically significant. In the post-MI heart, the percentages of PH3+, BrdU/EdU+, and AurkB+ cardiomyocytes were not significantly different between the infarct border zone and remote zone. The percentage of Ki67+ cardiomyocytes in the infarct border zone was statistically higher than that in the remote zone. Most of the studies (6 out of 10) using genetic reporter/tracing mouse systems showed that the difference in cardiomyocyte proliferation between MI and Sham groups was not statistically significant. Among the other 4 studies, at least 3 studies could not demonstrate that MI stimulates bona fide cardiomyocyte proliferation because of methodological shortages.
Conclusions
MI injury increases Ki67+ cycling adult mouse cardiomyocytes in infarct border zone. Very little overwhelming evidence shows that MI stimulates bona fide proliferation in the adult heart.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03822-0.
Keywords: Cardiomyocyte proliferation, Myocardial infarction, Cell cycle, Heart, Lineage tracing
Background
Myocardial infarction (MI) is a life-threatening heart disease characterized by the occlusion in the coronary arteries, leading to a great loss of functional cardiomyocytes. The current therapies such as reperfusion therapy and medications can only partially delay the pathological remodeling process, but they do not replenish cardiomyocyte numbers to replace the lost myocardium [1].
Fetal and neonatal cardiomyocytes have a remarkable ability to proliferate. When MI or apex resection injury was induced in a postnatal day 1 (P1) mice/pigs, the remaining cardiomyocytes can proliferate to repair the injury and fully restore the cardiac function after 8 weeks. It implies that injuries could stimulate cardiomyocyte proliferation and generate certain amounts of new cardiomyocytes. However, this regenerative capacity diminishes within the first week after birth [2]; injuries at P7 could not recover but instead replaced with scar tissue [3]. In both normal and injured hearts, accumulating evidence has revealed that there were still cardiomyocyte proliferation events, but the efficacies were very low. However, it is not clear whether, in adult heart, MI injury can trigger cardiomyocyte proliferation.
Most studies investigating cardiomyocyte proliferation have been performed using methodology of immunostaining of cell cycle indicator Ki67+, PH3+, AurkB+ or BrdU/EdU incorporation [4]. Some studies showed that MI in adult mice induced marginal cardiomyocytes to reenter the cell cycle and proliferate [5]. In other studies, the absence of changes in cell cycle indicators suggested that MI did not sufficiently induce cardiomyocyte proliferation [6]. However, in adult cardiomyocytes, these methods usually confuse bona fide proliferation with bi/multinucleation and polyploidization. In recent years, genetic reporter or lineage-tracing systems, i.e., mosaic analysis with double markers (MADM) mice [7] and rainbow mice [8], have been used for more precise detection of new cardiomyocyte formation. Since the golden-standard methodology is still lacking, the results by genetic reporter or lineage-tracing mouse systems are also controversial. For example, by combining genetic lineage tracing with stable isotope labeling and multi-isotope imaging mass spectrometry, it showed that the rate of new myocyte formation increases about 3 folds following MI injury, with pre-existing cardiomyocytes in the border zone being the dominant source of new cardiomyocytes [9]. Inconsistent results were found in MI model of MADM mice, which showed MI did not stimulate new cardiomyocyte formation [10].
By performing this meta-analysis and systematic review, we analyzed the available data from independent research groups to evaluate whether MI can induce cardiomyocyte proliferation in adult mice. The findings are of significance in understanding the features of cardiac regeneration post-injury and exploring underlying mechanisms for repairing the injured heart.
Methods
Literature search strategy
We conducted the procedures for this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [11]. The PRISMA checklist is presented in Additional file 1. The registration number is 2,024,120,002 in the International Platform of Registered Systematic Review and Meta-analysis Protocols. A comprehensive literature search was conducted through PubMed/Medline, Embase, and Web of Science databases from 1 January 2000 to 21 December 2023. A series of in vivo studies on estimating the cell cycle markers after MI in adult mice were collected. A full list of search terms for all databases was shown in Additional file 2: Table S1-3. We also checked reference lists of related reviews and all eligible studies for additional trials.
Eligibility and exclusion criteria
Studies were included if they met the following criteria: (1) MI models in adult mice were successfully completed; (2) there was information on cell cycle activity of cardiomyocytes in the MI group vs Sham group or infarct border zone vs remote zone in the same post-MI heart; (3) outcome indicators of proliferative cell cycle must include at least one of followings: Ki67, PH3, BrdU/EdU, and AurkB. Studies were excluded if they met any of the following criteria: (1) studies were reviews, comments, or meeting abstracts; (2) studies were conducted with animal models of neonatal mice; (3) there was missing data about predetermined outcome parameters. Two reviewers performed all screening processes independently (L.Y. and Z.P.). Discrepancies were resolved by discussion and consensus.
Data extraction and quality assessment
Information was recorded on the author, published year, country, sample size in control and experimental groups, mouse strains, age, outcome measures, and time points for outcome measures. The predefined outcomes were the proportions of Ki67+, PH3+, BrdU/EdU+, or AurkB+ [12, 13]. The mean and standard deviation (SD) of outcome measures were extracted from the eligible articles. For articles with only figure information, the GetData software was used to extract the values from the figures. Two reviewers (L.L. and Z.P.) independently collected data from each report.
Two reviewers (L.Y. and Z.P.) independently appraised the potential risk of bias (ROB) and quality assessment using SYRCLE’s Risk of Bias tool [14], based on 10 domains: random sequence generation, baseline characteristics, allocation concealment, random housing, blinding, random outcome assessment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Discrepancies were resolved by consensus.
Statistical analysis
Mean difference (MD) was used as a summary statistic, calculated for each comparison with 95% CI. The standard error (SE) in the study was converted to SD. The pooled effect was calculated by a random or common effect model in this meta-analysis accounting for potential heterogeneity. I2 statistic was used to evaluate the heterogeneity among studies [15]. If I2 > 50%, a random effects model was applied.
Subgroup analysis was conducted for different time points for outcome measures post-MI (within 14 days or more than 14 days) and different mouse strains (C57BL/6 J and other strains) considering potential causes of heterogeneity. A leave-one-out meta-analysis conducted sensitivity analyses to evaluate the robustness of results for decisions made.
For all analyses, the statistical significance was set as p-value < 0.05. All statistical analyses used the R packages meta, robvis, forestplot, and ggplot2 (version 4.3.1).
Results
Characteristics of the selected studies with both methodologies of cell cycle indicators and genetic reporter/tracing mouse systems
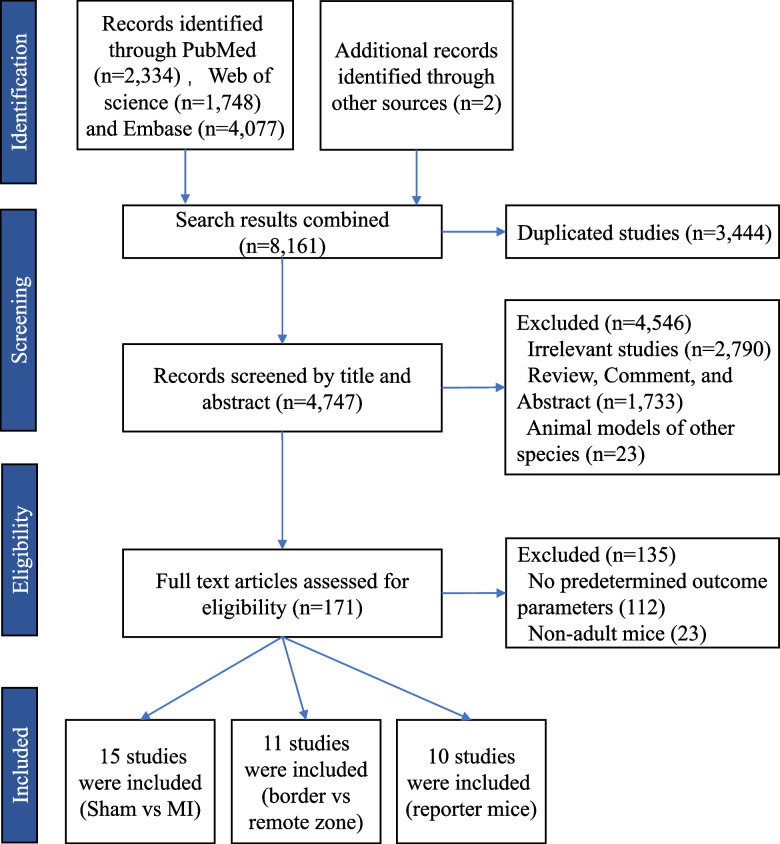
The detailed search strategy and selection results are shown in Fig. 1. The comprehensive searches of PubMed/Medline, Embase, and Web of Science databases yielded 8159 records, and manual searches of reference of reviews and eligible studies identified an additional 2 articles. The publication dates of the included studies ranged from 2013 to 2023. After removing duplicates, 4747 records were screened by title and abstract, and 171 articles were screened by full text. A total of 26 studies with methodologies of cell cycle indicators (Ki67+, PH3+, AurkB+, or BrdU/EdU incorporation) were included in the meta-analysis, and 10 studies with methodology of genetic reporter/tracing systems were collected for a systematic review. For all the studies, time points for outcome measurements differed substantially among these studies, ranging from 3 to 63 days post-MI.
Fig. 1.
Flowchart of study. The comprehensive searches of PubMed/Medline, Embase, and Web of Science databases yielded 8159 records, and manual searches of reference of reviews and eligible studies identified an additional 2 articles. After removing duplicates, 4747 records were screened by title and abstract, and 171 article texts were screened full text. A total of 26 studies with methodologies of cell cycle indicators (Ki67+, PH3+, AurkB+ or BrdU/EdU incorporation) were included in the meta-analysis
Studies using cell cycle indicators showed no significant increase in cardiomyocyte cell cycle activation in MI heart versus Sham heart
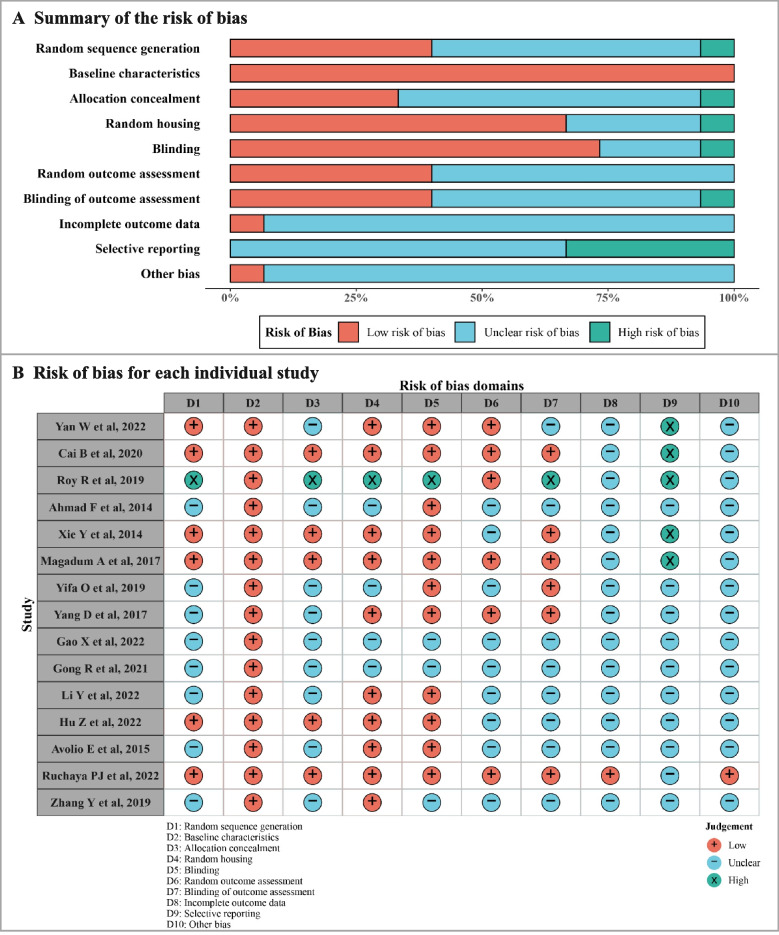
In the 26 studies, the methodologies used in cell cycle indicators showed that most of the mice strains were C57BL/6J. Among those studies, 15 studies were included to analyze the effects of MI on cardiomyocyte proliferation between the MI group and the Sham group (Table 1) [16–30]. The results of the ROB and quality assessment are shown in Fig. 2. All studies described the baseline characteristics. However, about half of the studies did not describe the process of random sequence generation, allocation concealment, random outcome assessment, and blinding of outcome assessment. Apart from that, most studies did not report whether there were incomplete outcome data, selective reporting or other biases.
Table 1.
Characteristics of the identified studies on cardiomyocyte proliferation between the MI and Sham groups
| Study | Years | Country | Sham (n) | MI (n) | Strains | Age | Time-points for outcome measures | Outcome measures |
|---|---|---|---|---|---|---|---|---|
| Ahmad F et al. [16] | 2014 | USA | 4 | 6 | Gsk3αfl/fl | 8w | 21d post-MI | PH3 |
| Avolio E et al. [17] | 2015 | UK | 3 | 6 | SCID Beige | 8w | 14d post-MI | Ki67; BrdU/EdU |
| Cai B et al. [18] | 2020 | China | 4 | 3 | C57BL/6 | 8w | 14d post-MI | PH3; Aurora B |
| Gao X et al. [19] | 2022 | China | 3 | 3 | Kun Ming | 8w | 14d post-MI | Ki67; PH3 |
| Gong R et al. [20] | 2021 | China | 3 | 3 | C57BL/6 | 5-6w | 28d post-MI | Ki67; PH3; Aurora B |
| Hu Z et al. [21] | 2022 | China | 5 | 5 | C57BL/6 | 8w | 14d post-MI | Ki67; BrdU/EdU; Aurora B |
| Li Y et al. [22] | 2022 | China | 4 | 4 | ICR | 8w | 7d post-MI | BrdU/EdU |
| Magadum A et al. [23] | 2017 | Germany | 4 | 4 | TMCM | 8w | 14d post-MI | BrdU/EdU |
| Roy R et al. [24] | 2019 | USA | 5 | 5 | C57BL/6 J | 8w | 14d post-MI | PH3; BrdU/EdU |
| Ruchaya PJ et al. [25] | 2022 | UK | 5 | 5 | C57BL/6 | 8w | 42d post-MI | BrdU/EdU |
| Xie Y et al. [26] | 2014 | USA | 6 | 7 | SCID-beige | 8w | 7d post-MI | Ki67;PH3 |
| Yan W et al. [27] | 2020 | China | 6 | 6 | C57BL/6 J | 8w | 3d post-MI | Ki67; BrdU/EdU |
| Yang D et al. [28] | 2017 | China | 4 | 4 | C57BL/6 J | 8w | 28d post-MI | BrdU/EdU |
| Yifa O et al. [29] | 2019 | Israel | 3 | 5 | C57BL/6 J | 12w | 4d post-MI | Ki67 |
| Zhang Y et al. [30] | 2019 | USA | 3 | 3 | C57Bl/6 | Adults | 24d post-MI | BrdU/EdU |
Fig. 2.
Assessment of risk of bias in the included studies on cardiomyocyte proliferation between MI group and Sham group. A Summary of the risk of bias for the included studies. B Risk of bias for each individual study
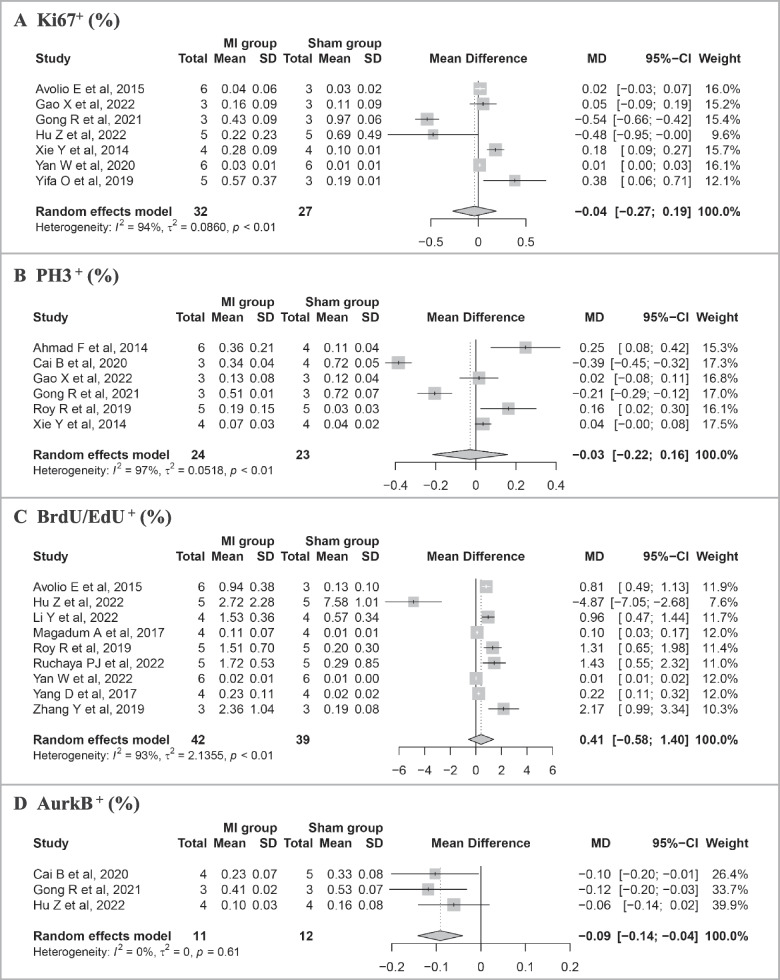
Pooled results of the meta-analysis on cell cycle markers between the MI group and the Sham group are shown in Fig. 3. Among the 15 studies, there were 7 records of cell cycle marker Ki67, 6 of PH3, 9 of BrdU/EdU, and 3 of AurkB. The results of meta-analysis did not find statistically significant differences in percentages of Ki67+ (MD = − 0.04%, 95% confidence interval [CI]: − 0.27 to 0.19), PH3+ (MD = − 0.03%, 95% CI: − 0.22 to 0.16), and BrdU/EdU+ (MD = 0.41%, 95%CI: − 0.58 to 1.40). The percentage of AurkB+ (MD = − 0.09%, 95% CI: − 0.14 to − 0.04) cardiomyocyte was lower in the MI group than that in the Sham group.
Fig. 3.
Meta-analysis of proportion of cell cycle markers between MI and Sham group in identified studies. A Proportion of Ki67+. B Proportion of PH3+. C Proportion of BrdU/EdU+. D Proportion of AurkB+. Study-specific mean differences (MD) are represented by squares (with their 95% confidence intervals [CIs] as lines). The size of the solid square reflects the weight of each eligible study, which represents the influence of each study on the overall effect. The overall effects are plotted as diamonds, and its intersection with an invalid line (X = 0) is considered statistically insignificant. Random effects models were used if I 2 > 50% and p < 0.05 in the heterogeneity test. Results showed no significant increase in cardiomyocyte cell cycle activation in the MI heart versus the Sham heart
Subgroup analyses were conducted to evaluate whether the time-points post-MI and mouse strains influenced the result (Additional file 3: Fig. S1-2). Firstly, we conducted a subgroup analysis at different time points post-MI (≤ 14 days vs. > 14 days) (Additional file 3: Fig. S1). The results showed statistical differences in pooled MD of Ki67+ (p < 0.01) between two subgroups. The proportion of BrdU/EdU+ cardiomyocytes in the MI group was statistically higher than that in the Sham group over 14 days post-MI. However, we did not find significant differences in pooled MD of PH3+ (p = 0.81), BrdU/EdU+ (p = 0.20), or AurkB+ (p = 0.47) between the two subgroups. In addition, we conducted a subgroup analysis in different mouse strains (C57BL/6 J vs. other strains). The results showed that there were no significant differences in pooled MD of Ki67+ (p = 0.30), PH3+ (p = 0.19), or BrdU/EdU (p = 0.67) between the two subgroups (Additional file 3: Fig. S2). The studies reporting AurkB+ used the same mouse strains and were unable to perform subgroup analysis.
Studies with the methodology of cell cycle indicators showed an increase of Ki67+cycling cardiomyocytes in infarct border zone versus remote zone in the same post-MI heart
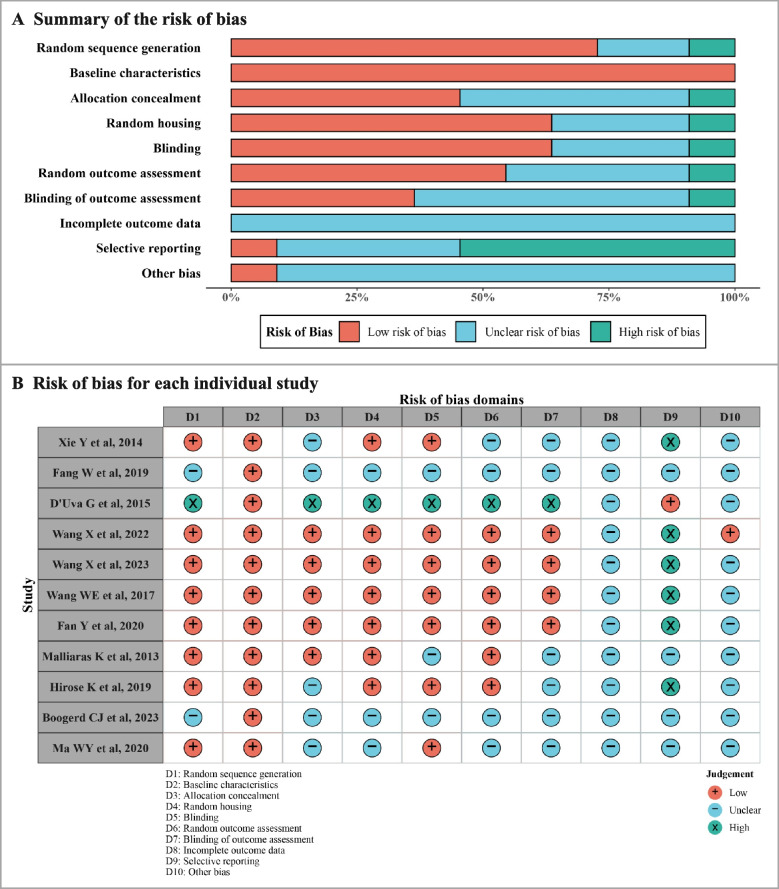
Among the 26 studies’ methodologies of cell cycle indicators, 11 studies were included between infarct border zone and remote zone in the same post-MI heart (Table 2) [6, 26, 31–39]. The results of ROB and quality assessment are shown in Fig. 4. All studies described the baseline characteristics. A few studies did not describe the process of random sequence generation, random housing, and blinding. About half of the studies did not explain the process of allocation concealment, random outcome assessment, blinding of outcome assessment, and selective reporting. Apart from that, only one study described other biases, and none of the studies reported whether there were incomplete outcome data, considering missing data may affect the authenticity of results.
Table 2.
Characteristics of the identified studies on cardiomyocyte proliferation between infarct border zone and remote zone in post-MI hearts
| Study | Years | Country | n | Strains | Age | Time-points for outcome measures | Outcome measures |
|---|---|---|---|---|---|---|---|
| Boogerd CJ et al. [31] | 2023 | Netherlands | 8 | C57BL/6 J | 9 w | 7 days post-MI | BrdU/EdU |
| D’Uva G et al. [32] | 2015 | Israel | 6 | C57BL/6 J | 6 w | 21 days post-MI | Ki67; Aurora B |
| Fan Y et al. [33] | 2020 | China | 3–5 | C57BL/6 J | 8 w | 14 days post-MI | Ki67; PH3; BrdU/EdU; Aurora B |
| Fang W et al. [34] | 2019 | China | 10 | C57BL/6 J | Adult | 7 days post-MI | Ki67 |
| Hirose K et al. [35] | 2019 | USA | 4 | C57BL/6 J | 6–13 w | 10 days post-MI | Ki67 |
| Ma WY et al. [36] | 2020 | China | 5 | C57BL/6 J | 6–8 w | 14 days post-MI | PH3; Aurora B |
| Malliaras K et al. [37] | 2013 | USA | 5 | MerCreMer/ZEG | 6–8 w | 35 days post-MI | BrdU/EdU |
| Wang WE et al. [38] | 2017 | China | 8 | C57BL/6 J | 16 w | 21 days post-MI | BrdU/EdU |
| Wang X et al. [6] | 2022 | USA | 6 | C57BL/6 J | 10–14 w | 14 days post-MI | Ki67; PH3; BrdU/EdU |
| Wang X et al. [6] | 2022 | USA | 5 | C57BL/6 J | 10–14 w | 28 days post-MI | Ki67; PH3; BrdU/EdU |
| Wang X et al. [39] | 2023 | China | 6 | C57BL/6 J | 10–12 w | 10 days post-MI | Ki67; PH3; BrdU/EdU |
| Wang X et al. [39] | 2023 | China | 6–10 | C57BL/6 J | 10–12 w | 28 days post-MI | Ki67; PH3; BrdU/EdU |
| Xie Y et al. [26] | 2014 | USA | 7 | C57BL/6 J | 8 w | 7 days post-MI | Ki67 |
Fig. 4.
Assessment of risk of bias in the included studies on cardiomyocyte proliferation between infarct border zone and remote zone. A Summary of the risk of bias for the included studies. B Risk of bias for each individual study
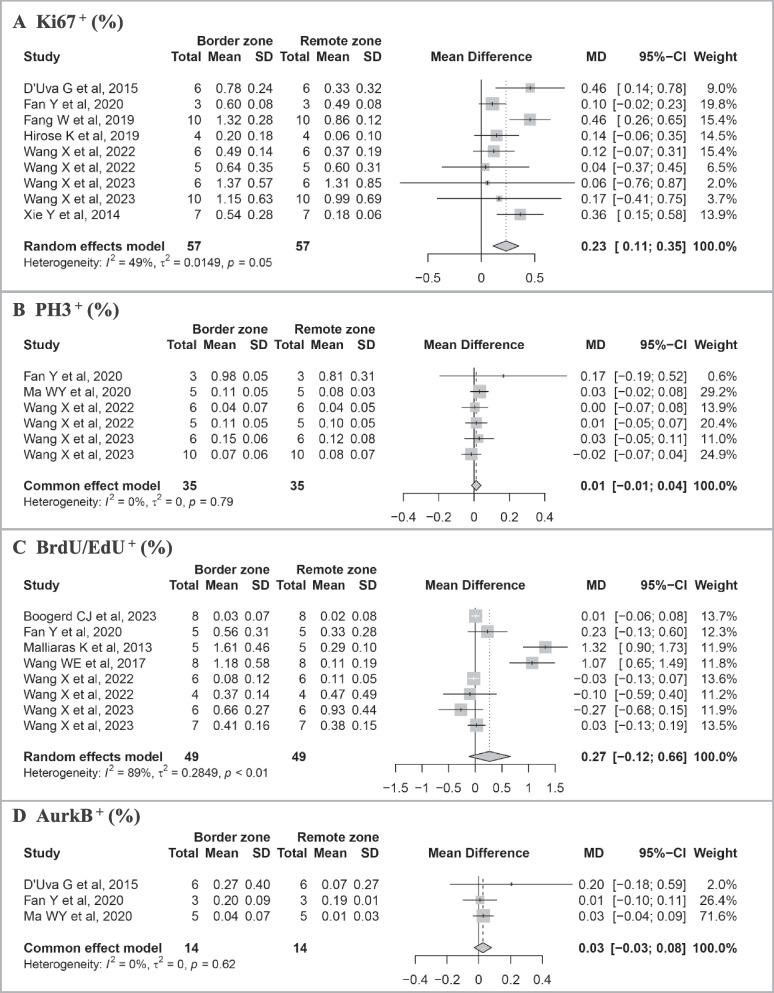
Pooled results of the meta-analysis on cell cycle markers between the border zone and remote zone are shown in Fig. 5. Among the 11 studies, 9 records of cell cycle marker Ki67 were found. Results of the meta-analysis revealed that the percentage of Ki67+ cardiomyocytes in the infarct border zone was statistically higher than that in the remote zone (MD = 0.23%, 95% CI: 0.11 to 0.35). There were 6 records of PH3, 8 of BrdU/EdU, and 3 of AurkB. Results of meta-analysis showed that the proportion of PH3+ (MD = 0.01%, 95% CI: − 0.01 to 0.04), BrdU/EdU+ (MD = 0.27%, 95% CI: − 0.12 to 0.66), and AurkB+ (MD = 0.03%, 95% CI: − 0.03 to 0.08) cardiomyocytes in the infarct border zone was higher than that in the remote zone, though the difference was not statistically significant.
Fig. 5.
Meta-analysis of the proportion of cell cycle markers between infarct border and remote zone in identified studies. A Proportion of Ki67+. B Proportion of PH3+. C Proportion of BrdU/EdU+. D Proportion of AurkB+. Study-specific mean differences (MD) are represented by squares (with their 95% confidence intervals [CIs] as lines). The size of the solid square reflects the weight of each eligible study, which represents each study’s influence on the overall effect. The overall effects are plotted as diamonds, and its intersection with an invalid line (X = 0) is considered statistically insignificant. Random effects models were used if I2 > 50% and p < 0.05 in the heterogeneity test. Results showed an increase of Ki67+ cycling cardiomyocytes in the infarct border zone versus the remote zone in the same post-MI heart
Results of subgroup analysis in different time points (≤ 14 days and > 14 days) were shown in (Additional file 3: Fig. S3). The percentage of Ki67+ cardiomyocytes in the border zone was statistically higher than that in the remote zone within 14 days post-MI (MD = 0.22%, 95%CI: 0.09 to 0.36), while there was no statistically significant difference over 14-days post-MI. However, we did not find significant differences in pooled MD of Ki67+ (p = 0.85), PH3+ (p = 0.32), BrdU/EdU+ (p = 0.11), or AurkB+ (p = 0.36) between two subgroups.
Studies with the methodology of genetic reporter/tracing mouse systems showed very few overwhelming evidence that MI stimulates bona fide proliferation in the adult heart
In addition, we conducted a systematic review of 10 studies with the methodology of genetic reporter/tracing systems (Table 3) [5, 8–10, 12, 40–44]. The mouse systems including MADM, FUCCI, Rainbow, αDKRC, Mki67, and MerCreMer+/ZEG+ were included in this study. In most studies (6 out of 10), the difference in cardiomyocyte proliferation between MI and Sham groups was not statistically significant.
Table 3.
Systematic review for cardiomyocyte proliferation post-MI evaluated by genetic reporter/tracing systems
| Study | Country | Sham (n) | Injury (n) | Age | Mice | Injury | Time-points for outcome measures | Cardiomyocyte proliferation | Sig |
|---|---|---|---|---|---|---|---|---|---|
| Ali SR et al. [10] | USA | 3 | 5 | 8 w | β-actinCreER; MADM GT/TG | LAD ligation | 4 w | MI did not increase the rate of cardiomyocyte division compared with Sham | No |
| Alvarez R et al. [12] | USA | / | / | 8 w | FUCCI | LAD ligation | 7,10, 14,21 d | Border zone cardiomyocytes exhibit signs of cell cycle re-entry but fail to show new CMs formation (%CMs nuclei) | No |
| Bradley LA et al. [40] | USA | 9 | 8 | 8 w | αDKRC (cardiomyocyte-specific αMHCMerDreMer-Ki67p-RoxedCre)::RLTG (Rox-Lox-tdTomato-eGFP) | I/R | 2 w | Cycling CMs increase almost 2 folds (especially in the border zone) after I/R (eGFP + CMs per ten-micron short-axis section) (MI vs. Sham) | Yes |
| Fu W et al. [41] | China | 5 | 5 | 8 w | Tnnt2-Dre; Aurkb-rox-tdTomato | LAD ligation | 2 w | MI did not activate CM proliferation in adult (MI vs. Sham) | No |
| Kretzschmar K et al. [42] | Netherlands | 3 | 3 | 8–9 w | Mki67(IRES-CreERT2); LSL-tdTomato | LAD ligation | 1 w | Ischemic injury did not stimulate significant generation of new CMs (%tdTomato + CMs of the total cells) (border zone vs. remote area) | No |
| Lima Correa B et al. [43] | France | 6 | 6 | adult | MADM | LAD ligation | 7–9 w | No significant difference of CM proliferation between infarct border zone and remote area (% single-colored CMs of the total labeled cells) | No |
| Liu X et al. [5] | China | 10 | 10 | 8–10 w | Ki67-CrexER; R26-GFP | LAD ligation | 8 w | Increased 3–7 folds of GFP + CMs in MI compared with Sham hearts and in border regions compared with remote region (GFP + CMs %) | Yes |
| 5 | 5 | Ccna2-CrexER; R26-GFP | 6 w | ||||||
| Mohamed TMA et al. [44] | USA | 6 | 6 | 8–9 w | Myh6-cre/Esr1; MADM | LAD ligation | 2 w | Increased less than 1 fold of single-colored CMs in the MI group compared with Sham (based on the overexpression of four cell-cycle regulators) | Yes |
| Senyo SE et al. [9] | USA | 3 | 4 | adult | MerCreMer + /ZEG + | LAD ligation | 8 w | Approximately 3.2% of the CMs adjacent to the infarct zone had undergone division (total 15N + × mononucleated diploid fraction) (MI vs. Sham) | Yes |
| Sereti KI et al. [8] | USA | 6 | 6 | 8 w | Rainbow: β-actinCreER; R26VT2/GK; Nkx2.5CreER; R26VT2/GK; αMHCCreER; R26VT2/GK | LAD ligation | 3 w | MI did not activate CM proliferation in adult (border zone vs. remote area) (percent of total clones) | No |
Abbreviations: MI myocardial infarction, I/R ischemia–reperfusion, CM cardiomyocytes, MADM mosaic analysis with double markers, LAD left anterior descending artery
Interestingly, among the other 4 studies showing MI stimulated cardiomyocyte proliferation, very few showed overwhelming evidence that MI stimulated bona fide proliferation in the adult heart because of methodological shortages. A study using the Ki67-based genetic system ProTracer for continuous recording of cycling cardiomyocytes documented a 3 ~ 7 folds increase of proliferative cardiomyocytes in MI hearts compared with Sham hearts [5]. However, the Ki67-based ProTracer could only record cycling cardiomyocytes but not dividing cardiomyocytes. Three studies showed a mild increase (1 ~ 3 folds) of proliferative cardiomyocytes in MI hearts compared with Sham hearts. A study used co-registration of cardiomyocyte fluorescent labeling and [15N] thymidine labeling of DNA replication and showed that 3.2% of infarct zone cardiomyocytes initiate DNA replication and nuclear division. However, most DNA synthesis that occurs in these cardiomyocytes does not complete cell division [9]. Another study showed that overexpression of four cell-cycle regulators robustly increased adult cardiomyocyte proliferation in MADM mouse heart; the proliferation was ~ 1 fold more in infarcted heart than Sham, and the proliferation was significantly more in border zone and infarct zone than in remote zone [44]. However, the data was based on manipulation of cell-cycle regulator overexpression. The study neither describes the comparison between MI versus Sham baseline level nor describes the comparison between different regions of the post-MI heart in mice without intervention.
Sensitivity analysis
After eliminating each study, sensitivity analysis of the cell cycle markers (Ki67+, PH3+, BrdU/EdU+, and AurkB+) showed that the effects between the MI group and Sham group were still stable in general (Additional file 3: Fig. S4). Sensitivity analyses of the effects between the border zone and remote zone were stable after excluding each study (Additional file 3: Fig. S5).
Discussion
The present study analyzed 36 studies in a wide range of 8161 records. Firstly. Meta-analysis of 26 studies with cell cycle activity assays showed that MI did not significantly increase cell cycle activity compared with Sham. But in post-MI heart, cardiomyocyte cell cycle activity was higher in the infarct board zone compared with the remote zone. Secondly, among the 10 studies with genetic reporter/tracing mouse systems, 6 studies showed MI did not significantly increase new cardiomyocyte formation compared with Sham. In contrast, the other 4 studies showed MI increased it by 1 ~ 7 folds.
It is known that the proliferation efficacy of cardiomyocytes is very low in the adult normal heart. In a previous study, human samples were examined from the border zone and remote zone of 13 patients who had died 4 to 12 days after infarction. Ki67 expression was detected in 4% of cardiomyocyte nuclei in the border zone and 1% of those in the remote zone. The re-entry of cardiomyocytes into the cell cycle resulted in mitotic indexes of 0.08% and 0.03% respectively in the border zone and remote zone [45]. Since a lack of normal hearts as controls, the observation did not compare cell cycle activation between normal and MI. It showed significant differences in distribution between border zone and remote zone in post-MI heart, indicating that MI might stimulate cell cycle activation. Interestingly, another study analyzed 15 human post-MI hearts by detecting Ki67 expression, mitotic bodies, and ploidy status, which indicated that in human infarcts, the entrance of cardiomyocytes into the cell cycle is transient and that endomitosis, leading to polyploidy, rather than mitosis, leading to karyokinesis, is the final fate of cycling cells [46]. These observations in human tissues raised important questions: Does MI stimulate the bona fide proliferation of mammalian adult cardiomyocytes? Does cardiomyocyte proliferation distribute differently in the post-MI heart?
There have been discrepancies among previous studies in terms of the intrinsic level of cardiomyocyte cell cycle activity post-injury. Some studies showed that MI robustly stimulates the division of pre-existing cardiomyocytes. Combining two different pulse-chase approaches—genetic fate-mapping with stable isotope labeling and multi-isotope imaging mass spectrometry—it showed a robust increase of proliferation in the scar border region compared to sham-operated mice [9]. EdU incorporation showed that the number of EdU+ cardiomyocytes was over 10 folds compared to remote areas [38]. On the other hand, by using the MADM mouse system which allows unambiguous identification of progeny cells, it showed that MI did not increase the rate of cardiomyocyte division compared with Sham [10]. Either by using EdU incorporation or MADM mouse system, another study showed no significant difference in cardiomyocyte proliferation between infarct border zones and remote areas [43].
The conventional approaches to evaluate the proliferation of adult cardiomyocytes usually include the indicators of DNA replication and nuclear division, such as Ki67, PH3, AurkB staining, and BrdU/EdU incorporation [47]. Distinguishing cell cycle marker staining from non-specific staining or the staining of non-cardiomyocytes (such as fibroblast or inflammatory cells) can be difficult, which may lead to the potential for methodologic bias and measurement errors [44, 48]. Incomplete cell division in adult cardiomyocytes results in the formation of polyploid or multinuclear cells without any increase in cell number [49]; thus, it often mistakenly equates multinucleation with genuine cell division. Immunostaining of AurkB presents a more specific cell division but can only show a snapshot of very rare proliferating cardiomyocytes at a single time point. The efficacy of BrdU/EdU+ labeling is influenced by the parameters of its administration, such as the timing post-administration, number of injections, and dose. We analyzed the proportion of BrdU/EdU+ between the MI group and Sham group with different labeling durations. The studies with a labeling duration time < 14 days showed no difference in BrdU/EdU+ cardiomyocytes between the MI group vs. the Sham group, while the studies with a labeling time ≥ 14 days showed an increased proportion of BrdU/EdU+ cardiomyocytes between the MI and Sham groups. The administration injections and concentrations of BrdU/EdU+ labeling by different research groups might also influence the results, but it is difficult to get a statistical conclusion due to the limited number of studies in each group.
This meta-analysis and systematic review can enhance the statistical power by pooling multiple small-sample preclinical studies, the data from different labs and mixed methodologies which helps assess the effects of MI on cardiomyocyte cell-cycle reentry and proliferation. In analyzing 26 mouse studies with cell cycle activity assays, our meta-analysis showed that MI did not significantly increase cell cycle activation compared with Sham. In post-MI hearts, Ki67+ cardiomyocyte was significantly more in the infarct board zone compared with the remote zone, while other cell cycle activity indicators did not differ between the two regions. In analyzing 10 studies with genetic reporter/tracing mouse systems, 6 studies showed MI did not significantly increase cardiomyocyte proliferation compared with Sham. Experiments using the FUCCI [12] and AurkB [41] systems have shown that MI could not increase the number of cardiomyocytes.
Interestingly, among the other 4 studies, at least 3 studies could not demonstrate that MI stimulates bona fide cardiomyocyte proliferation because of methodological shortages. For example, the Ki67-based genetic system ProTracer only records cycling cardiomyocytes in adult hearts [5], since many of the Ki67-expressing cardiomyocytes in the adult heart do not necessarily undergo cell division. The finding in the Ki67-based genetic system ProTracer mouse that more accumulated Ki67+ cycling cardiomyocytes in border zone compared with the remote zone is consistent with the meta-analysis results of Ki67+ cardiomyocyte distribution in the post-MI heart.
MADM lineage tracing could unequivocally label post-cell division daughter cardiomyocytes, although it may underestimate the cell division events. In the three studies using the MADM lineage tracing system, two studies showed no difference in the frequency of single-colored cardiomyocytes. For example, when Cre was induced by sequentially daily injection of tamoxifen for 14 days post-MI, it showed MI did not increase the MADM labeled daughter cardiomyocytes compared with Sham in 56-day-old mice [10]. Only one study with the MADM system reported an increase of post-cytokinesis new cardiomyocytes in the MI heart compared with sham, but the data was based on manipulation rather than the baseline level.
There are several limitations of our study. First, although having screened all relevant literature in recent years, the final included studies in our meta-analysis potentially introduce some bias. We excluded those studies that documented a pro-proliferative effect on post-MI mice but lacked experimental control of the Sham group. Second, current labeling approaches for adult cardiomyocyte proliferation have certain methodological limitations as they may mix the true proliferation of cardiomyocytes with incomplete mitosis like polyploidy and endomitosis. Besides, separating the true cardiomyocyte-positive signals from other surrounding entangled cell signals, especially fibroblasts after myocardial injury, is difficult. Third, the different experiment protocols from different investigators may lead to data complexity.
Conclusions
In conclusion, although the “gold standard method” for determining the proliferation of adult cardiomyocytes is still lacking, this meta-analysis and systematic review try to conclude current understandings of cardiomyocyte cell cycling and proliferation in the post-MI heart. We showed that MI injury might stimulate cardiomyocyte cell cycle reentry in the adult heart, especially in infarct border zone and labeled with Ki67 expression and that very little overwhelming evidence showed that MI stimulates bona fide proliferation in adult heart.
Supplementary Information
Additional file 2: Table S1. Search strategy of PubMed/Medline; Table S2. Search strategy of EMBASE; Table S3. Search strategy of Web of Science.
Additional file 3: Fig. S1 Subgroup analysis of the proportion of cell cycle markers between MI group and Sham group in different observation time-point post-MI; Fig. S2 Subgroup analysis of the proportion of cell cycle markers between border zone and remote zone in different observation time-point post-MI; Fig. S3 Subgroup analysis of the proportion of cell cycle markers between MI group and Sham group in different mouse strains; Fig. S4 Sensitivity analysis of the proportion of cell cycle markers between MI and Sham group. Fig. S5 Sensitivity analysis of the proportion of cell cycle markers between border zone and remote zone.
Acknowledgements
The author would like to thank Prof. Zhide Hu for his assistance with statistical issues in this paper.
Abbreviations
- MI
Myocardial infarction
- P1
Postnatal day 1
- MADM
Mosaic analysis with double markers
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SD
Standard deviation
- MD
Mean difference
- ROB
Risk of bias
- SE
Standard error
- CI
Confidence interval
Authors’ contributions
Ya Liu: Original manuscript preparation, Literature search; Lingyan Liu: Data extraction and analysis, Original manuscript preparation; Pengcheng Zhuang: Literature search, Data extraction; Jiamin Zou, Xiaokang Chen, Hao Wu and Bingjun Lu: Data correction and curation; Wei Eric Wang: Supervision, Review & Editing. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Key Research & Development Program of China (2021YFA0805002) and National Natural Science Foundation of China (U20A20344).
Data availability
The data are available from the corresponding authors on reasonable requests.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All co-authors have provided consent for the final accepted version of the manuscript to be considered for publication in BMC Medicine.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ya Liu and Lingyan Liu contributed equally to this work.
References
- 1.Reuter SP, Soonpaa MH, Field D, Simpson E, Rubart-von der Lohe M, Lee HK, Sridhar A, Ware SM, Green N, Li X, et al. Cardiac troponin I-interacting kinase affects cardiomyocyte S-phase activity but not cardiomyocyte proliferation. Circulation. 2023;147(2):142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye L, D’Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, Tee GZ, Pua CJ, Pena EM, Cheng RB, et al. Early regenerative capacity in the porcine heart. Circulation. 2018;138(24):2798–808. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc. 2014;9(2):305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigaud VOC, Hoy RC, Kurian J, Zarka C, Behanan M, Brosious I, Pennise J, Patel T, Wang T, Johnson J, et al. RNA-binding protein LIN28a regulates new myocyte formation in the heart through long noncoding RNA-H19. Circulation. 2023;147(4):324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Pu W, He L, Li Y, Zhao H, Li Y, Liu K, Huang X, Weng W, Wang QD, et al. Cell proliferation fate mapping reveals regional cardiomyocyte cell-cycle activity in subendocardial muscle of left ventricle. Nat Commun. 2021;12(1):5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wan TC, Lauth A, Purdy AL, Kulik KR, Patterson M, Lough JW, Auchampach JA. Conditional depletion of the acetyltransferase Tip60 protects against the damaging effects of myocardial infarction. J Mol Cell Cardiol. 2022;163:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121(3):479–92. [DOI] [PubMed] [Google Scholar]
- 8.Sereti KI, Nguyen NB, Kamran P, Zhao P, Ranjbarvaziri S, Park S, Sabri S, Engel JL, Sung K, Kulkarni RP, et al. Analysis of cardiomyocyte clonal expansion during mouse heart development and injury. Nat Commun. 2018;9(1):754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111(24):8850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed). 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez R Jr, Wang BJ, Quijada PJ, Avitabile D, Ho T, Shaitrit M, Chavarria M, Firouzi F, Ebeid D, Monsanto MM, et al. Cardiomyocyte cell cycle dynamics and proliferation revealed through cardiac-specific transgenesis of fluorescent ubiquitinated cell cycle indicator (FUCCI). J Mol Cell Cardiol. 2019;127:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auchampach J, Han L, Huang GN, Kühn B, Lough JW, O’Meara CC, Payumo AY, Rosenthal NA, Sucov HM, Yutzey KE, et al. Measuring cardiomyocyte cell-cycle activity and proliferation in the age of heart regeneration. Am J Physiol Heart Circ Physiol. 2022;322(4):H579-h596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad F, Lal H, Zhou J, Vagnozzi RJ, Yu JE, Shang X, Woodgett JR, Gao E, Force T. Cardiomyocyte-specific deletion of Gsk3α mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. J Am Coll Cardiol. 2014;64(7):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez-Arabaolaza I, Dang Z, Mitchell K, et al. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116(10):e81-94. [DOI] [PubMed] [Google Scholar]
- 18.Cai B, Ma W, Wang X, Sukhareva N, Hua B, Zhang L, Xu J, Li X, Li S, Liu S, et al. Targeting LncDACH1 promotes cardiac repair and regeneration after myocardium infarction. Cell Death Differ. 2020;27(7):2158–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Li H, Zhang W, Wang X, Sun H, Cao Y, Zhao Y, Ji H, Yang F, Ma W, et al. Photobiomodulation drives MiR-136-5p expression to promote injury repair after myocardial infarction. Int J Biol Sci. 2022;18(7):2980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong R, Wang X, Li H, Liu S, Jiang Z, Zhao Y, Yu Y, Han Z, Yu Y, Dong C, et al. Loss of m(6)A methyltransferase METTL3 promotes heart regeneration and repair after myocardial injury. Pharmacol Res. 2021;174:105845. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z, Chen P, Wang L, Zhu Y, Chen G, Chen Y, Hu Z, Mei L, You W, Cong W, et al. FGF6 promotes cardiac repair after myocardial infarction by inhibiting the Hippo pathway. Cell Prolif. 2022;55(5):e13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Yang M, Tan J, Shen C, Deng S, Fu X, Gao S, Li H, Zhang X, Cai W. Targeting ACSL1 promotes cardiomyocyte proliferation and cardiac regeneration. Life Sci. 2022;294:120371. [DOI] [PubMed] [Google Scholar]
- 23.Magadum A, Ding Y, He L, Kim T, Vasudevarao MD, Long Q, Yang K, Wickramasinghe N, Renikunta HV, Dubois N, et al. Live cell screening platform identifies PPARδ as a regulator of cardiomyocyte proliferation and cardiac repair. Cell Res. 2017;27(8):1002–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy R, Leigh T, Gao E, Zhang X, Tian Y: Activation or inhibition of PPARα-mediated fatty acid β-oxidation does not active cardiomyocyte proliferation in normal or infarcted adult mice. 2019:667964.
- 25.Ruchaya PJ, Lewis-McDougall FC, Sornkarn N, Amin S, Grimsdell B, Shaalan A, Gritti G, Soe KT, Clark JE, Ellison-Hughes GM. Transplantation of skeletal muscle-derived Sca-1(+)/PW1(+)/Pax7(-) interstitial cells (PICs) improves cardiac function and attenuates remodeling in mice subjected to myocardial infarction. Cells. 2022;11(24):4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells. 2014;32(9):2397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan W, Lin C, Guo Y, Chen Y, Du Y, Lau WB, Xia Y, Zhang F, Su R, Gao E, et al. N-Cadherin overexpression mobilizes the protective effects of mesenchymal stromal cells against ischemic heart injury through a β-catenin-dependent manner. Circ Res. 2020;126(7):857–74. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Fu W, Li L, Xia X, Liao Q, Yue R, Chen H, Chen X, An S, Zeng C, et al. Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction. Clin Sci (Lond). 2017;131(24):2919–32. [DOI] [PubMed] [Google Scholar]
- 29.Yifa O, Weisinger K, Bassat E, Li H, Kain D, Barr H, Kozer N, Genzelinakh A, Rajchman D, Eigler T, et al. The small molecule Chicago Sky Blue promotes heart repair following myocardial infarction in mice. JCI Insight. 2019;4(22):e128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Gago-Lopez N, Li N, Zhang Z, Alver N, Liu Y, Martinson AM, Mehri A, MacLellan WR. Single-cell imaging and transcriptomic analyses of endogenous cardiomyocyte dedifferentiation and cycling. Cell Discov. 2019;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boogerd CJ, Perini I, Kyriakopoulou E, Han SJ, La P, van der Swaan B, Berkhout JB, Versteeg D, Monshouwer-Kloots J, van Rooij E. Cardiomyocyte proliferation is suppressed by ARID1A-mediated YAP inhibition during cardiac maturation. Nat Commun. 2023;14(1):4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–38. [DOI] [PubMed] [Google Scholar]
- 33.Fan Y, Cheng Y, Li Y, Chen B, Wang Z, Wei T, Zhang H, Guo Y, Wang Q, Wei Y, et al. Phosphoproteomic analysis of neonatal regenerative myocardium revealed important roles of checkpoint kinase 1 via activating mammalian target of rapamycin C1/ribosomal protein S6 kinase b-1 pathway. Circulation. 2020;141(19):1554–69. [DOI] [PubMed] [Google Scholar]
- 34.Fang W, He A, Xiang MX, Lin Y, Wang Y, Li J, Yang C, Zhang X, Liu CL, Sukhova GK, et al. Cathepsin K-deficiency impairs mouse cardiac function after myocardial infarction. J Mol Cell Cardiol. 2019;127:44–56. [DOI] [PubMed] [Google Scholar]
- 35.Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364(6436):184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma WY, Song RJ, Xu BB, Xu Y, Wang XX, Sun HY, Li SN, Liu SZ, Yu MX, Yang F, et al. Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway. Acta Pharmacol Sin. 2021;42(6):921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbán E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5(2):191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, et al. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation. 2017;136(9):834–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Wan TC, Kulik KR, Lauth A, Smith BC, Lough JW, Auchampach JA. Pharmacological inhibition of the acetyltransferase Tip60 mitigates myocardial infarction injury. Dis Model Mech. 2023;16(5):dmm049786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley LA, Young A, Li H, Billcheck HO, Wolf MJ. Loss of endogenously cycling adult cardiomyocytes worsens myocardial function. Circ Res. 2021;128(2):155–68. [DOI] [PubMed] [Google Scholar]
- 41.Fu W, Liao Q, Li L, Shi Y, Zeng A, Zeng C, Wang WE. An Aurora kinase B-based mouse system to efficiently identify and analyze proliferating cardiomyocytes. Front Cell Dev Biol. 2020;8:570252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kretzschmar K, Post Y, Bannier-Hélaouët M, Mattiotti A, Drost J, Basak O, Li VSW, van den Born M, Gunst QD, Versteeg D, et al. Profiling proliferative cells and their progeny in damaged murine hearts. Proc Natl Acad Sci U S A. 2018;115(52):E12245-e12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima Correa B, El Harane N, Desgres M, Perotto M, Alayrac P, Guillas C, Pidial L, Bellamy V, Baron E, Autret G, et al. Extracellular vesicles fail to trigger the generation of new cardiomyocytes in chronically infarcted hearts. Theranostics. 2021;11(20):10114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173(1):104-116.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750–7. [DOI] [PubMed] [Google Scholar]
- 46.Meckert PC, Rivello HG, Vigliano C, González P, Favaloro R, Laguens R. Endomitosis and polyploidization of myocardial cells in the periphery of human acute myocardial infarction. Cardiovasc Res. 2005;67(1):116–23. [DOI] [PubMed] [Google Scholar]
- 47.Broughton KM, Sussman MA. Adult cardiomyocyte cell cycle detour: off-ramp to quiescent destinations. Trends Endocrinol Metab. 2019;30(8):557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hesse M, Raulf A, Pilz GA, Haberlandt C, Klein AM, Jabs R, Zaehres H, Fügemann CJ, Zimmermann K, Trebicka J, et al. Direct visualization of cell division using high-resolution imaging of M-phase of the cell cycle. Nat Commun. 2012;3:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157(4):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Table S1. Search strategy of PubMed/Medline; Table S2. Search strategy of EMBASE; Table S3. Search strategy of Web of Science.
Additional file 3: Fig. S1 Subgroup analysis of the proportion of cell cycle markers between MI group and Sham group in different observation time-point post-MI; Fig. S2 Subgroup analysis of the proportion of cell cycle markers between border zone and remote zone in different observation time-point post-MI; Fig. S3 Subgroup analysis of the proportion of cell cycle markers between MI group and Sham group in different mouse strains; Fig. S4 Sensitivity analysis of the proportion of cell cycle markers between MI and Sham group. Fig. S5 Sensitivity analysis of the proportion of cell cycle markers between border zone and remote zone.
Data Availability Statement
The data are available from the corresponding authors on reasonable requests.