Abstract
873140 is a novel CCR5 antagonist with potent in vitro anti-human immunodeficiency virus (HIV) activity. This study was a double-blind, randomized, placebo-controlled, single- and repeat-dose escalation investigation of the safety, pharmacokinetics, and food effect of 873140 in 70 adult subjects. During single-dose escalation, three cohorts (each composed of 10 subjects, with 8 subjects receiving the active drug and 2 subjects receiving the placebo [8 active and 2 placebo]) received doses of 50, 200, 400, 800, and 1,200 mg after an overnight fast, or 400 mg plus a standard high-fat breakfast in an alternating panel design. During repeat-dose escalation, four cohorts (each with 8 active and 2 placebo) received doses of 200, 400, 600, or 800 mg every 12 h (BID) for 8 days. Laboratory safety tests, vital signs, and electrocardiograms (ECGs) were performed at regular intervals, and blood samples were obtained for pharmacokinetics. Single and repeat doses of 50 mg to 800 mg were well tolerated, with no serious adverse events and no grade 3 or 4 adverse events. The mild-to-moderate side effects were primarily gastrointestinal and included abdominal cramping, nausea, and diarrhea. No specific trends in laboratory parameters or clinically significant ECG changes were noted. Plasma 873140 concentrations increased rapidly; the median time to maximum concentration of drug in serum was 1.75 to 5 h. The median area under the plasma concentration-time profile (AUC) and the maximum concentration of drug in serum (Cmax) ranged from 127 ng · h/ml and 24 ng/ml at 200 mg BID to 329 ng · h/ml and 100 ng/ml at 800 mg BID, respectively. Food consumption increased the AUC and Cmax by a mean of 1.7- and 2.2-fold, respectively. The pharmacokinetic and safety profile supports the continued investigation of 873140 with HIV-infected subjects.
Despite an improvement in morbidity and mortality attributable to highly active antiretroviral therapy (HAART), the emergence of multiclass drug-resistant strains and the problematic toxicities associated with HAART warrant the development of new classes of therapies for human immunodeficiency virus (HIV) infection. Most of the currently approved antiretroviral drugs are targeted toward the inhibition of viral enzymes. The identification of the HIV coreceptors CC chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4) on the cell surface has resulted in an improved understanding of viral entry and fusion. These discoveries have led to promising new targets for drug development (7).
873140 is a CCR5 antagonist in Phase 2 clinical development as a viral entry inhibitor for the treatment of HIV infection. 873140 has potent in vitro antiviral activity, with a 50% inhibitory concentration (IC50) against CCR5-tropic HIV type 1 (HIV-1) in the subnanomolar range (J. Demarest, S. Shibayama, R. Ferris, C. Vavro, M. St Clair, and L. Boone, XV Int. AIDS Conf., abstr. WeOrA1231, 2004). 873140 selectively inhibits MIP1α and MIP1β binding in the 2 to 10 nM range and does not cause internalization of the receptor (5). 873140 demonstrates a slow receptor offset rate with the half-life of dissociation from receptor exceeding 150 h in vitro (J. Demarest, S. Sparks, C. Watson, C. McDanal, S. Shibayama, and T. Kenakin, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-211, 2004).
Preclinical pharmacokinetic and in vitro metabolism and protein binding studies have been conducted. Following single intravenous administration to rats and monkeys, 873140 has a moderate clearance (averaging ∼50% of liver blood flow) and a rapid terminal elimination half-life ranging from 0.4 to 1.6 h. Oral bioavailability of various 837140 formulations in rats and monkeys ranged from 3 to 30%. 873140 is extensively metabolized to multiple oxidative and glucuronide metabolites in rat and monkey and in vitro studies with human liver microsomes and recombinant CYP450 enzymes systems suggest that 873140 is predominantly metabolized by CYP3A4. 873140 binding in human plasma is at 93%, and in vitro cell culture studies showed that 873140 was a P-glycoprotein substrate.
The properties described above suggest that 873140 could have therapeutic utility as an HIV entry inhibitor. The purpose of this study was to evaluate the pharmacokinetics and safety of 873140 following single and repeat doses in healthy adult subjects.
(These data were presented in part at the 11th Conference on Retroviruses and Opportunistic Infections, San Francisco, Calif., February 2004.)
MATERIALS AND METHODS
Study design.
A double-blind, randomized, placebo-controlled single and repeat oral dose escalation study was conducted with healthy male and female subjects. Doses were selected based on allometric scaling of preclinical pharmacokinetic data and consideration of toxicology of no-observed-adverse-effect-level exposures. 873140 tablets were available in 10-mg and 100-mg strengths. The study design is shown in Table 1. The single-dose escalation phase had an alternating panel design and included three cohorts with 10 subjects each (with eight subjects receiving the active drug and two subjects receiving the placebo [8 active and 2 placebo]); subjects received doses of 50, 200, 400, 800, or 1,200 mg after a 10-hour fast or 400 mg with a standard high-fat breakfast (869 cal; 53% fat, 32.1 g protein, 70.2 g carbohydrate, and 51.1 g fat). Subjects consumed the meal within 25 min, and 873140 was administered within 5 min of meal completion. Subjects were admitted to the research unit the evening prior to dosing and remained there until the 48-h postdose study assessments were completed. Vital signs, electrocardiograms (ECGs), laboratory tests, and serial blood samples for 873140 plasma concentrations were collected prior to dosing and through 24 h postdose.
TABLE 1.
Study design
| Regimen and cohorta | Dosing schedule |
|---|---|
| Single-dose escalation | |
| A | 50 mg for period 1; 800 mg for period 2 |
| B | 200 mg for period 1; 1,200 mg for period 2 |
| C | 400 mg for period 1; 400 mg + food for period 2 |
| Repeat-dose escalation | |
| D | 200 mg on day 1; 200 mg BID on days 2 to 8 |
| E | 400 mg on day 1; 400 mg BID on days 2 to 8 |
| F | 600 mg on day 1; 600 mg BID on days 2 to 8 |
| G | 800 mg on day 1; 800 mg BID on days 2 to 8 |
For each cohort, n = 10 (8 active and 2 placebo).
During the repeat-dose escalation phase, four cohorts (each with 8 active and 2 placebo) received doses of 200, 400, 600, or 800 mg as a single dose on day 1 and then every 12 h (BID) on days 2 to 8. The morning dose was administered after a 10-h fast, and fasting was maintained for 4 h after dosing; the evening dose was administered approximately 3 h after the evening meal. Vital signs, ECGs, and laboratory tests were obtained throughout the 8-day period. Serial blood samples for 873140 plasma concentrations were collected prior to dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 16, and 24 h postdose on day 1 and prior to dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, and 12 h following the morning dose on day 8. In addition, blood samples for trough plasma concentrations were obtained immediately prior to the morning dose on days 3, 4, 5, 6, and 7. Samples were processed within 1 h of collection, and plasma was stored at −20°C.
All subjects provided written informed consent, and the protocol was approved by the Institutional Review Board of Covance in Madison, WI.
Bioanalytical methods.
873140 was extracted from 50 μl of plasma using 150 μl of acetonitrile containing a stable isotopically labeled internal standard, [2H4]873140. The sample was briefly mixed and centrifuged, and 100 μl of the supernatant was added to 100 μl 0.1% formic acid. A small aliquot of the extracted sample was injected into a 50- by 2.1-mm (inside diameter) Polaris C18 3-μm column. The mobile phase consisted of two eluents, mobile phase A (0.1% formic acid in water) and mobile phase B (acetonitrile). 873140 was eluted from the column using a fast gradient at a flow rate of 0.65 ml/min. Starting at time zero, the mobile phase changes from 70/30 A:B to 40/60 A:B over 1 min. A Sciex API4000 with a turbo-ion spray source was used to monitor the precursor to product ion transition (m/z, 578 to 227) to ensure high selectivity of the compound. A calibration plot of analyte/internal standard peak area ratio versus 873140 concentration was constructed, and a weighted 1/x2 linear regression was applied to the data. Concentrations of 873140 in study samples and quality control samples at concentrations of 5, 75, and 850 ng/ml were determined from the calibration line. The calibration range of the method was 1 to 1,000 ng/ml.
Performance of the method, as assessed from 873140 determination in quality control samples, showed that the average within-run precision (coefficient of variation) was less than or equal to 5.5% and the between-run precision (coefficient of variation) was less than or equal to 0.8%.
Pharmacokinetic analyses.
A noncompartmental pharmacokinetic analysis of concentration-time data was performed by standard methods with WinNonlin Professional Version 4.1 (Pharsight Corporation, Mountain View, CA). Plasma pharmacokinetic parameters were calculated for each treatment, when possible, as follows: for the single-dose escalation phase, the area under the plasma concentration-time profile from time zero to the last measurable concentration [AUC(0-last)], the area under the plasma concentration-time profile extrapolated to infinity [AUC(0-∞)], the maximum observed plasma concentration (Cmax), the time of maximum observed plasma concentration (Tmax), the terminal elimination phase rate constant (λz), the terminal elimination phase half-life (t1/2), the apparent oral clearance (CL/F), and the observed time point immediately prior to the first quantifiable concentration (Tlag); for day 1 of the repeat-dose escalation phase, AUC(0-last), area under the plasma concentration-time profile from 0 to 12 h postdose [AUC(0-12)], AUC(0-∞), Cmax, Tmax, λz, t1/2, CL/F, and Tlag; and for day 8 of the repeat-dose escalation phase, AUC(0-12), Cmax, Tmax, C12, λz, t1/2, CL/F, and Tlag. Actual elapsed time from dosing was used to estimate all individual pharmacokinetic properties. The AUC was determined using the linear-up-log-down trapezoidal rule. The λz was estimated using only those data points judged to describe the terminal log-linear decline. The λz and other parameters that rely on λz [e.g., t1/2 and AUC(0-∞)] were accepted only if a minimum of three data points were used to estimate λz, and the duration of time over which λz was estimated was at least twice the subsequently estimated t1/2.
Statistical analyses.
Descriptive statistics, including geometric mean and 95% confidence intervals, were calculated for all pharmacokinetic parameters and summarized by treatment. The effect of food on the pharmacokinetics of a single 400-mg dose of GW873140 was evaluated for subjects with pharmacokinetic parameters during both the fasted and fed periods (n = 8) by analysis of variance (ANOVA), with subject as a random effect and treatment as a fixed effect. The impact of food was estimated by the ratio of geometric least square (GLS) means for AUC(0-∞) and Cmax and the corresponding 90% confidence intervals (CI). Dose proportionality of 873140 AUC(0-∞) and Cmax values during single-dose escalation and days 1 and 8 of the repeat-dose escalation was assessed using the power model y = α × doseβ, where y denotes the PK parameter being analyzed and α depends on subject and period. Dose proportionality was assessed by estimating β along with its confidence interval. A β value of 1 implied dose proportionality.
For the repeat-dose escalation, the time-invariance ratios [AUC(0-12) on day 8 to AUC(0-∞) on day 1] and accumulation ratios [AUC(0-12) on day 8 to AUC(0-12) on day 1] were calculated and assessed by ANOVA with terms for subjects as random effect and day as fixed effect. In addition, achievement of plasma 873140 steady-state trough concentrations was assessed by calculating the 90% CI of the slope of the linear regression between day 3 and day 8 pre-morning dose concentration (i.e., C12 following evening dose) versus day for each treatment. Pharmacokinetic parameters, except Tmax and Tlag, were log-transformed prior to statistical testing. All statistical analyses were performed using SAS Version 8.2 (SAS, Cary, NC).
RESULTS
Demographics. (i) Single-dose escalation.
Twenty-four of the 30 subjects enrolled in the study were male, and 6 subjects were female. Twenty-eight subjects were white, one subject was Asian, and one subject was Hispanic. The median age, weight, height, and body mass index were 33 years (range, 18 to 55 years), 74.95 kg (range, 51.2 to 98.7 kg), 172.5 cm (range, 155 to 187 cm), and 25.6 kg/m2 (range, 19.5 to 30.6 kg/m2), respectively.
(ii) Repeat-dose escalation.
Thirty-three of the 40 subjects enrolled in the study were male, and 7 subjects were female. Thirty-six subjects were white, one subject was black, one subject was Hispanic, and two subjects were classified as “Other.” The median age, weight, height, and body mass index were 34.5 years (range, 20 to 53 years), 74.3 kg (range, 57.5 to 94 kg), 174 cm (range, 152 to 193 cm), and 25.1 kg/m2 (range, 20 to 29.3 kg/m2), respectively.
Safety. (i) Single-dose escalation.
873140 was well tolerated following single doses of 50 mg to 800 mg. The 1,200-mg dose was associated with both upper and lower gastrointestinal events and a single grade-4 serum lipase that was asymptomatic and resolved within 24 h. Twenty-six adverse events (AEs) considered by the investigator to be related to study drug were reported by 11 of 30 subjects (37%) in the single-dose phase. The most commonly reported drug-related AEs were loose stools (four subjects), headache (three subjects), abdominal pain (two subjects), and nausea (two subjects). There were no grade 3/4 AEs. The incidence of AEs was greater in subjects who received the 873140 1,200-mg single dose than in subjects who received the placebo or any of the other single doses of 873140. There were no clinically significant changes in systolic or diastolic blood pressure, heart rate, or ECG interval in subjects who received single doses of 873140.
(ii) Repeat-dose escalation.
873140 was well tolerated at doses of 200 mg to 800 mg following repeat dosing for 8 days. Seventy-one AEs that were considered by the investigator to be related to the study drug were reported by 27 of 40 subjects (68%). The most commonly reported drug-related AEs were diarrhea (14 subjects), abdominal pain (7 subjects), dizziness (7 subjects), loose stools (6 subjects), nausea (5 subjects), and lower abdominal pain (3 subjects). All adverse events resolved without treatment. There were no grade 3/4 AEs. One subject to whom the placebo was administered was withdrawn from the repeat-dose phase of the study due to repeated grade 2 increases in both alanine aminotransferase and aspartate aminotransferase, a protocol-defined endpoint. There were no clinically significant changes in systolic or diastolic blood pressure, heart rate, or ECG waveforms or intervals in subjects who received repeat doses of 873140 over 8 days.
Pharmacokinetics. (i) Single-dose escalation.
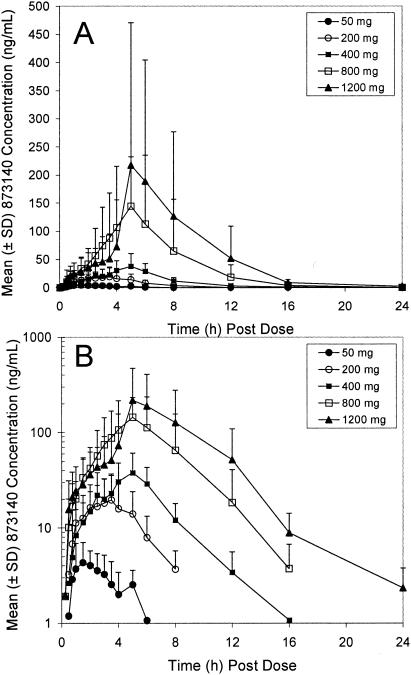
The mean plasma concentration-time profiles are shown in Fig. 1, and the resulting pharmacokinetic parameters are presented in Table 2. After a short lag time (median Tlag of 0.5 h at 50 mg to 0.13 h at 1,200 mg), 873140 plasma concentrations increased rapidly. The majority of individual 873140 plasma-concentration time profiles were characterized by the presence of multiple peaks, with the first peak generally occurring within 3 h of dosing and the second peak occurring 4 to 8 h after dosing. The median 873140 Tmax values ranged from 1.75 to 5 h over the 50-mg to 1,200-mg dose range, with the later Tmax being associated with doses of ≥400 mg. The 873140 AUC(0-last), AUC(0-∞), and Cmax values increased with dose; the increase was slightly greater than dose proportional. The geometric mean t1/2 values ranged from 1.8 to 2.9 h over the 200-to-1,200 mg dose range.
FIG. 1.
Rectilinear (A) and semilog (B) plots of mean 873140 plasma concentration-time profiles for healthy volunteers during the single-dose escalation phase.
TABLE 2.
Plasma 873140 pharmacokinetic parameters after single escalating oral doses of 873140 hydrochloride salta
| Treatment | Geometric mean for pharmacokinetic parameter indicated (95% CI)
|
|||||
|---|---|---|---|---|---|---|
| AUC(0-∞) (ng · h/ml) | Cmax (ng/ml) | Tmax (h) | Tlag (h) | t1/2 (h) | CL/F (liters/min) | |
| 50 mg (n = 8) | NC | 5.41 (3.64-8.04) | 1.75 (1.00-3.00) | 0.5 (0-1.0) | NC | NC |
| 200 mg (n = 8)b | 95.0 (58.5-154) | 18.6 (11.4-30.4) | 2.75 (1.00-3.50) | 0.38 (0-0.5) | 1.83 (1.39-2.41) | 33.0 (20.3-53.6) |
| 400 mg (n = 8) | 187 (122-288) | 39.5 (25.7-60.6) | 5.00 (2.50-6.00) | 0.25 (0-0.75) | 2.50 (1.94-3.21) | 33.5 (21.8-51.5) |
| 800 mg (n = 7) | 630 (312-1270) | 139 (64.5-298) | 5.00 (4.00-8.00) | 0.25 (0-0.25) | 2.54 (2.17-2.97) | 19.9 (9.9-40.1) |
| 1,200 mg (n = 6) | 918 (369-2282) | 190 (64.4-564) | 5.00 (5.00-8.00) | 0.13 (0-0.25) | 2.91 (1.79-4.71) | 20.8 (8.3-50.9) |
| 400 mg + food (n = 8) | 314 (204-482) | 78.0 (49.3-124) | 3.75 (2.00-6.00) | 0.5 (0.25-1.0) | 2.38 (1.90-2.98) | 20.0 (13.0-30.7) |
Dose strengths represent mg of 873140 in hydrochloride salt form. Medians and ranges are presented for Tlag and Tmax.
AUC(0-∞) and t1/2 are based on cohorts of seven subjects. NC, not calculated.
Eight subjects in the cohort that received a single 400-mg dose under fasting conditions during period 1 also received a 400-mg single dose after the standard high-fat breakfast in period 2. Single-dose administration of 873140 (400-mg dose) with a standard high-fat breakfast increased the 873140 AUC(0-∞) by 68% (ratio of GLS means, 1.68; 90% CI, 1.43 to 1.97) and the Cmax by 98% (ratio of GLS means, 1.98; 90% CI, 1.48 to 2.65).
(ii) Repeat-dose escalation.
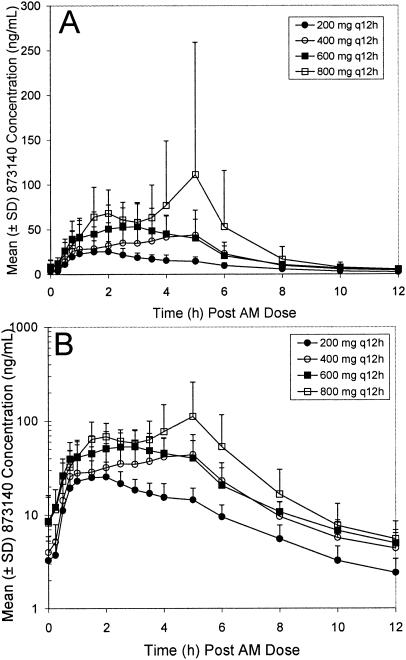
The day 1 and day 8 pharmacokinetic parameters are shown in Table 3, and the mean day 8 plasma concentration time profiles are shown in Fig. 2. A short lag time was noted following the single dose, but not after the repeat dose. After a single dose on day 1, the median 873140 Tmax values ranged from 2.75 to 5 h over the 200- to 800-mg dose range. The increase in 873140 AUC(0-last), AUC(0-∞), and Cmax values was approximately proportional (Table 3) to the increase in dose across this dose range. On day 8, plasma 873140 concentration profiles after the morning dose were characterized by rapid absorption with no observable lag time. The median Tmax tended to be earlier on repeat dosing than on single dosing and ranged from a low of 1.75 h (200 mg) to a high of 4 h (400 mg). The increase in Cmax was dose proportional, whereas the increase in AUC(0-12) and C12 values tended to be less than dose proportional. The accumulation ratios [day 8 AUC(0-12)/day 1 AUC(0-12)] for the 200-, 400-, 600-, and 800-mg BID doses were 1.52 (90% CI, 1.21 to 1.91), 1.37 (90% CI, 0.94 to 2.02), 1.13 (90% CI, 0.77 to 1.66), and 1.06 (90% CI, 0.71 to 1.59), respectively. Accumulation was greater at the lower doses than at the higher doses. The time-invariance ratios [day 8 AUC(0-12)/day 1 AUC(0-∞)] were 1.39 (90% CI, 1.09 to 1.78), 1.1 (90% CI, 0.78 to 1.52), 0.93 (90% CI, 0.61 to 1.42), and 0.97 (90% CI, 0.59 to 1.58) for the 200-, 400-, 600-, and 800-mg BID doses, respectively, and suggested that the pharmacokinetics did not change over time for the 400-, 600-, and 800-mg dose groups. For the 200-mg BID dose group, the AUC(0-12) on day 8 was 39% higher than the AUC(0-∞) on day 1, suggesting a modest time-dependent accumulation at the lower dose. Pre-morning trough plasma concentrations were similar from day 5 to day 8 for all doses, based on the statistical analysis of slope and visual inspection.
TABLE 3.
Plasma 873140 pharmacokinetic parameters after single and repeat oral doses of 873140 hydrochloride saltd
| Day and treatment | Geometric mean for pharmacokinetic parameter indicated (95% CI)
|
|||||
|---|---|---|---|---|---|---|
| AUC(0-∞) (ng · h/ml) | Cmax (ng/ml) | Tmax (h) | Tlag (h) | t1/2 (h) | CL/F (liters/min) | |
| Day 1 | ||||||
| 200 mg (n = 8)b | 90.6 (56.3-146) | 18.8 (13.31-26.42) | 2.75 (1.00-6.00) | 0.25 (0.25-0.50) | 1.97 (1.59-2.45) | 34.6 (21.5-55.7) |
| 400 mg (n = 8)b | 206 (113-377) | 34.9 (19.3-63.3) | 3.75 (2.00-5.00) | 0.13 (0-0.75) | 1.92 (1.58-2.32) | 30.4 (16.6-55.4) |
| 600 mg (n = 8)b | 292 (164-520) | 47.9 (31.4-73.1) | 5.00 (4.00-12.00) | 0.13 (0-1.00) | 2.49 (1.53-4.07) | 32.2 (16.6-55.4) |
| 800 mg (n = 8)b | 438 (181-1057) | 80.0 (39.2-163) | 5.00 (4.00-10.00) | 0.00 (0-0.50) | 1.99 (1.49-2.65) | 28.7 (11.9-69.2) |
| Day 8 | ||||||
| 200 mg BID (n = 8)c | 125 (95.2-163) | 27.3 (19.3-38.6) | 1.75 (1.00-4.00) | 0.0 | 2.48 (2.01-3.05) | 25.2 (19.2-32.9) |
| 400 mg BID (n = 7)c | 228 (156-333) | 55.2 (38.3-79.5) | 4.00 (2.00-5.00) | 0.0 | 2.45 (2.05-2.92) | 27.5 (18.9-40.1) |
| 600 mg BID (n = 8)c | 283 (204-392) | 64.5 (47.5-87.5) | 2.75 (2.00-5.00) | 0.0 | 2.23 (1.56-3.19) | 33.3 (24.0-46.1) |
| 800 mg BID (n = 8)c | 409 (253-660) | 123 (67.8-223) | 2.02 (1.50-5.00) | 0.0 | 2.09 (1.59-2.76) | 30.7 (19.0-49.5) |
Dose strengths represent mg of 873140 in hydrochloride salt form. Medians and ranges are presented for Tlag and Tmax.
AUC(0-∞) and t1/2 values are based on cohorts of 5 (400 mg, 600 mg), 6 (200 mg), or 7 (800 mg) subjects.
t1/2 values are based on cohorts of 5 (200, 400, 800 mg BID) or 4 (600 mg BID) subjects.
Data for day 1 are for single doses; data for day 8 are for repeat (twice daily) doses.
FIG. 2.
Rectilinear (A) and semilog (B) plots of mean 873140 plasma concentration-time profiles for healthy volunteers during the repeat-dose escalation phase.
DISCUSSION
CCR5 is the coreceptor used by the majority of HIV strains detected at the time of transmission and throughout progression (6) and is considered an attractive target for HIV therapy. CCR5 antagonists target a discrete step in the viral entry pathway. HIV entry into the host CD4+ T cells involves a cascade of molecular interactions between the virion envelope glycoprotein (Env) and host cell surface receptors. The gp120 subunit of Env binds to CD4, and it is the subsequent binding of HIV to the host cell's coreceptors, CCR5 or CXCR4, which initiates a conformational change leading to membrane fusion into the host cell (2). Allosteric binding of a CCR5 antagonist results in a receptor conformation that the virus cannot bind, thus interfering with the fusion process.
This study describes the first administration of 873140, a potent CCR5 antagonist, to humans. A favorable short-term safety and tolerability profile was observed in this study. 873140 was well tolerated at single doses from 50 mg to 800 mg and repeat doses of 200 mg to 800 mg twice daily for 7 days. The 1,200-mg single dose was associated with tolerability issues, primarily gastrointestinal in nature. The most commonly reported AEs across all doses were minor gastrointestinal complaints that resolved without treatment interruption. There were no significant changes in resting ECG waveform or intervals with 873140 administration. This is of note, as prolongation of the QT interval in both healthy volunteers and HIV-infected subjects was demonstrated with a previous investigational agent in this class, Sch-C (3). However, as with any new class, the development of CCR5 antagonists, including 873140, will involve long-term monitoring to establish safety and tolerability in chronic dosing.
The pharmacokinetic properties of 873140 were characterized by the rapid appearance of detectable plasma concentrations, a prolonged absorption phase, a relatively high apparent clearance (CL/F), and an apparent elimination phase half-life of approximately 2 to 3 h. A high-fat meal was shown to approximately double the compound's systemic exposure. The intersubject variability in AUC and Cmax tended to be high, with higher doses showing greater variability than low doses and single doses showing greater variability than repeat doses. Intersubject variability in AUC and Cmax did not change with the administration of food, which may point toward first-pass metabolism rather than gastrointestinal absorption as the main source of variability. In vitro studies have shown that 873140 is substrate of CYP3A, the most abundant CYP450 enzyme subfamily in human liver and intestine. High interindividual variability in the expression of CYP3A, which may contribute to the high variability 873140 pharmacokinetics, has been noted (4). CYP3A intestinal and liver metabolism also likely contributes to the relatively high CL/F of 873140, and clinical studies investigating the interaction of 837140 with CYP3A inhibiting and inducing antiretrovirals are needed.
Statistical analysis of the trough 873140 plasma concentrations indicated that steady-state trough concentrations were reached by day 5. Time-invariant pharmacokinetics were observed for the three higher dose groups; however, for the 200-mg dose group, the mean AUC(0-τ) on day 8 was 39% higher than the AUC(0-∞) on day 1. The delay in time to steady-state and greater-than-expected accumulation at the low dose was surprising given the estimated 2-h half-life of 873140 and may be a reflection of high intersubject variability or some unknown time-dependent mechanism.
The unique characteristics of CCR5 antagonists pose new challenges for predicting antiviral activity based on plasma concentrations. Unlike traditional antiretroviral classes, CCR5 antagonists target a host receptor and not the virus. In addition, the activity of 873140 and other compounds in the CCR5 class may be a function of prolonged receptor binding. For example, the offset rate of 873140 from CCR5 has been shown to be in excess of 150 h, while the plasma elimination half-life is only approximately 3 h (J. Demarest, S. Sparks, C. Watson, C. McDanal, S. Shibayama, and T. Kenakin, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-211, 2004). Prolonged receptor binding may explain the continued antiviral effect observed with the investigational CCR5 antagonist SCH-C (25 mg) for up to 4 days after cessation of therapy (J. Reynes, R. Rouzier, T. Kanouni, V. Baillat, B. Baroudy, A. Keung, C. Hogan, M. Markowitz, and M. Laughlin, 9th Conf. Retrovir. Opportunistic Infect., abstr. 1, 2002). Therefore, the antiviral activity of durably bound CCR5 antagonists may be more closely related to Cmax or AUC than trough concentrations, as seen with HIV protease inhibitors and nonnucleoside reverse transcriptase inhibitors (1). Plasma concentrations above the protein binding corrected the IC90 (∼24 ng/ml, estimated as four times the IC50 in peripheral blood mononuclear cells), suggesting that therapeutic concentrations can be achieved with oral doses of ≥200 mg BID. The specific 873140 pharmacokinetic parameter that best predicts antiviral activity remains to be determined. Ongoing studies with 873140 in HIV-infected patients have been designed to specifically address this issue.
The development of entry inhibitors, such as CCR5 antagonists, will offer new avenues for constructing HAART regimens and for HIV therapy sequencing. The presence of escape mutations associated with reverse transcriptase or protease inhibitor failures should not limit the efficacy of CCR5 antagonists, such as 873140, suggesting that this class of drugs will be of use in both antiretroviral-naïve and treatment-experienced patients (7; M. Westby, C. Napier, R. Mansfield, D. Collins, W. Huang, N. Hellman, Y. Lie, and M. Perros, XII Int. HIV Drug Resist. Workshop, abstr. 23, 2003). In addition, in vitro studies suggest potential synergy among entry inhibitors, providing an opportunity for combinations of entry inhibitors as well as use of entry inhibitors in conjunction with other antiretroviral therapy classes, such as reverse transcriptase or protease inhibitors (8, 9). Future combinations may provide opportunities for improved sequencing and improved antiviral durability.
REFERENCES
- 1.Acosta, E. P., and J. G. Gerber. 2002. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res. Hum. Retrovir. 18:825-834. [DOI] [PubMed] [Google Scholar]
- 2.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry tropism and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 3.Este, J. A. 2002. Sch-351125 and Sch-350634. Curr. Opin. Investig. Drugs 3:379-383. [PubMed] [Google Scholar]
- 4.Gibbs, M. A., and N. A. Hosea. 2003. Factors affecting the clinical development of cytochrome P450 3A substrates. Clin. Pharmacokinet. 42:969-984. [DOI] [PubMed] [Google Scholar]
- 5.Maeda, K., H. Nakata, T. Miyakawal, H. Ogata, Y. Takaoka, S. Shibay, K. Sagawa, D. Fukushima, J. Moravek, Y. Koyanagi, and H. Mitsuya. 2004. Spirodiketopiperazine-based CCR5 inhibitor which preserves C chemokine/CCR5 interactions and exerts potent activity against human immunodeficiency virus type 1 in vitro. J. Virol. 78:8654-8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philpott, S. M. 2003. HIV-1 coreceptor usage, transmission, and disease progression. Curr. HIV Res. 1:217-227. [DOI] [PubMed] [Google Scholar]
- 7.Pierson, T. C., R. W. Doms, and S. Pohlmann. 2004. Prospects of HIV-1 entry inhibitors as novel therapeutics. Rev. Med. Virol. 14:255-270. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay, C. L., F. Giguel, C. Kollmann, Y. Guan, T. C. Chou, B. M. Baroudy, and M. S. Hirsch. 2002. Anti-human immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob. Agents Chemother. 46:1336-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay, C. L., C. Kollmann, F. Giguel, T. C. Chou, and M. S. Hirsch. 2000. Strong in vitro synergy between the fusion inhibitor T-20 and the CXCR4 blocker AMD-3100. J. Acquir. Immune Defic. Syndr. 25:99-102. [DOI] [PubMed] [Google Scholar]