Abstract
Fifteen healthy adult volunteers received in their drinking water a lower Escherichia coli phage T4 dose (103 PFU/ml), a higher phage dose (105 PFU/ml), and placebo. Fecal coliphage was detected in a dose-dependent way in volunteers orally exposed to phage. All volunteers receiving the higher phage dose showed fecal phage 1 day after exposure; this prevalence was only 50% in subjects receiving the lower phage dose. No fecal phage was detectable a week after a 2-day course of oral phage application. Oral phage application did not cause a decrease in total fecal E. coli counts. In addition, no substantial phage T4 replication on the commensal E. coli population was observed. No adverse events related to phage application were reported. Serum transaminase levels remained in the normal range, and neither T4 phage nor T4-specific antibodies were observed in the serum of the subjects at the end of the study. This is, to our knowledge, the first safety test in the recent English literature which has measured the bioavailability of oral phage in humans and is thus a first step to the rational evaluation of phage therapy for diarrheal diseases.
Antibiotic treatment of Escherichia coli diarrhea is frequently problematic, which raises interest in alternative approaches. Felix d'Hérelle, the codiscoverer of phages, advocated the idea of exploiting the lytic effect of phages on bacteria for therapeutic purposes. Phage therapy has a colorful history but became a common therapy for intestinal and skin infections only in the Soviet Union (17). Currently, we see a renewed interest in phage therapy (13). The present study describes the oral administration of phages to human volunteers and the subsequent clinical and microbiological analyses. This safety test is a follow-on from ecology studies of T4-like phages isolated from the stools of pediatric diarrhea patients (7), the analysis of their genomes (6), and their behavior in experimental animals (8).
MATERIALS AND METHODS
Subjects.
Fifteen healthy adult volunteers between 23 and 54 years of age (six women and nine men) were recruited from the personnel at the Nestlé Research Center. Their heights ranged from 150 to 187 cm, and their weights ranged from 56 to 85 kg (body mass index range, 21.4 to 32.1 kg/m2). All subjects were Caucasians. Exclusion criteria for enrollment were immunosuppression, gastric problems, raised serum transaminase levels, antibiotic treatment during the preceding 4 weeks, laxative use, pregnancy, and participation in other trials. The protocol was approved by the local ethical committee, and the participants provided written consent.
Study design.
The study was designed as a single-center, randomized, and placebo-controlled study. The trial was a double-blinded, three-period crossover comparison of two dosages of oral T4 phage conducted in June 2003 at our research center. Each subject received a higher phage dose (dose A, with 105 PFU/ml), a lower phage dose (dose B, with 103 PFU/ml), and placebo (dose C). The subjects were randomly assigned to one of the following treatment sequences: ABC, BCA, and CAB. The vehicle was 150 ml of mineral water (Vittel; pH 7.3; HCO3−, 258 mg/liter). For safety concerns, our medical advisor asked for the use of a lower phage dose in our first human safety trial.
The study was divided into four 1-week intervals. The first week served as the baseline, during which two random stool samples were taken. In the second week the subjects were randomized and received 150 ml of the allotted mineral water three times per day for 2 consecutive days (days 1 and 2), followed by 5 consecutive days without the test mineral water (washout). This procedure was repeated for 3 consecutive weeks. During the study period the subjects provided all stool samples produced each day. The code was broken only after the complete acquisition of clinical and laboratory data. A clinical examination by a physician was done at the start (day 0) and at the end (day 30) of the study, and the volunteers received forms on which they could report any type of adverse events.
Phage preparation.
Hershey medium was inoculated with E. coli K803, a strain K-12 derivative lacking bacteriophage lambda prophage, and was then infected with bacteriophage T4 (obtained from C. Georgopoulos, Geneva University, Geneva, Switzerland) at 37°C. The completely lysed culture was centrifuged at 4,000 × g for 15 min to remove bacterial debris. In parallel, mock-infected K803 was treated with lysozyme and then sonicated. The supernatants were filtered through a 0.22-μm-pore-size Millipore filter. The phage was pelleted from the medium by centrifugation (35,000 × g for 25 min), resuspended in 0.5 ml, and then diluted to the desired titer with mineral water. Phage solutions were kept at 4°C, and no decrease in the phage titer was observed during the 1-month study period in the plaque assay.
Polyacrylamide gel electrophoresis, followed by silver staining, revealed in the resuspended T4 phage pellet no proteins which comigrated with proteins from the “phage” preparation of the mock-infected K803 cell. Negative-staining electron microscopy of the phage pellet showed a relatively pure phage fraction only minimally contaminated with small cellular debris. In the Limulus test (E-toxate kit; Sigma), 2 μg endotoxin/ml was detected in the phage pellet; 2 μg carbohydrate/ml was measured as described by Dubois et al. (9). No viable bacteria were detected in the phage preparation on Drigalski agar.
Laboratory evaluation.
Stools were stored at 4°C immediately after defecation and were analyzed at the latest on the following working day. We verified that phage titers did not decrease after overnight storage. Five grams of each individual stool specimen was resuspended in 10 ml of TS (8.5 g/liter NaCl, 1 g/liter tryptone), homogenized on a Vortex agitator, and cleared by centrifugation at 4,000 × g for 15 min. One milliliter of the supernatant was filtered through a Millex AP20 prefilter and through a 0.45-μm-pore-size Minisart filter. Fecal phage and E. coli were counted as described previously (8). T4-specific antibodies were tested by immunoglobulin G (IgG), IgM, and IgA isotype-specific enzyme-linked immunosorbent assay (ELISA), performed as described previously (4).
RESULTS
Safety.
All treatments were well tolerated. Five mild adverse events were reported. Four were related to the gut (stomach pain, nausea, increased peristalsis), and one volunteer reported a sore throat. None of the gut-related events were severe enough to necessitate treatment. Adverse events were not reported more often with the higher phage dose than with the lower phage dose or placebo and were rated by the study physician as unrelated to the study intervention.
Normal serum alanine aminotransferase and aspartate aminotransferase levels were measured by an accredited clinical laboratory before and after the study. No significant increases in the levels of these two liver enzymes were observed. At the beginning and at the end of the 1-month study period, the plasma samples from all 15 volunteers were negative for T4 phage in the plaque assay (detection limit, 10 PFU/ml), and no volunteer showed an increase in optical density by the IgG-, IgM-, or IgA-specific ELISA with T4 phage coated on the plate (data not shown).
Fecal phage excretion.
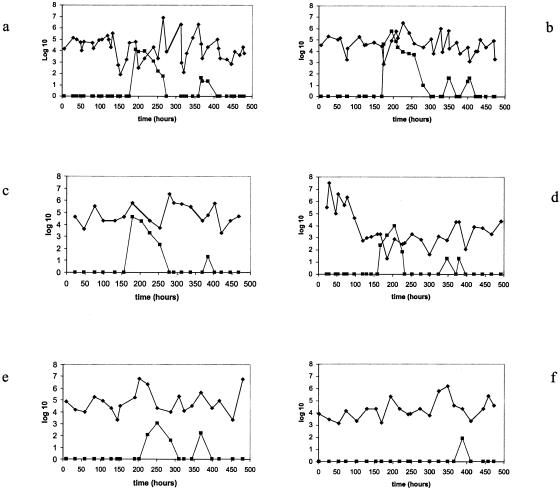
During the baseline evaluation, none of the 15 volunteers excreted coliphage in their stool samples, which could be detected on the K803 indicator. In contrast, 15 of 15 subjects showed fecal phage when they drank the mineral water with the higher phage dose. We did not verify the identity of the orally ingested and fecally excreted phage beyond its ability to grow on the indicator strain K803. Thirteen of 15 subjects demonstrated at least one phage-positive stool sample when they were exposed to the lower phage dose, while none of the volunteers excreted phage when they were receiving the placebo (Fig. 1a to e). The mean number of phage-positive stool samples differed significantly between the two treatment groups: 4.4 and 1.3 positive stool samples were observed for the 15 volunteers during the higher and lower phage dose applications, respectively. Two subjects showed fecal phage only when they received the high dose (Fig. 1f).
FIG. 1.
Fecal phage and E. coli counts in the five individual volunteers receiving the products in the sequence placebo-higher phage dose-lower phage dose during the study period (time in hours after start of treatment) (panels a to e). (f) Excretion pattern of a possible “low phage responder” receiving the sequence lower phage dose-placebo-higher phage dose. The fecal phage titers (expressed as log10 PFU/g stool) are represented by squares, whereas the fecal E. coli counts (expressed as log10 CFU/g stool) are depicted with diamonds.
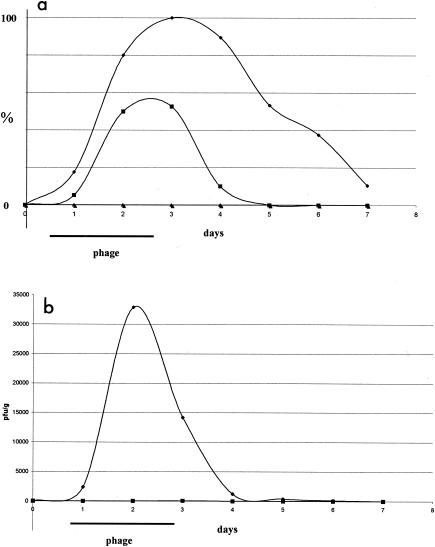
Figure 2a presents the time course of phage prevalence in the stool samples for all subjects. In both the group receiving the higher phage dose and the group receiving the lower phage dose, all stool samples were phage negative on the day before oral phage exposure. On the first day of phage exposure, less than 20% of the subjects were phage positive. On the second day of exposure to oral phage, the prevalence increased to 80 and 50% for the higher and the lower phage dose groups, respectively. In the group receiving the higher phage dose, the stool phage prevalence rose to 100% on the third day, when the volunteers were no longer receiving oral phage; remained at 90% on the following day; and dropped only slowly over the next few days, with 38% phage-positive stools 3 days after the cessation of oral phage application. A different type of kinetics was seen in the group receiving the lower phage dose. A 50% prevalence was still maintained on the day after phage exposure, but it then dropped off very quickly. In both groups the oral phage took approximately 1 day to reach maximal prevalence in the feces. In the group receiving the lower phage dose, the time for the washout of oral phage was again approximately 1 day, reflecting a passive transit of the phage through the gut.
FIG. 2.
Gastrointestinal phage transit in human volunteers. Fifteen adult volunteers received the lower phage T4 dose (squares), the higher phage T4 dose (diamonds), and no phage (triangles) in their drinking water for 2 days (the treatment period is marked by a horizontal bar). The stool samples were tested for the presence of phages starting 1 day preceding the intervention. (a) The prevalence (in percent) of phage-positive stool samples; (b) the time development of the mean phage titer in the feces of the volunteers (expressed as PFU/g stool). Each datum point represents the results from all 15 volunteers. The mean titers for the recipients of the lower phage dose were 17 and 68 at days 2 and 3, respectively; they are too small to be visible at the chosen scale.
Figure 2b presents the mean fecal phage titers. The highest titers were found on the second day after the subjects drank the mineral water with the higher phage dose, but significant titers were still detected in the stools 1 day after the subjects drank the last phage dose. Only very low mean fecal phage titers were seen in volunteers receiving the lower phage dose; the peak mean titer was 68; i.e., it was 500-fold lower than the peak mean titer in those receiving the higher phage dose. The phage titers in the daily stool samples describe a time profile. The area under the curve of this time profile is the total amount of surviving T4 phage and was statistically analyzed as the primary outcome. The difference between the groups receiving the higher and the lower phage doses was statistically significant (P < 0.0001 in a linear mixed model, P = 0.003 by the Kruskal-Wallis test).
Fecal E. coli count.
Each stool sample was evaluated for its bacterial count on Drigalski agar. We determined previously that the vast majority of the fecal colonies observed on this medium are E. coli (8). The E. coli counts for volunteers who were exposed to the sequence placebo-higher phage dose-lower phage dose are shown in Fig. 1a to e. There were substantial variations in these counts between subjects and within the same subject. However, none of the subjects showed a significant decrease in fecal E. coli counts that was associated in time with application of the higher phage dose.
The fecal E. coli counts in the daily stool samples describe a time profile. The area under the curve of this time profile is the total amount of excreted fecal E. coli and was statistically analyzed as the secondary outcome. The analysis was performed by using a linear mixed-effect model (with Bonferroni's correction). No differences were detected between the treatment and the placebo groups (P > 0.99).
Random E. coli colonies from the 15 volunteers were tested for their susceptibilities to phage T4 before and after the 3-week intervention period. Before the intervention, 12 of 168 colonies (7.1%) were susceptible to T4 phage, while after the intervention only 1 of 116 colonies (0.9%) lysed in the presence of T4 phage.
DISCUSSION
One might question the use of phage T4 in a safety trial for phage therapy since T4 has only a narrow host range on E. coli. In fact, when T4 was tested for its lytic properties on a collection of 42 E. coli strains representing 23 different somatic O antigens, 10 different capsular K antigens, and 10 distinct H antigens (8), only 2 strains were lysed in the cell tube test (S. Chibani-Chennoufi, unpublished results). T4 is thus not a likely candidate for phage therapy but was chosen as a surrogate for a first safety trial because it has been completely sequenced and extensively characterized (12). Recent genome analysis (16) and 40 years of genetic research with T4 have not identified virulence genes on its chromosome. In that respect, T4 differs clearly from lambda-like phages, which frequently carry virulence genes (2, 3). This difference between T4 and lambda phages is perhaps not surprising since T4 is the paradigm of an obligate lytic phage. Evolutionary reasoning suggests that it is the coexistence of phage and bacterium in lysogeny which selects for prophage genes that are of survival benefit to the bacterial host (5, 14).
In this small trial of oral phage use, no significant adverse events were observed and the treatments were well tolerated. Inchley (11) showed that Kupffer cells of the liver phagocytized more than 99% of labeled T4 phage within 30 min after intravenous injection. The release of liver enzymes should thus be a sensitive measure of liver toxicity from oral phages. However, the serum levels of two liver transaminases did not increase with the intervention. This observation is not so surprising, since with the low phage doses used, no transit of the oral phage into the bloodstream was observed. Mice fed a much higher oral dose of 1012 PFU of phage lambda showed only between 10 and 1,000 PFU per ml of blood directly after exposure (10). Within 10 h this phage was removed from the circulation. Likewise, phage lambda injected into the bloodstream of mice was also quickly cleared in the spleen (10, 15). However, clinical trials conducted in Eastern Europe showed that oral phage enters the bloodstream (1, 18). Yet, the absence of an anti-T4 immune response in our volunteers suggests that no substantial amounts of T4 phage ever appeared in their circulation. The ELISA protocol was sensitive enough to measure the serum antibody response 2 weeks after primary exposure of humans to oral rotavirus (4).
The fecal phage counts demonstrated a dose-dependent appearance of the orally applied phage. While they were on the “high” dose, the volunteers received a total of 9 × 107 PFU. Approximately 107 PFU phage was excreted into the stool, as calculated from the mean fecal phage titers and the estimated stool volumes. This value is strikingly close to the orally applied phage number, suggesting a remarkable stability of the phage during gastrointestinal transit and only small, if any, phage replication during the gut transit. This interpretation concurs with the maintenance of the total fecal E. coli counts. However, the possibility of destruction of the phage in the stomach, followed by intestinal replication of the oral phage, cannot formally be excluded by the current data. This interpretation is, however, less likely in view of previous experiments with germfree mice (8).
It is clear that the present study is only a first step in the assessment of the safety of oral phages. Higher phage titers with a phage cocktail covering multiple E. coli serotypes need to be tested. It will also be necessary to assess in more detail the effect of phage on the composition of the commensal E. coli gut microbiota.
Acknowledgments
We thank our colleagues at the Nestle Research Center who served as volunteers, A. Blondel and S. Oguey-Araymon for the daily care of the volunteers at our Metabolic Unit, J. Sidoti for technical assistance, A. de Batz and D. Grathwohl for the statistical analysis, A. Constable for critical reading of the report, and finally, Marianna Giarre for the elaboration of the study master file.
REFERENCES
- 1.Babalova, E. G., K. T. Katsitadze, L. A. Sakvarelidze, N. S. Imnaishvili, T. G. Sharashidze, V. A. Badashvili, G. P. Kiknadze, A. N. Meipariani, N. D. Gendzekhadze, E. V. Machavariani, K. L. Gogoberidze, E. I. Gozalov, and N. G. Dekanosidze. 1968. Preventive value of dried dysentery bacteriophage. Zh. Mikrobiol. Epidemiol. Immunobiol. 45:143-145. (In Russian.) [PubMed] [Google Scholar]
- 2.Boyd, E. F., and H. Brüssow. 2002. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 10:521-529. [DOI] [PubMed] [Google Scholar]
- 3.Brüssow, H., C. Canchaya, and W. D. Hardt. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüssow, H., H. Werchau, W. Liedtke, L. Lerner, C. Mietens, J. Sidoti, and J. Sotek. 1988. Prevalence of antibodies to rotavirus in different age-groups of infants in Bochum, West Germany. J. Infect. Dis. 157:1014-1022. [DOI] [PubMed] [Google Scholar]
- 5.Canchaya, C., C. Proux, G. Fournous, A. Bruttin, and H. Brüssow. 2003. Prophage genomics. Microbiol. Mol. Biol. Rev. 67:238-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chibani-Chennoufi, S., C. Canchaya, A. Bruttin, and H. Brüssow. 2004. Comparative genomics of the T4-like Escherichia coli phage JS98: implications for the evolution of T4 phages. J. Bacteriol. 186:8276-8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibani-Chennoufi, S., J. Sidoti, A. Bruttin, M. L. Dillmann, E. Kutter, F. Qadri, S. A. Sarker, and H. Brüssow. 2004. Isolation of Escherichia coli bacteriophages from the stool of pediatric diarrhea patients in Bangladesh. J. Bacteriol. 186:8287-8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chibani-Chennoufi, S., J. Sidoti, A. Bruttin, E. Kutter, S. Sarker, and H. Brüssow. 2004. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob. Agents Chemother. 48:2558-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 10.Geier, M. R., M. E. Trigg, and C. R. Merril. 1973. Fate of bacteriophage lambda in non-immune germ-free mice. Nature 246:221-223. [DOI] [PubMed] [Google Scholar]
- 11.Inchley, C. J. 1969. The activity of mouse Kupffer cells following intravenous injection of T4 bacteriophage. Clin. Exp. Immunol. 5:173-187. [PMC free article] [PubMed] [Google Scholar]
- 12.Karam, J. D. 1994. Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 13.Kutter, E., and A. Sulakvelidze. 2005. Bacteriophages: biology and applications. CRC Press, Inc., Boca Raton, Fla.
- 14.Lawrence, J. G., R. W. Hendrix, and S. Casjens. 2001. Where are the pseudogenes in bacterial genomes? Trends Microbiol. 9:535-540. [DOI] [PubMed] [Google Scholar]
- 15.Merril, C. R., B. Biswas, R. Carlton, N. C. Jensen, G. J. Creed, S. Zullo, and S. Adhya. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, E. S., E. Kutter, G. Mosig, F. Arisaka, T. Kunisawa, and W. Ruger. 2003. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67:86-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber-Dabrowska, B., M. Dabrowski, and S. Slopek. 1987. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch. Immunol. Ther. Exp. (Warsaw) 35:563-568. [PubMed] [Google Scholar]